Abstract
The aim of this study was to assess the efficacy and tolerability of balneotherapy (BT) in patients with primary fibromyalgia syndrome (FS). In a prospective, randomized, controlled, double-blind trial with a 6-month follow-up, 100 FS patients were randomized to receive a cycle of BT with highly mineralized sulfate water (BT group) or with tap water (control group). Clinical assessments were performed at screening visit, at basal time, and after treatment (2 weeks, 3 and 6 months). The primary outcome measures were the change of global pain on the Visual Analogue Scale (VAS) and Fibromyalgia Impact Questionnaire total score (FIQ-Total) from baseline to 15 days. Secondary outcomes included Widespread Pain Index, Symptom Severity Scale Score, Short Form Health Survey, State-Trait Anxiety Inventory (STAI), and Center for Epidemiologic Studies Depression Scale. We performed an intent-to-treat analysis. The Kolmogorov-Smirnov test was applied to verify the normality distribution of all quantitative variables and the Student’s t test to compare sample data. In the BT group, we observed a significant improvement of VAS and FIQ-Total at the end of the treatment that persisted until 6 months, while no significant differences were found in the control group. The differences between groups were significant for primary parameters at each time point. Similar results were obtained for the other secondary outcomes except for the STAI outcome. Adverse events were reported by 10 patients in the BT group and by 22 patients in the control group. Our results support the short- and long-term therapeutic efficacy of BT in FS. Trial registration: NCT02548065
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibromyalgia syndrome (FS) is a musculoskeletal disorder characterized by chronic widespread pain, tenderness at marked sites of the body called tender points, and fatigue. It is often accompanied by a broad array of subjective symptoms such as sleep disturbances, headache, irritable bowel syndrome, morning stiffness, paresthesia of the extremities, frequent psychological distress, and depressed mood [1]. The prevalence of FS ranges from 2.1 to 2.4% in women and 1.8% in men [2]. Patients with FS experience disability and poor quality of life (QoL) [3]. Various pharmacological treatments including analgesic or non-steroidal anti-inflammatory drugs, opioids, anticonvulsivants, serotonin and noradrenaline reuptake inhibitors, selective serotonin reuptake inhibitors, growth hormone, and muscle relaxants have been used to treat FS [4]. However, the efficacy of these different drugs has been modest or poor.
Optimal management of FS consists of a multidisciplinary approach with a combination of pharmacological modalities and non-pharmacological treatments, such as hot and cold applications, balneotherapy (BT), patient education, and physical exercises [4,5,6]. BT represents a possible non-pharmacological and complementary intervention into the therapeutic strategy for patients with FS.
BT traditionally involves immersion in mineral and/or thermal waters from natural springs and should not be confused with bathing in tap water (hydrotherapy).
Various randomized clinical trials (RCT), meta-analysis, and systematic review showed a significant improvement in pain and tender point counts (TPC) at the end of a cycle of BT lasting in the medium long-term follow-up (3–6 months) [7,8,9,10]. Furthermore, despite low tolerance of physical treatments by FS patients, BT seems to be well-tolerated and have a lower percentage of side effects, which also are less severe, than those associated with pharmacological treatments. However, some aspects of these studies are criticizable and could be a source of bias such as the lack of double blind experimental design and the small number of patients included in the trials.
The aim of the present randomized double-blind controlled study was to investigate the efficacy of BT with a highly mineralized sulfate water on global pain and Fibromyalgia Impact Questionnaire total score (FIQ-Total) as primary end points in patients with primary FS. Secondary objectives were to evaluate the effects on QoL, anxiety, and depressive symptoms and to analyze the safety profile of BT. The follow-up period was extended to 6 months following study start to determine maintenance of the efficacy.
Our hypothesis is that BT is safe and superior to bath in tap water in short- and long-term follow-up in patients with FS.
Materials and methods
Trial design
This 24-weeks prospective randomized, controlled, parallel group, double-blind trial was conducted between April 2015 and November 2015. All potential participants were recruited at Rheumatology Unit of Trento (Italy) by a rheumatologist; treatments and all following assessments were performed at Levico Terme Spa Center (Trento, Italy). All eligible patients provided a written informed consent.
The study protocol followed the Principles of the Declaration of Helsinki 1964 and later amendments and was approved by the Ethics Committee of Santa Chiara-Trento (Italy) (decision no. 2/2014, March 20, 2014) and registered on http://www.clinicaltrials.gov (NCT02548065).
Participants
Inclusion criteria
Patients of both sexes who met the 2010 American College of Rheumatology (ACR) criteria for primary FS [11] and aged between 18 and 65 years were eligible for this study. All subjects resided in the rural area near the spa of Levico Terme within a 30-km radius, continued to live at home, and carried out their daily routines during the study period. Furthermore, entry criteria included (1) Widespread Pain Index (WPI) ≥ 7 and Symptom Severity Scale Score (SS) ≥ 5 or WPI 3–6 and SS ≥ 9 at screening (7 days before enrollment) and at baseline visit (time of enrollment); (2) stable doses of drugs for at least 4 weeks; and (3) are able to understand and willing to follow the study protocol.
Exclusion criteria
Exclusion criteria from the study included (1) severe comorbidity of the heart, liver, kidney, systemic blood disease, and neoplasms in the last 5 years; (2) concurrent autoimmune or inflammatory diseases (rheumatoid arthritis, systemic lupus erythematous, inflammatory bowel disease, etc.); (3) severe psychiatric illnesses (as current schizophrenia, major depression with suicidal ideation) within 2 years; (4) routine daily use of narcotic analgesics or history of substance abuse; and (5) pregnancy and nursing.
Also, we excluded patients taking part in another interventional trial or who had spa treatments 6 months previously prior to the screening visit and patients unable to understand consent form and the questionnaires.
Treatments
After the screening, patients were randomly assigned to receive a cycle of BT with Vetriolo’s water (VW) (BT group) or with tap water (control group). VW is a highly mineralized (fixed residue at 180 °C 1702 mg/l) strongly acidic (pH 5.7) sulfate (1100 mg/l) water, rich in calcium (111.0 mg/l), magnesium (65.5 mg/l), and iron (315 mg/l). The patients in the BT group were treated with daily immersion in a bathtub containing 150 l of VW at 36 °C for 15 min. The control group was treated with 36 °C heated tap water. The color of the tap water was changed to resemble that of the VW through the addition of powdered red bush (rooibos) tea. An independent blinded physician supervised the treatment to detect possible adverse events.
All patients in both groups had 15 min of bed rest after each treatment and received BT six times a week, for 2 consecutive weeks in the same place (spa center of Levico Terme).
Patients in both groups continued their established pharmacological and non-pharmacological treatments (physical exercise and/or physical and cognitive behavioral therapy) started before the screening visit.
Washout of concomitant symptomatic drugs was required for a week before the assessment of basal time with the exception of acetaminophen (≤ 3 g/day) taken as rescue therapy. However, all symptomatic drugs should be stopped 24 h before each visit to avoid masking symptoms of pain. Patients were advised to take acetaminophen only if needed.
Outcomes
The primary outcome measures were global pain during the last 24 h prior to assessment on a 0–10-cm Visual Analogue Scale (VAS) with 0 = “no pain” and 10 = “the worst pain possible” and FIQ-Total. FIQ, an extensively validated patient self-report questionnaire, evaluates the impact of FS on daily life [12, 13]. In this work, we used the Italian version of the FIQ [14]. It ranges from 0 to 100, with higher scores indicating greater impact of FS on functioning [12,13,14].
The secondary outcome measures included WPI, SS, Short Form Health Survey (SF-12), State-Trait Anxiety Inventory (STAI), and Center for Epidemiologic Studies Depression Scale (CES-D). WPI identifies body areas where pain or tenderness was felt during the previous 7 days, with a total of 19 body areas identified; the maximum score is 19 [11]. The SS score includes two parts (SS score 2a and SS score 2b): The first one evaluates three major symptoms (fatigue, trouble thinking or remembering and waking up tired, which can be coded 0–3 (0 = no problem;1 = slight or mild problems; 2 = moderate, considerable problems; and 3 = severe, continuous, life-disturbing problems), and the second one investigates three additional symptoms (pain or cramps in lower abdomen, depression, headache), which can be coded as present (1) or absent (0) (total sub-score 0–3). These three items are surrogates for the somatic symptom burden item of the ACR 2010 criteria [11]. The total SS score ranges from 0 to 12. The 12-item SF-12 is a short version of SF-36 that is a widely used measure of health and well-being, validated in multiple countries, including Italy [15]. SF-12 comprises two main domains: the physical component score (PCS) and the mental component score (MCS), and eight scales for assessing eight dimensions: physical functioning, physical role, social role, emotional role, bodily pain, general health, vitality, and mental health. Scores range from “0 to 100” where “0” indicates the worst condition and “100” indicates the best possible condition [16].
STAI is a self-report questionnaire with two independent 20-item scales (STAI T-Anxiety Scale or Form X-2 and STAI S-Anxiety Scale or Form X-1) for measuring state-related or trait-related anxiety [17]. A high score on the STAI corresponds to a high level of anxiety symptoms. We used the Italian validated version [18]. The 20-item CES-D Scale is frequently used to estimate the prevalence of depressive symptomatology in the general population. Respondents rate the frequency with which they have experienced particular depressive symptoms during the past week. Responses to each item range from 0 (less than 1 day) to 3 (5–7 days) and are summed to compute a total score [19]. The Italian version measures one depression factor scoring from 0 to 60; scores of 16 or above are considered cases of depression [20].
Clinical assessments of the patients were performed at the screening visit, at basal time, after 2 weeks, and after 3 and 6 months following the beginning of the study.
All assessments were performed by two blinded investigators (ST and RB).
Safety parameters
All adverse events, whether reported spontaneously by the patients or observed by the physician, were reported in a diary, noting the severity and any possible correlations with the treatment. Serious adverse events were to be reported immediately to the University of Siena’s Rheumatology Unit and resulted in the patient’s exclusion from the treatment.
Sample size
The sample size was determined for the primary outcomes, VAS, and overall score of FIQ-Total. According to previous studies [21, 22], we considered a difference of 2.0 points on the VAS scale and a change of 20% in the FIQ-Total at 15 days as a clinically meaningful difference in pain intensity and function impairment. Assuming a maximum dropout rate of 15% with a standard deviation of 2 cm for the VAS score and of 20 points for the FIQ-Total, a sample size of 50 patients in each group was required to obtain a power analysis of 0.80 with α = 0.05.
Randomization and blinding
Patients fulfilled the screening criteria after signed the informed consent were randomized 1:1 and allocated to one of two groups using a computer-generated table of random numbers.
Randomization was performed immediately before the basal time visit.
The principal investigator (AF) was provided with individual envelopes, each containing patient codes, corresponding to the treatment assignment. The investigator who assigned patients to their randomized treatment (GP), the assessors who performed the patient evaluation, the medical team and the nurses of the spa center, and the statistician were blinded to the treatment allocation. The result of the randomization was known exclusively by the bath assistant who filled the bathtubs with the mineral water or tap water.
The patients did not know which treatment they received, and to verify if the blinding was successful, the bath assistant asked them, at the end of the course.
Statistical analysis
The main analysis for efficacy and safety was an intent-to-treat (ITT) analysis. Missing follow-up assessments were replaced using the last observation carried forward (LOCF) method.
Descriptive statistics for each group was performed by calculating mean and standard deviation for quantitative variables, frequency counts, and percentage for qualitative variables.
The Kolmogorov-Smirnov test was applied to verify the normality distribution of all quantitative variables. The Student’s t test was used to compare sample data for normally distributed quantitative variables. The corresponding non-parametric tests of Wilcoxon or Mann-Whitney were used for non-normal dependent or independent data, respectively.
A significance level of 95% was chosen for all statistical analyses executed using the SPSS statistical software, version 10.
Results
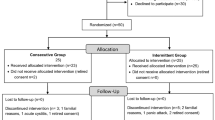
Between 1 April 2015 and 1 November 2015, 273 patients were screened and 100 met the inclusion criteria. These patients were enrolled in the clinical trial and included in the ITT analysis (50 patients in the BT group and 50 patients in the control group) (Fig. 1).
The baseline demographic and clinical characteristics of the study participants were similar between the two groups (Table 1). Most study patients were female (95%), slightly overweight, with a median BMI of 25.49 ± 4.70 and with a mean duration of FS of 15.55 ± 11.73.
As shown in Table 2, there were no significant differences at baseline between the two groups concerning the outcome measures, except for SF-12 PCS (P = 0.009).
The changes from baseline in patient’s assessment of global pain measured by VAS and in FIQ total score are reported in Fig. 2a, b. Concerning measurement of pain by VAS in the BT group, we observed a statistically significant (P < 0.0001) reduction at the end of treatment (2 weeks) that persisted at 3- and 6-month follow-up (Fig. 2a) (Table 3). In the control group, there were no significant differences at the end of treatment and during the entire follow-up period. The differences between the BT and control group were already significant at week 2 (P < 0.01) and were maintained after 3 (P < 0.0001) and 6 (P < 0.001) months. The FIQ total score in the BT group resulted significantly improved after 2 weeks (P < 0.001) and persisted significantly until 6 months (P < 0.0001) (Fig. 2b), while in the control group, no significant differences were observed. The difference between the two groups was significant at the end of the treatment (P < 0.05) and during the follow-up period (P < 0.001) (Fig. 2b) (Table 2).
Changes in Global pain using a Visual Analogue Scale (VAS) (a) and in Fibromyalgia Impact Questionnaire (FIQ) total score (b) during follow-up period by treatment group. Data are expressed as mean ± SD. ***P < 0.001; ****P < 0.0001 2 weeks vs baseline vs; 3 months vs baseline vs; 6 months vs baseline. °P < 0.05, °°P < 0.01; °°°P < 0.001; °°°°P < 0.0001 BT vs control groups
WPI significantly decreased at the end of the treatment both in the BT and control group, and this reduction persisted during the follow-up (P < 0.0001 and P < 0.01, respectively). The decrease of WPI was more significant in BT in comparison to the control group after 2 weeks (P < 0.05) and until 6 months (P < 0.0001). For what concerns SS total score and the sub-scores 2a and 2b, they significantly reduced only in the BT group at the end of the BT cycle (P < 0.01 for SS 2a and SS total; P < 0.05 for SS 2b) and until 6 months (P < 0.0001 for SS 2a and SS total; P < 0.001 for SS 2b). SF-36 PCS resulted at baseline significantly higher in the BT group than in the control group (P < 0.01), and the difference between groups persisted during the follow-up (P < 0.0001). SF-12 MCS and CES-D significantly improved in the BT group at the end of the treatment (P < 0.01) and were sustained until 6 months of follow-up (P < 0.0001 and P < 0.05, respectively); the differences between groups were significant after 3 (P < 0.05) and 6 months (P < 0.0001 for SF-12 MCS; P < 0.05 for CES-D) from the treatment. No significant differences between the basal time and all other times were observed in both groups for STAI score (Table 2).
Generally, the treatment was well-tolerated; during the cycle of BT, adverse events occurred in 10 (20%) patients of the BT group and in 22 (44%) patients within the control group. However, in the BT group, the side effects observed were transient and of light intensity, and no patient discontinued the therapy, while in the control group, two patients interrupted the therapy for exacerbation of symptoms (after five BT sessions in one case and after seven sessions in the other) (Table 3).
Furthermore, we notified that during the follow-up period, no adverse events or comorbidities able to influence our results, in particular the indexes of QoL, occurred.
Discussion
This paper aimed to assess the efficacy and tolerability of a cycle of BT with highly mineralized sulfate water in patients affected by primary FS. The results obtained indicate that BT provides a rapid and persistent belief in controlling the global pain and improving the function; indeed, the decrease of VAS score and FIQ was already significant at the end of the BT cycle and persisted after 6 months. Significant improvements were also reported in the BT group for WPI, SS, SF-12 MCS, and CES-D.
Nowadays, the management of FS is still a challenge, and it is generally accepted that a multidisciplinary approach with a combination of non-pharmacological and pharmacological treatments should be preferred and shared with the patient. At this regard, an increasing number of health professionals consider the non-pharmacological therapy the first choice of treatment and encourage a patient-tailored approach. In this context, BT represents an interesting alternative, and it was included in the European League Against Rheumatology (EULAR) revised recommendations for the management of FS [4].
The results of the current study strongly supported this evidence with a superior methodological quality than the previous studies. Indeed, this is the first double-blind randomized clinical trial that investigates the effect of BT in FS patients. The double-blind design is particularly important, and it was considered the main limitation of the previous trials, considering that a treatment such as BT requires active participation in the study for the patients and medical staff [10].
However, in literature, many review or meta-analysis suggest the positive effect of BT in FS considering the pain severity, FIQ scores, and indexes of QoL [7,8,9,10, 23]. Our data are in agreement with previous RCTs, although a lot of them were methodologically flawed and had small sample sizes [24,25,26]. Neumann et al. [24] and Buskila et al. [25] reported in two different papers the beneficial effect of BT at the Dead Sea on severity and frequency of FS-related symptoms and the significant improvement of QoL indexes. Other authors referred, as observed in our trial, a decrease of FIQ at the end of BT cycle that persisted until 6 months [26, 27]. In 2005, Zijlstra et al. [28] experimented a combination treatment of thalassotherapy techniques (including physical therapy and hydrotherapy), exercise, and patient education in FS patients and reported a medium-term (3 months) improvement of SF-36 and FIQ. More recently, the beneficial effect of BT, alone or in association to patient education or exercise, was confirmed by others [29,30,31,32].
In the present study, BT resulted safe and well-accepted by the patients without the frequent side effects associated to the pharmacological therapy of FS.
The efficacy of BT can be partially explained by heat which increases the pain threshold in the nerve endings and reduces muscle spasm [33]; indeed, thermal stress stimulates the secretion of beta endorphin helping to induce analgesic and anti-spastic effects of BT [34]. In addition to the physical properties, the minerals dissolved in the water can have a key role in the mechanism of action of BT [33, 35]. This assumption could be substantiated by the significantly better and longer effects of BT on pain and function in comparison to tap water bath at the same temperature in different musculoskeletal disorders [36,37,38,39]. Indeed, the persistent clinical efficacy of BT after the treatment constitutes another indirect demonstration of the specific chemical effect of mineral water [35].
Various in vitro and in vivo studies demonstrated that the mineral waters affect the oxidant-antioxidant balance with an enhancement of total antioxidant status and a decrease of oxidant release [40, 41]. Oxidative stress seems to play an important role in the pathogenesis and in the clinical symptoms of FS patients [42, 43]; the effect on oxidant/antioxidant system could contribute to explain the therapeutic efficacy of BT in FS.
Furthermore, BT seems to induce a reduction in circulating levels of Interleukin (IL)-1, prostaglandin E2 (PGE2) and leukotriene B4 (LTB4), important mediators of inflammation and pain, in FS patients, contributing to explain the mechanism of clinical benefits of the mineral waters in this disorder [44].
Another possible mechanism of action of VW in FS could be attributed to the high concentration of magnesium in this water; indeed, several studies have reported a deficiency of magnesium in FS subjects and a significant improvement of FS symptoms after magnesium supplementation [45].
Finally, additional factors such as environmental change, pleasant scenery, and absence of work duties can contribute to induce the clinical improvement obtained by BT; however, in the present study, in order to remove these unspecific factors, all patients lived in the areas near the thermal center and continued their routine activities.
Furthermore, other physical or rehabilitation treatments were not included in the spa program and during the follow-up period for both studied groups to eliminate all potential confounding factors.
Others strengths of this paper are the long-term follow-up, the use of the consolidate standards of reporting trials (CONSORT), and the assessment of the end points (e.g., pain VAS, SF-36, and FIQ) suggested by outcome measure in rheumatology (OMERACT) [46].
In conclusion, the results obtained from our study support the evidence of tolerability and long-term effectiveness of BT in controlling pain and improving functionality in patients affected by FS. Considering the usual side effects often associated with pharmacological therapy, this conclusion underlines the practical utility and the growing importance of BT among other complementary and non-pharmacological modalities in the management of FS.
References
Mease P (2005) Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures and treatment. J Rheumatol Suppl 75:6–21
Wolfe F, Brahler E, Hinz A, Häuser W (2013) Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care Res 65:777–785. https://doi.org/10.1002/acr.21931
Spaeth M (2009) Epidemiology, costs, and the economic burden of fibromyalgia. Arthritis Res Ther 11:117. https://doi.org/10.1186/ar2715
Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluß E et al (2017) EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 76:318–328. https://doi.org/10.1136/annrheumdis-2016-209724
Goldenberg DL, Burckhardt C, Crofford L (2004) Management of fibromyalgia syndrome. JAMA 292:2388–2395
Ablin J, Fitzcharles MA, Buskila D, Shir Y, Sommer C, Häuser W (2013) Treatment of fibromyalgia syndrome: recommendations of recent evidence-based interdisciplinary guidelines with special emphasis on complementary and alternative therapies. Evid Based Complement Alternat Med 2013:485272. https://doi.org/10.1155/2013/485272
McVeigh JG, McGaughey H, Hall M, Kane P (2008) The effectiveness of hydrotherapy in the management of fibromyalgia syndrome: a systematic review. Rheumatol Int 29:119–130. https://doi.org/10.1007/s00296-008-0674-9
Langhorst J, Musial F, Klose P, Häuser W (2009) Efficacy of hydrotherapy in fibromyalgia syndrome—a meta-analysis of randomized controlled clinical trials. Rheumatology 48:1155–1159. https://doi.org/10.1093/rheumatology/kep182
Guidelli GM, Tenti S, De Nobili E, Fioravanti A (2012) Fibromyalgia syndrome and spa therapy: myth or reality? Clin Med Insights Arthritis Musculoskelet Disord 5:19–26. https://doi.org/10.4137/CMAMD.S8797
Naumann J, Sadaghiani C (2014) Therapeutic benefit of balneotherapy and hydrotherapy in the management of fibromyalgia syndrome: a qualitative systematic review and meta-analysis of randomized controlled trials. Arthritis Res Ther 16:R141. https://doi.org/10.1186/ar4603
Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 62:600–610. https://doi.org/10.1002/acr.20140
Burckhardt CS, Clark SR, Bennett RM (1991) The fibromyalgia impact questionnaire: development and validation. J Rheumatol 18:728–733
Bennett R (2005) The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol 5(Suppl 39):S154–S162
Sarzi-Puttini P, Atzeni F, Fiorini T, Panni B, Randisi G, Turiel M, Carrabba M (2003) Validation of an Italian version of the Fibromyalgia Impact Questionnaire (FIQ-I). Clin Exp Rheumatol 21:459–464
Apolone G, Mosconi P (1998) The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol 51:1025–1036
Ware J Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Inc, Palo Alto
Lazzari R, Pancheri P (1980) Questionario di autovalutazione per l’ansia di stato e di tratto. Manuale di istruzioni. Ed. Organizzazioni Speciali, Firenze
Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401
Fava GA (1983) Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. J Clin Psychol 39:249–251
Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL (2000) Defining the clinically important difference in pain outcome measures. Pain 88:287–294
Bennett RM, Bushmakin AG, Cappelleri JC, Zlateva G, Sadosky AB (2009) Minimal clinically important difference in the fibromyalgia impact questionnaire. J Rheumatol 36:1304–1311. https://doi.org/10.3899/jrheum.081090
Fraioli A, Grassi M, Mennuni G, Geraci A, Petraccia L, Fontana M, Conte S, Serio A (2013) Clinical researches on the efficacy of spa therapy in fibromyalgia. A systematic review. Ann Ist Super Sanita 49:219–229. https://doi.org/10.4415/ANN_13_02_13.
Neumann L, Sukenik S, Bolotin A, Abu-Shakra M, Amir M, Flusser D, Buskila D (2001) The effect of balneotherapy at the Dead Sea on the quality of life of patients with fibromyalgia syndrome. Clin Rheumatol 20:15–19
Buskila D, Abu-Shakra M, Neumann L, Odes L, Shneider E, Flusser D, Sukenik S (2001) Balneotherapy for fibromyalgia at the Dead Sea. Rheumatol Int 20:105–108
Evcik D, Kizilay B, Gökçen E (2002) The effects of balneotherapy on fibromyalgia patients. Rheumatol Int 22:56–59
Dönmez A, Karagülle MZ, Tercan N, Dinler M, Işsever H, Karagülle M et al (2005) SPA therapy in fibromyalgia: a randomised controlled clinic study. Rheumatol Int 26:168–172
Zijlstra TR, van de Laar MA, Bernelot Moens HJ, Taal E, Zakraoui L, Rasker JJ (2005) Spa treatment for primary fibromyalgia syndrome: a combination of thalassotherapy, exercise and patient education improves symptoms and quality of life. Rheumatology (Oxford) 44:539–546
Fioravanti A, Perpignano G, Tirri G, Cardinale G, Gianniti C, Lanza CE, Loi A, Tirri E, Sfriso P, Cozzi F (2007) Effects of mud-bath treatment on fibromyalgia patients: a randomized clinical trial. Rheumatol Int 27:1157–1161
Ozkurt S, Dönmez A, Zeki Karagülle M, Uzunoğlu E, Turan M, Erdoğan N (2012) Balneotherapy in fibromyalgia: a single blind randomized controlled clinical study. Rheumatol Int 32:1949–1954. https://doi.org/10.1007/s00296-011-1888-9
Bağdatlı AO, Donmez A, Eröksüz R, Bahadır G, Turan M, Erdoğan N (2015) Does addition of 'mud-pack and hot pool treatment' to patient education make a difference in fibromyalgia patients? A randomized controlled single blind study. Int J Biometeorol 59:1905–1911. https://doi.org/10.1007/s00484-015-0997-7
Koçyiğit BF, Gür A, Altındağ Ö, Akyol A, Gürsoy S (2016) Comparison of education and balneotherapy efficacy in patients with fibromyalgia syndrome: a randomized, controlled clinical study. Agri 28:72–78. https://doi.org/10.5505/agri.2015.77699
Tenti S, Fioravanti A, Guidelli GM, Pascarelli NA, Cheleschi S (2014) New evidence on mechanisms of action of spa therapy in rheumatic diseases. TANG 4:31–38
Cozzi F, Lazzarin P, Todesco S, Cima L (1995) Hypothalamic-pituitary-adrenal axis dysregulation in healthy subjects undergoing mud-bath applications. Arthritis Rheum 38:724–726
Morer C, Roques CF, Françon A, Forestier R, Maraver F (2017) The role of mineral elements and other chemical compounds used in balneology: data from double-blind randomized clinical trials. Int J Biometeorol 61:2159–2173. https://doi.org/10.1007/s00484-017-1421-2
Bálint GP, Buchanan WW, Adám A, Ratkó I, Poór L, Bálint PV et al (2007) The effect of the thermal mineral water of Nagybaracska on patients with knee joint osteoarthritis—a double blind study. Clin Rheumatol 26:890–894
Kovács C, Pecze M, Tihanyi Á, Kovács L, Balogh S, Bender T (2012) The effect of sulphurous water in patients with osteoarthritis of hand. Double-blind, randomized, controlled follow-up study. Clin Rheumatol 31:1437–1442. https://doi.org/10.1007/s10067-012-2026-0
Kulisch A, Bender T, Németh A, Szekeres L (2009) Effect of thermal water and adjunctive electrotherapy on chronic low back pain: a double-blind, randomized, follow-up study. J Rehabil Med 41:73–79. https://doi.org/10.2340/16501977-0291
Karagülle M, Karagülle MZ (2015) Effectiveness of balneotherapy and spa therapy for the treatment of chronic low back pain: a review on latest evidence. Clin Rheumatol 34:207–214. https://doi.org/10.1007/s10067-014-2845-2
Prandelli C, Parola C, Buizza L, Delbarba A, Marziano M, Salvi V, Zacchi V, Memo M, Sozzani S, Calza S, Uberti D, Bosisio D (2013) Sulphurous thermal water increases the release of the anti-inflammatory cytokine IL-10 and modulates antioxidant enzyme activity. Int J Immunopathol Pharmacol 26:633–646
Bender T, Bariska J, Vághy R, Gomez R, Kovács I (2007) Effect of balneotherapy on the antioxidant system-a controlled pilot study. Arch Med Res 38:86–89
Cordero MD, Alcocer-Gómez E, Cano-García FJ, De Miguel M, Carrión AM, Navas P et al (2011) Clinical symptoms in fibromyalgia are better associated to lipid peroxidation levels in blood mononuclear cells rather than in plasma. PLoS One 6:e26915. https://doi.org/10.1371/journal.pone.0026915
Fatima G, Das SK, Mahdi AA (2017) Some oxidative and antioxidative parameters and their relationship with clinical symptoms in women with fibromyalgia syndrome. Int J Rheum Dis 20:39–45. https://doi.org/10.1111/1756-185X.12550
Ardiç F, Ozgen M, Aybek H, Rota S, Cubukçu D, Gökgöz A (2007) Effects of balneotherapy on serum IL-1, PGE2 and LTB4 levels in fibromyalgia patients. Rheumatol Int 27:441–446. https://doi.org/10.1007/s00296-006-0237-x
Sendur OF, Tastaban E, Turan Y, Ulman C (2008) The relationship between serum trace element levels and clinical parameters in patients with fibromyalgia. Rheumatol Int 28:1117–1121. https://doi.org/10.1007/s00296-008-0593-9
Mease PJ, Clauw DJ, Christensen R, Crofford LJ, Gendreau RM, Martin SA, OMERACT Fibromyalgia Working Group et al (2011) Toward development of a fibromyalgia responder index and disease activity score: OMERACT module update. J Rheumatol 38:1487–1495. https://doi.org/10.3899/jrheum.110277
Acknowledgements
The authors want to thank the Thermal Resort of Levico and Vetriolo, Levico Terme (Trento), Italy.
Funding
This study was supported by “Provincia Autonoma di Trento”. The sponsors of this study did not participate in the study design, in the collection, analysis, or the interpretation of data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Fioravanti, A., Manica, P., Bortolotti, R. et al. Is balneotherapy effective for fibromyalgia? Results from a 6-month double-blind randomized clinical trial. Clin Rheumatol 37, 2203–2212 (2018). https://doi.org/10.1007/s10067-018-4117-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4117-z