Abstract
Fumonisins (FBs) are widespread Fusarium toxins commonly found as corn contaminants. FBs could cause a variety of diseases in animals and humans, such as hepatotoxic, nephrotoxic, hepatocarcinogenic and cytotoxic effects in mammals. To date, almost no review has addressed the toxicity of FBs in relation to oxidative stress and their metabolism. The focus of this article is primarily intended to summarize the progress in research associated with oxidative stress as a plausible mechanism for FB-induced toxicity as well as the metabolism. The present review showed that studies have been carried out over the last three decades to elucidate the production of reactive oxygen species (ROS) and oxidative stress as a result of FBs treatment and have correlated them with various types of FBs toxicity, indicating that oxidative stress plays critical roles in the toxicity of FBs. The major metabolic pathways of FBs are hydrolysis, acylation and transamination. Ceramide synthase, carboxylesterase FumD and aminotransferase FumI could degrade FB1 and FB2. The cecal microbiota of pigs and alkaline processing such as nixtamalization can also transform FB1 into metabolites. Most of the metabolites of FB1 were less toxic than FB1, except its partial (pHFB1) metabolites. Further understanding of the role of oxidative stress in FB-induced toxicity will throw new light on the use of antioxidants, scavengers of ROS, as well as on the blind spots of metabolism and the metabolizing enzymes of FBs. The present review might contribute to reveal the toxicity of FBs and help to protect against their oxidative damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fumonisins (FBs), belong to the relatively recently discovered group of mycotoxins mainly produced by Fusarium verticillioides and Fusarium proliferatum, are widespread mycotoxins commonly found as corn contaminants (Marschik et al. 2013). A. niger is also a source of fumonisins, such as Fumonisin2 (FB2), Fumonisin4 (FB4) and Fumonisin6 (FB6) (Mansson et al. 2010). In addition, FBs have also been found in wheat, barley, sorghum, rice, wine, raisins, black tea leaves, asparagus, pine nuts and Thai coffee beans (Logrieco et al. 2010; Marin et al. 2007; Martins et al. 2001; Mateo and Jimenez 2000; Nielsen et al. 2015). They are structurally characterized by an eicosane backbone, esterified with two tricarballylic acid groups (Fig. 1). Fumonisin1 (FB1) and FB2 are the most important representatives of FBs (European Commission 2003). In most cases from field samples, FB1, FB2 and Fumonisin3 (FB3) constituted approximately 70, 20 and 10 % of FBs, respectively (Howard et al. 2002).
FBs are found all around the world. In Hebei Province of China during 2011–2013, the incidence of FBs (FB1 + FB2) for all samples was 46.4 % (Li et al. 2015b). In Shandong Province of China in 2014, the incidences of FB1, FB2 and FB3 in the corn products were 98.1 %, and the simultaneous occurrence of FB1, FB2 and FB3 was observed in 76.7 % of the corn products (Li et al. 2015a). The incidence of FBs in nine provinces of China was reported to be from 81.0 to 10.3 % in maize samples in 2012 (Fu et al. 2015). A recent study revealed that FB2 was also detected in 37.5 % of the onion samples from the Taif region in Saudi Arabia (Gherbawy et al. 2015). In animal feeds distributed in South Korea in 2011, the contamination rate of feed ingredients with FB1 and FB2 was 50 and 40 %, respectively (Seo et al. 2013). FB2 was found in 29 % of the raisin samples at levels ranging from 7.1 to 25.5 µg/kg in raisins from Western Greece regions in 2011 (Perrone et al. 2013). In Iran, the natural occurrences of FB1, FB2 and FB3 were 68.2, 42.6 and 31.7 %, respectively (Sorensen et al. 2009). A prevalence survey in Taiwan showed that there was a contamination rate of 40 % in domestic animal feeds, and the average contaminated level was 4.5 mg/kg (Cheng et al. 2006).
FBs could cause a variety of diseases in animals and humans, such as hepatotoxic, nephrotoxic, hepatocarcinogenic and cytotoxic effects in mammals (Dall’Asta et al. 2009). FB1 is the most abundant of the naturally occurring analogues, and it was classified as possibly carcinogenic to humans by the International Agency for Research on Cancer (IARC 2002). It was reported that purified FB1 could cause both liver and kidney tumors in rodents in long-term feeding studies (Creppy 2002). The high incidence of human esophageal cancer was reported to be associated with FBs, such as in Cixian Country of Hebei Province in China (Wang et al. 2000), and in the former Transkei region of the Eastern Cape Province of South Africa (Rheeder et al. 1992). FB1 was reported to inhibit embryonic sphingolipid synthesis and has been implicated as a risk factor for neural tube defects (NTDs) in cultured mouse embryos (Sadler et al. 2002) and in the sensitive LM/Bc mouse (Gelineau-van Waes et al. 2005, 2009; Marasas et al. 2004; Sadler et al. 2002; Voss et al. 2009). Furthermore, FB1 could cause cardiotoxicity and pulmonary edema in swine associated with F. verticillioides exposure (Colvin and Harrison 1992; Smith et al. 1999). Horses were the more susceptible species to FBs, and the main mycotoxicosis of horses is leukoencephalomalacia caused by the FB1 and FB2 produced by Fusarium spp. (Riet-Correa et al. 2013; Voss et al. 2007).
FBs could inhibit ceramide synthases with the subsequent disruption of sphingolipid metabolism and sphingolipid-dependent physiological processes (Gelineau-van Waes et al. 2009; Voss et al. 2009; Zitomer et al. 2009). FB1 modulates immunity in animals, and it has immunocytotoxic effects by increasing IL-10 and IL-4 mRNA levels in the spleen of Balb/c mice together with the decrease in mRNA levels of IFN-γ and TNF-α compared with controls (Abbes et al. 2015). Similarly, FB1 could significantly inhibit the expression of IL-1β, IL-2, IFN-α and IFN-γ when broilers were exposed to 15 mg FB1 per kg for 3 weeks (Cheng et al. 2006). Together with AFB1 or not, FB1 increased levels of IL-4 and decreased levels of IL-10 in spleen mononuclear cells (SMC) in both in vivo and in vitro studies (Theumer et al. 2002, 2003). Moreover, recent studies indicated that the generation of reactive oxygen species (ROS) and oxidative stress was closely related to the immunotoxic effect elicited by the mycotoxins (Kotan et al. 2011; Mary et al. 2012; Theumer et al. 2010). There has been considerable focus for the last several years on the potential of FB1 to induce oxidative stress. In vitro studies conducted on several cell lines have suggested the possible role of oxidative stress on FB1-induced cytotoxicity and apoptosis (Bernabucci et al. 2011; Domijan et al. 2015; Ferrante et al. 2002; Kang and Alexander 1996; Klaric et al. 2007; Kouadio et al. 2005; Mary et al. 2012; Mobio et al. 2003; Stockmann-Juvala et al. 2004a, b). Similar results were also observed in mice (Abbes et al. 2015), rats (Hassan et al. 2014) and broiler chicks (Poersch et al. 2014). Oxidative stress will occur in cells when the concentration of ROS exceeds the antioxidant capacity (Mishra et al. 2014). High concentrations of ROS lead to the disruption of redox homeostasis, initiation of the process of lipid peroxidation in the lipid membrane causing damage to the cell membrane, and DNA damage by propagating a chain reaction (Mishra et al. 2014; Zuo et al. 2015). In the toxicity of FBs, oxidative stress and ROS might play important roles in the induction of FB-induced damage to lipids, DNA and proteins. To date, several reviews have been published on the determination method of FBs, biomarkers of FB-induced toxicity, apoptosis and carcinogenesis (Arranz et al. 2004; Scott 2012; Shephard et al. 2007; Stockmann-Juvala and Savolainen 2008). Although oxidative stress was briefly mentioned in one of the reviews 7 years ago (Stockmann-Juvala and Savolainen 2008), some new articles about the role of oxidative stress induced by FBs have been published. Therefore, it is necessary to review the new progress of the toxic effects on oxidative stress induced by FBs. The scope of this review is primarily intended to summarize the evidence associated with a role of oxidative stress as a plausible mechanism for FB-induced toxicity. The studies related to FBs toxicity under in vitro and in vivo conditions are summarized in Tables 1 and 2, respectively. Furthermore, the metabolizing routes, metabolizing enzymes, factors affecting the metabolism of FBs and the toxicity of metabolites of FBs were also reviewed. The present review shows that over the past two decades, oxidative stress and ROS generation might correlate closely with various types of toxicity, as well as with the metabolism of FBs, and the antioxidant mechanism contributed greatly to finding more effective antioxidants to inhibit FBs-induced toxicity.
Oxidative stress and toxicity
Generation of reactive oxygen, oxidative stress and reactive nitrogen species
Oxidative stress is initiated by ROS such as perhydroxy radical (HOO−), hydroxyl radical (HO·) and superoxide anion (O ·−2 ), and by reactive nitrogen species (RNS) including nitric oxide (Mishra et al. 2014).
Mary et al. (2012) documented that when SMC from Wistar rats were exposed to 10 μM FB1 for up to 48 h, the treatment increased total ROS. The mitochondrial complex I, CYP450, the arachidonic acid metabolism and the NADPH oxidase system were involved in the ROS generation induced by the FB1 group (Mary et al. 2012). In cell cultures of rat primary astrocytes and human neuroblastoma (SH-SY5Y), FB1 (0.5, 5 and 50 µM), as a neurodegenerative mycotoxin, decreased the rate of mitochondrial and cellular respiration, and increased ROS production in mitochondria by inhibiting mitochondrial complex I (Domijan and Abramov 2011). However, the effect of FB1 on ROS over-production is a consequence rather than a mechanism of its toxicity (Domijan and Abramov 2011; Galvano et al. 2002). FB1 increased the production of ROS in human U-118MG glioblastoma (U-118MG) cells, showing significant dose- and time-level effects after incubation with 100 µM FB1 for 48–144 h and with 10 µM FB1 for 72 and 144 h (Stockmann-Juvala et al. 2004b). After cells were treated with FB1 (0.1–100 µM) for 0–144 h, FB1 caused a dose-dependent increase in ROS production in mouse C6 glioblastoma and rat GT1-7 hypothalamic cells, but was without any effect in human SH-SY5Y cells (Stockmann-Juvala et al. 2004a). It was interesting to note that ROS production was observed in SH-SY5Y cells by Domijan and Abramov, whereas it was not noted by Stockmann-Juvala (Domijan and Abramov 2011; Stockmann-Juvala et al. 2004a). However, to date, there has been no generation of RNS reported in the study of FB1. When birds were fed diets containing FB1 (5, 10 and 15 mg/kg) for 3 weeks, 15 mg FB1/kg feed had no effect on inducible nitric oxide synthase (iNOS) (Cheng et al. 2006).
FBs-mediated oxidative damage
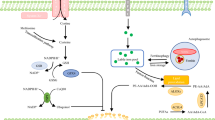
Oxidative stress up-regulates the antioxidant defense system and resulted in damage to cellular macromolecules, such as DNA, lipids and proteins. Subsequently, oxidative stress led to cell death by apoptotic or necrotic mechanisms. During the progress, DNA damage, enhanced lipid peroxidation and protein damage occurred (Tables 1, 2). The schematic representation of FBs induced the damage to DNA, lipids and proteins is shown in Fig. 2.
Oxidative stress-mediated mode of action proposed for fumonisins. The increased generation of ROS as well as a decrease in antioxidant status may induce lipid, protein and DNA oxidation, leading to toxicity and apoptosis via ERK and the intrinsic mitochondrial pathway. The heat shock proteins might be induced by oxidative stress and protect cells against oxidative damage
Damage to lipids
Oxidative damage to lipids of the cell membrane increases lipid peroxidation. Lipid peroxidation can be measured by monitoring the changes in thiobarbituric acid-reacting substances (TBARS) and malondialdehyde (MDA). As one part of TBARS, MDA was the most abundant individual aldehyde resulting from lipid peroxidation, and its level is a marker of lipid oxidation (Klaric et al. 2007; Pirinccioglu et al. 2010; Zhou et al. 2015).
FBs could significantly increase lipid peroxidation. FB1 (35 and 70 µg/mL) significantly increased MDA production in bovine peripheral blood mononuclear cells (Bernabucci et al. 2011). The exposure of SMC from Wistar rats to FB1 (10 µM) for 48 h significantly raised the formation of MDA relative to the control (Mary et al. 2012). Applied for 48 h, only the highest dose of FB1 (5 µg/mL) significantly increased TBARS (57 %) (Klaric et al. 2007). Compared with other Fusarium toxins such as deoxynivalenol (DON) and zearalenone (ZEN), FB1 showed the highest production of MDA in Caco-2 cells, identifying that FB1 causes the highest level of oxidative damage to lipids (Kouadio et al. 2005). MDA production induced by FB1 was also noted in the study of oxidative stress in SH-SY5Y neuroblastoma, rat C6 glioblastoma, mouse GT1-7 hypothalamic cells, C6 glioma cells (with normal p53 status) and p53-null mouse embryonic fibroblasts, indicating that lipid peroxidation induced by FB1 might be a common phenomenon (Mobio et al. 2003; Stockmann-Juvala et al. 2004a). A similar report showed that the treatment of FB1 (5 and 10 µM) for 24 h increased MDA production in the macrophage cell line J774A.1 (Ferrante et al. 2002). An in vivo study also found that FB1 (100 µg/kg b.w.) resulted in a significant increase in MDA level in liver and kidney tissues (Hassan et al. 2014). After feeding a diet contaminated with FB1 (100 mg/kg) for 21 days, hepatic TBARS levels in male broiler chicks were also increased (Poersch et al. 2014). Theumer et al. (2010) reported that after exposure to experimental diets contaminated with a culture material containing 100 mg/kg of FB1 for 90 days, the MDA levels were increased in SMC (Theumer et al. 2010). The level of MDA in serum was elevated in SD rats treated with FB1 (100 µg/kg b.w.) by gavage for 30 days (Sun et al. 2006). Abel and Gelderblom (1998) reported that dietary FB1 levels of 250 and 500 mg FB1/kg increased the level of TBARS significantly in the liver of rats fed FB1 over 21 days. Also, in the 250 mg FB1/kg group, subcellular fractionation of the liver of the rats showed a significant increase in TBARS in the microsomes. Furthermore, the level of TBARS was increased in a dose-dependent manner, associated with an increase in cytotoxicity in primary rat hepatocytes (Abel and Gelderblom 1998). Another study revealed that female Sprague–Dawley (SD) rats fed FB-contaminated diet (200 mg/kg diet) showed a significant increase in MDA compared with the control group (Hassan et al. 2010). However, MDA concentration did not change from control after the treatment with FB1 (5, 50 and 500 µg/kg b.w.) for 4, 24 and 48 h in the liver of male Wistar rats. The low FB1 dose and short exposure time were suggested as the possible reasons (Domijan et al. 2008). In contrast, MDA level in the liver of male SD rats was significantly increased when the rats were fed FB-contaminated diet (200 mg/kg diet) for 3 weeks, indicating that a sufficient exposure time of FBs could also lead to lipid peroxidation, even at a low concentration of FBs (El-Nekeety et al. 2007). In a study to determine whether very low doses of FB1 induce oxidative stress in the rat kidney and liver, rats were treated orally with FB1 (200 ng/kg b.w. and 50 µg/kg b.w.). The results found that FB1 did not affect MDA concentration in the liver, while both FB1 doses increased MDA concentration in the kidney, suggesting that the kidney was more sensitive to the toxicity of FB1 (Domijan et al. 2007).
Damage to DNA and RNA
The threat of oxidative damage is particularly significant to DNA. DNA damage can be measured by alkaline comet (SCGE) assay, cytokinesis-block micronucleus (CBMN) assay and Fpg-modified comet assay (Domijan et al. 2006, 2015). The formation of the major oxidative DNA damage product 8-hydroxydeoxyguanosine (8-OH-dG) was also used as an indicator of oxidative DNA damage (Ihsan et al. 2011).
Domijan revealed that FB1 (20 µg/mL) already induced DNA damage in the primary human peripheral blood lymphocytes (HPBLs) after 1-h exposure (Domijan et al. 2015). An 8-h exposure of SMC to the FB1 (10 µM) significantly increased the 8-OH-dG levels, compared with the control (Mary et al. 2012). In a study to compare the cytotoxic effect of DON, ZEN and FB1 (1–150 µM) and the pathways leading to cell death and related to oxidative stress in Caco-2 cells, DNA synthesis is inhibited by FB1 with the highest IC50 of 20 µM among three kinds of toxins (Kouadio et al. 2005). DNA fragmentation was studied in U-118MG cells exposed to 10–100 µM FB1 for 144 h, and FB1 increased inter-nucleosomal DNA fragmentation as the formation of DNA ladders (Stockmann-Juvala et al. 2004b). Mobio et al. (2003) reported that the treatment with FB1 (3 and 36 µM) for 24 h induced a significant and dose-related increase in 8-OH-dG and DNA fragmentation in both C6 glioma and MEF cells. The maximum DNA breakage was already observed at 9 µM of FB1 in both C6 glioma and MEF cells. However, MEF cells showed a higher sensitivity to FB1 even at lower FB1 concentrations. In addition, FB1 (9 and 18 µM) incubation significantly increased the rate of deoxycytosine methylation in C6 glioma cells but not in MEF cells, suggesting that different cells showed different sensitivity to FBs (Mobio et al. 2003). DNA fragmentation in the liver and kidney was increased after female SD rats were exposed to FB1 (100 µg/kg b.w.) for 12 weeks (Hassan et al. 2014). Another study showed that the DNA damage was found in 13.7 and 81.7 % (comet assay) and in 2.8 and 7.0 % (micronucleus technique) in control and 100 mg/kg FB1 groups in a subchronic study in male Wistar rats, respectively (Theumer et al. 2010). Similarly, another study suggested that FB1-induced micronucleated polychromatic erythrocytes (Mn-PCEs) in bone marrow increased DNA and RNA in the liver after female SD rats were fed an FB-contaminated diet (200 mg/kg diet) for 4 weeks (Hassan et al. 2010).
The role of oxidative stress in FB1-induced DNA damage has been illustrated by Domijan et al. (2008) in male adult Wistar rats treated with single FB1 doses (5, 50 and 500 µg/kg b.w.) and killed at 4, 24 and 48 h after treatment. There was a significant increase in the DNA mobility of liver cells after 48 h following treatment with 50 and 500 µg/kg b.w. compared to the control. Moreover, tail intensity appeared to be a more sensitive parameter for detecting DNA damage, even at 5 µg/kg b.w. after 48 h. This study proved that FB1-induced DNA damage is time- and dose-dependent, and Wistar rats were sensitive to FB1 (Domijan et al. 2008). To investigate DNA damage induced by FB1, rats orally received FB1 (0.2, 50 and 500 µg/kg b.w., respectively) for 5 days. The results showed that even at the lowest dose of FB1, the tail length, tail intensity and olive tail moment (OTM) obtained with the standard comet assay and Fpg-modified comet assay were significantly higher in treated animals than in controls. Furthermore, the Fpg-modified comet assay showed significantly greater tail length, tail intensity and OTM in all treated animals than in the standard comet assay, indicating that oxidative stress is likely to be responsible for DNA damage induced by FB1. However, DNA damage detected by the standard comet assay at all FB1 doses indicated that some other mechanism was also involved (Domijan et al. 2006). Additionally, treatment with FB1 led to splenic and hepatic DNA fragmentation in 85 % of the test animals after fasted rats were injected intravenously with a single dose of FB1 at 1.55 mg/kg b.w. into the tail vein, suggesting that oxidative damage caused by FB1 may be one of the underlining mechanisms of FB1-induced cell injury and DNA damage (Atroshi et al. 1999).
Damage to proteins
Apart from lipids and DNA, the possible major target of oxidative damage is proteins that can be transformed into protein carbonyls (PCs) (Domijan et al. 2007). Mary et al. identified that the 48-h incubation of SMC with FB1 (10 µM) significantly raised the carbonyl levels compared to the control (Mary et al. 2012). The inhibition of protein synthesis has been reported in a few studies. In one study to compare the inhibition of protein synthesis among DON, ZEN and FB1 in Caco-2 cells, IC50 of 5, 8.8 and 19 µM for DON, FB1 and ZEN identified that protein synthesis is not a specific target of FB1. The toxic effects of FB1 act through lipid peroxidation rather than protein synthesis (Kouadio et al. 2005). In the FB1 concentration range (3–30 µM), protein synthesis was inhibited in both C6 glioma (IC50 = 6 µM) and MEF cells (IC50 = 5 µM) after 24-h incubation with a superimposable kinetic (Mobio et al. 2003). Domijan et al. (2007) reported that FB1 (200 ng/kg b.w. and 50 µg/kg b.w.) could cause oxidative damage of proteins in the kidney of male Wistar rats. FB1 did not affect PCs concentrations in the liver, while in the kidney, both FB1 doses increased the concentration of PCs, indicating that proteins are also more susceptible to oxidative lesions in the kidney than in the liver (Domijan et al. 2007).
Alterations in antioxidant status
The enzymatic antioxidant defense systems are responsible for protecting from ROS such as O ·−2 , HO· and hydrogen peroxide (H2O2) (Yang and Lee 2015). The primary antioxidant enzymes are SOD, CAT, glutathione reductase (GR) and GPx. These antioxidant enzymes can serve as redox biomarkers because they are the first to indicate the antioxidant state through oxidation/reduction processes (Yang and Lee 2015). GSH is the most abundant intracellular antioxidant involved in the protection of cells against oxidative damage and in various detoxification mechanisms (Shi et al. 2015).
The antioxidant enzymes were regarded as important mediators of the immunotoxicity induced by FB1. After Balb/c mice were treated daily for 2 weeks by oral gavage with FB1 (100 µg/kg), the results showed that in the spleen, exposure to mycotoxins led to an increase in lipid peroxidation, IL-10 and IL-4 mRNA levels, but decreased GSH content and down-regulated expression of GPx and SOD, and of IFN-γ and TNF-α mRNA (Abbes et al. 2015). FB1 might significantly increase intracellular reactive oxygen metabolites (ROM) and decrease both cytoplasmic SOD and GPx mRNA abundance, suggesting that FB1 might induce cytotoxicity through impairment of the oxidative status of bovine peripheral blood mononuclear cells (PBMCs) (Bernabucci et al. 2011).
The activities of antioxidant enzymes in oxidative stress are implicated as a mechanism of FB1 neurotoxicity (Domijan and Abramov 2011; Stockmann-Juvala et al. 2004a). After 24-h treatment of FB1 (0.5–200 µM), the level of GSH in astrocytes was significantly increased in SH-SY5Y cells (Domijan and Abramov 2011). The ability of FB1 to increase GSH level was explained by its direct action on the proteins responsible for GSH synthesis or/and by its mild ROS production that can stimulate GSH synthesis (Dinkova-Kostova and Talalay 2008; Domijan and Abramov 2011; Galvano et al. 2002). However, long-term exposure of U-118MG cells to FB1 resulted in decreased GSH levels. Significant depletion of GSH was noted after incubation with 1, 10 and 100 µM FB1 for 144 h and after incubation with 100 µM FB1 for 48 and 72 h (Stockmann-Juvala et al. 2004b). Similarly, FB1 decreased GSH levels in mouse C6 glioblastoma and rat GT1-7 hypothalamic cells but had no effects in human SH-SY5Y cells (Stockmann-Juvala et al. 2004a). It was interesting to note that the trend of GSH level induced by FB1 was increased in SH-SY5Y cells, as reported by Domijan and Abramov, whereas it was not affected in the study of Stockmann-Juvala (Domijan and Abramov 2011; Stockmann-Juvala et al. 2004a). The difference in the trend of GSH level in both studies might be related to the different concentrations and incubation time of FB1.
The misbalance of the enzymatic antioxidant defense systems was noted in vivo when animals were administered with FB1. Abbes et al. (2015) found that FB1 decreased GSH content and down-regulated the expression of GPx and SOD in spleen when Balb/c mice were treated daily for 2 weeks by oral gavage with FB1 (100 µg/kg) (Abbes et al. 2015). Similarly, female SD rats receiving FB1 (100 µg/kg b.w.) for 12 weeks decreased GSH content in the liver and kidney and down-regulated the gene expression of antioxidants enzymes such as GPX, SOD and CAT (Hassan et al. 2014). When broiler chicks were administered the FB1 diet (100 mg/kg) for 21 days, the hepatic CAT activity in the liver was significantly increased, showing that subacute exposure of FB1 induced liver oxidative stress in broiler chicks (Poersch et al. 2014). In SMC of male Wistar rats subchronically (90 days) fed with culture material containing 100 mg/kg of FB1, the CAT and SOD activities were increased in the poisoned animals, indicating the hypothesis of an oxidative stress-mediated genotoxicity induced by FB1 (Theumer et al. 2010). FB1 showed hepatotoxicity in C57BL/6 N mice accompanied by decreased GSH in the liver following the subcutaneous injection of 2.25 mg FB1/kg b.w. once daily for 5 days (He et al. 2006). Intravenous injection of the tail vein with FB1 (1.55 mg/kg) markedly decreased GSH levels in the liver (20 % of the control) and spleen (26 % of the control) (Atroshi et al. 1999). However, in the research of early toxic effects of FB1 in rat liver, GSH concentration did not change when male adult Wistar rats were treated with single FB1 doses (5, 50 and 500 µg/kg b.w.) and killed at 4, 24 and 48 h after treatment. The reason for this was suggested to be the low FB1 dose and short exposure time (Domijan et al. 2008). However, with a longer exposure time of FB1 study, FB1 did not affect the activity of CAT and SOD in the liver and kidney when rats were treated orally with FB1 (200 ng/kg b.w. and 50 µg/kg b.w.) for 5 days (Domijan et al. 2007). Rumora et al. (2007) noted that cellular GSH content in the liver of rats exposed for 2 days did not differ from control animals when Wistar rats were treated intraperitoneally (i.p.) with FB1 (0.5 mg/kg b.w.) for 2 or 7 days, whereas in the liver of rats treated with FB1 for 7 days, GSH concentrations increased significantly (24 % increase), and kidney GSH levels in animals treated for 2 and 7 days decreased significantly (15 and 27 % decrease, respectively); this indicates that the enzymatic antioxidant defense systems in the kidney seemed to be more sensitive to oxidative stress than that in the liver (Rumora et al. 2007).
Stress-mediated biological response
Oxidative stress plays important roles in a large number of biological responses and cell signaling pathways. Thus, the significant changes in gene expression and the stimulation or inhibition of signal transduction usually result in many toxicological effects. The role of FB-mediated oxidative stress in the induction of apoptosis and their respective cell signaling pathways has been studied widely in different cell systems (Tables 1, 2).
Apoptosis and cell signaling
Apoptosis is regarded as a common result of oxidative stress caused by ROS production, disturbed GSH defense system and lipid peroxidation (Higuchi 2003). The caspase-dependent pathway was usually stimulated and resulted in cell apoptosis (Stockmann-Juvala et al. 2004b). It was suggested that human U-118MG glioblastoma cells treated with FB1 underwent caspase-dependent apoptosis. The highest dose of FB1 (100 µM) caused a significant increase in caspase-3-like protease activity in cells at 12, 48 and 72 h of exposure; caspase-3-like protease activity was not affected at other time points (24 and 144 h) or with lower concentrations of FB1 (1 and 10 µM), indicating that the activation of caspase-3 might be one of the events causing an increase in ROS production, and subsequent lipid peroxidation and reduction in intracellular GSH levels (Stockmann-Juvala et al. 2004b). Abbes et al. (2015) reported that increased caspase-3 activity was noted in the spleens of rats after exposure to FB1 (100 µg/kg) daily for 2 weeks by oral gavage (Abbes et al. 2015).
The p53 gene is known to suppress cell cycle progression in response to DNA damage, thus preventing the transmission of damaged genetic information from one cell generation to the next (Yin et al. 1997). An interesting study found that the suppression of cell cycle progression was observed in C6 glioma but not in p53-null MEF cells incubated with FB1, indicating that cells lacking mechanisms governed by the p53 gene would be more susceptible to neoplastic cascade or mutation following DNA lesions induced by this mycotoxin (Mobio et al. 2003).
The perturbations of the cellular redox state or the generation of ROS stimulate redox-sensitive signaling molecules, such as mitogen-activated protein kinases (MAPKs) and heat shock proteins (Hsps). The MAPK superfamily of serine/threonine kinases is activated by a number of extracellular stimuli and is involved in signal transduction cascades that play an important regulatory role in cell apoptosis (Matsuzawa and Ichijo 2005). Three major mammalian MAPKs were extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 MAPK (Wang et al. 2012; Wu et al. 2014). The expression of Hsp25/27 and Hsp70 might affect cell survival by interfering with apoptotic machinery and inhibiting apoptosis both upstream and downstream of the caspase cascade in a caspase-independent manner. These proteins could also decrease ROS production, affect cellular GSH levels and neutralize the toxic effects of oxidized proteins (Escobedo et al. 2004; Ferns et al. 2006). FB1 could also increase MAPKs activation and the expression of Hsp25 and Hsp70 in the liver and kidney of male Wistar rats given 0.5 mg FB1/kg b.w. intraperitoneally for 2 or 7 days. Phospho-JNK signal was activated even when cells were treated with FB1 for 2-day treatment. However, FB1 increased Hsp25 expression and decreased Hsp70 expression in the rat kidney after a 7-day treatment with FB1. These findings documented that FB1 could lead to tissue-specific activation and expression of redox-sensitive signaling molecules, and kidney cells were more sensitive to adverse effects of FB1 (Rumora et al. 2007).
Ca2+ homeostasis of the cells is one of the many important functions. The proliferation of cells, to undergo apoptosis, induction of oxidative stress and physiological functions such as signal transduction, is part of Ca2+ homeostasis (Cig and Naziroglu 2015). A recent study revealed that FB1 (0.5, 5 and 50 µM) resulted in mitochondrial injury and decreased calcium uptake in SH-SY5Y cells and led to the sustained deregulation of calcium homeostasis and presumably cell death (Domijan and Abramov 2011). FB1 (1.55 mg/kg b.w.) administration caused total Ca2+ in liver to increase within 4 h (204 % of control), while pretreatment with CoQ10 (30 mg CoQ10/kg diet) together with l-carnitine (2.8 mg carnitine/kg diet), alpha-tocopherol (30 IU vitamin E/kg diet) and selenium (1 mg selenium as sodium selenite/kg diet) could decrease the activities of Ca2+ in the liver (Atroshi et al. 1999).
Prevention of FB-mediated oxidative stress
Various compounds, including free radical scavengers, have been shown to combat FB-induced cell damage (Fig. 3). As an antioxidant, sodium copper chlorophyllin (CHL) (0.1–100 µg/mL) exhibited free radical scavenging activity in the DPPH assay and protected against cell and DNA damage induced by FB1 (20 µg/mL) (Domijan et al. 2015). Co-treatment using lactic acid bacteria (LAB) (2 × 109 CFU/L, approximately 2 mg/kg) with FB1 (100 µg/kg) suppressed levels of DNA fragmentation, normalized splenic lipid peroxidation and increased GSH levels, up-regulated the expression of GPx and SOD and normalized mRNA levels of the analyzed cytokines (Abbes et al. 2015). Panax ginseng extract (PGE) exerted antioxidant and anti-apoptotic effects, which resulted from its ability to play a role in increasing the antioxidant status as well as lowering the oxidative damage of nucleic acids in the body (Abdel-Wahhab et al. 2010). PGE contains many classes of compounds, including salicylic acid, caffeic acid, vitamins C and E, which have antioxidant activity and are important to enhance the non-enzymatic antioxidants of the body such as vitamins C and E, and the antioxidant capacity including the enzymatic antioxidants such as CAT, GPx, GSH and SOD (Ramesh et al. 2012a, b). Research has shown that PGE (20 mg/kg b.w.) combined treatments with FB1 (100 µg/kg b.w.) could suppress DNA fragmentation in the liver of female SD rats, normalize lipid peroxidation and increase GSH in the liver and kidney and up-regulate the expression of GPx, SOD1 and CAT mRNA (Hassan et al. 2014).
Schematic illustration of preventive effect of different compounds including antioxidants and free radical scavengers on fumonisins-induced oxidative stress. The use of different antioxidants such as lactic acid bacteria (LAB), panax ginseng extract (PGE) and the ethanol extract of Aquilegia vulgaris L. suppressed levels of DNA fragmentation, improved the total antioxidant status and led to the prevention of apoptosis. Sodium copper chlorophyllin (CHL) exhibited free radical scavenging activity and protected against DNA damage induced by FB1. Similarly, royal jelly (RJ) has a protective effect against FB toxicity by decreasing lipid oxidation as well as improving antioxidant status. The mixture of CoQ10 together with l-carnitine, alpha-tocopherol and selenium decreased DNA damage and the activities of Ca2+, ASAT and ALAT in the liver, thus leading to the prevention of apoptosis
In another study, the ethanol extract of Aquilegia vulgaris L. (5 and 10 mg/kg b.w.) was identified to be against the oxidative stress and the genotoxicity in female SD rats fed with FB-contaminated diet (200 mg/kg diet). A. vulgaris L. (Ranunculaceae) is a perennial herb indigenous in central and southern Europe, Africa and Asia (Hassan et al. 2010). The FBs within corn powder consisted of 68 % FB1, 21 % FB2 and 11 % FB3 based on the total FBs in the corn powder. The combined treatment showed significant improvements in GSH, total antioxidant capacity and MDA and succeeded to normalize PCEs, NCEs, PCEs/NCEs ratio, DNA and RNA in the liver and histological pictures in the liver tissues (Hassan et al. 2010). It was interesting to note that different teas showed varying effects on the hepatotoxicity, cancer promotion and activities of CAT, GPx and GR, as well as GSH status in the rat liver. Unfermented rooibos and honeybush significantly reduced the total number of foci, respectively, while fermentation seems to reduce the protective effect of the herbal teas (Marnewick et al. 2009). A study showed that royal jelly (RJ) has a protective effect against FBs toxicity, and this protection was dose-dependent. Co-treatment with FBs (200 mg/kg diet) plus RJ (100 or 150 mg/kg b.w.) resulted in a significant improvement in the histological and histochemical pictures of the liver and kidney, and all of the tested parameters such as ALT, AST, triglycerides, cholesterol, HDL, LDL, creatinine, MDA, GPx and SOD and uric acid levels (El-Nekeety et al. 2007). Mobio et al. (2003) reported that vitamin E (25 mM) inhibited MDA production in both C6 glioma and p53-null MEF cells, while transresveratrol (3 mM) and epicatechin (15 mM) decreased MDA formation in C6 glioma cells (Mobio et al. 2003). The prevention of DNA damage and cell injury has also been reported by Atroshi et al. (1999) in FB1-treated rats (1.55 mg/kg b.w.). The study demonstrated that pretreatment with CoQ10 (30 mg CoQ10/kg diet) together with l-carnitine (2.8 mg carnitine/kg diet), alpha-tocopherol (30 IU vitamin E/kg diet) and selenium (1 mg selenium as sodium selenite/kg diet), decreased DNA damage and the activities of Ca2+, aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) in the liver. However, the CoQ10 alone did not significantly protect against toxic cell death and glutathione depletion induced by FB1 (Atroshi et al. 1999). Another study indicated that S-adenosylmethionine (SAM) and methylthioadenosine (MTA) were ineffective in protecting against FB1 hepatotoxicity in C57BL/6 N mice. Neither SAM (intraperitoneal injection of 25 mg/kg b.w.) nor MTA (intraperitoneal injection of 25 mg/kg b.w.) modified the FB1-induced expression of TNFα, IL-1α or IL-1 receptor antagonist. Both agents prevented an increase in free sphingosine but not sphinganine, and they could not improve the GSH level in liver following FB1 treatment (subcutaneous injection of 2.25 mg/kg b.w.) (He et al. 2006).
Metabolism of FBs
Metabolic pathways
The metabolism of FBs has already been studied since the 1990s, and some metabolites have been identified in the past years. The metabolism of FBs can notably occur in the liver and the digestive tract. The major concerned metabolic pathways of FBs are hydrolysis, acylation and transamination (Fig. 4) (Hartinger et al. 2011; Humpf et al. 1998; Seiferlein et al. 2007).
HFB1 (also called AP1), the major metabolite of FB1, originates from FB1 by the hydrolysis of tricarballylic acid side chains at carbon 14 and 15, which are replaced by hydroxyl groups (Hartinger et al. 2011). HFB1 (42–121 µg/kg) was detected in the liver of weaned piglets fed a diet with FB1 at 30 mg/kg feed for 6 weeks based on high-performance liquid chromatography (HPLC) combined with fluorescence detection (FL), indicating that the appearance of HFB1 in the liver clearly originates from the breakdown of FB1 from dietary origin (Pagliuca et al. 2005). Humpf et al. (1998) reported that HFB1 (but not FB1, under the conditions tested) can be acylated to N-palmitoyl-HFB1 (also called N-Pal-HFB1 or pHFB1) with a V max/km of approximately 1 by ceramide synthase (Humpf et al. 1998). As the hydrolyzed forms of FB1 and FB2, HFB1 and HFB2 (also called AP2), respectively, can be metabolized by ceramide synthase to their respective N-acylated metabolites (nervonoyl- or palmitoyl-HFB1 and -HFB2) using rat liver microsomes and palmitoyl-CoA or nervonoyl-CoA as cosubstrates; the formation of N-acyl-HFB1 was further identified in vivo using a HPLC-MS/MS method to detect tissues from rats given intraperitoneal doses of HFB1 (Seiferlein et al. 2007). N-acyl-FB1 derivatives were also detected as in cultured cells at levels of up to 10 pM/mg of protein, and the acyl-chain length of the N-acyl-FB1 depends on the ceramide synthase isoform acylating them (Harrer et al. 2013). A recent study found that the N-acyl chain length of the FB1 metabolites varied in a tissue-dependent manner with C16 derivatives predominating in the kidney and C24 derivatives being prevalent in the liver when male rats were dosed with intraperitoneal injection with 0.5, 1 or 2 mg/kg body weight FB1 on five consecutive days (Harrer et al. 2015).
Metabolizing enzymes
Encoded as part of a gene cluster of Sphingopyxis sp. MTA144, the aminotransferase FumI could degrade FB1 and catalyze deamination of the first intermediate of the catabolic pathway, hydrolyzed FB1. FumI showed activity in the range of pH 6–10 with an optimum at pH 8.5, and in the range of 6–50 °C with an optimum at 35 °C, and at low salt concentration (Hartinger et al. 2011). Ceramide synthase also acylates HFB1 to pHFB1 with a V max/km of approximately 1 (Humpf et al. 1998). Additionally, HFB1 can be obtained by de-esterification of FB1 with a carboxylesterase and the toxicity of HFB1 was much lower than FB1, indicating that conversion to HFB1 could be a good strategy to reduce FB1 exposure (Grenier et al. 2012).
The metabolizing enzymes for FB1 in ruminants were also investigated. However, it appeared that no metabolizing enzyme was involved in the metabolism of FB1 because no biodegradation or metabolism of FB1 by liver microsomes was detectable after incubating FB1 with bovine microsomes in the presence of a regenerating system for 1 h (Spotti et al. 2001). Additionally, an in vitro study showed that no hydrolyzed metabolites (aminopolyols or aminopentol) were detected when FB1 (1 µg/mL) was incubated in ruminal fluid for up to 72 h, in the presence or absence of alfalfa as a substrate for microbial growth, using a model rumen, suggesting that FB1 is poorly metabolized in the rumen and such metabolism is not the cause of the tolerance to this toxin displayed by ruminants (Caloni et al. 2000).
Strong differences were noted concerning the effects of FB1 on drug-metabolizing enzyme activities. FB1 (0, 5, 15 and 45 mg/kg/day) can affect drug-metabolizing enzyme activities when four groups of five growing ducks were treated with daily oral administration over 12 days. It can significantly increase benzphetamine, ethylmorphine, erythromycin N-demethylases and ethoxyresorufin O-deethylase activities (respectively, by 114, 242, 57 and 27 % with 5 mg/kg/day and 1024, 969, 200 and 147 % with 45 mg/kg/day) except glutathione S-transferase (GST) and significantly increase aminopyrine and nitrosodimethylamine N-demethylases, methoxyresorufin and pentoxyresorufin O-dealkylases and UDP-glucuronyltransferase activities at the dose of 45 mg/kg/day; this suggests that phase 1 drug-metabolizing enzyme activities appeared more sensitive to the toxin than phase 2 (Raynal et al. 2001). When rats were given intraperitoneal administration at three dose levels (0.125, 0.25 and 2.5 mg/kg) once a day for 6 days, FB1 increased the renal and hepatic N-demethylation of erythromycin (CYP3A1) and the hepatic O-deethylation of ethoxyresorufin (CYP1A1) at the two highest doses and increased the renal O-deethylation of ethoxyresorufin at the highest dose of 2.5 mg/kg. Furthermore, CYP4A subfamily, the beta-oxidation of palmitoyl-coenzyme A (CoA) and trans-2-enoyl-CoA hydratase were also increased in the treatment of FB1 at the two highest doses in the liver (Fig. 4) (Martinez-Larrañaga et al. 1996).
Other factors on the metabolism of FBs
The cecal microbiota of pigs can also transform FB1 to the metabolites. In an in vitro study, pure FB1 was treated with the suspensions of cecal contents and McDougall buffer solution under anaerobic conditions for 0, 12, 24, 48 and 72 h. After 48 h, the conversion of FB1 to partially hydrolyzed FB1 (pHFB1) (46 %) was nearly equal to the percentage ratio of FB1, while it was 49 % by 72 h. In vitro, the conversion of FB1 to HFB1 was less than 1 %, indicating that the most probable metabolic process is the removal of one propane-1,2,3-tricarboxylic acid side chain by esterase action in the mammalian gut, while the conversion of FB1 to HFB1 is not a priority during metabolism (Fodor et al. 2007). Alkaline processing like nixtamalization of maize generates pHFB1 and HFB1 and thermal treatment in the presence of reducing sugars leads to the formation of N-(1-deoxy-d-fructos-1-yl) fumonisin1 (NDF) (Grenier et al. 2012; Hahn et al. 2015). The investigated alkaline and thermal processing products of FB1 were non-toxic for rats at the tested concentrations (10 mg/kg FB1, 7.8 mg/kg pHFB1, 5.6 mg/kg HFB1 and 12.2 mg/kg NDF), indicating that appropriate food processing can reduce fumonisin toxicity for humans (Hahn et al. 2015).
The toxicity of FBs and their metabolites
FB1, pHFB1, pHFB2 and HFB1 metabolites presented poor toxicity for the human intestinal cell line Caco-2 when cells were treated with them (in the range of 1–138 mM) for 48 h (Caloni et al. 2002). Although FB1 appears the most toxic to cells, HFB1, losing the tricarballylic acid chain is more bioavailable than FB1 on intestinal cells, indicating that risk evaluations of the metabolites of FBs are also necessary (Caloni et al. 2002). In another study, a significant increase in sphinganine (up to 7000 % compared to control cells) was observed with FB1, FB2, FB3, HFB1 and pHFB1, and sphingosine levels remained almost unchanged, indicating that all substrates effectively inhibited ceramide synthase. Furthermore, only exposure to 10 µM/L FB1 for 24 h led to a significant increase in caspase-3 activity, chromatin condensation and DNA fragmentation, suggesting that FB2, FB3 and the metabolites of FB1 could not induce apoptosis of human proximal tubule-derived cells (IHKE cells), whereas FB1 could (Seefelder et al. 2003). A previous study revealed that HFB1 showed much lower toxicity than FB1. When piglets were exposed by gavage for 2 weeks to 2.8 µM FB1 or HFB1/kg body weight/day, FB1 induced significant toxicity as well as hepatotoxicity and the toxic effects on the morphology of the different segments of the small intestine, decrease in villi height and modification of intestinal cytokine expression, whereas HFB1 did not trigger hepatotoxicity, did not impair intestinal morphology and slightly modified the intestinal immune response (Grenier et al. 2012). However, another in vivo study, where rats received diets supplemented with FB1 or HFB1, showed that the latter can be more toxic than the parent compound and induces fumonisin-like liver and kidney lesions (Voss et al. 1996). Furthermore, HFB1 shows cancer-promoting activity in liver (Hendrich et al. 1993). The different toxicity induced by FB1 in piglets and rats suggested that there are species differences in the toxicity of FB1, which was far from clear and the reason was worthy of further investigation.
pHFB1 was found to be at least ten times more toxic than FB1 or HFB1 and caused sphinganine accumulation as an inhibitor of ceramide synthase using HT29 cells, showing that pHFB1 was a toxic metabolite and may play a role in the diseases caused by FBs (Humpf et al. 1998). To investigate the toxic effect on sphingolipid metabolism, high purity FB1, pHFB1a + b, HFB1 and NDF were fed to male SD rats for 3 weeks, and the results showed that the Sa/So ratios were significantly increased in the FB1 positive control group, and the Sa/So ratios of pHFB1, HFB1 and NDF were indifferent from the negative control group (Hahn et al. 2015). Considering the fact that 2-keto-HFB1 cannot be acylated in a similar way to HFB1 (Humpf et al. 1998; Seiferlein et al. 2007), 2-keto-HFB1 was speculated to be non-toxic (Hartinger et al. 2011). The acylation products of HFB1 (C16-HFB1 and C24:1-HFB1) and HFB2 (C16-HFB2 and C24:1-HFB2) also inhibited ceramide synthase and significantly reduced the number of viable cells in an in vitro MTT assay using HT29 cells, suggesting that acylation apparently does not detoxify hydrolyzed fumonisins (Seiferlein et al. 2007). A recent study confirmed that acylation of FB1 was even more toxic than the parent FB1 when comparing the cytotoxicity of 20 µM N-acyl-FB1 derivatives with that of FB1 at the same concentration for 8 h in human embryonic kidney (Hek) cells, Hep3B cells and human skin fibroblasts, respectively (Harrer et al. 2013).
In summary, FBs was metabolized mainly through hydrolysis, acylation and transamination progress. The metabolites of FBs were different in species. Differences in regulated enzymatic profiles among the analyzed species may be the reasons for the different metabolism of FBs. Enzymatic detoxification of FB1 may be a suitable approach with which to increase feed and food safety, because the main metabolites of FB1 showed lower toxicity than FB1. Furthermore, the cooking progress was also a good strategy to reduce FBs exposure.
Conclusion
FBs were considered to be one of the most important contaminants of corn and corn-based food worldwide. The most potent and abundant of the class of FBs is FB1. FBs could cause a variety of species-specific toxicological effects in human, domestic and laboratory animals. Therefore, it was necessary to investigate the toxic effect of FBs and therefore to protect from the injury of these toxins.
Most of the studies regarding oxidative stress induced by FBs (mainly FB1) have been carried out on in vitro models and in vivo studies. A recent study found that GSH level was increased in a dose-dependent manner after 24-h incubation of astrocytes with FB1. Furthermore, in experiments in which the pre-incubation of cells with a conjugator of GSH did not affect ROS production induced by FB1, this indicated that ROS is not involved in FB1 toxicity, at least in astrocytes (Domijan and Abramov 2011). Similar conclusions were drawn by the previous studies (Abel and Gelderblom 1998; Galvano et al. 2002). However, no similar study has been carried out in other cells. However, in some cells, such as SMC, SH-SY5Y cells, U-118MG cells, mouse C6 glioblastoma cells and rat GT1-7 hypothalamic cells, ROS production was suggested to play important roles in the toxic effects of FB1; it was not clear whether the ROS over-production was a consequence rather than a mechanism of toxicity. Moreover, discrepancies were noted in ROS generation or not in SH-SY5Y cells by Domijan and Abramov (2011) and by Stockmann-Juvala et al. (2004a, b), suggesting that there are a few studies that should be carried out to determine the role of ROS in the toxic mechanism of FB1.
The mechanism of toxicity of FBs is complex, but the major biochemical effect of FBs is the inhibition of ceramide synthase (sphinganine- and sphingosine-N-acyl transferase), a key enzyme in the sphingolipid metabolism (Seefelder et al. 2003). ROS and oxidative stress played important roles in cellular apoptosis. However, few studies were carried out to elucidate the role of the inhibition of ceramide synthase induced by FB1 in the oxidative stress and ROS generation in vitro and in vivo. The inhibition of ceramide synthase is sufficient for the induction of apoptosis only in some cell types (Seefelder et al. 2003). Then, the factors playing a critical role in the different induction of cellular apoptosis induced by FB1 were worthy of investigation. Moreover, the research into the relationship between oxidative stress, ROS production and other factors also contribute to reveal the toxic mechanism of FB1.
In a study to evaluate the toxic effects of FB1 and other FBs, including FB2, FB3, fumonisin P1, HFB1, N-acetyl-FB1 and N-carboxymethyl-FB1 (approximately 0, 14, 70 and 140 µM/kg diet) in mice for 28 days, only FB1 showed hepatotoxic effects (Howard et al. 2002). However, FB2 could cause equine leukoencephalomalacia (Ross et al. 1994). Furthermore, short-term carcinogenesis studies in the rat liver bioassay indicated that FB2 and FB3 closely mimic the toxicological and cancer-initiating activity of FB1 and thus could contribute to the toxicological effects of FBs in animals (Gelderblom et al. 1992). Therefore, the toxic effects of other FBs, such as FB2 and FB3, in vitro and in vivo seemed to be worthy of evaluation. However, few studies were carried out to reveal the potentially toxic effects of other FBs besides FB1.
Various studies, including in vitro models and in vivo studies, have identified that the induction of an oxidative stress response induced by FBs exposure was considered one of the mechanisms responsible for its toxicity (Tables 1, 2). However, most in vivo studies were carried on rats or mice, and only one study was performed in chicks. Thus, greater emphasis should be placed on in vivo studies of livestock and poultry with the doses, which are commonly encountered in food crops or in feed.
The in vitro effects of hydrolyzed FBs were reported to range from less to more toxic than those of the intact FBs. The N-acyl-metabolites are even more cytotoxic than FB1, suggesting that the 2-amino group of hydrolyzed FBs might play a critical role for toxicity. As for the metabolism of FBs in animals, the metabolism of FB1 in cattle was significantly different from that in piglets. In the rumen, some studies found that FB1 was apparently not dependent on its detoxification in the liver, and it still remains unclear why the tolerance of ruminants to FB1 was higher than that of pigs. HFB1 was found to show much lower toxicity than FB1 in piglets, whereas it was more toxic in rats than the parent compound and induced fumonisin-like liver and kidney lesions and showed cancer-promoting activity in the liver. It was necessary to compare the different metabolizing progress among the rumen of piglets and rats, and even more species. However, until now, the metabolizing difference in species was far from clear.
It was interesting to note that oxidative stress occurred in rats, mice and chicks in the toxicology studies of FB1, and various compounds, including free radical scavengers, can efficiently combat FB-induced damage, suggesting that the toxic effects of FB1 might be closely related to its metabolizing progress and oxidative stress. The main metabolizing route of FBs was hydrolysis, acylation and transamination. However, it was far from clear which kind of metabolizing progress was closely related to oxidative stress, which was worthy of further investigation. Understanding the relationship between the metabolism of FBs and oxidative stress and identifying the related enzymes will contribute more to combating the toxicity induced by FBs.
To protect cells from FB1-induced oxidative stress, a variety of compounds have been evaluated for their anti-oxidative effects. Further understanding the role of oxidative stress as well as the metabolism of FBs in FB-induced toxicity will throw new light onto the use of antioxidants and scavengers of ROS. Considering the worldwide containment of FBs, the work focused on looking for highly effective antioxidants and efficient detoxification enzymes seems to be very meaningful to reduce the various toxic effects induced by FBs.
Abbreviations
- 8-OH-dG:
-
8-Hydroxydeoxyguanosine
- ALAT:
-
Alanine aminotransferase
- aminopolyols:
-
Hydrolyzed fumonisins
- ASAT:
-
Aspartate aminotransferase
- CAT:
-
Catalase
- CBMN:
-
Cytokinesis-block micronucleus assay
- CHL:
-
Sodium copper chlorophyllin
- CoQ10:
-
Coenzyme Q10
- CYP1A1:
-
O-deethylation of ethoxyresorufin
- CYP3A1:
-
N-demethylation of erythromycin
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- DON:
-
Deoxynivalenol
- ERK:
-
Extracellular signal-regulated kinase
- FB:
-
Fumonisin
- FL:
-
Fluorescence detection
- GE:
-
Ginseng extract
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- GST:
-
Glutathione S-transferases
- H2O2 :
-
Hydrogen peroxide
- HFB1/2:
-
Hydrolyzed fumonisin B1 and/or B2
- HPBLs:
-
Human peripheral blood lymphocytes
- HPLC:
-
High-performance liquid chromatography
- HO· :
-
Hydroxyl radical
- HOO− :
-
Perhydroxy radical
- Hsps:
-
Heat shock proteins
- HT29:
-
Human colonic cell line
- IARC:
-
The International Agency for Research on Cancer
- IHKE cells:
-
Human proximal tubule-derived cells
- iNOS:
-
Inducible nitric oxide synthase
- i.p.:
-
Intraperitoneally
- JNK:
-
c-Jun N-terminal kinase
- LAB:
-
Lactic acid bacteria
- MAPKs:
-
Mitogen-activated protein kinases
- MDA:
-
Malondialdehyde
- MEF:
-
Mouse embryonic fibroblasts
- Mn-PCEs:
-
Micronucleated polychromatic erythrocytes
- MTA:
-
Methylthioadenosine
- NCEs:
-
Normochromatic erythrocytes
- NDF:
-
N-(1-deoxy-d-fructos-1-yl) fumonisin1
- N-Pal-HFB1 (PAP1):
-
N-palmitoyl-hydrolyzed fumonisin1, NTD, neural tube defects
- O ·−2 :
-
Superoxide anion
- OTA:
-
Ochratoxin A
- OTM:
-
Olive tail moment
- PBMC:
-
Peripheral blood mononuclear cells
- PCEs:
-
Polychromatic erythrocytes
- PCs:
-
Protein carbonyls
- PGE:
-
Panax ginseng extract
- pHFB1:
-
Partially hydrolyzed FB1
- RJ:
-
Royal jelly
- RNS:
-
Reactive nitrogen species
- ROM:
-
Reactive oxygen metabolites
- ROS:
-
Reactive oxygen species
- SAM:
-
S-adenosylmethionine
- SCGE:
-
Alkaline comet assay
- SMC:
-
Spleen mononuclear cells
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid-reacting substances
- U-118MG:
-
Human U-118MG glioblastoma
- ZEN:
-
Zearalenone
References
Abbes S, Ben Salah-Abbes J, Jebali R, Younes RB, Oueslati R (2015) Interaction of aflatoxin B and fumonisin B in mice causes immunotoxicity and oxidative stress: possible protective role using lactic acid bacteria. J Immunotoxicol. doi:10.3109/1547691X.2014.997905
Abdel-Wahhab MA, Hassan NS, El-Kady AA et al (2010) Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats. Food Chem Toxicol 48(2):733–742. doi:10.1016/j.fct.2009.12.006
Abel S, Gelderblom WC (1998) Oxidative damage and fumonisin B1-induced toxicity in primary rat hepatocytes and rat liver in vivo. Toxicology 131(2–3):121–131
Arranz I, Baeyens WR, Van der Weken G, De Saeger S, Van Peteghem C (2004) Review: HPLC determination of fumonisin mycotoxins. Crit Rev Food Sci Nutr 44(3):195–203. doi:10.1080/10408690490441604
Atroshi F, Rizzo A, Biese I et al (1999) Fumonisin B1-induced DNA damage in rat liver and spleen: effects of pretreatment with coenzyme Q10, l-carnitine, alpha-tocopherol and selenium. Pharmacol Res 40(6):459–467. doi:10.1006/phrs.1999.0529
Bernabucci U, Colavecchia L, Danieli PP et al (2011) Aflatoxin B1 and fumonisin B1 affect the oxidative status of bovine peripheral blood mononuclear cells. Toxicol In Vitro 25(3):684–691. doi:10.1016/j.tiv.2011.01.009
Caloni F, Spotti M, Auerbach H, den Camp HO, Gremmels JF, Pompa G (2000) In vitro metabolism of fumonisin B1 by ruminal microflora. Vet Res Commun 24(6):379–387
Caloni F, Spotti M, Pompa G, Zucco F, Stammati A, De Angelis I (2002) Evaluation of Fumonisin B(1) and its metabolites absorption and toxicity on intestinal cells line Caco-2. Toxicon 40(8):1181–1188
Cheng YH, Ding ST, Chang MH (2006) Effect of fumonisins on macrophage immune functions and gene expression of cytokines in broilers. Arch Anim Nutr 60(4):267–276. doi:10.1080/17450390600785079
Cig B, Naziroglu M (2015) Investigation of the effects of distance from sources on apoptosis, oxidative stress and cytosolic calcium accumulation via TRPV1 channels induced by mobile phones and Wi-Fi in breast cancer cells. Biochim Biophys Acta. doi:10.1016/j.bbamem.2015.02.013
Colvin BM, Harrison LR (1992) Fumonisin-induced pulmonary edema and hydrothorax in swine. Mycopathologia 117(1–2):79–82
Creppy EE (2002) Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol Lett 127(1–3):19–28
Dall’Asta C, Mangia M, Berthiller F et al (2009) Difficulties in fumonisin determination: the issue of hidden fumonisins. Anal Bioanal Chem 395(5):1335–1345. doi:10.1007/s00216-009-2933-3
Dinkova-Kostova AT, Talalay P (2008) Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res 52(Suppl 1):S128–S138. doi:10.1002/mnfr.200700195
Domijan AM, Abramov AY (2011) Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis—implication to mechanism of cell toxicity. Int J Biochem Cell Biol 43(6):897–904. doi:10.1016/j.biocel.2011.03.003
Domijan AM, Zeljezic D, Kopjar N, Peraica M (2006) Standard and Fpg-modified comet assay in kidney cells of ochratoxin A- and fumonisin B(1)-treated rats. Toxicology 222(1–2):53–59. doi:10.1016/j.tox.2006.01.024
Domijan AM, Peraica M, Vrdoljak AL, Radic B, Zlender V, Fuchs R (2007) The involvement of oxidative stress in ochratoxin A and fumonisin B1 toxicity in rats. Mol Nutr Food Res 51(9):1147–1151. doi:10.1002/mnfr.200700079
Domijan A, Zeljezic D, Peraica M et al (2008) Early toxic effects of fumonisin B1 in rat liver. Hum Exp Toxicol 27(12):895–900. doi:10.1177/0960327108100418
Domijan AM, Gajski G, Jovanovic IN, Geric M, Garaj-Vrhovac V (2015) In vitro genotoxicity of mycotoxins ochratoxin A and fumonisin B(1) could be prevented by sodium copper chlorophyllin—implication to their genotoxic mechanism. Food Chem 170:455–462. doi:10.1016/j.foodchem.2014.08.036
El-Nekeety AA, El-Kholy W, Abbas NF, Ebaid A, Amra HA, Abdel-Wahhab MA (2007) Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon 50(2):256–269. doi:10.1016/j.toxicon.2007.03.017
Escobedo J, Pucci AM, Koh TJ (2004) HSP25 protects skeletal muscle cells against oxidative stress. Free Radic Biol Med 37(9):1455–1462. doi:10.1016/j.freeradbiomed.2004.07.024
European Commission (2003) Updated opinion of the Scientific Committee on Food (SCF) on Fumonisin B1, B2 and B3. http://ec.europa.eu/food/fs/sc/scf/out185_en.pdf
Ferns G, Shams S, Shafi S (2006) Heat shock protein 27: its potential role in vascular disease. Int J Exp Pathol 87(4):253–274. doi:10.1111/j.1365-2613.2006.00484.x
Ferrante MC, Meli R, Mattace Raso G et al (2002) Effect of fumonisin B1 on structure and function of macrophage plasma membrane. Toxicol Lett 129(3):181–187
Fodor J, Meyer K, Gottschalk C et al (2007) In vitro microbial metabolism of fumonisin B1. Food Addit Contam 24(4):416–420. doi:10.1080/02652030701216461
Fu M, Li R, Guo C, Pang M, Liu Y, Dong J (2015) Natural incidence of Fusarium species and fumonisins B1 and B2 associated with maize kernels from nine provinces in China in 2012. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32(4):503–511. doi:10.1080/19440049.2014.976846
Galvano F, Russo A, Cardile V, Galvano G, Vanella A, Renis M (2002) DNA damage in human fibroblasts exposed to fumonisin B(1). Food Chem Toxicol 40(1):25–31
Gelderblom WC, Marasas WF, Vleggaar R, Thiel PG, Cawood ME (1992) Fumonisins: isolation, chemical characterization and biological effects. Mycopathologia 117(1–2):11–16
Gelineau-van Waes J, Starr L, Maddox J et al (2005) Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Res A 73(7):487–497. doi:10.1002/bdra.20148
Gelineau-van Waes J, Voss KA, Stevens VL, Speer MC, Riley RT (2009) Maternal fumonisin exposure as a risk factor for neural tube defects. Adv Food Nutr Res 56:145–181. doi:10.1016/S1043-4526(08)00605-0
Gherbawy Y, Elhariry H, Kocsube S et al (2015) Molecular characterization of black Aspergillus species from onion and their potential for ochratoxin A and fumonisin B2 production. Foodborne Pathog Dis. doi:10.1089/fpd.2014.1870
Grenier B, Bracarense AP, Schwartz HE et al (2012) The low intestinal and hepatic toxicity of hydrolyzed fumonisin B(1) correlates with its inability to alter the metabolism of sphingolipids. Biochem Pharmacol 83(10):1465–1473. doi:10.1016/j.bcp.2012.02.007
Hahn I, Nagl V, Schwartz-Zimmermann HE et al (2015) Effects of orally administered fumonisin B(1) (FB(1)), partially hydrolysed FB(1), hydrolysed FB(1) and N-(1-deoxy-d-fructos-1-yl) FB(1) on the sphingolipid metabolism in rats. Food Chem Toxicol 76:11–18. doi:10.1016/j.fct.2014.11.020
Harrer H, Laviad EL, Humpf HU, Futerman AH (2013) Identification of N-acyl-fumonisin B1 as new cytotoxic metabolites of fumonisin mycotoxins. Mol Nutr Food Res 57(3):516–522. doi:10.1002/mnfr.201200465
Harrer H, Humpf HU, Voss KA (2015) In vivo formation of N-acyl-fumonisin B1. Mycotoxin Res 31(1):33–40. doi:10.1007/s12550-014-0211-5
Hartinger D, Schwartz H, Hametner C, Schatzmayr G, Haltrich D, Moll WD (2011) Enzyme characteristics of aminotransferase FumI of Sphingopyxis sp. MTA144 for deamination of hydrolyzed fumonisin B(1). Appl Microbiol Biotechnol 91(3):757–768. doi:10.1007/s00253-011-3248-9
Hassan AM, Mohamed SR, El-Nekeety AA, Hassan NS, Abdel-Wahhab MA (2010) Aquilegia vulgaris L. extract counteracts oxidative stress and cytotoxicity of fumonisin in rats. Toxicon 56(1):8–18. doi:10.1016/j.toxicon.2010.03.006
Hassan AM, Abdel-Aziem SH, El-Nekeety AA, Abdel-Wahhab MA (2014) Panax ginseng extract modulates oxidative stress, DNA fragmentation and up-regulate gene expression in rats sub chronically treated with aflatoxin B and fumonisin B. Cytotechnology. doi:10.1007/s10616-014-9726-z
He Q, Suzuki H, Sharma RP (2006) S-adenosylmethionine or 5′-methylthioadenosine are unable to prevent fumonisin B1 hepatotoxicity in mice despite increased oxidation in liver. J Appl Toxicol 26(6):509–516. doi:10.1002/jat.1170
Hendrich S, Miller KA, Wilson TM, Murphy PA (1993) Toxicity of Fusarium proliferatum fermented nixtamalized corn-based diets feed to rats: effect of nutritional status. J Agric Food Chem 41:1649–1654
Higuchi Y (2003) Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem Pharmacol 66(8):1527–1535. doi:10.1016/S0006-2952(03)00508-2
Howard PC, Couch LH, Patton RE et al (2002) Comparison of the toxicity of several fumonisin derivatives in a 28-day feeding study with female B6C3F(1) mice. Toxicol Appl Pharmacol 185(3):153–165
Humpf HU, Schmelz EM, Meredith FI et al (1998) Acylation of naturally occurring and synthetic 1-deoxysphinganines by ceramide synthase. Formation of N-palmitoyl-aminopentol produces a toxic metabolite of hydrolyzed fumonisin, AP1, and a new category of ceramide synthase inhibitor. J Biol Chem 273(30):19060–19064
Ihsan A, Wang X, Liu Z et al (2011) Long-term mequindox treatment induced endocrine and reproductive toxicity via oxidative stress in male Wistar rats. Toxicol Appl Pharmacol 252(3):281–288. doi:10.1016/j.taap.2011.02.020
International Agency for Research on Cancer (IARC) (2002) Fumonisin B1. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. Monographs on the evaluation of carcinogenic risks to humans, vol 82. IARC, Lyon, pp 301–366. doi:10.1016/j.fct.2009.12.006
Kang YJ, Alexander JM (1996) Alterations of the glutathione redox cycle status in fumonisin B1-treated pig kidney cells. J Biochem Toxicol 11(3):121–126
Klaric MS, Pepeljnjak S, Domijan AM, Petrik J (2007) Lipid peroxidation and glutathione levels in porcine kidney PK15 cells after individual and combined treatment with fumonisin B(1), beauvericin and ochratoxin A. Basic Clin Pharmacol Toxicol 100(3):157–164. doi:10.1111/j.1742-7843.2006.00019.x
Kotan E, Alpsoy L, Anar M, Aslan A, Agar G (2011) Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB(1) in human lymphocytes in vitro. Toxicol Ind Health 27(7):599–605. doi:10.1177/0748233710394234
Kouadio JH, Mobio TA, Baudrimont I, Moukha S, Dano SD, Creppy EE (2005) Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 213(1–2):56–65. doi:10.1016/j.tox.2005.05.010
Li F, Jiang D, Zheng F, Chen J, Li W (2015a) Fumonisins B, B and B in corn products, wheat flour and corn oil marketed in Shandong province of China. Food Addit Contam Part B Surveill. doi:10.1080/19393210.2015.1028480
Li R, Guo C, Zhang Q, Pang M, Liu Y, Dong J (2015b) Fumonisins B1 and B2 in maize harvested in Hebei province, China, during 2011–2013. Food Addit Contam Part B Surveill 8(1):1–6. doi:10.1080/19393210.2014.940401
Logrieco A, Ferracane R, Visconti A, Ritieni A (2010) Natural occurrence of fumonisin B2 in red wine from Italy. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27(8):1136–1141. doi:10.1080/19440041003716547
Mansson M, Klejnstrup ML, Phipps RK et al (2010) Isolation and NMR characterization of fumonisin B2 and a new fumonisin B6 from Aspergillus niger. J Agric Food Chem 58(2):949–953. doi:10.1021/jf902834g
Marasas WF, Riley RT, Hendricks KA et al (2004) Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr 134(4):711–716
Marin S, Ramos AJ, Vazquez C, Sanchis V (2007) Contamination of pine nuts by fumonisin produced by strains of Fusarium proliferatum isolated from Pinus pinea. Lett Appl Microbiol 44(1):68–72. doi:10.1111/j.1472-765X.2006.02028.x
Marnewick JL, van der Westhuizen FH, Joubert E, Swanevelder S, Swart P, Gelderblom WC (2009) Chemoprotective properties of rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) herbal and green and black (Camellia sinensis) teas against cancer promotion induced by fumonisin B1 in rat liver. Food Chem Toxicol 47(1):220–229. doi:10.1016/j.fct.2008.11.004
Marschik S, Hepperle J, Lauber U et al (2013) Extracting fumonisins from maize: efficiency of different extraction solvents in multi-mycotoxin analytics. Mycotoxin Res 29(2):119–129. doi:10.1007/s12550-013-0163-1
Martinez-Larrañaga MR, Anadon A, Diaz MJ et al (1996) Induction of cytochrome P4501A1 and P4504A1 activities and peroxisomal proliferation by fumonisin B1. Toxicol Appl Pharmacol 141(1):185–194. doi:10.1006/taap.1996.0275
Martins ML, Martins HM, Bernardo F (2001) Fumonisins B1 and B2 in black tea and medicinal plants. J Food Prot 64(8):1268–1270
Mary VS, Theumer MG, Arias SL, Rubinstein HR (2012) Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology 302(2–3):299–307. doi:10.1016/j.tox.2012.08.012
Mateo JJ, Jimenez M (2000) Trichothecenes and fumonisins produced in autoclaved tiger nuts by strains of Fusarium sporotrichioides and Fusarium moniliforme. Food Microbiol 17(2):167–176. doi:10.1006/fmic.1999.0301
Matsuzawa A, Ichijo H (2005) Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal 7(3–4):472–481. doi:10.1089/ars.2005.7.472
Minervini F, Garbetta A, D’Antuono I et al (2014) Toxic mechanisms induced by fumonisin b1 mycotoxin on human intestinal cell line. Arch Environ Contam Toxicol 67(1):115–123. doi:10.1007/s00244-014-0004-z
Mishra S, Dwivedi PD, Pandey HP, Das M (2014) Role of oxidative stress in Deoxynivalenol induced toxicity. Food Chem Toxicol 72:20–29. doi:10.1016/j.fct.2014.06.027
Mobio TA, Tavan E, Baudrimont I et al (2003) Comparative study of the toxic effects of fumonisin B1 in rat C6 glioma cells and p53-null mouse embryo fibroblasts. Toxicology 183(1–3):65–75
Nielsen KF, Ngemela AF, Jensen LB, de Medeiros LS, Rasmussen PH (2015) UHPLC-MS/MS determination of ochratoxin A and fumonisins in coffee using QuEChERS extraction combined with mixed-mode SPE purification. J Agric Food Chem 63(3):1029–1034. doi:10.1021/jf504254q
Pagliuca G, Zironi E, Ceccolini A, Matera R, Serrazanetti GP, Piva A (2005) Simple method for the simultaneous isolation and determination of fumonisin B1 and its metabolite aminopentol-1 in swine liver by liquid chromatography—fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 819(1):97–103. doi:10.1016/j.jchromb.2005.01.035
Perrone G, De Girolamo A, Sarigiannis Y, Haidukowski ME, Visconti A (2013) Occurrence of ochratoxin A, fumonisin B2 and black aspergilli in raisins from Western Greece regions in relation to environmental and geographical factors. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 30(7):1339–1347. doi:10.1080/19440049.2013.796594
Pirinccioglu AG, Gokalp D, Pirinccioglu M, Kizil G, Kizil M (2010) Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin Biochem 43(15):1220–1224. doi:10.1016/j.clinbiochem.2010.07.022
Poersch AB, Trombetta F, Braga AC et al (2014) Involvement of oxidative stress in subacute toxicity induced by fumonisin B1 in broiler chicks. Vet Microbiol 174(1–2):180–185. doi:10.1016/j.vetmic.2014.08.020
Ramesh T, Kim SW, Hwang SY, Sohn SH, Yoo SK, Kim SK (2012a) Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res 32(9):718–726. doi:10.1016/j.nutres.2012.08.005
Ramesh T, Kim SW, Sung JH et al (2012b) Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp Gerontol 47(1):77–84. doi:10.1016/j.exger.2011.10.007
Raynal M, Bailly JD, Benard G, Guerre P (2001) Effects of fumonisin B1 present in Fusarium moniliforme culture material on drug metabolising enzyme activities in ducks. Toxicol Lett 121(3):179–190
Rheeder JP, Marasas WFO, Thiel TG, Sydenham EW, Shephard GS, Van Schalkwyk DJ (1992) Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathology 82:353–357
Riet-Correa F, Rivero R, Odriozola E, Adrien Mde L, Medeiros RM, Schild AL (2013) Mycotoxicoses of ruminants and horses. J Vet Diagn Invest 25(6):692–708. doi:10.1177/1040638713504572
Ross PF, Nelson PE, Owens DL, Rice LG, Nelson HA, Wilson TM (1994) Fumonisin B2 in cultured Fusarium proliferatum, M-6104, causes equine leukoencephalomalacia. J Vet Diagn Invest 6(2):263–265
Rumora L, Domijan AM, Grubisic TZ, Peraica M (2007) Mycotoxin fumonisin B1 alters cellular redox balance and signalling pathways in rat liver and kidney. Toxicology 242(1–3):31–38. doi:10.1016/j.tox.2007.09.006
Sadler TW, Merrill AH, Stevens VL, Sullards MC, Wang E, Wang P (2002) Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology 66(4):169–176. doi:10.1002/tera.10089
Scott PM (2012) Recent research on fumonisins: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 29(2):242–248. doi:10.1080/19440049.2010.546000
Seefelder W, Humpf HU, Schwerdt G, Freudinger R, Gekle M (2003) Induction of apoptosis in cultured human proximal tubule cells by fumonisins and fumonisin metabolites. Toxicol Appl Pharmacol 192(2):146–153
Seiferlein M, Humpf HU, Voss KA et al (2007) Hydrolyzed fumonisins HFB1 and HFB2 are acylated in vitro and in vivo by ceramide synthase to form cytotoxic N-acyl-metabolites. Mol Nutr Food Res 51(9):1120–1130. doi:10.1002/mnfr.200700118
Seo DG, Phat C, Kim DH, Lee C (2013) Occurrence of Fusarium mycotoxin fumonisin B1 and B2 in animal feeds in Korea. Mycotoxin Res 29(3):159–167. doi:10.1007/s12550-013-0172-0
Shephard GS, Van Der Westhuizen L, Sewram V (2007) Biomarkers of exposure to fumonisin mycotoxins: a review. Food Addit Contam 24(10):1196–1201. doi:10.1080/02652030701513818
Shi J, Sun B, Shi W et al (2015) Decreasing GSH and increasing ROS in chemosensitivity gliomas with IDH1 mutation. Tumour Biol 36(2):655–662. doi:10.1007/s13277-014-2644-z
Smith GW, Constable PD, Tumbleson ME, Rottinghaus GE, Haschek WM (1999) Sequence of cardiovascular changes leading to pulmonary edema in swine fed culture material containing fumonisin. Am J Vet Res 60(10):1292–1300
Sorensen LM, Lametsch R, Andersen MR, Nielsen PV, Frisvad JC (2009) Proteome analysis of Aspergillus niger: lactate added in starch-containing medium can increase production of the mycotoxin fumonisin B2 by modifying acetyl-CoA metabolism. BMC Microbiol 9:255. doi:10.1186/1471-2180-9-255
Spotti M, Pompa G, Caloni F (2001) Fumonisin B1 metabolism by bovine liver microsomes. Vet Res Commun 25(6):511–516
Stockmann-Juvala H, Savolainen K (2008) A review of the toxic effects and mechanisms of action of fumonisin B1. Hum Exp Toxicol 27(11):799–809. doi:10.1177/0960327108099525
Stockmann-Juvala H, Mikkola J, Naarala J, Loikkanen J, Elovaara E, Savolainen K (2004a) Oxidative stress induced by fumonisin B1 in continuous human and rodent neural cell cultures. Free Radic Res 38(9):933–942. doi:10.1080/10715760412331273205
Stockmann-Juvala H, Mikkola J, Naarala J, Loikkanen J, Elovaara E, Savolainen K (2004b) Fumonisin B1-induced toxicity and oxidative damage in U-118MG glioblastoma cells. Toxicology 202(3):173–183. doi:10.1016/j.tox.2004.05.002
Sun GJ, Wang SK, Wang JS (2006) The combined toxity of two kinds of mycotoxin in Sprague–Dawley rats. Zhonghua yu fang yi xue za zhi (Chinese J Prev Med) 40(5):319–323
Theumer MG, Lopez AG, Masih DT, Chulze SN, Rubinstein HR (2002) Immunobiological effects of fumonisin B1 in experimental subchronic mycotoxicoses in rats. Clin Diagn Lab Immunol 9(1):149–155
Theumer MG, Lopez AG, Masih DT, Chulze SN, Rubinstein HR (2003) Immunobiological effects of AFB1 and AFB1-FB1 mixture in experimental subchronic mycotoxicoses in rats. Toxicology 186(1–2):159–170
Theumer MG, Canepa MC, Lopez AG, Mary VS, Dambolena JS, Rubinstein HR (2010) Subchronic mycotoxicoses in Wistar rats: assessment of the in vivo and in vitro genotoxicity induced by fumonisins and aflatoxin B(1), and oxidative stress biomarkers status. Toxicology 268(1–2):104–110. doi:10.1016/j.tox.2009.12.007
Voss KA, Bacon CW, Meredith FI, Norred WP (1996) Comparative subchronic toxicity studies of nixtamalized and water-extracted Fusarium moniliforme culture material. Food Chem Toxicol 34(7):623–632
Voss KA, Smith GW, Haschek WM (2007) Fumonisins: toxicokinetics, mechanism of action and toxicity. Anim Feed Sci Tech 137(3–4):299–325. doi:10.1016/j.anifeedsci.2007.06.007
Voss KA, Riley RT, Snook ME, Waes JG (2009) Reproductive and sphingolipid metabolic effects of fumonisin B(1) and its alkaline hydrolysis product in LM/Bc mice: hydrolyzed fumonisin B(1) did not cause neural tube defects. Toxicol Sci 112(2):459–467. doi:10.1093/toxsci/kfp215
Wang H, Wei H, Ma J, Luo X (2000) The fumonisin B1 content in corn from North China, a high-risk area of esophageal cancer. J Environ Pathol Toxicol Oncol 19(1–2):139–141
Wang X, Liu Q, Ihsan A et al (2012) JAK/STAT pathway plays a critical role in the proinflammatory gene expression and apoptosis of RAW264.7 cells induced by trichothecenes as DON and T-2 toxin. Toxicol Sci 127(2):412–424. doi:10.1093/toxsci/kfs106
Wu QH, Wang X, Yang W et al (2014) Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update. Arch Toxicol 88(7):1309–1326. doi:10.1007/s00204-014-1280-0
Yang HY, Lee TH (2015) Antioxidant enzymes as redox-based biomarkers: a brief review. BMB Rep 48(4):200–208
Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T (1997) Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385(6617):637–640. doi:10.1038/385637a0
Zhou F, Sun W, Zhao M (2015) Controlled formation of emulsion gels stabilized by salted myofibrillar protein under malondialdehyde (MDA)-induced oxidative stress. J Agric Food Chem 63(14):3766–3777. doi:10.1021/jf505916f
Zitomer NC, Mitchell T, Voss KA et al (2009) Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J Biol Chem 284(8):4786–4795. doi:10.1074/jbc.M808798200
Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM (2015) Biological and physiological role of ROS-the good, the bad and the ugly. Acta Physiol. doi:10.1111/apha.12515
Acknowledgments
This work was supported by Grants from 948 of the Ministry of Agriculture Project (2014-S12), International Cooperation Project (4002-122002), National Basic Research Program of China (2009C118800), Project of Excellence FIM UHK and Project Ref. S2013/ABI-2728 (ALIBIRD-CM Program) from Comunidad de Madrid, Spain.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wang, X., Wu, Q., Wan, D. et al. Fumonisins: oxidative stress-mediated toxicity and metabolism in vivo and in vitro. Arch Toxicol 90, 81–101 (2016). https://doi.org/10.1007/s00204-015-1604-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1604-8