Abstract
Purpose
Thyroglobulin (Tg) may be released from damaged residual thyroid tissues after radioactive iodine (RAI) therapy in patients with differentiated thyroid carcinoma (DTC). We investigated whether altered levels of serum Tg after recombinant human thyrotropin (rhTSH)-aided RAI therapy could be a prognostic marker in patients with DTC.
Methods
We evaluated 68 patients who underwent RAI therapy after total thyroidectomy. Serum Tg levels were measured just before RAI administration (D0Tg) and 7 days after RAI therapy (D7Tg). Patients with a D0Tg level greater than 2.0 ng/mL were excluded to more precisely evaluate the injury effect of RAI in small remnant tissues. The ratioTg was defined as the D7Tg level divided by that on D0Tg. The therapeutic responses were classified as acceptable or non-acceptable. Finally, we investigated which clinicopathologic parameters were associated with therapeutic response.
Results
At the follow-up examination, an acceptable response was observed in 50 patients (73.5%). Univariate analysis revealed significant differences in N stage (P = 0.003) and ratioTg (acceptable vs. non-acceptable responses, 21.9 ± 33.6 vs. 3.8 ± 6.5; P = 0.006). In multivariate analysis, only ratioTg significantly predicted an acceptable response (odds ratio 1.104; 95% confidence interval 1.005–1.213; P = 0.040). A ratioTg above 3.5 predicted an acceptable response with a sensitivity of 66.0%, specificity of 83.3%, and accuracy of 70.6% (area under the curve = 0.718; P = 0.006).
Conclusions
Altered levels of serum Tg after RAI therapy, calculated as the ratioTg (D7Tg/D0Tg), significantly predicted an acceptable response in patients with DTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroglobulin (Tg) is a representative biomarker used to predict outcome in patients with differentiated thyroid carcinoma (DTC) who have undergone total thyroidectomy followed by radioactive iodine (RAI) therapy [1,2,3,4,5,6,7,8]. High levels of stimulated Tg before RAI therapy aided by thyroid hormone withdrawal (THW) were reported to predict a poor prognosis [2,3,4,5].
In DTC patients who received RAI therapy prepared with recombinant human thyrotropin (rhTSH), stimulated Tg measured before or after RAI therapy (72 h after the final injection of rhTSH) could be also used as a prognostic marker [7, 8]. However, rhTSH-stimulated Tg levels before RAI therapy could be underestimated because 72 h after the second injection of rhTSH corresponds to peak serum Tg levels [9]. rhTSH-stimulated Tg levels checked 72 h after the second injection could be overestimated through the addition of Tg released from damaged residual thyroid tissues after RAI-induced cellular injury [10,11,12,13].
Several studies have suggested that altered levels of serum Tg after THW-aided RAI therapy could predict the prognosis of patients with DTC [1, 3, 5, 6]. Therefore, we investigated whether altered levels of serum Tg 7 days after rhTSH-aided RAI therapy had prognostic value in these patients.
Materials and Methods
Patient Selection
From January 2015 to October 2015, 202 patients with DTC who underwent total or near-total thyroidectomy and received a first RAI therapy prepared with rhTSH in our institution were initially included. Among these patients, those with uncertain T stage (n = 1), missing Tg measurements (n = 2), levels of serum anti-Tg antibody (TgAb) above 100 IU/mL (to exclude the effect on serum Tg, n = 4), insufficient follow-up (n = 51), and undetectable serum Tg (< 0.1 ng/mL) on admission day (n = 45) were excluded. There were no patients with distant metastasis. To precisely evaluate the injury effect in small remnant tissues after RAI therapy, we determined the cutoff value of rhTSH-stimulated Tg that corresponded to a THW-stimulated Tg level less than 10 ng/mL [14]. Therefore, patients with serum Tg level just before RAI administration above 2.0 ng/mL (n = 31) were excluded. Finally, 68 patients were enrolled in this study.
This retrospective study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments and ethical approval was obtained from the local ethics committee (CNUHH-2016-069). For this type of study, the need for informed consent was waived.
Radioactive Iodine Therapy
RAI therapy for all patients consisted of two consecutive daily doses of 0.9 mg rhTSH (Thyrogen, Genzyme Transgenics Corp., Cambridge, USA) administered by intramuscular injection prior to RAI therapy (Fig. 1). The patients consumed a low-iodine diet for 2 weeks prior to RAI therapy. Twenty-four hours after the second injection of rhTSH, a therapeutic dose of RAI (3.70 GBq) was administered. A whole-body scan was performed 7 days after the oral ingestion of I-131.
Serum Thyroglobulin, Anti-Thyroglobulin Antibody, and TSH Measurement
Serum Tg levels were checked just before RAI administration (D0) and 2 days (D2) and 7 days (D7) after RAI therapy (Fig. 1). The levels were measured by immunoradiometric assay (IRMA) (RIA Tg-plus, BRAHMS GmbH, Hennigsdorf, Germany) with an analytical sensitivity (AS) and functional sensitivity (FS) of 0.08 and 0.2 ng/mL, respectively. Serum TgAb and TSH levels were also checked at the same time. Serum TgAbs levels were measured by radioimmunoassay (RIA anti-Tgn, BRAHMS GmbH, Hennigsdorf, Germany) with an AS of 5.5 U/mL and FS of 20 U/mL. Serum TSH levels were determined by IRMA (TSH-CTK-3, DiaSorin, Saluggia, Italy) with an AS and FS of 0.04 and 0.07 mU/L, respectively.
Follow-up Study
The first post-ablation follow-up examination was performed 6–24 months after RAI therapy. The follow-up protocol included serum Tg measurement (with or without TSH stimulation), neck ultrasound (US), and I-123 whole-body scan (WBS). In 43 patients, suppressed Tg with neck US was checked and 14 patients underwent stimulated Tg and I-123 WBS. The remaining patients (n = 11) underwent suppressed Tg with neck US and I-123 WBS with stimulated Tg.
The therapeutic responses were evaluated based on previous study and ATA guidelines mainly reflecting altered Tg levels [15, 16], as follows. (1) Acceptable response: negative or non-specific imaging findings and either suppressed Tg level < 1 ng/mL or stimulated Tg level < 10 ng/mL; (2) non-acceptable response: persistent or newly identified disease on imaging study and suppressed Tg level ≥ 1 ng/mL or stimulated Tg level ≥ 10 ng/mL or increased TgAb values.
Data Analysis
The ratioTg was defined as the D7Tg level divided by the D0Tg level to correct for the injury effect of remnant thyroid tissues from RAI from the initial remnant volume. We investigated which factors, including patient age, patient sex, T stage, N stage, tumor size, multiplicity, extrathyroidal extension, serum Tg, TgAb, TSH level, and ratioTg, were associated with therapeutic response. Tumors and lymph node metastases were classified using the staging system of the Union for International Cancer Control (UICC) and the seventh edition of the American Joint Committee on Cancer (AJCC) cancer staging manual. The differences in variables between the two groups were evaluated by Student’s t tests and chi-square tests for continuous and categorical variables, respectively. Variables with P values less than 0.25 were further analyzed by multivariate logistic regression analysis using the stepwise backward method. The results are presented as means ± standard deviations and P values less than 0.05 were considered statistically significant. The analysis was performed using IBM SPSS for Windows®, version 21.0 (IBM Corp., Armonk, NY, USA).
Results
The patients’ characteristics are summarized in Table 1. The study population comprised more women than men (45 women [66.2%]; 23 men [33.8%]), and the patients were 23–79 years of age (mean 48.9) at the time of surgery. Most pathologic results were T1 (n = 36, 52.9%) or T3 (n = 27, 39.7%) stages and N1a (n = 48, 70.6%). The histological DTC subtype was papillary in all 68 cases (100%). At the time of RAI therapy (3.70 GBq), the D0Tg level ranged from 0.1 to 1.9 ng/mL (mean 0.7), while the D2Tg and D7Tg ranged from 0.1 to 8.2 ng/mL (mean 1.6) and from 0.1 to 67.3 ng/mL (mean 2.4), respectively.
The patients’ characteristics are summarized in Table 1. The study population comprised more women than men (45 women [66.2%]; 23 men [33.8%]), and the patients were 23–79 years of age (mean 48.9) at the time of surgery. Most pathologic results were T1 (n = 36, 52.9%) or T3 (n = 27, 39.7%) stages and N1a (n = 48, 70.6%). The histological DTC subtype was papillary in all 68 cases (100%). At the time of RAI therapy (3.70 GBq), the D0Tg level ranged from 0.1 to 1.9 ng/mL (mean 0.7), while the D2Tg and D7Tg ranged from 0.1 to 8.2 ng/mL (mean 1.6) and from 0.1 to 67.3 ng/mL (mean 2.4), respectively.
At the follow-up examination, an acceptable response was observed in 50 (73.5%) patients, while 18 (26.5%) patients had a non-acceptable response. In univariate analysis, N stage (P = 0.003) and ratioTg (acceptable response vs. non-acceptable response, 21.9 ± 33.6 vs. 3.8 ± 6.5; P = 0.006) showed statistically significant differences, while tumor size (acceptable response vs. non-acceptable response, 1.2 ± 0.7 vs. 1.4 ± 0.6 cm; P = 0.202), D0Tg (acceptable response vs. non-acceptable response, 0.6 ± 0.4 vs. 0.9 ± 0.6 ng/mL; P = 0.063), and D7Tg (acceptable response vs. non-acceptable response, 10.5 ± 15.5 vs. 4.7 ± 12.5 ng/mL; P = 0.054) showed a statistical trend between the two groups (Table 2). In multivariate logistic regression analysis, the ratioTg (odds ratio [OR] 1.104; 95% confidence interval [CI] 1.005–1.213; P = 0.040) significantly predicted an acceptable response (Table 3). However, N stage did not show statistical significance for response prediction after RAI therapy (OR 3.223; 95% CI 0.808–12.852; P = 0.097).
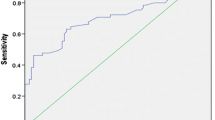
A receiver-operating characteristic (ROC) curve analysis showed that the optimal ratioTg cutoff value was 3.5, with a sensitivity of 66.0%, specificity of 83.3%, and accuracy of 70.6% for predicting an acceptable response (area under the curve (AUC) = 0.718; P = 0.006) (Fig. 2). Among 36 patients with a ratioTg > 3.5, an acceptable response was observed in 33 patients (91.7%), whereas 15 of the 32 (46.9%) patients with ratioTg ≤ 3.5 showed a non-acceptable response.
Receiver operating characteristic (ROC) curve analysis of ratioTg (D7Tg/D0Tg) for the prediction of acceptable response after rhTSH-aided radioactive iodine therapy. The optimal cutoff value of ratioTg was 3.5, with a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 66.0, 83.3, 91.7, 46.9, and 70.6%, respectively
Discussion
Tg, a homodimeric glycoprotein of 660 kDa (TG 19S), functions as the highly specialized matrix for thyroid hormone biosynthesis as well as the storage of the inactive forms of the thyroid hormones and iodine [17]. In patients with DTC, the stimulated Tg level measured just before RAI therapy was shown to be a biochemical tumor marker that could predict persistent or recurrent disease after THW [2,3,4,5]. Kim et al. [2] reported that serum Tg levels measured at the time of RAI therapy in low-risk patients with DTC were correlated with persistent or recurrent disease as well as serum Tg measured 6–12 months later prepared with THW.
rhTSH represents a safe and effective means of increasing serum TSH levels in preparation for RAI therapy, avoiding the signs and symptoms of hypothyroidism associated with THW [18, 19]. I-131 is usually administered on the day after the second injection of rhTSH, according to the conventional schedule. Serum Tg levels should be measured 72 h after the final injection of rhTSH, which corresponds to peak serum Tg levels [9]. rhTSH-stimulated Tg has been studied as a prognostic marker in DTC patients [7, 8]. Park et al. [8] showed that these levels just before RAI therapy were an independent predictor of excellent response on follow-up (OR 2.012; 95% CI 1.384–2.925; P < 0.001), and the optimal cutoff for early Tg before RAI therapy was 2.0 ng/mL for predicting a non-excellent response (sensitivity 46.2%, specificity 95.3%, positive predictive value 88.2%, negative predictive value 69.8%, area under the curve [AUC] 0.704; P < 0.001). However, serum Tg levels checked before RAI were not fully stimulated in patients aided by rhTSH. Melo et al. [7] demonstrated that serum Tg level measured 3 days after rhTSH-aided RAI therapy had a predictive value for disease persistence or recurrence 1 year later in 131 consecutive DTC patients. In this study, Tg levels ranged from 0 to 1927 ng/mL and were significantly lower in the disease-free group (17.9 ± 49.1 vs. 136.3 ± 341.0 ng/mL, P < 0.001), with an optimal Tg cutoff level of 7.2 ng/mL.
However, serum Tg level measured 72 h after the second injection of rhTSH is not a reliable prognostic factor because elevated serum Tg level could be related to the release of stored Tg from thyroid follicles through radiation-induced cellular damage after RAI therapy. Serum Tg and thyroid hormone levels are elevated due to cellular damage after incidental external irradiation to the thyroid through increased cellular membrane permeability and apoptosis after radiation exposure [10,11,12,13]. Several studies have reported that altered serum Tg levels after THW-aided RAI therapy could predict the prognosis of patients with DTC [1, 3, 5, 6]. Kim et al. [5] showed that a high ratioTg (Tg checked 3 days after RAI ablation/stimulated Tg just before RAI therapy), as well as a low level of stimulated Tg just before RAI therapy, showed significant results for the prediction of ablation effects. Bernier et al. [3] showed that a lower or higher ratioTg (serum Tg sampled 5 days after RAI therapy/stimulated Tg just before RAI therapy) was independently associated with successful ablation. Therefore, precise parameters are necessary to overcome the limitations of Tg measurement.
Our study showed that the ratioTg, which consisted of released Tg (D7Tg) levels corrected according to early Tg (D0Tg) levels to reflect early remnant volume, was a significant predictor of an acceptable response in selected patients with D0Tg ≤ 2.0 ng/mL of RAI therapy prepared with rhTSH (OR 1.104; 95% CI 1.005–1.213; P = 0.040) (Table 3). In particular, a ratioTg of 3.5 was a good predictor for an acceptable response (AUC = 0.718; P = 0.006). There are several reasons why we selected patients with D0Tg ≤ 2.0 ng/mL. The cutoff level of rhTSH-stimulated Tg differed significantly from that of THW-stimulated Tg. Kowalska et al. [14] reported that values rhTSH-stimulated Tg of 0.6 and 2.3 ng/mL corresponded to THW-stimulated Tg values of 2.0 and 10.0 ng/mL, respectively, indicating that rhTSH-stimulated Tg levels less than 2.0 ng/mL could have a wide range of Tg in THW-stimulated status. In previous studies, patients with stimulated serum Tg levels prepared with THW > 10 ng/mL [2, 4] and stimulated serum Tg level aided by rhTSH > 2 ng/mL [8] had a poor prognosis. Therefore, we focused on patients with rhTSH-stimulated Tg levels corresponding to THW-stimulated Tg levels less than 10 ng/mL although rhTSH-stimulated Tg levels could not be directly compared with THW-stimulated Tg levels.
Our study has several limitations. First, this study was retrospective, so a selection bias was inevitable. Especially, it was difficult to apply RAI dose differently based on the risk stratification or pathologic features. Second, the follow-up protocols were not consistent for all patients. Some patients underwent I-123 WBS with stimulated Tg, and some underwent neck US with suppressed Tg. In order to reduce errors caused by different imaging test modalities, we tried to focus on changes in serum Tg levels after RAI therapy by adjusting for response assessment. Third, we did not perform long-term follow-up with ratioTg. Therefore, further studies are required to validate ratioTg as a prognostic marker for recurrence.
Conclusion
Tg elevation after RAI therapy, calculated as ratioTg, could significantly predict therapeutic response in DTC patients with relatively small remnant thyroid tissue. A higher ratioTg (> 3.5) had a favorable diagnostic accuracy for the prediction of an acceptable response. This parameter could be useful in DTC patients undergoing RAI therapy aided by rhTSH because rhTSH-stimulated Tg (both D0Tg and D2Tg) have limitations as prognostic markers compared to THW-stimulated Tg.
References
Muratet JP, Giraud P, Daver A, Minier JF, Gamelin E, Larra F. Predicting the efficacy of first iodine-131 treatment in differentiated thyroid carcinoma. J Nucl Med. 1997;38:1362–8.
Kim TY, Kim WB, Kim ES, Ryu JS, Yeo JS, Kim SC, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:1440–5.
Bernier MO, Morel O, Rodien P, Muratet JP, Giraud P, Rohmer V, et al. Prognostic value of an increase in the serum thyroglobulin level at the time of the first ablative radioiodine treatment in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2005;32:1418–21.
Park HJ, Jeong GC, Kwon SY, Min JJ, Bom HS, Park KS, et al. Stimulated serum thyroglobulin level at the time of first dose of radioactive iodine therapy is the most predictive factor for therapeutic failure in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014;48:255–61.
Kim YI, Im HJ, Paeng JC, Cheon GJ, Kang KW, Lee DS, et al. Serum thyroglobulin level after radioiodine therapy (day 3) to predict successful ablation of thyroid remnant in postoperative thyroid cancer. Ann Nucl Med. 2015;29:184–9.
Jeong GC, Song M, Park HJ, Min JJ, Bom HS, Cho SG, et al. Iodine uptake patterns on post-ablation whole body scans are related to elevated serum thyroglobulin levels after radioactive iodine therapy in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2016;50:329–36.
Melo M, Costa G, Ribeiro C, Carrilho F, Martins MJ, da Rocha AG, et al. Stimulated thyroglobulin at recombinant human TSH-aided ablation predicts disease-free status one year later. J Clin Endocrinol Metab. 2013;98:4364–72.
Park HJ, Min JJ, Bom HS, Kim J, Song HC, Kwon SY. Early stimulated thyroglobulin for response prediction after recombinant human thyrotropin-aided radioiodine therapy. Ann Nucl Med. 2017;31:616–22.
Rosario PW, Salles DS, Purisch S. Area under the curve of TSH after levothyroxine withdrawal versus administration of recombinant human TSH (rhTSH): possible implications for tumor growth. Arq Bras Endocrinol Metabol. 2009;53:767–70.
Nishiyama K, Kozuka T, Higashihara T, Miyauchi K, Okagawa K. Acute radiation thyroiditis. Int J Radiat Oncol Biol Phys. 1996;36:1221–4.
Cramp WA, Yatvin MB, Harms-Ringdahl M. Recent developments in the radiobiology of cellular membranes. Acta Oncol. 1994;33:945–52.
Ramakrishnan N, McClain DE, Catravas GN. Membranes as sensitive targets in thymocyte apoptosis. Int J Radiat Biol. 1993;63:693–701.
Bachelot A, Cailleux AF, Klain M, Baudin E, Ricard M, Bellon N, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12:707–11.
Kowalska A, Pałyga I, Gąsior-Perczak D, Walczyk A, Trybek T, Słuszniak A, et al. The cut-off level of recombinant human TSH-stimulated thyroglobulin in the follow-up of patients with differentiated thyroid cancer. PLoS One. 2015;10:e0133852.
Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–9.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2015;26:1–133.
Rivolta CM, Targovnik HM. Molecular advances in thyroglobulin disorders. Clin Chim Acta. 2006;374:8–24.
Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–7.
Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–85.
Funding
This research was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2015M3C1A3056410).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors Minchul Song, Subin Jeon, Sae-Ryung Kang, Zeenat Jabin, Su Woong Yoo, Jung-Joon Min, Hee-Seung Bom, Sang-Geon Cho, Jahae Kim, Ho-Chun Song, and Seong Young Kwon declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
Rights and permissions
About this article
Cite this article
Song, M., Jeon, S., Kang, SR. et al. Response Prediction of Altered Thyroglobulin Levels After Radioactive Iodine Therapy Aided by Recombinant Human Thyrotropin in Patients with Differentiated Thyroid Cancer. Nucl Med Mol Imaging 52, 287–292 (2018). https://doi.org/10.1007/s13139-018-0528-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-018-0528-7