Abstract
Extremophilic microorganisms have adopted a variety of ingenious strategies for survival under high or low temperature, extreme pressure, and drastic salt concentrations. A novel application area for extremophiles is the use of “extremolytes,” organic osmolytes from extremophilic microorganisms, to protect biological macromolecules and cells from damage by external stresses. In extremophiles, these low molecular weight compounds are accumulated in response to increased extracellular salt concentrations, but also as a response to other environmental changes, e.g., increased temperature. Extremolytes minimize the denaturation of biopolymers that usually occurs under conditions of water stress and are compatible with the intracellular machinery at high (>1 M) concentrations. The ectoines, as the first extremolytes that are produced in a large scale, have already found application as cell protectants in skin care and as protein-free stabilizers of proteins and cells in life sciences. In addition to ectoines, a range of extremolytes with heterogenous chemical structures like the polyol phosphates di-myoinositol-1,1′-phosphate, cyclic 2,3-diphosphoglycerate, and α-diglycerol phosphate and the mannose derivatives mannosylglycerate (firoin) and mannosylglyceramide (firoin-A) were characterized and were shown to have protective properties toward proteins and cells. A range of new applications, all based on the adaptation to stress conditions conferred by extremolytes, is in development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effectiveness of salt treatment against the growth of harmful microorganisms has been used for food preservation for thousands of years and it was therefore long believed that at a salt concentration in access of 100 g NaCl/l, microbial growth is impossible. The discovery of extremophiles, organisms that thrive under high salt concentration, high temperature, and other stresses has changed this picture (for review, see Rothschild and Mancinelli 2001). Organisms that are capable of growing at high salt concentration achieve osmotic balance across the cell membrane by two different strategies. The first mechanism, which is typical for the archaeal Halobacteriaceae, uses inorganic solutes (potassium and chloride ions) to achieve osmotic equilibrium. This method leads to a relatively narrow adaptation for a specific high-salt environment. The second mechanism is widely used in eubacterial halophiles and employs the accumulation of small organic molecules of low molecular weight to counterbalance the salt concentration in the environment. These small molecules are chemically diverse and can be zwitterionic, uncharged, or anionic (K+ is often used as a counterion). In mesophilic microorganisms, a variety of chemically diverse osmolytes is found. These osmolytes can be amino acids like proline, amino sulfone acids (taurine), sugars (trehalose), inositols (myo-inositol), and betaines (glycine betaine). All these compounds have a relatively low molecular weight and are very polar. Some osmolytes, like proline, are also components of primary metabolic pathways.
The mechanism of using osmolytes has the advantage of being more flexible and suitable over a wider range of salinity. Organic osmolytes play an essential role in maintaining and protecting intracellular homeostasis in organisms ranging from bacteria to humans. Their primary function is the adaptation to changing extracellular osmolarity. Osmolytes are accumulated as a consequence of increased extracellular salinity and their intracellular concentration is reduced. The concentration of osmolytes in the cell can range from millimolars to 1–2 M in response to the extracellular osmolarity. This means that some osmolytes are tolerated by the macromolecular machinery of the cell over a wide concentration range, hence, these compounds were also termed compatible solutes (Brown 1976). The biosynthesis and release of osmolytes is tightly regulated. In general, the microorganism can accumulate osmolytes either by de novo synthesis or by uptake from the environment. Specific uptake systems exist, e.g., for ectoine in Marinococcus marinus, where the BCCT type ectoine transporter EctM serves as a high-affinity uptake system for ectoine (Vermeulen and Kunte 2004). Often, a mixture of several osmolytes is produced, and the composition of the osmolyte pool changes with environmental conditions: Halomonas elongata predominantly accumulates potassium glutamate at intermediate salinity (0.5 M NaCl) and ectoine at higher salinity (Kraegeloh and Kunte 2002).
Extremolytes: compatible solutes from extremophilic microorganisms
Cells exposed to changing and elevated osmolarities have developed an osmolyte strategy. In the case of extremophilic microorganisms, which are adapted to extremely high salinities and other environmental extremes like high (or low) temperature, a specific set of osmolytes is found. We have termed these compounds extremolytes (osmolytes from extremophiles, Fig. 1).
The ability of extremolytes to compensate osmotic pressure and to stabilize macromolecules was studied extensively and a model for their mode of action of macromolecule stabilization was proposed. The preferential exclusion model postulates that the extremolytes are less likely to be found at the surface of proteins, thus leading to an increased (preferential) hydration of the protein (Arakawa and Timasheff 1985). Thus preferential hydration favors the native state of the protein by making the status of unfolding less favorable in the presence of extremolytes (Fig. 2). Because osmolytes do not interact directly with proteins, the catalytic activity of enzymes is largely undisturbed. The effect of the osmolyte glycine on the stabilization of chymotrypsin inhibitor 2 was studied by NMR and it was found that the presence of a high concentration of glycine has a stabilizing effect on chymotrypsin inhibitor 2, which is accompanied by a large reduction of the exchange rate constants of most slowly exchanging amide protons (Foord and Leatherbarrow 1998). Molecular dynamics simulation performed for a model ectoine–water mixture using chymotrypsin inhibitor 2 as a target protein are consistent with the idea that the ectoine does not interact with the protein surface or change the hydration structure (Yu and Nagaoka 2004). Instead, the solvent (water) property is altered leading to a large slowdown of water diffusion and thus stabilization of protein conformation. Therefore, in line with the compatibility with the cellular machinery at high molar concentrations, the general mode of action of extremolytes is a physical effect on the water properties.
Mode of action of extremolytes. The hydrated tertiary structure of globular proteins in aqueous solution (a) is stabilized in the presence of extremolytes (b), which build solute hydrate clusters (Galinski et al. 1997) that are preferentially excluded from the hydrate shell of the protein (Arakawa and Timasheff 1985). This leads to a more compact tertiary structure with reduced surface area (c). Thus, the solute does not interact directly with the protein, the stabilizing effects are rather due to modification of the solvent (water) properties
Extremolytes exhibit protective properties not only toward globular proteins but also toward whole cells and nucleic acids (see Table 1). These protective effects may partially be secondary effects of protein stabilization (e.g., stabilization of membrane proteins) or are due to, e.g., the replacement of water by hydroxyl group bearing extremolytes like hydroxyectoine upon drying (Lippert and Galinski 1992).
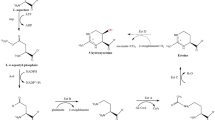
Ectoine ((4S)-2-methyl-1,4,5,6-tetrahydropyrimidine-4-carboxylic acid) was originally discovered by Galinski et al. (1985) in Halorhodospira (formerly Ectothiorhodospira) halochloris, an extremely halophilic phototrophic eubacterium, isolated from Wadi Natrun, Egypt. Later the substance was found in a wide range of halophilic and halotolerant bacteria. The ability to accumulate ectoine is also widespread among organisms that are unable to synthesize ectoines like Escherichia coli (Jebbar et al. 1992), Bacillus subtilis (Jebbar et al. 1997), Corynebacterium glutamicum (Steger et al. 2004), and Sinorhizobium melilotii (Jebbar et al. 2005). The hydroxy derivative hydroxyectoine ((4S,5S)-2-methyl-5-hydroxy-1,4,5,6-tetrahydropyrimidine-4-carboxylic acid) was first discovered in an actinomycin-D-producing Streptomyces strain (Inbar and Lapidot 1988) and is present in many strains that also produce ectoine. The relative amount of hydroxyectoine often increases with growth temperature, e.g., in Streptomyces.
Mannosylglycerate (firoin) and mannosylglyceramide (firoin-A) are examples for carbohydrate extremolytes. As typical for this group of extremolytes, the chemically reactive end of the sugar forms a glycosidic bond with a hydroxyl group of glyceric acid or glyceramide. Rhodothermus marinus synthesizes both mannosylglycerate and mannosylglyceramide. Whereas the anionic mannosylglycerate is accumulated in response to heat stress, the uncharged mannosylglyceramide increases with elevated NaCl levels (Silva et al. 1999). It is interesting to note that mannosylglycerate is also found in eukaryotic red algae (Karsten et al. 1993).
Several archaea accumulate negatively charged derivatives of inositol and glycerol. Di-myoinositol-1,1′-phosphate (DIP), a phosphodiester derivative of the uncharged osmolyte myo-inositol found in eukaryotes, is typical for halotolerant hyperthermophilic archaea like Pyrococcus furiosus and Thermotoga maritima (Scholz et al. 1992; Ciulla et al. 1997). In Thermotogales, the concentration of DIP increases at NaCl levels above the optimum for growth (Martins et al. 1996). In many cases, however, an even stronger accumulation of DIP is observed in response to temperatures above the optimum for growth (Santos and da Costa 2002) reaching 20-fold in P. furiosus at 101 °C (Martins and Santos 1995).
α-Diglycerol phosphate (DGP) also belongs to the anionic phosphodiesters found in halotolerant archaea. DGP was identified as a new extremolyte in Archaeoglobus fulgidus and shown to be an effective protein stabilizer in vitro (Lamosa et al. 2000, Pais et al. 2005). A. fulgidus also synthesizes DIP and while DGP is accumulated in response to elevated external NaCl, increase in temperature leads to enhanced DIP accumulation (Lamosa et al. 2000).
Cyclic 2,3-diphosphoglycerate (cDPG), a cyclic trianionic pyrophosphate, is accumulated in some archaea and was first detected in the thermophilic methanogen Methanothermobacter thermoautotrophicus (Kanodia and Roberts 1983). cDPG exhibits a somewhat unusual behavior because its intracellular concentration is not modulated in response to high NaCl concentrations (Ciulla et al. 1997). The primary role in Methanothermobacter may therefore be as a phosphate storage compound. A role in thermoadaptation was proposed for Methanothermus fervidus, which accumulates high levels of cDPG (Matussek et al. 1998). In line with this, cDPG was shown to stabilize archaeal glyceraldehyde-3-phosphate dehydrogenases at high temperature (Hensel and Jakob 1994).
Production of extremolytes
The high level of accumulation (up to 25% of cell dry mass) of extremolytes makes the development of bioprocesses for large-scale production feasible. A prerequisite for this approach is the ability to cultivate the corresponding microorganism in fermenter scale and high cell densities at nonstandard conditions, e.g., >10% (w/v) NaCl and high temperature. Alternatively, extremolytes can be produced in recombinant hosts, e.g., E. coli or yeast, which are more common in bioprocesses. A prerequisite for this approach is the availability of the synthetic pathway genes for cloning and the construction of a corresponding recombinant organism. This approach was chosen for mannosylglycerate where the bifunctional mannosylglycerate synthase from Dehalococcoides ethenogenes was overproduced in E. coli and Saccharomyces cerevisiae (Empadinhas et 2al. 2004). In S. cerevisiae, a moderate NaCl-dependent accumulation of mannosylglycerate was observed. Another approach was chosen for DGP where chemical synthesis is the most feasible way of production (Santos et al. 1998).
For cDPG, a production process using a recombinant E. coli harboring the genes for 2-phosphoglycerate kinase and cDPG synthetase was outlined (Moritz 2003).
zDIP can be produced by fermentation of P. furiosus at 98 °C under anaerobic conditions, the productivity of this continuous process, however, is comparably low. A more economically feasible, recombinant approach to DIP production has to rely on the more complete characterization of the biosynthetic pathway, which is under way (Chen and Roberts 1998; Chen et al. 1998).
H. elongata was originally isolated from a solar saltern (Vreeland et al. 1980) and can adapt to a wide range of salinities. This is due to the adaptation to an environment with a gradual increase of salinity due the evaporation and fast dilution due to rainfall or flooding of evaporation ponds by tidal wave. This robustness of H. elongata, together with the ability to achieve high cell densities (>40 g dry weight/l corresponding to >10 g/l ectoine) and the safety of the microorganism indicated by its use in food processing (Hinrichsen et al. 1994), have led to its use in the manufacturing process for ectoines. Strains of H. elongata, which continuously release ectoine, can also accumulate ectoine in the culture broth at concentrations >20 g/l (Grammann et al. 2002).
A proprietary industrial bioprocess, termed “bacterial milking” was established for the industrial-scale production of the extremolyte ectoine (Sauer and Galinski 1998). In this process H. elongata, a moderately halophilic ectoine producer, is grown under high-salt conditions (15–20% w/v NaCl) and the intracellularly accumulated ectoines are released by applying an osmotic down-shock, leading to the opening of mechanosensitive channels in the inner membrane of H. elongata. The biomass is returned to the fermenter for the next round of fermentation while the product solution is further purified by electrodialysis, chromatography, filtration, evaporation, and crystallization.
An ectoine bioprocess with an even higher productivity, based on the continuous fermentation of H. elongata, was developed recently (Lentzen and Schwarz 2005). This continuous “permanent milking” process is now used by bitop AG for the production of ectoines in metric ton scale (Fig. 3).
Ectoine bioprocess scheme: H. elongata is cultivated in a continuous fermentation and the culture broth is separated from the biomass by microfiltration. The concentrated biomass is exposed to an osmotic down-shock and again concentrated by microfiltration. The ectoine containing filtrates are the starting material for the purification process with the key steps electrodialysis, chromatography, and crystallization yielding a highly pure, crystalline product
For the production of hydroxyectoine, a bioprocess using the Marinococcus strain M52 was described (Frings et al. 1995). Alternatively, hydroxyectoine can be produced with H. elongata by changing the fermentation conditions in the bacterial milking process.
Protection of proteins by extremolytes
The physicochemical mode of action of extremolytes (Fig. 2) by modifying the water properties is in line with the many experimental data for protein stabilization by extremolytes, which at the same time do not interfere with the enzymatic or binding activity. The stabilization against heat denaturation is a direct measure for increased protein stability in solution. Because some extremolytes are accumulated in their natural producers upon increase of temperature, e.g., hydroxyectoine in Streptomyces (Malin and Lapidot 1996), it was of great interest to study heat protection effects in vitro. In a study involving a variety of solutes, 1 M of hydroxyectoine showed the best performance in heat stabilization, increasing the midpoint of thermal inactivation by 14 °C for the model enzymes phosphofructokinase and lactate dehydrogenase (LDH) (Lippert and Galinski 1992). In a study comparing mannosylglycerate (firoin) with trehalose, mannosylglycerate was the superior thermoprotectant for model enzymes from both hyperthermophilic and mesophilic origin, in line with its role as thermostabilizer under physiological conditions (Ramos et al. 1997). A comparative study of extremolytes, together with some other more common osmolytes like glycerol, showed for the model enzyme LDH that mannosylglycerate and hydroxyectoine were the best protectants against heat stress, while other compounds like ectoine or glycerol showed a much lower degree for stabilization (Borges et al. 2002). At least for mannosylglycerate the stabilization does not depend on the basic net charge of LDH because it was also a good thermoprotectant of the acidic glucose oxidase from Aspergillus niger. DGP, which originates from a thermophilic archaeon, was found to protect a range of enzymes (LDH, alcohol dehydrogenase, glutamate dehydrogenase, and rubredoxin) against heat inactivation (Lamosa et al. 2000). An NMR study indicates that the stabilization of rubredoxin by DGP is mainly due to a stabilization of the closed, more compact form of the protein (Lamosa et al. 2003). Staphylococcus aureus nuclease A was used as a model enzyme to test the effect of the extremolyte mannosylglycerate (Faria et al. 2004). Mannosylglycerate at 0.5 M increases the thermal denaturation transition point (T m) by 7 °C and increases the heat capacity by a factor of 2, but does not influence the (un)folding pathway of the nuclease.
Hydroxyectoine was shown to be an especially effective cryoprotectant of antibody conjugates (Barth et al. 2000). When stored in a 1 M solution of hydroxyectoine, an antibody conjugate was shown to maintain 90% of binding activity even after four freeze–thaw cycles, whereas binding activity was completely lost after only two freeze–thaw cycles in the absence of hydroxyectoine.
Many proteins are inactivated by freeze-drying, which combines the freezing stress with desiccation stress. When comparing the remaining activity after freeze-drying for betaine, ectoine, hydroxyectoine, trehalose, maltose, and sucrose, hydroxyectoine and trehalose turned out as the best protectants against dehydration, whereas ectoine resulted in a lower remaining activity (Lippert and Galinski 1992). Here, the presence of hydroxyl groups, which are able to replace water upon desiccation, seems to be essential. When comparing residual activities after freeze-drying of a mesophilic LDH and a thermophilic glutamate dehydrogenase, mannosylglycerate was superior to trehalose in both cases (Ramos et al. 1997).
Probably as a consequence of the stabilization of the tertiary structure of proteins, ectoine was also shown to protect proteins from proteolysis by trypsin and trypsinogen (Kolp et al. 2003) and proteolytic cleavage of antibodies (Bersch et al. 2000). Hydroxyectoine is able to protect LDH from metal-catalyzed oxidation and against oxidation by hydrogen peroxide (Andersson et al. 2000), indicating that the protection is independent of the mechanism of oxidation. Also, ectoine is able to protect against metal-catalyzed oxidation of LDH. In addition to this potentially indirect antioxidative effect (due to the protection of oxidizable groups in the protein), we have also assessed the antioxidative properties of extremolytes (Table 2). cDPG, DGP, and DIP strongly protect plasmid DNA from damage by hydroxyl radicals, whereas the control compounds proline and trehalose are inactive even at a 20-fold higher concentration. Using the superoxide generating phenazine methosulfate/NADH system (Valentao et al. 2002), we could show that cDGP is also a very efficient scavenger of superoxide radicals with one third of the activity of the strong antioxidant ascorbic acid.
Use of extremolytes in protein chemistry and protein expression
Due to their stabilization of the native structure of proteins, some extremolytes can inhibit protein aggregation seen in different aggregation pathways. One such pathway is the formation of inclusion bodies that occurs upon overexpression of recombinant proteins. Often, the formation of inclusion bodies is desired because it simplifies the extraction of pure protein from the biomass. It is then critical to develop a protocol for efficient refolding of the inclusion body aggregates. Using a standard refolding protocol based on dissolving the inclusion bodies in the chaotropic reagent guanidinium chloride and diluting the dissolved protein to achieve refolding, we could show that ectoine improves the refolding yield of a model protein (Fig. 4). Addition of ectoine, hydroxyectoine, or firoin-A (mannosylglyceramide) to the refolding reaction increases the refolding yield by several folds, while addition of betaine, trimethylamine-N-oxide, or firoin is not beneficial. The effect of ectoine is concentration-dependent up to 1 M and additive to the effect of arginine, an amino acid sometimes used in refolding protocols. The data show that the use of extremolytes in refolding protocols can improve yields of active protein.
The use of extremolytes may be especially useful in the expression and purification of problematic, aggregation-prone proteins. As shown with immunotoxins (Barth et al. 2000), osmolytes allow increased yields when used in the periplasmatic expression of proteins in E. coli (Barth et al. 2000) and furthermore stabilize the protein during the purification process.
Ectoine was also successfully used for the optimization of protein crystallization conditions for X-ray crystallography, resulting in larger and more regular protein crystals (Harjes et al. 2004).
Taken together the data on protein stabilization by extremolytes in vitro and in vivo, these low molecular weight compounds do combine many properties of ideal protein protectants, i.e., high stability and no interference with binding and enzymatic activity. Due to the increasing sensitivity for the potential propagation of transmissible spongiform encephalopathies by stabilizers containing animal protein, extremolytes offer an attractive novel approach to the protein-free and animal-free stabilization and storage of sensitive proteins.
Amyloid inhibition by ectoines
The number of diseases associated with the misfolding of proteins is growing fast (Dobson 2003). In an important subgroup of misfolding diseases, protein aggregation leading to the formation of highly regular aggregates termed amyloids plays a key role and includes diseases like Alzheimer’s disease and spongiform encephalopathies. In a study that used the formation of insulin amyloid in vitro as a model system, it was shown that ectoine is a very effective inhibitor of amyloid formation decreasing both the initiation and elongation phase of amyloid formation (Arora et al. 2004). In a further study, ectoine and hydroxyectoine were investigated for their effect on Aβ peptide amyloid formation (Kanapathipillai et al. 2005). Aβ peptide is the major constituent of senile plaques, the key pathological feature of Alzheimer’s disease. Ectoine and hydroxyectoine were both effective inhibitors on the inhibition of Aβ42 aggregation and toxicity to human neuroblastoma cells. Protein misfolding is also a key event in the pathogenesis of polyglutamine diseases such as Machado–Joseph disease (MJD). Looking at apoptotic cell death produced by the truncated MJD gene product with an expanded polyglutamine tract in cultured neuro2a cells, Furusho et al. (2005) showed that ectoine reduces the total amount of aggregates and changes its intracellular distribution. In consequence, apoptotis is decreased after ectoine application.
Stabilization of nucleic acids
Although most research on stabilization of macromolecules by extremolytes has focused on proteins, there is also evidence for the stabilization of other cellular macromolecules, especially DNA. In general, high concentrations of zwitterionic solutes increase the dielectric constant of the solution, thus decreasing ionic interactions and affecting the DNA duplex (Flock et al. 1996). For ectoine it was experimentally shown that it lowers the melting temperature of double-stranded DNA (Lapidot et al. 1999). Furthermore, ectoine and hydroxyectoine increase the thermal stability of DNA polymerases at elevated temperatures and can thus be used to improve primer extension, sequencing and polymerase chain reactions (Lapidot et al. 1999). Ectoine and hydroxyectoine were also implicated in the self-defense mechanism of antitumor antibiotic-producing Streptomyces strains: Here, the ectoines may protect Streptomyces DNA from intercalation by actinomycin D (Inbar and Lapidot 1988).
Another DNA-protective effect is seen in the inhibition of hydroxyl radical cleavage of DNA by extremolytes (Table 2) in vitro and in the protection of mitochondrial DNA in human dermal fibroblast against UVA-irradiation-induced damage (Bünger and Driller 2004).
Protection of cells by extremolytes
Extremolytes do not only stabilize proteins and other macromolecules; they are also potent cell protectants. The protective effect is obvious in the extremolyte-producing strains, but is also seen with other prokaryotes and even eukaryotic cells (Table 1). Louis et al. (1994) showed that ectoine and hydroxyectoine do stabilize air-dried and freeze-dried E. coli during drying. Ectoines added to the culture medium reversed the inhibition of E. coli growth caused by osmotic stress and increased temperature (Malin and Lapidot 1996). Manzanera et al. (2004) showed that E. coli dried in hydroxyectoine exhibited a high degree of desiccation tolerance, similar to that achieved using the more common trehalose as an extracellular protectant. Ectoine also serve as a specific osmoprotectant for the lactic acid bacterium Tetragenococcus halophila, which is used in soy sauce fermentation (Baliarda et al. 2002). In the gram-negative organism, Pseudomonas putida, which is used for bioremediation due to its ability to degrade organic solvents, hydroxyectoine was superior to trehalose as a drying excipient for storage (Manzanera et al. 2002). Hydroxyectoine is therefore well-suited for the improvement of desiccation tolerance and as a useful excipient for dry storage and shipment of sensitive microorganisms. Ectoine was also used in fermentation technology to increase the osmotolerance and yield in the production of amino acids by coryneform bacteria (Yasuhiko et al. 1997). The positive effects of extremolytes on the expression of recombinant proteins and the fermentative yield of amino acids indicate a more general potential for the use of extremolytes as fermentation additives. In particular, bioprocesses that involve high salt concentrations, like the production of fermented food, could be improved by the use of extremolytes.
Cytoprotection by ectoines, however, is not limited to bacteria but is also seen with eukaryotic cells, leading to some of the most interesting applications. The effect of ectoine on membranes was tested with red blood cell (RBC) assay. This test is a biological in vitro test for the rapid estimation of membrane and protein denaturing properties of surfactants. The standard protocol uses erythrocytes, nonnucleated blood cells containing hemoglobin. In the event that the cell membrane is damaged, hemoglobin is released from the cell and can be quantified by a standard photometric determination. RBCs were incubated with ectoine before the addition of surfactant as the lytic agent. The action of ectoine was compared to the well-known membrane stabilizer phosphatidylcholine. Five different surfactants were used and ectoine showed membrane stabilization for all detergents tested, and for four out of five detergents, the effect was stronger than with phosphatidylcholine (Bünger et al. 2001). When applying UVA irradiation as a stress to human keratinocytes pretreated with ectoine, cells are protected from damage. The effect seems to be exerted via a modulation of the keratinocyte cell membrane because the release of ceramides from sphingomyelin is decreased in skin cells pretreated with ectoine (Bünger and Driller 2004). This leads to the inhibition of the release of proinflammatory cytokines like intercellular adhesion molecule-1 and processes that are involved in UVA-induced accelerated skin aging. In the same study it was also shown that ectoine protects mitochondrial DNA from UVA-induced mutation in human cell culture. A possible mechanism for the ectoine effects could be the stabilization of membrane structures, leading to an increased resistance to potentially damaging environmental influences like UVA. The cytoprotective effect of ectoine even extend to plant cells: Transgenic tobacco (Nicotiana tabacum) cells expressing the genes for ectoine biosynthesis ectA, ectB, and ectC accumulated small levels of ectoine, showed increased tolerance to hyperosmotic shock, and could grow at elevated salt levels (Nakayama et al. 2000).
Protection of skin by ectoine
Because of it’s origin from water-stressed organisms and the accessibility of bulk amounts due to an economical biotechnological production process, ectoine raised commercial interest as a moisturizer for skin care products. Initial studies did focus on the protection of the skin by of ectoine against water loss and desiccation (Bünger 1999). The influence of ectoine on the transepidermal water loss was measured in a test with human volunteers where skin was pretreated with an emulsion containing 0, 2, or 5% ectoine. With increasing amounts of ectoine, the skin becomes less susceptible to damage by the detergent sodium dodecyl sulfate and the subsequent water loss. Furthermore, ectoine treatment in a cosmetic formulation protects the skin from drying out subsequent to application of a hygroscopic silica gel and thus has a prophylaxic effect against dry skin. The ideal formulation properties of ectoine as a stable, crystalline white powder taken together with the lack of interference with cell metabolism and lack of irritative potential are further factors for its suitability as a cosmetic ingredient.
Ectoine was also tested for its ability to reduce the UV-induced damage to keratinocytes, also termed sunburn cells (SBCs), which are formed as a consequence of UV irradiation. Using a skin model system it was shown that preincubation of the skin equivalent for 24 h with ectoine significantly reduced the number of SBCs (Bünger et al. 2001). The photoprotective properties of ectoines seen in a skin model could also be shown in vivo. The effect is especially pronounced with the Langerhans cells (LC), which present antigens crossing the skin barrier and induce T cell immune response. LC are particularly sensitive to UV-induced stress and as a consequence of UV irradiation, the number of cells visible by ATPase staining is reduced, correlating with a loss of their antigen-presenting properties. Pretreatment with an emulsion containing 1% ectoine led, however, to a highly significant protective effect of the LC against UV-induced damage and destruction (Beyer et al. 2000). Thereby, ectoine exerts an immunoprotective effect, protecting skin from potentially damaging UV light. A recent study (Buommino et al. 2005) shows the cytoprotective effects of ectoine also when bacterial lipopolysaccharides are used as a stressor because pretreatment with ectoine prevents cell damage by maintaining an elevated level of the Hsp70.
Outlook: novel areas of application
A major application area for extremolytes established today is in cosmetics where ectoine (Ectoin™) is now used in a growing range of skin care products. Another established use of extremolytes is in stabilization of proteins and nucleic acids in life science and protein chemistry. The high compatibility with biological systems together with the macromolecule-stabilizing and cytoprotective properties of extremolytes raise possibilities for a much wider range of applications, for which we give a few examples below.
Many immunotoxins and immunomodulators do have a particularly severe side effect, termed “vascular leak syndrome” (VLS), which reduces the suitability of otherwise effective therapies because it strongly limits the applicable therapeutic dose (Baluna and Vitetta 1997). Hydroxyectoine, which is able to stabilize immunotoxins in vitro (Barth et al. 2000), also ameliorates the highly toxic side effects of immunotoxins in an animal model when administered in combination with the immunotoxin (Barth 2000). Hydroxyectoine could therefore be a powerful functional excipient for cancer drugs with VLS toxic side effect. The high compatibility, lack of toxicity, and macromolecule-stabilizing properties of extremolytes make them promising candidates for the use as pharmaceutical excipients. Mannosylglycerate (firoin) allowed long-term storage (half-life >1 year) of adenoviral vectors, which are promising tools for gene therapy, but are hampered by loss of vector infectivity during storage and transport (Cruz et al. 2006).
The chiral ectoines, which are now available in bulk quantities, could also serve as interesting starting points for the design of natural product chemical libraries. In particular, hydroxyectoine, with its two chiral centers, provides an interesting starting point for the synthesis of chiral compounds.
It was recently shown that ectoine is accumulated in certain cheeses up to 89 mg/100 g of cheese (J. Klein et al., submitted for publication). The occurrence in cheese is due to the growth of Brevibacterium linens, an ectoine producer traditionally used in surface ripening of red-smear cheeses such as Limburger, Munster, French cheese, Swiss cheese, and Tilsiter (Rattray and Fox 1999). The occurrence of ectoine in food is probably not limited to cheese, but could extend to other food where the production involves the fermentation with ectoine-producing microorganisms under high salt conditions like in soy sauces, Natto (a Japanese soy fermentation product produced by a Bacillus species), fermented fish sauces, and cured meat. The occurrence in food, taken together with the known protein and cell protection properties of ectoine, open a new area of application for extremolytes as functional food ingredients. One application could be the use of ectoine to increase the stability and freshness of foods by stabilizing food components with additional potential health benefits due to the cytoprotective properties of ectoines.
References
Andersson MM, Breccia JD, Hatti-Kaul R (2000) Stabilizing effect of chemical additives against oxidation of lactate dehydrogenase. Biotechnol Appl Biochem 32(Pt 3):145–153
Arakawa T, Timasheff SN (1985) The stabilization of proteins by osmolytes. Biophys J 47:411–414
Arora A, Ha C, Park CB (2004) Inhibition of insulin amyloid formation by small stress molecules. FEBS Lett 564:121–125
Baliarda A, Robert H, Jebbar M, Blanco C, Deschamps A, Marrec C (2002) Potential osmoprotectants for the lactic acid bacteria Pediococcus pentosaceus and Tetragenococcus halophila. Int J Food Microbiol 2607:1–8
Baluna R, Vitetta ES (1997) Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology 37:117–132
Barth S (2000) Pharmaceutical preparation. European Patent EP000001183047
Barth S, Huhn MF, Matthey B, Klimka A, Galinski EA, Engert A (2000) Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions. Appl Environ Microbiol 66:1572–1579
Bersch S, Vangala M, Schwarz T, Kaufmann M (2000) Protection of antibodies against proteolytic degradation by compatible solutes. In: 2nd international conference on protein stabilisation/biomolecule stabilisation, Lissabon
Beyer N, Driller H, Bünger J (2000) Ectoin—a innovative, multi-functional active substance for the cosmetic industry. SÖFW-J 126:27–29
Borges N, Ramos A, Raven ND, Sharp RJ, Santos H (2002) Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 6:209–216
Brown AD (1976) Microbial water stress. Bacteriol Rev 40:803–846
Bünger J (1999) Ectoine added protection and care for the skin. Eurocosmetics 7:22–24
Bünger J, Driller H (2004) Ectoin: an effective natural substance to prevent UVA-induced premature photoaging. Skin Pharmacol Physiol 17:232–237
Bünger J, Degwert J, Driller H (2001) The protective function of compatible solute ectoine on the skin cells and its biomolecules with respect to UV-radiation, immunosuppression and membrane damage. IFSCC Mag 4:1–6
Buommino E, Schiraldi C, Baroni A, Paoletti I, Lamberti M, De Rosa M, Tufano MA (2005) Ectoine from halophilic microorganisms induces the expression of hsp70 and hsp70B′ in human keratinocytes modulating the proinflammatory response. Cell Stress Chaperones 10:197–203
Chen L, Roberts MF (1998) Cloning and expression of the inositol monophosphatase gene from Methanococcus jannaschii and characterization of the enzyme. Appl Environ Microbiol 64:2609–2615
Chen L, Spiliotis ET, Roberts MF (1998) Biosynthesis of di-myo-inositol-1,1′-phosphate, a novel osmolyte in hyperthermophilic archaea. J Bacteriol 180:3785–3792
Ciulla RA, Diaz MR, Taylor BF, Roberts MF (1997) Organic Osmolytes in Aerobic Bacteria from Mono Lake, an Alkaline, Moderately Hypersaline Environment. Appl Environ Microbiol 63:220–226
Cruz PE, Silva AC, Roldao A, Carmo M, Carrondo MJ, Alves PM (2006) Screening of novel excipients for improving the stability of retroviral and adenoviral vectors. Biotechnol Prog 22:568–576
Dobson CM (2003) Protein folding and disease: a view from the first Horizon Symposium. Nat Rev Drug Discov 2:154–160
Empadinhas N, Albuquerque L, Costa J, Zinder SH, Santos MA, Santos H, da Costa MS (2004) A gene from the mesophilic bacterium Dehalococcoides ethenogenes encodes a novel mannosylglycerate synthase. J Bacteriol 186:4075–4084
Faria TQ, Lima JC, Bastos M, Macanita AL, Santos H (2004) Protein stabilization by osmolytes from hyperthermophiles: effect of mannosylglycerate on the thermal unfolding of recombinant nuclease a from Staphylococcus aureus studied by picosecond time-resolved fluorescence and calorimetry. J Biol Chem 279:48680–48691
Flock S, Labarbe R, Houssier C (1996) 23Na NMR study of the effect of organic osmolytes on DNA counterion atmosphere. Biophys J 71:1519–1529
Foord RL, Leatherbarrow RJ (1998) Effect of osmolytes on the exchange rates of backbone amide protons in proteins. Biochemistry 37:2969–2978
Frings E, Sauer T, Galinski EA (1995) Production of hydroxyectoine: high cell-density cultivation and osmotic downshock of Marinococcus strain M52. J Biotechnol 43:53–61
Furusho K, Yoshizawa T, Shoji S (2005) Ectoine alters subcellular localization of inclusions and reduces apoptotic cell death induced by the truncated Machado–Joseph disease gene product with an expanded polyglutamine stretch. Neurobiol Dis 20:170–178
Galinski EA, Pfeiffer HP, Truper HG (1985) 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem 149:135–139
Galinski EA, Stein M, Amendt B, Kinder M (1997) The kosmotropic (structure-forming) effect of compensatory solutes. Comp Biochem Physiol 117A:357–365
Göller K, Galinski EA (1999) Protection of a model enzyme (lactate dehydrogenase) against heat, urea and freeze-thaw treatment by compatible solute additives. J Mol Catal B Enzym 7:37–45
Grammann K, Volke A, Kunte HJ (2002) New type of osmoregulated solute transporter identified in halophilic members of the bacteria domain: TRAP transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581(T). J Bacteriol 184:3078–3085
Grether-Beck S, Timmer A, Felsner I, Brenden H, Brammertz D, Krutmann J (2005) Ultraviolet A-induced signaling involves a ceramide-mediated autocrine loop leading to ceramide de novo synthesis. J Invest Dermatol 125:545–553
Harjes S, Scheidig A, Bayer P (2004) Expression, purification and crystallization of human 3′-phosphoadenosine-5′-phosphosulfate synthetase 1. Acta Crystallogr D Biol Crystallogr 60:350–352
Hensel R, Jakob I (1994) Stability of Glyceraldehyde-3-Phosphate Dehydrogenases from Hyperthermophilic Archaea at High Temperature. Syst Appl Microbiol 16:742–745
Hinrichsen LL, Montel MC, Talon R (1994) Proteolytic and lipolytic activities of Micrococcus roseus (65), Halomonas elongata (16) and Vibrio sp. (168) isolated from Danish bacon curing brines. Int J Food Microbiol 22:115–126
Inbar L, Lapidot A (1988) The structure and biosynthesis of new tetrahydropyrimidine derivatives in actinomycin D producer Streptomyces parvulus. Use of 13C- and 15N-labeled L-glutamate and 13C and 15N NMR spectroscopy. J Biol Chem 263:16014–16022
Jebbar M, Talibart R, Gloux K, Bernard T, Blanco C (1992) Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J Bacteriol 174:5027–5035
Jebbar M, von Blohn C, Bremer E (1997) Ectoine functions as an osmoprotectant in Bacillus subtilis and is accumulated via the ABC-transport system OpuC. FEMS Microbiol Lett 154:325–330
Jebbar M, Sohn-Bosser L, Bremer E, Bernard T, Blanco C (2005) Ectoine-induced proteins in Sinorhizobium meliloti include an Ectoine ABC-Type transporter involved in osmoprotection and ectoine catabolism. J Bacteriol 187:1293–1304
Kanapathipillai M, Lentzen G, Sierks M, Park CB (2005) Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer’s beta-amyloid. FEBS Lett 579:4775–4780
Kanodia S, Roberts MF (1983) Methanophosphagen: Unique cyclic pyrophosphate isolated from Methanobacterium thermoautotrophicum. Proc Natl Acad Sci USA 80:5217–5221
Karsten U, West JA, Ganesan EK (1993) Comparative physiological ecology of Bostrychia moritziana (Ceramiales, Rhodophyta) from freshwater and marine habitats. Phycologia 32:401–409
Kolp S, Pietsch M, Galinski EA, Gütschow M (2003) Protection against proteolytic cleavage by compatible solutes. In: Polish–Austrian–German–Hungarian–Italian joint meeting on medicinal chemistry, Krakow
Kraegeloh A, Kunte HJ (2002) Novel insights into the role of potassium for osmoregulation in Halomonas elongata. Extremophiles 6:453–462
Lamosa P, Burke A, Peist R, Huber R, Liu MY, Silva G, Rodrigues-Pousada C, LeGall J, Maycock C, Santos H (2000) Thermostabilization of proteins by diglycerol phosphate, a new compatible solute from the hyperthermophile Archaeoglobus fulgidus. Appl Environ Microbiol 66:1974–1979
Lamosa P, Turner DL, Ventura R, Maycock C, Santos H (2003) Protein stabilization by compatible solutes. Effect of diglycerol phosphate on the dynamics of Desulfovibrio gigas rubredoxin studied by NMR. Eur J Biochem 270:4606–4614
Lapidot A, Iakobashvili R, Malin G (1999) Methods for DNA amplification and sequencing. Patent WO1999041410
Lentzen G, Schwarz T (2005) Kompatible Solute: Mikrobielle Herstellung und Anwendung. In: Anthranikian G (ed) Angewandte Mikrobiologie. Springer, Berlin Heidelberg New York, pp 355–372
Lippert K, Galinski EA (1992) Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol 37:61–65
Louis P, Trüper HG, Galinski EA (1994) Survival of Escherichia coli during drying and storage in the presence of compatible solutes. Appl Microbiol Biotechnol 41:684–688
Malin G, Lapidot A (1996) Induction of synthesis of tetrahydropyrimidine derivatives in Streptomyces strains and their effect on Escherichia coli in response to osmotic and heat stress. J Bacteriol 178:385–395
Manzanera M, Garcia de Castro A, Tondervik A, Rayner-Brandes M, Strom AR, Tunnacliffe A (2002) Hydroxyectoine is superior to trehalose for anhydrobiotic engineering of Pseudomonas putida KT2440. Appl Environ Microbiol 68:4328–4333
Manzanera M, Vilchez S, Tunnacliffe A (2004) High survival and stability rates of Escherichia coli dried in hydroxyectoine. FEMS Microbiol Lett 233:347–352
Martins LO, Santos H (1995) Accumulation of mannosylglycerate and di-myo-inositol-phosphate by Pyrococcus furiosus in response to salinity and temperature. Appl Environ Microbiol 61:3299–3303
Martins LO, Carreto LS, Da Costa MS, Santos H (1996) New compatible solutes related to di-myo-inositol-phosphate in members of the order Thermotogales. J Bacteriol 178:5644–5651
Matussek K, Moritz P, Brunner N, Eckerskorn C, Hensel R (1998) Cloning, sequencing, and expression of the gene encoding cyclic 2, 3-diphosphoglycerate synthetase, the key enzyme of cyclic 2, 3-diphosphoglycerate metabolism in Methanothermus fervidus. J Bacteriol 180:5997–6004
Melo EP, Faria TQ, Martins LO, Gonçalves AM, Cabral JMS (2001) Cutinase unfolding and stabilization by trehalose and mannosylglycerate. Proteins 42:542–552
Moritz P (2003) Die Bedeutung der cyclischen 2,3-Diphosphoglycerat Synthetase für die temperaturabhänige Regulation der intrazellulären Konzentration von cyclischem 2,3-Diphosphoglycerat im hyperthermophilen Archaeum Methanothermus fervidus. Ph.D. thesis, Universität Duisburg-Essen
Nakayama H, Yoshida K, Ono H, Murooka Y, Shinmyo A (2000) Ectoine, the compatible solute of Halomonas elongata, confers hyperosmotic tolerance in cultured tobacco cells. Plant Physiol 122:1239–1247
Pais TM, Lamosa P, dos Santos W, Legall J, Turner DL, Santos H (2005) Structural determinants of protein stabilization by solutes. The important of the hairpin loop in rubredoxins. Febs J 272:999–1011
Ramos A, Raven ND, Sharp RJ, Bartolucci S, Rossi M, Cannio R, Lebbink J, Oost Jvd, Vos WMd, Santos H (1997) Stabilization of enzymes against thermal stress and freeze-drying by mannosylglycerate. Appl Environ Microbiol 63:4020–4025
Rattray FP, Fox PF (1999) Aspects of enzymology and biochemical properties of brevibacterium linens relevant to cheese ripening: a review. J Dairy Sci 82:891–909
Rothschild LJ, Mancinelli RL (2001) Life in extreme environments. Nature 409:1092–1101
Santos H, da Costa MS (2002) Compatible solutes of organisms that live in hot saline environments. Environ Microbiol 4:501–509
Santos H, Lamosa P, Burke A, Maycock C (1998) Thermostabilization, osmoprotection and protection against desiccation of enzymes, cell components and cells by di-glycerol-phosphate. European Patent EP0965268
Sauer T, Galinski EA (1998) Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng 57:306–313
Scholz S, Sonnenbichler J, Schafer W, Hensel R (1992) Di-myo-inositol-1,1′-phosphate: a new inositol phosphate isolated from Pyrococcus woesei. FEBS Lett 306:239–242
Silva Z, Borges N, Martins LO, Wait R, da Costa MS, Santos H (1999) Combined effect of the growth temperature and salinity of the medium on the accumulation of compatible solutes by Rhodothermus marinus and Rhodothermus obamensis. Extremophiles 3:163–172
Steger R, Weinand M, Kramer R, Morbach S (2004) LcoP, an osmoregulated betaine/ectoine uptake system from Corynebacterium glutamicum. FEBS Lett 573:155–160
Valentao P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, de Lourdes Bastos M (2002) Antioxidant activity of Hypericum androsaemum infusion: scavenging activity against superoxide radical, hydroxyl radical and hypochlorous acid. Biol Pharm Bull 25:1320–1323
Vermeulen V, Kunte HJ (2004) Marinococcus halophilus DSM 20408T encodes two transporters for compatible solutes belonging to the betaine-carnitine-choline transporter family: identification and characterization of ectoine transporter EctM and glycine betaine transporter BetM. Extremophiles 8:175–184
Vreeland RH, Litchfield CD, Martin EL, Elliot E (1980) Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Bacteriol 30:485–495
Yasuhiko Y, Yoshio K, Eiichiro K, Yasuhiro T, Seichi S (1997) Production of l-amino acid by fermentation method using ectoine. Japanese Patent JP9271382, 21 Oct 1997
Yu I, Nagaoka M (2004) Slowdown of water diffusion around protein in aqueous solution with ectoine. Chem Phys Lett 388:316–321
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lentzen, G., Schwarz, T. Extremolytes: natural compounds from extremophiles for versatile applications. Appl Microbiol Biotechnol 72, 623–634 (2006). https://doi.org/10.1007/s00253-006-0553-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0553-9