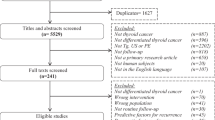
Abstract
Purpose
To discuss the role and limitations of serum thyroglobulin (Tg) assay in management of differentiated thyroid cancer (DTC) from the perspective of nuclear medicine (NM) physicians.
Methods
We performed a literature search in electronic databases PubMed, Embase, and the Cochrane Library up to January 2019 using the following key words: thyroid, cancer/carcinoma/neoplasm, and thyroglobulin. We included prospective and retrospective original and recent studies written in English regarding Tg in DTC.
Results
Serum Tg level can reflect disease status. It is a tumor marker of DTC. Many studies have confirmed the clinical roles of Tg in monitoring thyroid remnant, locoregional, and distant metastatic disease, tailoring radioactive iodine (RAI) therapy, and predicting persistence or recurrence as well as response to treatment. Most NM physicians in Asia are actively involved in the management of DTC. They are actively using serologic markers such as Tg, anti-Tg antibody, and thyroid stimulating hormone as well as imaging studies including neck ultrasound and RAI whole body scan. Authors suggest an algorithm for the use of serum Tg assay as an indicator of RAI therapy. Pitfalls of interpretation and complementary roles of Tg and imaging studies are discussed.
Conclusions
Serum Tg is a useful biomarker for risk stratification and decision-making in the management of DTC. It is expected to be used more widely in NM practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most common endocrine malignancy, with differentiated thyroid cancer (DTC) comprising more than 90% of all thyroid carcinomas [1]. Thyroid follicular cells are assumed to be the only source of thyroglobulin (Tg), a large glycoprotein (660,000 Daltons) in human body [2]. Serum Tg can be used as a tumor marker to evaluate disease status in the whole course of DTC. Most nuclear medicine (NM) physicians in Asia are actively involved in the management of DTC. Radioactive iodine (RAI) therapy for DTC is a common practice of NM physicians throughout Asia. They are actively using serologic markers such as Tg, anti-Tg antibody (TgAb), and thyroid stimulating hormone (TSH) as well as imaging studies including neck ultrasound (US) and RAI whole body scan (WBS) [3]. Of note, Tg measurement can be influenced by a variety of factors, including TgAb, TSH level, and different laboratory assay methods. Tg measurement also faces challenge of test interference and measurement heterogeneity due to different test methods. The present paper describes the clinical roles of Tg in the management of DTC through a wide systematic review. We performed a literature search in electronic databases PubMed, Embase, and the Cochrane Library up to January 2019 using the folowing keywords: thyroid, cancer/carcinoma/neoplasm, and thyroglobulin. Inclusion criteria were prospective and retrospective original studies written in English regarding thyroglobulin in DTC. Additional evidence was collected by identifying studies from respective references of selected articles. Reviews, expert opinion articles, and guidelines were also appraised. Due to the plethora of relative studies, the most recent and qualified studies in each clinical issue were selected and reviewed.
Preoperative measurement of Tg
Role of preoperative serum Tg assay is controversial. The American Thyroid Association (ATA) guideline in 2015 did not recommend measuring serum Tg routinely for initial evaluation of thyroid nodules [4]. A complementary role of preoperative serum Tg can be expected in selected cases. Serum Tg combined with nodule size on US can provide information to differentiate follicular cancer from benign nodules in indeterminate nodules [5]. Serum Tg is an insensitive marker for diagnosis of metastatic diseasese as its level is low in many DTC patients with locoregional metastasis. Malignant nodule topography such as location of the nodule was helpful to predict the presence of metastases [6]. Serum Tg level is correlated with the size of the thyroid gland and thyroid nodules rather than metastases [7]. Tg can be measured in washout of fine needle aspiration (FNA). It can be used for differential diagnosis. Multiple studies have shown high sensitivity and specificity of FNA washout Tg for the diagnosis of metastasis from thyroid cancer in neck mass and lymph nodes [8,9,10,11]. Various cutoff values of FNA washout Tg make this technique difficult to use in daily practice [12, 13]. False-positive result of FNA washout Tg can occur if there is blood contamination in the aspirate [14].
Postoperative Tg for selection of patients and dose for RAI treatment
Measurement of either stimulated TSH or non-stimulated serum Tg a few weeks after total thyroidectomy and before RAI therapy has been demonstrated to be clinically useful. ATA 2015 guideline recommends measurement of serum Tg at 3–4 weeks after operation when serum Tg level reaches its nadir [4]. It is regarded as a tool to aid in initial risk stratification and decision-making for adjuvant therapy as it helps assessment of the persistence of residual thyroid tissues either remnant normal thyroid tissue or thyroid cancer. The negative predictive value of postoperative and pre-ablation serum Tg is very high for successful RAI treatment and recurrence of DTC [15, 16]. RAI treatment is usually not recommended if postoperative Tg is undetectable or very low (less than 1.0 ng/mL for TSH stimulated status or less than 0.2 ng/mL for non-stimulated status) [17].
As today expressly stated or widely accepted Tg-based dose-decision rules are not available. Zhang et al. [18] have demonstrated that low-dose RAI (30 mCi) treatment is not inferior to high-dose (100 mCi or higher) in achieving excellent response when DTC patients have stimulated Tg of less than 5 ng/mL irrespective of lymph node metastases. Jin et al. [19] have recently compared adjusted dose group according to RAI uptake and serum Tg levels (n = 207) to fixed dose group (n = 58). RAI uptake and Tg-based activity was established based on four levels of RAI uptake (≤ 2%, 2–5%, 5–15%, and > 15%) and Tg levels (≤ 2 ng/mL, 2–5 ng/mL, 5–10 ng/mL, and > 10 ng/mL). Based on this, RAI doses of 1.1, 1.85, 3.7, and 5.55 GBq were administered. The dose in the adjusted group varied from 1.54 to 3.26 GBq. Although the administered dose was significantly lower than that in the fixed dose group, the successful response rate was significantly higher in the adjusted group (94.2% vs. 70.7%; p < 0.0001). Incidence of xerostomia was significantly lower in the adjusted group. Further multicenter studies are warranted to confirm whether serum Tg level can be used to select RAI dose.
Preablative Tg for prediction of treatment response and recurrence
Preablative Tg has a great value in predicting therapeutic response and recurrence [20]. A high preablative stimulated Tg level is the most significant predictor of therapeutic failure at the time of first RAI therapy in patients with DTC [21]. Several studies have shown that the optimal diagnostic threshold of Tg level is between 20 and 30 ng/mL in predicting resistance or recurrence of the disease [22,23,24]. Patients with preablative stimulated Tg level ≥ 10 ng/mL have 25.5 times greater chance of therapeutic failure than those with level < 10 ng/mL [25]. Yang et al. [26] have provided cutoff value of preablative stimulated Tg at 26.75 ng/mL for differentiating structural incomplete from either excellent, indeterminate, or biomedical incomplete responses. Preablative stimulated Tg is an independent and the most predictive variable of structural incomplete response (odds ratio: 42.312; P < 0.001) [26]. High postoperative TSH stimulated Tg values (> 10–30 ng/mL) are also associated with poor survival and treatment failure [27, 28].
There is a good probability that most low Tg (< 2 ng/mL) patients can achieve excellent response [29]. Although low level of pre-ablation stimulated Tg (less than 1–2 ng/mL) can indicate lower recurrence rate and better prognosis, it cannot completely eliminate the possibility of identifying a metastatic foci outside the thyroid bed on post-therapy WBS [30, 31]. In one study, RAI-avid metastatic foci outside the thyroid bed, mostly in cervical and mediastinal lymph nodes, were detected in 6.3% of intermediate-/high-risk patients with stimulated Tg < 2 ng/mL [32]. Hu et al. [33] have reported that the prognosis of patients showing positive post-therapy WBS but negative serum Tg is excellent. There was no recurrence or metastasis in such patients [33].
Timing of measuring serum Tg after recombinant human TSH (rhTSH) is controversial. Serum Tg is usually measured at 72 h after second injection of rhTSH as it corresponds to the peak serum Tg level [34, 35]. Serum Tg values measured after RAI therapy may include released Tg from thyroid follicles of normal thyroid tissue damaged by RAI. Thus, they might not be reliably predictive [36, 37]. Park et al. [38] have shown that stimulated Tg measured early after the second rhTSH injection, but before RAI administration is more predictive than Tg values measured after RAI therapy. The cutoff value to predict a non-excellent response was 2.0 ng/mL.
Combinations of serologic markers with imaging studies offer distinct advantages for diagnosis. Higher serum Tg level indicates presence of residual thyroid tissue with or without distant metastasis. Lin et al. [39] have reported that preablative stimulated Tg is significantly different between patients with and without distant metastasis (440.6 vs 5.3 ng/mL, p < 0.0001). It is important to evaluate the location of the lesion by imaging studies such as 123I WBS and 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) when serum Tg level is higher. Sometimes distant metastasis can be found in patients showing low serum Tg level. One study reported that 9 out of 573 patients (1.56%) had a discordant pattern with low level of Tg/positive WBS in post-surgical follow-up [40]. Of these 9 patients, four were metastatic at presentation while five with metastasis during follow-up persistently remained low levels of Tg (< 5 ng/mL) [40]. The risk of persistent disease was minimal when both stimulated Tg and post-therapy WBS were negative: only 2 of 93 had persistent diseases [41]. However, neither Tg level alone nor WBS alone can be considered as a reliable indicator for absence of disease. Diagnostic WBS contributes to risk stratification by defining residual nodal and distant metastatic disease. It has changed clinical management for 29.4% of patients [41]. Single photon emission computed tomography with computed tomography (SPECT/CT) could provide additional clinical information compared to planar WBS, which results to change in risk stratification after surgery or patient management. Avram et al. [42] demonstrated that pre-ablation SPECT/CT, combined with stimulated serum Tg, altered the risk stratification in 15% of the patients by detecting residual nodal or distant metastases, which led to changes in the further management plan in 29.4% of the patients with DTC. Jeong et al. [43] reported that SPECT/CT could detect additional findings in 8.6% of the patients and that the serum Tg levels at the time of RAI therapy were significantly higher in patients with additional imaging findings than in those without them. From this study, post-therapeutic SPECT/CT should be considered in patients with high level of serum Tg for the detection of hidden metastases.
Tg for risk stratification after RAI treatment
Serum Tg on thyroxine therapy is recommended to be measured every 6–12 months after RAI treatment for low and intermediate risk patients [4]. It can be lengthened to 12–24 months if patients show excellent response to therapy. Excellent response is defined as an unstimulated Tg below 0.2 ng/mL or stimulated Tg below 1.0 ng/mL with negative imaging findings based on ATA guideline 2015. Hu et al. [44] have shown that suppressed Tg < 0.2 ng/mL performs better than stimulated Tg < 1.0 ng/mL in defining an excellent response. More frequent Tg measurements may be appropriate for high-risk patients. Several studies have shown a strong correlation between follow-up Tg and recurrence or response to initial treatment. In patients with positive TgAb, serum Tg concentrations alone cannot be used as a marker to detect disease status. Disease recurrence can be heralded by a rise in TgAb with or without a corresponding rise in serum Tg. Changes in TgAb levels in the first year after surgery can predict the risk of persistent or recurrent disease of TgAb-positive DTC patients. Patients who have achieved negativization of TgAb have excellent prognosis [45].
RAI therapy algorithm using Tg as a gatekeeper along with other risk factors
Although many clinicians commonly measure serum Tg in the course of RAI treatment [3], there is no consensus for the timing of measurement or optimal cutoff value and such measurement is not adopted in guidelines as an indicator for decision-making [4]. In this review, we suggested new RAI therapy and follow-up algorithm considering both Tg and conventional risk factors as indicators (Fig. 1). RAI therapy might not be recommended if postoperative Tg is undetectable or very low (less than 0.2 ng/mL in unstimulated status or less than 1.0 ng/mL in stimulated status) in low risk group patients. It was reported that 17.4% of DTC patients with low- or low- to intermediate-risk showed metastases on post-therapy WBS [46]. Postoperative RAI treatment can be actively considered as an adjuvant therapy if serum Tg level is detectable. When patients show higher serum Tg levels, indicating the presence of residual thyroid tissue, the location of residual thyroid tissue should be evaluated by imaging studies such as 123I WBS and 18F-FDG PET/CT. According to authors’ experience, patients with pretreatment stimulated Tg levels ≥ 10 ng/mL have greater risk of therapeutic failure [25]. We recommend imaging studies when stimulated Tg level is 10 ng/mL or over. Serum Tg level of 10 ng/mL after TSH stimulation is equivalent to 2 ng/mL of non-stimulated Tg [4]. RAI-avidity is an important finding in the decision-making of RAI therapy. Surgical resection or external radiotherapy be considered first before RAI therapy when metastatic lesions show low RAI-avidity. Dose of RAI higher than 5.5 GBq is considered for high-risk group, especially for patients with distant metastasis showing RAI-avidity. Bone marrow dosimetry by Benua method [47] can be done by serially measuring blood activities. This information is important for the next high-dose RAI therapy, especially when treatment failure is suspected.
Algorithm for radioactive iodine therapy using thyroglobulin as a gatekeeper. TT total thyroidectomy, Tg thyroglobulin (along with anti-Tg antibody), Risk risk assessment using clinical and pathologic information, LR low risk, IHR intermediate to high-risk, THR thyroid hormone replacement, Img imaging studies, M malignancy or metastasis, RT external radiotherapy, RAI radioactive iodine, (−) negative, (+) positive, (++) strong positive
There is a trend to de-escalate the dose in patients with intermediate-risk group. We suggest administration of 1.1 GBq when non-stimulated serum Tg level is between 0.2 and 2.0 ng/mL or when stimulated serum Tg is between 1.0 and 10.0 ng/mL, if patients’ risks are favorable. This dose can be administered at an outpatient clinic in many countries. WBS at 3 days after RAI therapy is important to evaluate the extent of residual thyroid tissue in the body. A delayed imaging after several days is helpful when there is a discrepancy between serum Tg level and WBS result.
Response to therapy is evaluated between 6 and 18 months after RAI treatment by serum Tg, TgAb, TSH, neck US, and RAI WBS. If a patient shows complete response, i.e., serum Tg is undetectable and imaging studies are negative, a surveillance protocol in longer intervals can be applied. Undetectable Tg alone is insufficient to confirm complete response as some patients show metastatic lesions. Serial measurement of serum Tg is sufficient if complete response is confirmed by both serum Tg and imaging studies. Appropriate treatment plans should be applied when patient has either biochemically or structurally incomplete response.
Interpretation of Tg in various clinical conditions
Stimulated Tg measured after thyroid hormone withdrawal (THW) is different by level of TSH which can be increasing with duration of THW. Son et al. [48] have reported that change of TSH is positively correlated with change of Tg in each patient. They revealed that stimulated Tg of individual patient after THW was not identical. They recommended considering TSH level when interpreting serial stimulated Tg measurement. rhTSH becomes a safe and effective mean to increase serum TSH level for RAI therapy preparation while avoiding signs and symptoms of hypothyroidism associated with THW. Comparisons of Tg levels after THW and rhTSH administration were reported. Kowalska et al. [49] have reported that rhTSH-stimulated Tg values of 0.6 and 2.3 ng/mL correspond to THW-stimulated Tg levels of 2.0 and 10.0 ng/mL, respectively. Patients with THW-stimulated Tg > 10 ng/mL [20, 25] and rhTSH-aided Tg > 2 ng/mL [38] have been reported to have poor prognosis. THW-aided stimulated Tg > 5.22 ng/mL [50] and rhTSH stimulated Tg > 4.64 ng/mL [51] at the time of RAI therapy were associated with recurrence in a 6-year follow-up.
The clinical role of serial Tg measurement before and after RAI treatment has been reported for prediction of therapeutic response. High ratios of serum Tg measured at 3, 5, and 7 days after treatment to pretreatment Tg indicate the release of stored Tg in follicles of remnant thyroid tissue, which can reflect early thyroid tissue destruction and good response of RAI therapy [37, 52, 53]. Early released Tg indicated by high Tg-Day3/Tg-Day0 ratio (i.e., extensive release of Tg to the blood after RAI therapy) and low preablative Tg (i.e., small remnant thyroid tissue) constitute indices of successful ablation after RAI therapy [37]. Similar findings have been observed for Tg-Day5/Tg-Day0 and Tg-Day7/Tg-Day0 ratios [52, 53].
Patients who no longer respond to RAI therapy despite appropriate TSH stimulation and iodine preparation have been identified as RAI refractory DTC [4]. These patients often show no RAI uptake on WBS, but significantly elevated serum Tg levels or rapidly rising serum Tg. Wang et al. [54] have observed changes of stimulated Tg from the first and the second RAI treatments. If Tg level does not decrease appreciably after the first RAI therapy, patients may benefit little and suffer from elevated risk of RAI refractory DTC. Cutoff value of the first/second Tg ratio has been reported to be 0.544, with a sensitivity of 90.0% and a specificity of 47.7% [54].
18F-FDG PET/CT plays an emerging role in assessment of RAI refractory DTC (Fig. 2). A recent study has suggested that 18F-FDG PET/CT may be of great value in identifying metastases in postoperative DTC patients with elevated Tg before RAI administration [55]. 18F-FDG positive metastatic DTC with maximum standardized uptake value (SUVmax) of greater than 4.0 possesses higher probability of non-avidity to RAI [55]. Another study has shown that 18F-FDG PET/CT is useful for investigating DTC with high stimulated Tg levels (> 10 ng/mL) and negative WBS as a standard of care for RAI refractory DTC [56]. Higher Tg level is related to higher sensitivity of 18F-FDG PET/CT [56].
18F-FDG PET/CT (a) and 123I-whole body scan (b) images of a 51-years-old female patient with papillary thyroid cancer who underwent total thyroidectomy and two times of high-dose (5.55 GBq) 131I treatment. At follow-up, stimulated serum thyroglobulin level was 32.0 ng/mL, anti-thyroglobulin antibody level was 21 U/mL, and thyrotropin level was 35.79 uIU/mL. Recurrence was verified by fine needle aspiration cytology. 18F-FDG PET/CT shows sensitivity to recognize non-RAI-avid lesion in patients with elevated Tg
Tg measurements in concordance with response evaluation criteria in solid tumors (RECIST 1.1) are valuable in the evaluation of tumor responses to molecular targeted treatment, especially for earlier response evaluation. A decrease in serum Tg level following sorafenib therapy has been observed in patients with RAI-refractory DTC [57,58,59]. Lin et al. [60] have also demonstrated rapid metabolic and structural response to apatinib in RAI refractory DTC. Tg and 18F-FDG PET/CT could afford more informative and distinct biochemical and glucose metabolism changes. They could be used as early response indicators [60].
Pitfalls of thyroglobulin measurement
Over the last few decades, three main methods for Tg measurement have been developed: radioimmunoassay (RIA) since 1970s, immunoradiometric assays (IRMA) since 1980s, and liquid chromatography–tandem mass spectrometry (LC–MS/MS) since 2008. These three different methods have different sensitivities, specificities, and propensities for interference from TgAb and heterophile antibodies (HAb). Many NM departments in Asia are running laboratories to provide measurements for Tg, TgAb, and other thyroid hormones. Tg from test sample can competes with a radiolabelled (125I) human Tg for binding to a limited amount of a high-affinity rabbit polyclonal TgAb in RIA. The functional sensitivity of RIA can reach a level of 0.5 µg/L [61, 62]. RIA is advertised to be more resistant than other assays to TgAb interferences because polyclonal antibodies could recognize Tg epitopes bound to TgAb [61]. However, false result due to TgAb still occurs with overestimation or underestimation of Tg [63,64,65]. IRMA are based on a two-site reaction that involves Tg capture by a solid-phase antibody followed by addition of a labeled antibody that targets different epitopes on the captured Tg [66]. The second-generation IRMA tests have higher functional sensitivity (≤ 0.10 μg/L) than the first-generation tests with functional sensitivity of ~ 1.0 μg/L. The second-generation IMA has been widely used as the standard of care, especially for monitoring low basal Tg [67,68,69]. The main limitation of Tg-IRMA is its high propensity for TgAb [70, 71] and HAb interferences [72, 73]. The presence of TgAb might cause falsely low/undetectable result whereas HAb might cause falsely high result. TgAb interference is the most common problem that limits Tg utility in one-fourth of DTC patients [74]. HAb interferences occur only in approximately 0.5% of DTC patients [73, 75]. By cleaving all proteins including Tg and TgAb, MS is regarded as a solution to interference of TgAb [66]. Kushnir et al. [76] have demonstrated that 23% of TgAb-positive samples with undetectable Tg concentrations by immunoassay (< 0.1 μg/L) have Tg concentrations ranging from 0.7 to 11 µg/L on MS assay. The degree of underestimation effect on Tg measurement by TgAb is variable according to Tg assay kits [77]. Different methods can display a twofold difference in numeric thyroglobulin values reported for the same serum [61, 71]. In clinical practice, such discrepancies emphasize that Tg monitoring should be done using the same manufacturer’s method, the same kit, and preferably the same laboratory. Ahn et al. [78] have suggested that applying mathematical equation to estimate true Tg value can eliminate effect of TgAb on Tg measurement. Currently, MS utility is compromised by suboptimal functional sensitivity and longer turnaround time than the second generation IMA with limited availability and high instrumentation cost [69]. Discordances in measurement results can also be observed. TgAb is recommended to be tested along with every Tg measurement. When positive TgAb renders IRMA unreliable, serum TgAb concentration can be used as a surrogate of disease marker in TgAb-positive patient. Notably, the use of the TgAb trend as a surrogate of disease is only possible when the same assay is used longitudinally.
Future perspectives
Much has been learned about the clinical utility of Tg as a biomarker for DTC over the past decades. Newly developed assay methods with high sensitivity offer more accurate ways for disease surveillance. A careful assessment of interferences (from TgAb, HAb, and non-adequate TSH level) in Tg measurement is needed to overcome potential pitfalls. Tg measurement along with reasonable imaging authenticates the use of Tg. More well designed, prospective, and multicenter studies are needed to disclose more about the wholesome role of Tg in DTC.
Abbreviations
- ATA:
-
American Thyroid Association
- DTC:
-
Differentiated thyroid cancer
- FDG:
-
Fluorodeoxyglucose
- FNA:
-
Fine needle aspiration
- HAb:
-
Heterophile antibody
- IRMA:
-
Immunoradiometric assay
- LC–MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- NM:
-
Nuclear medicine
- PET/CT:
-
Positron emission tomography/computed tomography
- RAI:
-
Radioactive iodine
- rhTSH:
-
Recombinant human thyroid stimulating hormone
- RIA:
-
Radioimmunoassay
- SPECT/CT:
-
Single photon emission computed tomography/computed tomography
- SUVmax:
-
Maximum standardized uptake value
- THW:
-
Thyroid hormone withdrawal
- Tg:
-
Thyroglobulin
- TgAb:
-
Anti-thyroglobulin antibody
- US:
-
Ultrasound
- WBS:
-
Whole body scan
References
Sherman SI (2003) Thyroid carcinoma. Lancet 361:501–511
Rivolta CM, Targovnik HM (2006) Molecular advances in thyroglobulin disorders. Clin Chim Acta 374:8–24
Jabin Z, Kwon SY, Bom HS et al (2018) Clinico-social factors to choose radioactive iodine dose in differentiated thyroid cancer patients: an Asian survey. Nucl Med Commun 39:283–289
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133
Lee EK, Chung KW, Min HS et al (2012) Preoperative serum thyroglobulin as a useful predictive marker to differentiate follicular thyroid cancer from benign nodules in indeterminate nodules. J Korean Med Sci 27:1014–1018
Campennì A, Giovanella L, Siracusa M et al (2014) Is malignant nodule topography an additional risk factor for metastatic disease in low-risk differentiated thyroid cancer? Thyroid 24:1607–1611
Patell R, Mikhael A, Tabet M et al (2018) Assessing the utility of preoperative serum thyroglobulin in differentiated thyroid cancer: a retrospective cohort study. Endocrine 61:506–510
Pacini F, Fugazzola L, Lippi F et al (1992) Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab 74:1401–1404
Baskin HJ (2004) Detection of recurrent papillary thyroid carcinoma by thyroglobulin assessment in the needle washout after fine-needle aspiration of suspicious lymph nodes. Thyroid 14:959–963
Boi F, Baghino G, Atzeni F et al (2006) The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab 91:1364–1369
Chung J, Kim EK, Lim H et al (2014) Optimal indication of thyroglobulin measurement in fine-needle aspiration for detecting lateral metastatic lymph nodes in patients with papillary thyroid carcinoma. Head Neck 36:795–801
Moon JH, Kim YI, Lim JA et al (2013) Thyroglobulin in washout fluid from lymph node fine-needle aspiration biopsy in papillary thyroid cancer: large-scale validation of the cutoff value to determine malignancy and evaluation of discrepant results. J Clin Endocrinol Metab 98:1061–1068
Zhao H, Wang Y, Wang MJ et al (2017) Influence of presence/absence of thyroid gland on the cutoff value for thyroglobulin in lymph-node aspiration to detect metastatic papillary thyroid carcinoma. BMC Cancer 17:296
Sakamoto K, Imanishi Y, Tomita T et al (2016) Usefulness and limitation of thyroglobulin measurement in fine needle aspirates (FNA-Tg) for diagnosis of neck lymph node metastasis from thyroid carcinoma. Nihon Jibiinkoka Gakkai Kaiho 119:721–726
Ibrahimpasic T, Nixon IJ, Palmer FL et al (2012) Undetectable thyroglobulin after total thyroidectomy in patients with low- and intermediate-risk papillary thyroid cancer–is there a need for radioactive iodine therapy? Surgery 152:1096–1105
Rosario PW, Mourao GF, Siman TL et al (2015) A low postoperative nonstimulated serum thyroglobulin level excludes the presence of persistent disease in low-risk papillary thyroid cancer patients: implication for radioiodine indication. Clin Endocrinol (Oxf) 83:957–961
Rosario PW, Mineiro Filho AF, Prates BS et al (2012) Postoperative stimulated thyroglobulin of less than 1 ng/mL as a criterion to spare low-risk patients with papillary thyroid cancer from radioactive iodine ablation. Thyroid 22:1140–1143
Zhang Y, Liang J, Yang X et al (2015) Low-dose radioiodine ablation in differentiated thyroid cancer with macroscopic extrathyroidal extension and low level of preablative-stimulated thyroglobulin. Nucl Med Commun 36:553–559
Jin Y, Ruan M, Cheng L et al (2019) Radioiodine uptake and thyroglobulin guided radioiodine remnant ablation in patients with differentiated thyroid cancer: a prospective, randomized, open-label, controlled trial. Thyroid 29:101–110
Kim TY, Kim WB, Kim ES et al (2005) Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 90:1440–1445
Yang X, Liang J, Li TJ et al (2015) Postoperative stimulated thyroglobulin level and recurrence risk stratification in differentiated thyroid cancer. Chin Med J (Engl) 128:1058–1064
Ronga G, Filesi M, Ventroni G et al (1999) Value of the first serum thyroglobulin level after total thyroidectomy for the diagnosis of metastases from differentiated thyroid carcinoma. Eur J Nucl Med 26:1448–1452
Hall FT, Beasley NJ, Eski SJ et al (2003) Predictive value of serum thyroglobulin after surgery for thyroid carcinoma. Laryngoscope 113:77–81
Heemstra KA, Liu YY, Stokkel M et al (2007) Serum thyroglobulin concentrations predict disease-free remission and death in differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 66:58–64
Park HJ, Jeong GC, Kwon SY et al (2014) Stimulated serum thyroglobulin level at the time of first dose of radioactive iodine therapy is the most predictive factor for therapeutic failure in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging 48:255–261
Yang X, Liang J, Li T et al (2016) Preablative stimulated thyroglobulin correlates to new therapy response system in differentiated thyroid cancer. J Clin Endocrinol Metab 101:1307–1313
Piccardo A, Arecco F, Puntoni M et al (2013) Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med 38:18–24
Lin JD, Huang MJ, Hsu BR et al (2002) Significance of postoperative serum thyroglobulin levels in patients with papillary and follicular thyroid carcinomas. J Surg Oncol 80:45–51
Zhang Y, Hua W, Zhang X et al (2018) The predictive value for excellent response to initial therapy in differentiated thyroid cancer: preablation-stimulated thyroglobulin better than the TNM stage. Nucl Med Commun 39:405–410
Campenni A, Giovanella L, Pignata SA et al (2018) Undetectable or low (< 1 ng/mL) postsurgical thyroglobulin values do not rule out metastases in early stage differentiated thyroid cancer patients. Oncotarget 9:17491–17500
Shen CT, Wei WJ, Qiu ZL et al (2016) Value of post-therapeutic 131I scintigraphy in stimulated serum thyroglobulin-negative patients with metastatic differentiated thyroid carcinoma. Endocrine 51:283–290
Park EK, Chung JK, Lim IH et al (2009) Recurrent/metastatic thyroid carcinomas false negative for serum thyroglobulin but positive by posttherapy I-131 whole body scans. Eur J Nucl Med Mol Imaging 36:172–179
Hu S, Kuang A, Ji T (2015) Clinical outcomes of negative serum thyroglobulin but positive diagnostic I-131 whole-body scans in patients with well-differentiated thyroid cancer after first remnant ablation. J Nucl Med 56(supplement 3):395
Torres MS, Ramirez L, Simkin PH, Braverman LE, Emerson CH (2001) Effect of various doses of recombinant human thyrotropin on the thyroid radioactive iodine uptake and serum levels of thyroid hormones and thyroglobulin in normal subjects. J Clin Endocrinol Metab 86:1660–1664
Weiss R, Magner J (2015) Serial measurements of serum thyroglobulin in response to recombinant human thyrotropin stimulation. Thyroid 25:708–710
Taieb D, Lussato D, Guedj E, Roux F, Mundler O (2006) Early sequential changes in serum thyroglobulin after radioiodine ablation for thyroid cancer: possible clinical implications for recombinant human thyrotropin-aided therapy. Thyroid 16:177–179
Kim YI, Im HJ, Paeng JC et al (2015) Serum thyroglobulin level after radioiodine therapy (Day 3) to predict successful ablation of thyroid remnant in postoperative thyroid cancer. Ann Nucl Med 29:184–189
Park HJ, Min JJ, Bom HS et al (2017) Early stimulated thyroglobulin for response prediction after recombinant human thyrotropin-aided radioiodine therapy. Ann Nucl Med 31:616–622
Lin Y, Li T, Liang J et al (2011) Predictive value of preablation stimulated thyroglobulin and thyroglobulin/thyroid-stimulating hormone ratio in differentiated thyroid cancer. Clin Nucl Med 36(1102–1105):40
Montella L, Caraglia M, Abbruzzese A et al (2004) Molecular technology and the recombinant TSH have changed diagnostics of thyroid carcinoma with positive I-131 whole body scan but low serum thyroglobulin. Exp Mol Med 36:268–273
Bachelot A, Leboulleux S, Baudin E et al (2005) Neck recurrence from thyroid carcinoma: serum thyroglobulin and high-dose total body scan are not reliable criteria for cure after radioiodine treatment. Clin Endocrinol (Oxf) 62:376–379
Avram AM, Esfandiari NH, Wong KK (2015) Preablation 131-I scans with SPECT/CT contribute to thyroid cancer risk stratification and 131-I therapy planning. J Clin Endocrinol Metab 100:1895–1902
Jeong SY, Lee SW, Kim HW et al (2014) Clinical applications of SPECT/CT after first I-131 ablation in patients with differentiated thyroid cancer. Clin Endocrinol (Oxf) 81:445–451
Hu HY, Liang J, Zhang T et al (2018) Suppressed thyroglobulin performs better than stimulated thyroglobulin in defining an excellent response in patients with differentiated thyroid cancer. Nucl Med Commun 39:247–251
Ernaga-Lorea A, Hernandez-Morhain MC, Anda-Apinaniz E et al (2018) Prognostic value of change in anti-thyroglobulin antibodies after thyroidectomy in patients with papillary thyroid carcinoma. Clin Transl Oncol 20:740–744
Campennì A, Pignata SA, Baldari S (2018) Post-operative radioiodine therapy (RaIT) as adjuvant therapy in low–intermediate risk differentiated thyroid cancer. Clin Transl Imaging 6:347–355
Benua RS, Cicale NR, Sonenberg M, Rawson RW (1962) The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am J Roentgenol Radium Ther Nucl Med 87:171–182
Son SH, Lee CH, Jung JH et al (2017) Consideration of serum thyrotropin when interpreting serum thyroglobulin level in patients with differentiated thyroid cancer. Int J Thyroidol 10:5–13
Kowalska A, Palyga I, Gasior-Perczak D et al (2015) The cut-off level of recombinant human TSH-stimulated thyroglobulin in the follow-up of patients with differentiated thyroid cancer. PLoS One 10:e0133852
Kim MH, Ko SH, Bae JS et al (2012) Combination of initial stimulation thyroglobulins and staging system by revised ATA guidelines can elaborately discriminate prognosis of patients with differentiated thyroid carcinoma after high-dose remnant ablation. Clin Nucl Med 37:1069–1074
Ha J, Kim MH, Jo K et al (2017) Recombinant human TSH stimulated thyroglobulin levels at remnant ablation predict structural incomplete response to treatment in patients with differentiated thyroid cancer. Medicine (Baltimore) 96:e7512
Song M, Jeon S, Kang SR et al (2018) Response prediction of altered thyroglobulin levels after radioactive iodine therapy aided by recombinant human thyrotropin in patients with differentiated thyroid cancer. Nucl Med Mol Imaging 52:287–292
Bernier MO, Morel O, Rodien P et al (2005) Prognostic value of an increase in the serum thyroglobulin level at the time of the first ablative radioiodine treatment in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 32:1418–1421
Wang C, Zhang X, Li H et al (2017) Quantitative thyroglobulin response to radioactive iodine treatment in predicting radioactive iodine-refractory thyroid cancer with pulmonary metastasis. PLoS One 12:e0179664
Liu M, Cheng L, Jin Y et al (2018) Predicting (131)I-avidity of metastases from differentiated thyroid cancer using (18)F-FDG PET/CT in postoperative patients with elevated thyroglobulin. Sci Rep 8:4352
Nascimento C, Borget I, Al Ghuzlan A et al (2015) Postoperative fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography: an important imaging modality in patients with aggressive histology of differentiated thyroid cancer. Thyroid 25:437–444
Kloos RT, Ringel MD, Knopp MV et al (2009) Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27:1675–1684
Prince HE, Yeh C (2013) Reactivity of human IgM binding murine monoclonal 6B6C1 (IgG2a) with other murine monoclonal IgG antibodies. J Clin Lab Anal 27:27–30
Cabanillas ME, Waguespack SG, Bronstein Y et al (2010) Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab 95:2588–2595
Lin Y, Wang C, Gao W et al (2017) Overwhelming rapid metabolic and structural response to apatinib in radioiodine refractory differentiated thyroid cancer. Oncotarget 8:42252–42261
Cherk MH, Francis P, Topliss DJ et al (2012) Incidence and implications of negative serum thyroglobulin but positive I-131 whole-body scans in patients with well-differentiated thyroid cancer prepared with rhTSH or thyroid hormone withdrawal. Clin Endocrinol (Oxf) 76:734–740
Spencer CA, Platler BW, Nicoloff JT (1985) The effect of [125I]thyroglobulin tracer heterogeneity on serum Tg RIA measurement. Clin Chim Acta 153:105–115
Schneider AB, Pervos R (1978) Radioimmunoassay of human thyroglobulin: effect of antithyroglobulin autoantibodies. J Clin Endocrinol Metab 47:126–137
Weightman DR, Mallick UK, Fenwick JD et al (2003) Discordant serum thyroglobulin results generated by two classes of assay in patients with thyroid carcinoma: correlation with clinical outcome after 3 years of follow-up. Cancer 98:41–47
Jahagirdar VR, Strouhal P, Holder G et al (2008) Thyrotoxicosis factitia masquerading as recurrent Graves’ disease: endogenous antibody immunoassay interference, a pitfall for the unwary. Ann Clin Biochem 45:325–327
Algeciras-Schimnich A (2018) Thyroglobulin measurement in the management of patients with differentiated thyroid cancer. Crit Rev Clin Lab Sci 55:205–218
Giovanella L, Clark PM, Chiovato L et al (2014) Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. Eur J Endocrinol 171:R33–R46
Giovanella L, Treglia G, Sadeghi R et al (2014) Unstimulated highly sensitive thyroglobulin in follow-up of differentiated thyroid cancer patients: a meta-analysis. J Clin Endocrinol Metab 99:440–447
Spencer C, LoPresti J, Fatemi S (2014) How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes Obes 21:394–404
Spencer CA, Takeuchi M, Kazarosyan M et al (1998) Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 83:1121–1127
Zahn H, Folsche ET (1966) Reaction of nitrophenylpropionate with N alpha-acetyllysine methylamide. Hoppe Seylers Z Physiol Chem 345:215–220
Giovanella L, Keller F, Ceriani L et al (2009) Heterophile antibodies may falsely increase or decrease thyroglobulin measurement in patients with differentiated thyroid carcinoma. Clin Chem Lab Med 47:952–954
Verburg FA, Waschle K, Reiners C et al (2010) Heterophile antibodies rarely influence the measurement of thyroglobulin and thyroglobulin antibodies in differentiated thyroid cancer patients. Horm Metab Res 42:736–739
Hollowell JG, Staehling NW, Flanders WD et al (2002) Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499
Spencer C, Fatemi S, Singer P et al (2010) Serum Basal thyroglobulin measured by a second-generation assay correlates with the recombinant human thyrotropin-stimulated thyroglobulin response in patients treated for differentiated thyroid cancer. Thyroid 20:587–595
Kushnir MM, Rockwood AL, Roberts WL et al (2013) Measurement of thyroglobulin by liquid chromatography-tandem mass spectrometry in serum and plasma in the presence of antithyroglobulin autoantibodies. Clin Chem 59:982–990
Ahn BC, Seo JH, Bae JH et al (2005) Effects of anti-thyroglobulin antibody on the measurement of thyroglobulin: differences between immunoradiometric assay kits available. Kor J Nucl Med 39:252–256
Ahn BC, Lee WK, Jeong SY, Lee SW, Lee J (2013) Estimation of true serum thyroglobulin concentration using simultaneous measurement of serum antithyroglobulin antibody. Int J Endocrinol 2013:210639. https://doi.org/10.1155/2013/210639
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A3B01006631).
Author information
Authors and Affiliations
Contributions
SYK: Literature search and review, manuscript writing, and editing. YJZ: Literature search and review, manuscript writing, and editing. YSL: Manuscript writing, editing, and content planning. BCA: Manuscript writing and editing. HSB: Literature search and review, manuscript writing and editing, and content planning.
Corresponding author
Ethics declarations
Conflict of interest
Seong Young Kwon, Yingjie Zhang, Yansong Lin, Byeong-Cheol Ahn, and Hee-Seung Bom declare that they have no conflict of interest.
Ethical standard
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kwon, S.Y., Zhang, Y., Lin, Y. et al. Role of thyroglobulin in the management of patients with differentiated thyroid cancer. Clin Transl Imaging 7, 209–217 (2019). https://doi.org/10.1007/s40336-019-00325-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-019-00325-4