Abstract
A halophilic strain W33 showing lipolytic activity was isolated from the saline soil of Yuncheng Salt Lake, China. Biochemical and physiological characterization along with 16S rRNA gene sequence analysis placed the isolate in the genus Idiomarina. The extracellular lipase was purified to homogeneity by 75 % ammonium sulphate precipitation, DEAE-Sepharose anion exchange and Sephacryl S-200 gel filtration chromatography. The molecular mass of the purified lipase was estimated to be 67 kDa by SDS-PAGE. Substrate specificity test indicated that it preferred long-chain p-nitrophenyl esters. Optimal lipase activity was found to be at 60 °C, pH 7.0–9.0 and 10 % NaCl, and it was highly active and stable over broad temperature (30–90 °C), pH (7.0–11.0) and NaCl concentration (0–25 %) ranges, showing excellent thermostable, alkali-stable and halotolerant properties. Significant inhibition by diethyl pyrocarbonate and phenylarsine oxide was observed, implying histidine and cysteine residues were essential for enzyme catalysis. In addition, the lipase displayed high stability and activity in the presence of hydrophobic organic solvents with log P ow ≥ 2.13. The free and immobilized lipases produced by Idiomarina sp. W33 were applied for biodiesel production using Jatropha oil, and about 84 and 91 % of yields were achieved, respectively. This study formed the basic trials conducted to test the feasibility of using lipases from halophile for biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipases (EC 3.1.1.3) are ubiquitous hydrolytic enzymes that hydrolyze triglycerides to fatty acids and glycerol at the oil–water interface, and catalyze the reverse reaction in non-aqueous solvent systems (Teo et al. 2003). They have diverse applications in many biotechnological fields such as food, dairy, pharmaceutical and agrochemical industries (Jaeger et al. 1999). Most industrial lipases known to date are mostly obtained from bacteria and fungi (Hough and Danson 1999). However, most industrial processes are commonly carried out under harsh conditions, such as high temperature, pH, salinity, and in the presence of organic solvents, which may lead to the inactivation of the available enzymes. In this sense, novel lipases with better catalytic efficiency and specific properties suitable for harsh reaction conditions are highly demanded (Lima et al. 2004).
Halophilic microorganisms are extremophiles which are able to live in saline environments. They constitute an interesting group of microorganisms that are reported to be good candidates for selecting novel enzymes (Van den Burg 2003). Enzymes from these prokaryotes can function optimally under extreme conditions, making them robust biocatalysts with potential applications under harsh conditions (Margesin and Schinner 2011). Since salt tends to greatly reduce water activity, lipases from halophile may become the choice for biocatalytic processes performed in low water activity environments like aqueous/organic or non-aqueous media (Sellek and Chaudhuri 1999). Lipase production has been widely reported in halophile, however, only a few lipases were purified and characterized (Daoud et al. 2013; Pérez et al. 2011). Meanwhile, their potential application in biotechnological processes is still an open question.
During the past decades, biodiesel has received considerable attention as an alternative energy because of its favorable properties, environmental benefits and the fact that it is derived from the biological resources (Azocar et al. 2010). Biodiesel could be produced by chemical or enzymatic methods according to the catalysts employed in the process. Comparing with chemical method, enzymatic process using lipases was demonstrated to be more effective (Antczak et al. 2009). At present, biodiesel is mainly produced from edible oils (more than 95 %), such as soybean oil, rapeseed oil and palm oil, which leads to global imbalance to the food supply and demand market. Thus, using low cost non-edible oils for biodiesel production attracted much attention (Yu et al. 2013). Among non-edible oils, Jatropha curcas, which is toxic owing to the presence of carcinogenic phorbol esters, has great potential for biodiesel production (Abdulla et al. 2011).
In this paper, a halophilic strain W33 showing lipolytic activity was isolated and identified. The purification and characterization of its extracellular lipase, especially its activity and stability in the presence of organic solvents, were reported. Moreover, the lipase was applied for biodiesel production using the seed oil of Jatropha curcas.
Materials and methods
Strain isolation, identification and lipase production
The strain W33 was isolated from the saline soil of Yuncheng Salt Lake, China. Production of extracellular lipase was performed in the complex medium (CM) containing (g l−1): casein peptone 7.5; yeast extract 10.0; sodium citrate 3.0; MgSO4·7H2O 20.0; KCl 2.0; FeSO4·7H2O 0.01; NaCl 70.0 and pH 7.0. Morphological, physiological and biochemical characteristics of strain W33 were studied either on CM agar plate (2 % agar, w/v) or in CM broth. 16S rRNA gene was amplified using the general bacterial primers 8F and 1492R, and has been submitted to GenBank with the accession number JN112010. The strain was deposited at China Center of Industrial Culture Collection (CICC) with the registration number CICC 10891.
Lipase activity assay
Lipase activity was determined using p-nitrophenyl palmitate (p-NPP) as substrate according to Winkler and Stuckmann (1979), with some modifications. The substrate p-NPP was dissolved in 2-propanol (1 ml) to give a final concentration of 1 mM and mixed with 9 ml of Tris–HCl buffer (10 mM, pH 8.0). After pre-incubation for 5 min, the reaction was initiated by adding 20 μl of appropriately diluted enzyme solution to 240 μl of substrate solution, and incubation was carried out at 60 °C for 10 min. Following the addition of 100 μl of Na2CO3 solution (0.1 M) to stop the reaction, the amount of p-nitrophenol (p-NP) released was measured at 410 nm against a blank. One unit (U) was defined as the amount of enzyme liberating 1 μmol of p-NP per minute under the standard assay conditions.
Lipase purification
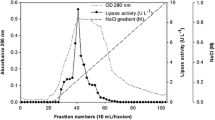
Culture supernatant obtained by centrifugation was treated with solid ammonium sulphate to 60 % saturation and stirred overnight at 4 °C. The precipitate collected was dissolved in buffer A (10 mM Tris–HCl containing 10 % NaCl, pH 8.0). After dialysis against buffer A overnight, the sample was applied to a DEAE-Sepharose column (2.5 cm × 30 cm). The column was eluted with a linear gradient of 0.1–0.8 M NaCl in Tris–HCl buffer at a flow rate of 0.5 ml min−1. Active fractions showing lipase activity were pooled and concentrated by freeze-drying. The resulting concentrate was dissolved in buffer A, and then loaded on a Sephacryl S-200 gel filtration column (1.8 cm × 100 cm). The sample was eluted with buffer A at a flow rate of 1.0 ml min−1. The protein elution profile was recorded spectrophotometrically at 280 nm. Fractions with lipase activity were pooled and concentrated using a Vivaspin centrifugal concentrator. Protein concentration was determined by the method of Bradford (1976), using bovine serum albumin as standard.
SDS-PAGE
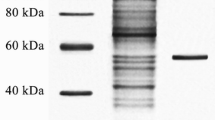
Molecular mass of the purified lipase was determined by SDS-PAGE using a 12 % cross-linked polyacrylamide gel, according to the method of Laemmli (1970). After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250.
Substrate specificity
To determine the substrate specificity of the lipase, p-nitrophenyl (p-NP) esters with different chain lengths (acetate, C2: butyrate, C4: hexanoate, C6: octanoate, C8: decanoate, C10: laurate, C12: myristate, C14: palmitate, C16: stearate C18) were used as the substrate with the final concentration of 1 mM, respectively, and then the released amount of p-nitrophenol was measured at 410 nm using p-NPP method described above. Data were expressed as the percentage of the observed maximal activity obtained with p-NPM (C14).
Effects of metal ions and chemical reagents
Effects of different metal ions and chemical reagents [ethylenediaminetetraacetic acid (EDTA), phenylmethylsulfonyl fluoride (PMSF), phenylarsine oxide (PAO), diethyl pyrocarbonate (DEPC), β-mercaptoethanol] on the lipase activity were examined by pre-incubating the enzyme with them in 10 mM phosphate buffer (pH 7.0) at 30 °C for 1 h, respectively. Residual activity was determined under the standard assay conditions. Lipase activity in the absence of any additives was taken as 100 %.
Effects of temperature, pH and NaCl concentration on lipase activity and stability
The temperature optimum of the purified lipase was determined at temperatures from 20 to 90 °C. To assess its thermostability, the enzyme was pre-incubated in Tris–HCl buffer containing 10 % NaCl (pH 8.0) under different temperatures for 24 h, and then residual activity was measured using p-NPP method. Optimal pH for the lipase activity was measured at different pH (5.0–11.0). The buffers (10 mM) used were as follows: sodium acetate (pH 5.0–5.5), sodium phosphate (pH 6.0–7.5), Tris–HCl (pH 8.0–9.0) and glycine–NaOH (pH 9.5–11.0). The pH stability was examined by pre-incubating the enzyme at different pH at 60 °C for 24 h, and residual activity was measured as described above. Effect of NaCl was tested by measuring the lipase activity in the reaction mixture containing different NaCl concentrations (0–25 %). To determine its halostability, the lipase was pre-incubated in Tris–HCl buffer (10 mM, pH 8.0) containing different NaCl concentrations at 60 °C for 24 h. Residual activity was measured under the standard assay conditions.
Effect of organic solvents on lipase activity and stability
Effect of organic solvents with different Log P ow values at 50 % (v/v) concentration on the lipase activity was determined by incubating the enzyme in different organic solvents at 30 °C with shaking, respectively. At different time intervals, aliquots were withdrawn and residual activity was measured under the standard conditions. If residual activity was more than 50 % after 10 days, half-life was taken as “>10 days”. While activity was less than 50 % after 1 days, half-life was taken as “<1 days”.
Immobilization of the purified lipase
The immobilization of the lipase was carried out as described by Ji et al. (2010). Briefly, one gram of macroporous anion exchange resin (Amberlite IRA-93, America) was added to 5 ml of solution containing 0.5 mg of purified lipase (about 60.2 U), and the mixture was stirred for 12 h at 20 °C with shaking at 150 rpm. The immobilized lipase was recovered through centrifugation, dried in a vacuum desiccator, and stored at 4 °C. The activity of the immobilized lipase was determined to be about 30.2 U g−1 using the p-NPP method.
Production of biodiesel catalyzed by the lipase
Jatropha oil was purchased from Shuyang Co. Ltd. (Henan, China). The composition of main fatty acids in this oil was reported by the manufacturer to be palmitic acid 4.3 %, oleic acid 61.2 %, linoleic acid 19.7 % and linolenic acid 10.2 %. It was used for lipase-catalyzed transesterification as described by Kawakami et al. (2011), with some modifications. Briefly, a typical reaction mixture was prepared by mixing 6.7 g Jatropha oil and 0.54 g methanol (molar ratio of methanol to oil, 4:1; and water content, 0.7 % (w/w) based on the total mass) in 20 ml screw-capped vials. The mixture was stirred at 180 rpm on a shaking incubator at 60 °C. The reaction was initiated by the addition of 0.25 mg free lipase powder or 1.0 g immobilized lipase. Aliquots (20 μl) of the reaction mixture were withdrawn at different time intervals, and then diluted with n-heptane for GC analysis.
The methyl ester contents of the reaction mixture were analyzed using a gas chromatograph (GC-14B, Shimadzu, Japan) equipped with a low polarity capillary column DB-5 (0.5 μm × 0.25 mm × 30 m, Agilent), according to the method of Kawakami et al. (2011). Concentrations of methyl esters of palmitic, stearic, oleic, and linoleic acids were quantified using calibration curves prepared by analyzing standard solutions of mixed methyl esters. The biodiesel yield was determined as a ratio of total concentration of these four methyl esters to total concentration of corresponding fatty acids in the initial reaction mixture.
Results and discussion
Strain identification and production of extracellular lipase
The strain W33 is a Gram-negative, strictly aerobic and rod-shaped bacterium. Colonies are light-yellow on CM agar plate. It is able to grow in media containing 0.5–25 % (w/v) NaCl with an optimum at 7 % NaCl. No growth was observed in the absence of NaCl. Thus, this strain was considered to be a halophilic bacterium (Kushner and Kamekura 1988). Optimal growth was observed at 37–40 °C and pH 7–8. Nitrate reduction, catalase, H2S production, tween 20 and starch hydrolysis are positive, while methyl red test, indole production, Voges–Proskauer test, oxidase, and casein hydrolysis are negative. Acid is produced from glucose, sucrose, starch, mannitol and inositol. Phylogenetic analysis based on 16S rRNA gene sequence comparisons revealed the strain W33 belonged to the genus Idiomarina, and was most close to Idiomarina loihiensis L2TRT (98.6 % 16S rRNA gene sequence similarity) (Fig. 1).
Phylogenetic tree based on 16S rRNA gene sequence of the strain W33 to other members of the genus Idiomarina. Accession numbers of the sequences used in this study are shown in parentheses after the strain designation. Numbers at nodes are percentage bootstrap values based on 1,000 replications. Bar 0.01 substitutions per nucleotide position
The extracellular lipase was produced from the early-exponential phase of strain growth (4 h), and reached a maximum level during the early-stationary phase (26 h). Moreover, lipase production was found to be strongly influenced by the salinity of the culture medium. Maximal lipase production (about 11.8 U ml−1) occurred when 10 % NaCl or 5 % Na2SO4 was added. These results clearly revealed the salt appeared to be a prerequisite for lipase production. Similar behavior was reported in other halophiles with the capability of producing extracellular enzymes (Amoozegar et al. 2003; Coronado et al. 2000).
Lipase purification
The extracellular lipase was purified by ammonium sulphate precipitation, DEAE-Sepharose anion exchange chromatography and Sephacryl S-200 gel filtration chromatography. It was purified 6.5-fold with recovery of 15.9 % and specific activity of 120.4 U mg−1 protein (Table 1). The purified lipase showed a single protein band on SDS-PAGE with an estimated molecular mass of 67 kDa (Fig. 2, lane 2), corresponding with that determined by gel filtration. Together these results indicated that the lipase was a single polypeptide chain. The molecular masses of microbial lipases reported previously mainly fell into two groups, 30–45 and 50–60 kDa (Ji et al. 2010). Therefore, the present lipase could be related to the higher molecular mass lipases.
Substrate specificity
The substrate specificity test showed p-NPM (C14) was most efficiently hydrolyzed by the lipase (Fig. 3). A trend of preferential specificity towards p-NP esters with acyl chain lengths longer than C10 is clearly evident. Enzyme activity declined with substrates having shorter chain-length, reaching 50.2 % activity with p-NPH (C8), 27.6 % activity with p-NPH (C6), 21.4 % with p-NPB (C4) and 15.2 % with p-NPA (C2), respectively. This preference for long-chain fatty acid esters was shown in most lipases, such as the lipases from Natronococcus sp. (Boutaiba et al. 2006) and Penicillium sp. DS-39 (Dheeman et al. 2011).
Effects of temperature, pH and NaCl concentration
As shown in Fig. 4a, the lipase from strain W33 displayed optimal activity at 60 °C. Most halophilic lipases were reported to show maximal activities between 45 and 65 °C (Boutaiba et al. 2006; Camacho et al. 2009; Ozcan et al. 2009). Thus, the present lipase can be rated among the higher thermoactive lipases. Moreover, excellent thermostability was observed under temperatures ranging from 20 to 80 °C, and the enzyme retained more than 50 % activity after treatment at 90 °C for 24 h. Such high stability in this temperature range has been previously described in the hyper-thermostable lipase from Pseudomonas sp. (Rathi et al. 2000). However, other thermostable lipases were reported to be neither active nor stable at temperatures above 70 °C (Dheeman et al. 2010, 2011; Ji et al. 2010). Thermostability is a desirable property in lipases for application in industrial processes operating under high temperatures (Janssen et al. 1994). The excellent thermostability might be due to the presence of NaCl, which had the capacity to increase the thermostability of enzymes from halophile (Boutaiba et al. 2006).
Effect of temperature (a), pH (b) and NaCl concentration (c) on activity (solid lines) and stability (dotted lines) of the purified lipase. Relative activity was defined as the percentage of activity detected with respect to the maximum enzyme activity. For determining the stability, the lipase activity without any treatment was taken as 100 %. Data are the average of three independent experiments. See “Materials and methods” for further details
As shown in Fig. 4b, optimal pH for the lipase activity was 7.0–9.0. Meanwhile, it was highly stable in the pH range of 7.0–11.0 after 24-h incubation at 60 °C, indicating the alkali-stable nature. Boutaiba et al. (2006) found that the highest lipolytic activity of strain Natronococcus TC6 was at pH 7.0; while in another study, Ozcan et al. (2009) reported optimal lipase activities of five halophilic archaeal strains were observed at pH 8.0. Alkaline enzymes have received considerable attention because of their tremendous potentiality in industrial processes (Chakraborty et al. 2011).
Lipase activity was measured in the presence of different NaCl concentrations, and optimal activity was found to be at 10 % NaCl (Fig. 4c). Meanwhile, the lipase showed strong tolerance to NaCl, as it was highly active and stable over a broad NaCl concentration range from 2.5 to 25 %. Similar extreme halotolerance has been reported in other lipases from halophiles (Boutaiba et al. 2006; Camacho et al. 2009). These results are quite interesting since they demonstrated that the lipase was completely adapted to high salinities and probably optimized to be fully efficient only when salt was present. In fact, many halophilic enzymes required the presence of NaCl or KCl for optimal activity and stability (Mevarech et al. 2000). Unlike other lipolytic enzymes from halophile, which lost their activity when exposed to low salt concentrations, the lipase from strain W33 retained 70 % of its total activity in the absence of NaCl (Boutaiba et al. 2006; Ozcan et al. 2009).
Effect of metal ions and chemical reagents
As shown in Table 2, the lipase activity was significantly stimulated in the presence of Ca2+ (131.7 %). Many lipases were reported to display enhanced activity in the presence of Ca2+. A possible explanation for this phenomenon is that Ca2+ binds to the active site of the lipase and changes the conformation of the protein (Rahman et al. 2005). Other metal ions tested, such as Mg2+, Fe2+, Cu2+, had little effects on the lipase activity.
After incubation with EDTA, about 91.9 % activity retained, indicating the lipase was not a metalloenzyme. The presence of β-mercaptoethanol and PMSF, led to only marginal reduction in lipase activity. However, significant inhibition by DEPC (a histidine modifier) and PAO (a cysteine modifier) on the lipase activity was observed, implying histidine and cysteine residues were essential for enzyme function. Such structural characteristics have not been reported in other previously described lipases.
Effect of organic solvents
High activity and stability of lipases in organic solvents is an essential prerequisite for applications in organic synthesis (Doukyu and Ogino 2010); hence, activity and stability in organic solvents are considered novel attributes in a lipase. As described above, the lipase from strain W33 was active and stable under high salinities, making it quite possible to remain stable in organic solvents.
Effect of various organic solvents on the activity and stability of the purified lipase is shown in Table 3. More than 80 % activity retained in the presence of glycerol, DMSO, toluene, cyclohexane or n-hexane compared to the control. Interestingly, n-hexane even increased the lipase activity to 110.1 %. The activation of lipase could be explained that organic solvent molecules could interact with hydrophobic amino acid residues present in the lid that covers the catalytic site of the enzyme, thereby maintaining the enzyme in its open conformation and conducting to catalyze (Rúa et al. 1993). Although there is a tendency for hydrophilic solvents to cause more significant enzyme inactivation than hydrophobic solvents (Doukyu and Ogino 2010), the lipase from strain W33 retained more than 60 % activity in the presence of hydrophilic organic solvents, such as methanol and acetonitrile (Table 3).
Furthermore, the lipase displayed considerable stability in the presence of hydrophobic organic solvents (log P ow 2.13–4.7), with longer half-lives in these solvents than in the absence of organic solvent or in the presence of hydrophilic solvents (Table 3). These results were in agreement with the general behavior of most lipases, which showed strong tolerance towards hydrophobic solvents with significant instability in hydrophilic solvents (Dheeman et al. 2011; Lima et al. 2004).
Application of the lipase for biodiesel production
Considering that the lipase was stable in organic solvents and that its preferential specificity towards long-chain substrates, it was deemed likely to be very suitable for biodiesel production. Short-chain alcohols, especially methanol, have poor solubility in oils, and therefore, a new liquid phase appears in the system leading to an inactivation of the enzyme and decreased yields of ester. Therefore, to minimize the enzyme inhibition, methanol was added in four steps at an interval of 6 h as described by Yu et al. (2013).
As shown in Fig. 5, biodiesel production with about 84 and 91 % yields was achieved using Jatropha oil by free and immobilized lipase, respectively. The immobilized lipase was found to catalyze biodiesel synthesis with higher yield, and this might be due to its larger surface area (Noureddini et al. 2005). Free lipases were known to have mass transfer problem since they form aggregates in low water media (Shah and Gupta 2007). In contrast to other lipases for biodiesel production (Ji et al. 2010; Sivaramakrishnan and Muthukumar 2012), the lipase from strain W33 showed relatively higher efficiency. Similarly, biodiesel production by the immobilized lipase from Burkholderia cepacia reached more than 90 % yield using Jatropha oil at the optimized conditions (Kawakami et al. 2011). However, the biodiesel yields obtained in this study are for an un-optimized process, and there are ample scopes to improve the efficiency of the process to obtain higher biodiesel yield. This study formed the basic trials conducted to test the feasibility of using lipases from halophile for biodiesel production.
Lipases from different sources have significant variation in properties, especially with respect to regiospecificity, substrate specificity, thermostability, pH optimum and kinetics in non-aqueous system (Gupta et al. 2004). A broad spectrum of substrate utilization coupled with enantioselectivity and enhanced efficiency in non-aqueous media is always desirable in new lipases. In this study, the lipase from Idiomarina sp. W33 displayed excellent thermostable, alkali-stable, halotolerant and organic solvent-tolerant properties. In addition, the lipase has been shown to be potentially useful for biodiesel production. These results make the lipase more potentially valuable for biotechnological applications in non-aqueous catalysis.
References
Abdulla R, Chan ES, Ravindra P (2011) Biodiesel production from Jatropha curcas: a critical review. Crit Rev Biotechnol 31:53–64
Amoozegar MA, Malekzadeh F, Malik KA (2003) Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J Microbiol Methods 52:353–359
Antczak MS, Kubiak A, Antczak T, Bielecki S (2009) Enzymatic biodiesel synthesis-key factors affecting efficiency of the process. Renew Energ 34:1185–1194
Azocar L, Ciudad G, Heipieper HJ, Navia R (2010) Biotechnological processes for biodiesel production using alternative oils. Appl Microbiol Biotechnol 88:621–636
Boutaiba S, Bhatnagar T, Hacene H, Mitchell DA, Baratti JC (2006) Preliminary characterization of a lipolytic activity from an extremely halophilic archaeon, Natronococcus sp. J Mol Catal B Enzym 41:21–26
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Camacho RM, Mateos JC, González-Reynoso O, Prado LA, Córdova J (2009) Production and characterization of esterase and lipase from Haloarcula marismortui. J Ind Microbiol Biotechnol 36:901–909
Chakraborty S, Khopade A, Biao R, Jian W, Liu XY, Mahadik K, Chopade B, Zhang LX, Kokare C (2011) Characterization and stability studies on surfactant, detergent and oxidant stable α-amylase from marine haloalkaliphilic Saccharopolyspora sp. A9. J Mol Catal B Enzym 68:52–58
Coronado M, Vargas C, Hofemeister J, Ventosa A, Nieto JJ (2000) Production and biochemical characterization of an alpha-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol Lett 183:67–71
Daoud L, Kamoun J, Ali MB, Jallouli R, Bradai R, Mechichi T, Gargouri Y, Ali YB, Aloulou A (2013) Purification and biochemical characterization of a halotolerant Staphylococcus sp. extracellular lipase. Int J Biol Macromol 57:232–237
Dheeman DS, Frias JM, Henehan GT (2010) Influence of cultivation conditions on the production of a thermostable extracellular lipase from Amycolatopsis mediterranei DSM 43304. J Ind Microbiol Biotechnol 37:1–17
Dheeman DS, Antony-Babu S, Frias JM, Henehan GTM (2011) Purification and characterization of an extracellular lipase from a novel strain Penicillium sp. DS-39 (DSM 23773). J Mol Catal B Enzym 72:256–262
Doukyu N, Ogino H (2010) Organic solvent tolerant enzymes. Biochem Eng J 48:270–282
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Hough DW, Danson DJ (1999) Extremozymes. Curr Opin Chem Biol 3:39–46
Jaeger KE, Dijkstra BW, Reetz MT (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Ann Rev Microbiol 53:315–351
Janssen PH, Monk CR, Morgan HW (1994) A thermophilic, lipolytic Bacillus sp., and continuous assay of its p-nitrophenyl-palmitate esterase activity. FEMS Microbiol Lett 120:195–200
Ji Q, Xiao S, He B, Liu X (2010) Purification and characterization of an organic solvent-tolerant lipase from Pseudomonas aeruginosa LX1 and its application for biodiesel production. J Mol Catal B Enzym 66:264–269
Kawakami K, Oda Y, Takahashi R (2011) Application of a Burkholderia cepacia lipase-immobilized silica monolith to batch and continuous biodiesel production with a stoichiometric mixture of methanol and crude Jatropha oil. Biotechnol Biofuels 4:42
Kushner D, Kamekura M (1988) Physiology of halophilic eubacteria. In: Rodriguez-Valera F (ed) Halophilic Bacteria. CRC Press, Boca Raton, pp 109–138
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lima VMG, Krieger N, Mitchell DA, Fontana JD (2004) Activity and stability of a crude lipase from Penicillium aurantiogriseum in aqueous media and organic solvents. Biochem Eng J 18:65–71
Margesin R, Schinner F (2011) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Mevarech M, Frolow F, Gloss LM (2000) Halophilic enzymes: proteins with a grain of salt. Biophys Chem 86:155–164
Noureddini H, Gao X, Philkana RS (2005) Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour Technol 96:769–777
Ozcan B, Ozyilmaz G, Cokmus C, Caliskan M (2009) Characterization of extracellular esterase and lipase activities from five halophilic archaeal strains. J Ind Microbiol Biotech 36:105–110
Pérez D, Martín S, Fernández-Lorente G, Filice M, Guisán JM, Ventosa A, Mellado E (2011) A novel halophilic lipase, LipBL, showing high efficiency in the production of eicosapentaenoic acid (EPA). PLoS One 6:e23325
Rahman RN, Baharum SN, Basri M, Salleh AB (2005) High-yield purification of an organic solvent-tolerant lipase from Pseudomonas sp. strain S5. Anal Biochem 341:267–274
Rathi P, Bradoo S, Saxena RK, Gupta R (2000) A hyper-thermostable, alkaline lipase from Pseudomonas sp. with the property of thermal activation. Biotechnol Lett 22(6):495–498
Rúa L, Díaz-Mauriño T, Fernández VM, Otero C, Ballesteros A (1993) Purification and characterization of two distinct lipases from Candida cylindracea. Biochim Biophys Acta 1156:181–189
Sellek GA, Chaudhuri JB (1999) Biocatalysis in organic media using enzymes from extremophiles. Enzyme Microb Technol 25:471–482
Shah S, Gupta MN (2007) Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochem 42:409–414
Sivaramakrishnan R, Muthukumar K (2012) Isolation of thermo-stable and solvent-tolerant Bacillus sp. lipase for the production of biodiesel. Appl Biochem Biotechnol 166:1095–1111
Teo JWP, Zhang LH, Poh CL (2003) Cloning and characterization of a novel lipase from Vibrio harveyi strain AP6. Gene 312:181–188
Van den Burg B (2003) Extremophiles as a source for novel enzymes. Curr Opin Microbiol 6:213–218
Winkler UK, Stuckmann M (1979) Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol 138:663–670
Yu XW, Sha C, Guo YL, Xiao R, Xu Y (2013) High-level expression and characterization of a chimeric lipase from Rhizopus oryzae for biodiesel production. Biotechnol Biofuels 6:29
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Grants No. 31300002), Natural Science Fund of Shanxi Province (Grants No. 2011021031-4) and PhD Start-up Foundation of Yuncheng University (Grants No. YQ-2011043).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Huang.
Rights and permissions
About this article
Cite this article
Li, X., Qian, P., Wu, SG. et al. Characterization of an organic solvent-tolerant lipase from Idiomarina sp. W33 and its application for biodiesel production using Jatropha oil. Extremophiles 18, 171–178 (2014). https://doi.org/10.1007/s00792-013-0610-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0610-0