Abstract
In recent decades, the greatest challenge facing the world has been protecting the environment from various forms of pollution. Water pollution is one of the most crucial environmental problems threatening living organisms’ lives and human health. Mostly anthropogenic, it undoubtedly originates from diverse sources, including agricultural, domestic, and industrial activities. Therefore, adopting sustainable and environmentally friendly practices constitutes an ideal solution for purifying contaminated water to be further used in industrial activities and so on. The valorization of lignocellulosic biomass for the production and conception of value-added products is an attractive and environmentally friendly way of preserving the environment. Lignocellulosic biomass, such as crops, agricultural wastes, forest residues, etc., is a sustainable and plentiful resource that can be valorized and used as robust material for eliminating different pollutants from sewage, including organic pollutants, heavy metals, inorganic compounds, and microorganisms. Indeed, the valorization of biomass wastes is among the most intelligent strategies. It is like killing two birds with one stone: reducing the quantity of biomass waste and benefiting from its physicochemical properties. Feedstocks are rich in cellulose, hemicellulose, and lignin, which have already been proven efficiency in removing persistent pollutants. Moreover, it can undergo physical, chemical, and thermal to prepare cellulose nanocrystals and biochar with high removal ability. The current review discusses the exploitation of lignocellulosic biomass to produce composite materials in the applications of wastewater purification, especially for the removal of different persistent organic and inorganic contaminants. It highlights the recent research studies and the mechanisms involved in eliminating pollutants using lignocellulosic-based materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, environmental issues, especially those related to water pollution, have become major current topics for the entire population and even constitute significant challenges for the future. Water pollution mainly originates from various socio-economic activities, including agricultural, domestic, and industrial activities. It is often accompanied by the discharge of effluents containing a variety of pollutants into the aquatic environment, namely, organic and inorganic contaminants, heavy metals, pesticides, pharmaceuticals, and many other substances [1, 2]. The release of these untreated and toxic pollutants, even at low concentrations in water without treatment, generates undesirable effects on the aquatic ecosystem and human life [3, 4]. Moreover, with the growing environmental awareness and new legislation, the discharged water must meet the minimum quality criteria to satisfy the requirements proposed by different countries [5]. Wastewater management is essential to underpin the rapidly expanding human population and reduce environmental pollution and health risks. According to the literature, several treatment processes have been implemented, more or less successfully, to control water pollution and to reduce the quantities of contaminants in aquatic environments, including flocculation and coagulation, flotation, activated sludge processing, membrane separation, adsorption, photocatalysis, and advanced oxidation processes [6,7,8,9,10]. The choice of the appropriate method is mainly based on the characteristics of the wastewater, the expertise for operation and maintenance, the costs implications, etc. [11, 12]. Thus, in terms of simplicity, efficiency, sustainability, and cost, the adsorption process remains the technique of most interest in cleaning polluted water. Indeed, many adsorbents have been used either for the elimination of organic and inorganic substances or persistent microorganisms [13, 14].
As part of a sustainable development policy, applying biotechnology in developing and designing new natural adsorbents, abundant economically and effective to treat ecosystems is an ideal solution [15, 16]. Biomaterials derived from renewable feedstocks such as lignocellulosic materials have raised more attention to substitute conventional sorbents because of their abundance, low cost, biodegradability, and ease to modify chemically and thermally to produce high-value products [17,18,19]. They display better adsorptive performances compared to some commercial resins [20]. The perspective of using biomass to produce meaningful products brings great economic benefit by preparing cheaper sorbents with high effectiveness and provides an environmental value through waste management and minimization. Lignocellulosic biomass is considered the most available renewable source on the earth, originating from plants, agricultural and forestry wastes, and annual and perennial dry energy grasses [21, 22]. It consists of cellulose, hemicellulose, and lignin as principal components and other substances such as pectin, proteins, and ashes as secondary products, besides the extractives and minerals. The compositional values of the significant features of lignocellulose biomass are relative to the type and source of the feedstock. Still, the cellulose content ranges between 40 and 60 wt.%, hemicellulose between 20 and 40 wt.%, and lignin between 10 and 25 wt.% [20, 23]. According to the literature, biomass such as natural fibers has shown good cost-effectiveness for capturing pollutants from sewage [24,25,26].
Nevertheless, lignocellulosic biomass requires additional thermal and chemical treatments to improve the removal of toxic pollutants. These treatments modify the surface characteristics and provide better accessibility to binding and active sites. This review seeks to bring an overview of recent studies on the exploitation of lignocellulosic biomass to produce highly effective composite materials to capture wastewater pollutants. Different physicochemical and thermal ways to convert biomass wastes to high-value products have been highlighted. The properties of the resulting materials have also been revealed. The mechanisms involved in removing different organic and inorganic contaminants have been discussed. And finally, the challenges and pitfalls of their use in large-scale applications are addressed.
2 Water pollution
Water pollution is one of the serious problems representing a real danger for human and aquatic life. Related to anthropogenic activities (e.g., domestic, urban, industrial, and agricultural), water contamination is generated by many substances, including organic, inorganic, and biological pollutants that can affect surface water and groundwater [27, 28]. Besides, identifying effective and inexpensive remediation methods and processes is always an essential issue in a comprehensive approach to controlling contaminant spreading and maintaining water quality. Indeed, many techniques are used today for the decontamination of polluted waters depending on the water to be treated [29, 30]. The adsorption process using eco-friendly materials is one of the efficient strategies for water quality management. This section aims to highlight the different sources of water contaminations and the categories of pollutants present in wastewater. Then, the emphasis will be put on the adsorption process as one of the powerful strategies for wastewater treatment.
2.1 Sources of water pollution and principal water contaminants
2.1.1 Sources of water pollution
Surface water pollution refers to the degradation of the water quality by changing its physical, chemical, and biological properties. Such pollution originates from various anthropogenic sources, namely:
-
Domestic water pollution: Domestic water contamination is mainly related to the disposal of household sludges rich in organic wastes, pharmaceutical compounds and personal care products, microorganisms, greases, mineral matters, nitrogen, and phosphorus, and also contains mineral salts and metals into surface waters. Domestic wastewater comprises two types of domestic water, black water loaded with fecal germs and various other organic matter full of nitrogen and greywater from various household activities full of organic debris, detergents, and grease [31]. The composition of domestic wastewater can be highly variable and depends on:
-
The original composition of the drinking water depends on the quality of the treatment of this water, the sanitary standards of the country concerned, the nature of the pipes, etc.
-
People use, which can bring an almost infinite number of pollutants: all the cleaning products, detergents but also, solvents, paints, thermometer mercury, glue, etc.
-
Discharge of organic matter such as urine and feces into sewer systems.
-
-
Industrial pollution: Industrial sewage consists mainly of effluents resulting from industrial activities such as food processing, textile, dyeing, mining and quarrying, pharmaceutical industry, etc. Thus, depending on the type of the industrial process, these waters can contain diverse pollutants, namely, organic matter and grease, chemical products, hydrocarbons, heavy metals, metallic trace elements, and so on. Water supplied by industrial activities is considered the most dangerous for the aquatic environment and human health since it contains harmful substances [32].
-
Agricultural pollution: Agricultural wastewater occurs because of processes on surrounding farms, agricultural activities, and sometimes contaminated groundwater. The principal causes of agricultural water pollution are:
-
Fertilizers on agricultural soil, especially nitrogenous fertilizers, are linked to cultivation practices.
-
Phytosanitary products (herbicides and pesticides).
-
Metals from food supplements for livestock, residues of antibiotics used against animal infections.
-
Animal manure cannot be recovered from grazing animals.
-
2.1.2 Principal water contaminants
The wastewater composition varies depending on its origin (agricultural, industrial, or domestic). It can contain many substances, either solid or dissolved form, and microorganisms. Depending on their physical, chemical, and biological characteristics, these compounds can be classified into three dominant classes, namely organic, inorganic, and microbiological contaminants. Table 1 summarizes the principal pollutants found in different polluted water.
2.2 Adsorption as an alternative approach for wastewater purification
For a long time, many techniques have been used in the decontamination and depollution of wastewater depending on the nature and concentration of the contaminant and the environment to be decontaminated. Adsorption technology is among the most efficient remediation techniques proposed for removing persistent pollutants from effluents due to its excellent removal efficiency, durability, low cost, and easy operation conditions [59,60,61,62]. Adsorption is a surface phenomenon involving the mass transfer of substances between two phases (from a liquid or gas phase to a solid phase) due to various interactions between the surface of the adsorbents and the adsorbed molecules. In general, we distinguish between two different types of adsorption mechanisms: physisorption and chemisorption [63, 64]. Physisorption is typically attributed to electrostatic forces between the substances in solution and the surface of the sorbent due to van der Waals forces, resulting in multilayer sorption. It is non-specific, reversible, usually rapid at low temperatures, and requires low activation energy, nearly 20 to 40 kJ/mol. Moreover, it is proportional to the adsorbent features (e.g., surface area and porosity). Chemisorption is typically related to covalent or electrostatic chemical bonds between the adsorbate and the adsorbent resulting in a unimolecular layer. It is an exothermic process that is specific, irreversible, and requires high activation energy, generally higher than 80 kJ/mol [65, 66].
Thus, the adsorption reaction can be performed generally in four main successive steps as follows [67, 68] (Fig. 1):
-
Transfer in solution phase, which can occur instantaneously (bulk diffusion).
-
Transfer the adsorbate from the bulk solution to the surface of the adsorbent through a hydrodynamic boundary layer (film or external mass diffusion).
-
Internal diffusion of the adsorbate inside the sorbent pores (pore or intraparticle diffusion).
-
Adsorption of the adsorbate on the external surface of the adsorbent through binding of the ions to the active sites.
The adsorption process can be achieved in several ways (e.g., batch adsorption, continuous fixed-bed, continuous fluidized bed, continuous pulsed bed continuous moving bed, and continuous-flow tank adsorption). As well as the most used systems are batch and continuous fixed bed modes due to their ease of implementation, affordability, and effectiveness [69]. Likewise, adsorption can be strongly affected by a variety of parameters, including the characteristics of the water being treated, the nature and properties of the material itself (surface area and porosity), and the environmental parameters such as pH, initial concentration of pollutant, temperature, contact time, etc. [70]. The choice of the appropriate adsorbent, in addition to the availability and the economic interest, is made according to its nature and the affinity that this adsorbent presents towards target pollutants. Commercial activated carbon is currently the most widely used adsorbent in wastewater treatment worldwide to remove dyes, aromatic and phenolic derivatives, pesticides, heavy metals, and so on. Nevertheless, the use of commercial activated carbon is often limited by the high cost and its difficulty to be regenerated [64, 71]. Therefore, researchers are now directed towards natural, inexpensive, and easily regenerable adsorbents to substitute commercial activated carbon such as clays, siliceous materials, biopolymers, industrial by-products, and lignocellulosic biomass [72,73,74,75,76]. Lignocellulosic biomass referring mainly to plant dry matter from agricultural and forest wastes is an attractive and efficient source for preparing highly effective and inexpensive materials for wastewater purification applications. Figure 2 illustrates the main types of lignocellulosic biomass; however, it is worth noting that we are particularly interested in lignocellulosic biomass of plant origin (agricultural and forestry waste), which are commonly composed of cellulose, hemicellulose, and lignin.
3 Generalities on lignocellulosic biomass
3.1 Structure and characteristics of lignocellulosic biomass
Lignocellulosic biomass, commonly known as natural or cellulosic fibers, has been used since antiquity in many applications. The main use is the reinforcement of polymers for developing new and highly mechanical composites for many purposes [77,78,79]. Since then, lignocellulosic biomass has been exploited in other fields, especially in environmental applications (e.g., energy, biofuel production, water purification, etc.). Compared with conventional materials, natural fibers offer the advantage of being biodegradable, lightweight, endowed with fascinating physicochemical properties, easy to be modified, and inexpensive [80,81,82]. Moreover, they are considered the most abundant and bio-renewable material on earth. According to the literature, lignocellulosic biomass is a plant cell wall of an assembly of cellulosic tissues, composed generally of polysaccharides (cellulose and hemicellulose) and aromatic polymer (lignin), as well as other minor components such as pectin, protein, salts, minerals, etc. which are linked to each other through both hydrogen and covalent bonds, forming a highly recalcitrant structure (Fig. 3) [83]. Their proportion varies depending on the type of raw material. Still, generally, cellulose is the major component of cell walls of lignocellulose biomass, representing 40–60% in weight, followed by hemicellulose acting as a binding adhesive between cellulose and lignin (20–40 wt%) and finally lignin which represents 10–25 in weight [84, 85]. Besides, according to their origin, lignocellulosic biomasses can be classified as seed, leaf, stems, xylem, bast, or core fibers [80]. Table 2 displays the content of cellulose, hemicellulose, and lignin of particular types of biomass species.
3.2 Lignocellulosic biomass as adsorbent for the removal of pollutants from wastewater
Different lignocellulosic biomass species have been used as adsorbents to retain a wide range of contaminants from wastewater due to their outstanding features, including abundance, low-cost, processability, non-toxicity, and high porosity and hydrophilicity, excellent mechanical properties, and high adsorption ability [92,93,94]. Kyzas et al. [26] investigated the adsorption behaviors of three natural fiber types, namely flax, ramie, and kenaf fibers, in removing dyes from aqueous solutions (Fig. 4). Adsorption experiments show that all three types of natural fibers effectively absorb basic yellow dye molecules 37 in alkaline media. This can be attributed to the presence of hydroxide groups on the outer surface of the natural fibers, which promote electrostatic interactions between the fibers and the amino groups of the dye. Moreover, the authors demonstrated that the adsorption behaviors toward the target pollutant differ for each natural fiber. Ramie fibers were more effective in absorbing the dye, followed by flax and then kenaf fibers. The authors attributed this difference to the amount of cellulose in each type of biomass, suggesting that natural fibers with high cellulose content are more effective for dye removal than fibers with a high amount of hemicellulose or lignin. Besides, fibers rich in cellulose can be regenerated and reused until several adsorption–desorption cycles without losing their adsorption capacity. Flax fibers present the worst reuse behaviors, while ramie had the best. Nevertheless, the correlation is not necessarily linear because of the many factors that affect adsorptive performances, such as porosity, surface area, etc. Banana fibers extracted from three different parts of the banana tree (leaves, stalk, and stem) were also used to absorb acid green dye from polluted water [95]. Adsorption results show that the adsorption capacity of the three sorbents increased with increasing contact time, initial dye concentration, and highly acidic conditions. Moreover, banana leaves displayed high adsorption capacity than banana stalk and stem fibers, which may be due to their high porosity. SEM micrographs of the three adsorbents revealed the smooth nature of banana stem and stalk fibers compared to banana leaf fibers. The sorption of all three sorbents closely follows pseudo-second-order kinetics, suggesting chemisorption occurs as a rate-controlling step. Similarly, Formosa papaya seed powder has been successfully used to absorb crystal violet dye from aqueous solutions [96]. Indeed, due to their porosity and the abundance of carbonyl and hydroxide groups on their surface, Formosa papaya seed fibers present a high affinity toward the amino groups of the cationic dye, favoring electrostatic interactions leading to high adsorption removal. The adsorption capacity of fibers was found strongly dependent on the pH of the dye solution, contact time, and the initial dye concentration. To improve the adsorptive performances of natural fibers towards anionic dyes, Idan et al. [97] investigate the modification of the external surface of kenaf core fibers using a (3-chloro-2-hydroxypropyl) trimethylammonium chloride surfactant. The purpose of such treatment is to enrich the external surface of natural fibers with NH4+ groups to increase their affinity towards anionic dyes through promoting ion-exchange adsorption. Adsorption results revealed the efficiency of modified kenaf core fibers in removing anionic reactive red-RB dye compared with neat fibers. Lignocellulosic fibers were also used for the removal of heavy metals from wastewater. In this context, Lee et al. [98] investigated the adsorption behaviors of a wide range of natural fibers, namely, coconut coir, cotton, kenaf bast, kenaf core, spruce, and sugarcane bagasse towards heavy metals. Different fibers’ ability to eliminate copper, nickel, and zinc was tested as a function of their lignin amount. Results show that the removal rate of heavy metal ions was independent of the lignin content. Kenaf bast fibers were found to be the best in the elimination of the three heavy metal ions from aqueous solutions, with high selectivity toward copper and zinc ions. While cotton presents low adsorption capacity, even its lignin content does not exceed 1% of its weight. Asadi et al. [99] assessed the effect of modifying two natural fibers, namely rice hull and sawdust, on heavy metal ions removal from aqueous media. Rice hull fibers exhibited a higher adsorption capacity for cadmium, lead, zinc, copper, and nickel ions than sawdust fibers. The adsorption capacity of the fibers increased with increasing the initial concentration and the pH of the solution until a pH equal to 5. The authors attributed this difference to the chemical behaviors of heavy metal ions, their sorption energy, and their affinity to sorbents. Moreover, it was observed that the uptake of the ions is more favorable in single-metal solutions than in two-metal solutions. Studies revealed that the sorption of different pollutants in lignocellulosic biomasses occurs through several mechanisms, including adsorption on the external surface through electrostatic interactions, chemisorption, complexation, diffusion through pores, and ion exchange [100,101,102]. Thus, to enhance the adsorption performances of lignocellulosic biomass and improve its physicochemical features, especially porosity, hydrophilicity, and surface area, a variety of physical, chemical, and thermal pathways have been proposed to convert raw fibers to high value-added products such as (i) cellulose/cellulose nanocrystals-based composites, (ii) hemicellulose-based composites, (iii) lignin-based composites, and (iv) biochar composites.
Pictures of fibers before and after adsorption experiments [26]
4 Cellulose-based composites for wastewater purification
As mentioned before, lignocellulosic biomass is constituted mainly of three compounds, namely, cellulose, hemicellulose, and lignin. The amount of each component depends on the nature of the feedstock. Moreover, to improve the adsorption capacity of natural fibers, many strategies have been proposed in the literature, including the extraction and separation of cellulose from other compounds [103]. Indeed, many physicochemical treatments have been used to obtain pure cellulose with high hydrophilicity, large surface area, and reactive hydroxyl groups on the outer surface. This section aims to highlight the use of cellulose and its derivatives as one of the world’s abundant and renewable materials for the preparation of efficacious composite materials for wastewater treatment.
4.1 Cellulose extraction from lignocellulosic biomass
Cellulose (C6H10O5)n, the major compound of the lignocellulose biomass, was first isolated by Anselme Payen in 1838 [24], while its chemical structure was described by Herman Staudinger [104], suggesting that cellulose is a homopolymer consisting of anhydroglucopyranose units linked by β-(1–4) glycosidic bonds. Since then, cellulose’s physical and chemical features have been intensively studied. Structurally, cellulose is a long linear polysaccharide polymer consisting of d-glucose subunits linked by β-(1,4)-glycosidic bonds, promoting a flat ribbon-like structure (Fig. 5) [105]. Each of the anhydroglucose units contains three free hydroxyl groups: primary alcohol on carbon 6 and two secondary alcohol on carbons 2 and 3. The d-glucose units display a chair-like conformation in which the three hydroxyl groups are in an equatorial position in the ring plane. At the same time, the hydrogen atoms are in an axial position. This spatial structure favors the formation of a very dense network of inter-and intramolecular hydrogen bonds, which are responsible for its remarkable resistance and highly organized structure. Cellulose microfibrils possess a semi-crystalline structure, characterized by an alternation of crystalline and amorphous zones in which the chains are less organized [106,107,108]. Native cellulose generally exhibited a degree of crystallinity within the range of 40–70% [109]. The degree of polymerization of cellulose is relatively high and varies depending on the nature of the raw feedstock. Thus, in plant walls, most cellulosic fibers are in the form of native cellulose. These fibers generally adopt a structure commonly called type I, which comprises two allomorphs, Iα and Iβ, in different ratios. Cellulose II is the regenerated cellulose or the cellulose treated by alkaline treatment (NaOH). Other allomorphs of cellulose with varying sizes of lattice, chain orientation, and conformation can also be obtained by different treatments such as cellulose III1, III2, IV1, and IV2 [110, 111].
The cellulose can be separated easily from other components through mechanical treatment to extract cellulose microfibrils (MFC) and undergoes chemical treatments, including alkaline and bleaching treatments to obtain cellulose nanofibrils (CNF). Alkaline treatment is widely used to eliminate waxes covering the fibers and hydrolyze hemicellulose and lignin. It is usually performed in the presence of sodium hydroxide (NaOH) at ambient temperatures and for long residence times up to 24 h. Alkaline solutions break the cross-link of ferulic acid binding between lignin and hemicelluloses and degrade them. Whilst bleaching treatment using hydrogen peroxide or sodium chlorite aims frequently to solubilize lignin by breaking the bonds binding it to other hydrocarbon compounds, promoting access to the rich cellulose fraction and also increasing the exposed surfaces area, the porosity, etc. [112, 113]. Moreover, to disseminate the amorphous regions and allow longitudinal cutting of the cellulosic microfibrils, acid hydrolysis is proposed to release the rod-shaped nanocrystalline cellulose (CNC). One of the main differences between the microfibrillated cellulose and the nanocrystalline cellulose is the distribution of the fiber size, which is large in the microfibrillated cellulose and close or drastically shorter in the nanocrystalline cellulose.
4.2 Cellulose nanocrystals extraction
While cellulose is a crystalline molecule, its crystallinity is flawed; a significant part of the cellulose structure is loosely ordered and can best be described as amorphous. Pure semi-crystalline cellulose, commonly known as cellulose pulp, can further undergo other treatments to produce highly crystalline cellulose, known as cellulose nanocrystals (CNC), cellulose nanowhiskers (CNW), or nanocrystalline cellulose (NCC) [114]. Compared to cellulose, nanocellulose exhibited high porosity and aspect ratio and large surface area, which are important for wastewater treatment applications. According to the nature of the feedstock, morphological structure, and preparation process, nanocellulose can be divided into three types, namely cellulose nanocrystals (CNC), cellulose nanofibrils (CNF), and bacterial nanocellulose (BNC) (Fig. 6).
Different types of nanocellulose [115]
Cellulose nanocrystals (CNCs) in general consist of rod-shaped nanoparticles with a width of 4–70 nm, a length of 100 to 250 nm (Fig. 7), a crystallinity index greater than 50%, and are usually derived out of various renewable sources through acid hydrolysis process [116]. Acid hydrolysis using a strong acid like sulfuric acid (H2SO4), hydrochloric acid (HCl), or phosphoric acid (H3PO4) involves the breakdown of the β-1,4-glycosidic bonds of amorphous regions, which is an essential step for the dissolving of cellulose and its hydrolysis to short-chain glucose-polymers, leading to cellulose nanocrystals. Nanofibrillated cellulose (NFC), also known as nanofibrous cellulose, exhibits an interlocking lattice structure with more extended and broader flexible nanofibers, widths varying from 10 to a few hundred nanometers or lengths are in the micron range are mainly produced through mechanical processing such as grinding, homogenization, enzymatic treatments, or a combination of them. Certain enzymes could degrade hemicellulose and lignin without disturbing the cellulose content. Compared to CNC, NFC exhibited low crystallinity since it comprises a mixture of crystalline cellulose and amorphous regions inside the fibers.
SEM micrographs and TEM image of CNC from different sources [121]
In contrast, several microorganisms and cell-free systems produce the bacterial nanocellulose consisting of ribbon-shaped nanofibers with 20–100-nm diameter and micrometer lengths. Besides, BNC represents the purest form of cellulose; however, large-scale production of BC and ECNF is difficult and remains a challenge [117,118,119]. The principle, advantages, and disadvantages of each preparation method were already addressed in the literature [120].
4.3 Cellulose and its derivative-based composites for wastewater purification
The unique and attractive characteristics of cellulose and its derivatives (CNC, NFC, and BCN) have already been extensively demonstrated, prompting the scientific communities to focus on the use of these materials, either alone or in the form of composite, for the removal of organic, inorganic and microbiological contaminants from sewage [122, 123]. Besides, the abundance of functional groups such as hydroxyl and carboxyl on its external surface enhances the adsorption and the binding of different contaminants. Cellulose-based composites are generally manufactured in two manners, the first consists of the dissolution of the cellulose fibers or powder in the suitable solvent, followed by a mixture with the material and the crosslinking of the chains, in order to obtain a three-dimensional network capable of adsorbing and retaining pollutants, while the second approach involves the dissolving the cellulose in the appropriate solvent, then crosslinking it to form the 3D network and subsequently impregnating it with the required material to obtain the composite with high retention capacity. In this context, Santoso et al. [124] successfully embedded a three-dimensional cellulose hydrogel with bentonite clay for the removal of anionic dye from wastewater. Cellulose from Whatman filter paper was deprotonated using sodium hydroxide and urea to prepare hydrogel solution and then embedded with bentonite with a different ratio, resulting in composites with high surface area and porous structure. Compared to neat filter paper, composite exhibited rough surfaces and a large surface area ranging between 29 to 51 m2/g. The surface area increased with increasing the bentonite clay content, suggesting the role of clay in improving the physicochemical of cellulose hydrogel. The presence of bentonite has a positive effect also on the hydrogel thermal stability and swelling features. Moreover, FTIR analysis shows that the bentonite is loaded in between the cross-linked cellulose networks and not attached to it with chemical interactions. Adsorption experiments confirmed the high adsorption capacity of all cellulosic composites compared to the neat Whatman filter paper. Thus, cellulose hydrogel embedded with a high amount of bentonite exhibits the highest adsorption performances toward Congo red dye, which is naturally attributed to its high surface area, and porous structure. Moreover, the adsorption of dye onto the composite occurred by physisorption. Similarly, Chong et al. [125] investigated the adsorption behaviors of CaCO3-decorated cellulose aerogel extracted from commercial cotton linter through the same technique previously described by Santoso and his co-authors toward anionic Congo red dye. The composite with a highly porous and interconnected three-dimensional framework structure (Fig. 8) displayed high adsorption capacity than neat hydrogel. A maximum adsorption capacity of 75.81 mg/g is higher than that of neat hydrogel due to the availability of additional sorption active sites after CaCO3 embedding. Likewise, adsorption kinetics show that the adsorption of congo red dye onto CaCO3-decorated cellulose aerogel occurred through chemisorption via valency forces through sharing or exchange of electrons. Chen et al. [126] investigated the preparation of cellulose/hPEI composite hydrogel using glutaraldehyde as a cross-linking agent to remove dyes from aqueous environments. The cellulose composite hydrogel with abundant amino groups on the surface was found efficient for the removal of cationic bright yellow M-7G, anionic reactive yellow X-RG, and nonionic disperse brown S-3RL dyes, with maximum adsorption capacities of 571.43, 970.87, and 581.40 mg/g, respectively, within 120 min. The adsorption of dyes onto the cellulose hydrogel was favorized by electrostatic interactions and hydrogen bonds. Similarly, Yu et al. [127] reported the modification of cellulose nanofibril aerogel with graphene nanoplates and carbon nanotube, with different mass ratios of cellulose to carbon for the uptake of dye molecules from wastewater. Results demonstrate that the cellulose composite with a cellulose/graphene ratio of 3:1 exhibited a maximum adsorption capacity of 1178.5 and 585.3 mg/g for methylene blue and congo red dyes, respectively, due to the larger surface area of the composite and the available site for adsorption. Likewise, the difference in adsorptive behaviors of aerogels towards the two dyes is mainly attributed to the high electrostatic interactions between the negatively charged carboxyl groups present on the external surface of the composite and cationic dye. Besides, the sorption of anionic dyes into the composite is performed through π–π interactions. Wei et al. [128] investigated the removal of methylene blue using microcrystalline cellulose (MCC) aerogel coated with different polydopamine (PDA) contents. The composite was prepared by self-polymerization of polydopamine in the solution mixture of MCC and LiBr, followed by freeze-drying technology. The findings show that the composite of microcrystalline cellulose with a high quantity of PDA showed a high adsorption behavior toward MB dye with an adsorption capacity of 153.4 mg/g. The explication for the increase in MB adsorption by the elaborated aerogel composite upon increasing the PDA content is related to the average pore size and the specific surface area. Based on SEM analysis, the average pore size of the MCC/PDA aerogels decreased with the increase in PDA content whereas their specific surface area rose with the increment in the content of PDA. Likewise, authors report that the developed composite was more effective than other materials such as PDA, GO/PVA microspheres, and graphene/CNT/Ag aerogels, suggesting the contribution of microcrystalline cellulose in taking up MB molecules from an aqueous medium. Adsorption kinetics and isotherms revealed that the adsorption of MB molecules occurred through π–π interactions relating to aromatic groups and electrostatic interaction between the negative charge feature of PDA and the cation feature of MB. Sharma and his co-author successfully functionalized cellulose with hydroxyethyl methacrylate (HEMA) and glycidyl methacrylate (GMA) for the removal of dyes and heavy metal ions from wastewater [129]. Cellulose was firstly extracted from rice husk fibers using total chlorine-free and ultrasonic physicochemical processes and then grafted with hydroxyethyl methacrylate and glycidyl methacrylate through a copolymerization approach under well-moderate conditions. The structural, morphological, and thermal properties of cellulose have been changed after copolymers grafting, resulting in a change in its adsorptive properties. Cellulose-graft-HEMA-co-GMA copolymer was found more efficient than neat cellulose for the removal of heavy metal ions from aqueous solutions, namely, nickel, copper, and lead ions, with a removal rate of 81.4, 71.6, and 75.5%, respectively. The lower sorption of copper ions on the graft copolymer is related to its strong acidic character, which weakly attaches to the softer sorption sites of the composite. Adsorption kinetics and isotherms revealed that the metal ion's adsorption occurred through monolayer sorption via chemisorption. The resulting composite was also found useful for removing organic dyes from aqueous solutions. In fact, under well moderate conditions of temperature, pH, initial dye concentration, and contact time, the elimination rates of congo red, crystal violet, and malachite green dyes dye were 70.38, 67.74, and 49.42%, respectively. The graft copolymer exhibits a higher affinity to anionic dye than cationic ones, which may be attributed to high electrostatic interactions between adsorbent sites of the composite and negatively charged molecules of Congo red. Sun et al. [130] reported the adsorption of mercury ions from wastewater using an amide functionalized cellulose from sugarcane bagasse. The cellulose-based adsorbents present outstanding adsorption performances in removing mercury ions from an aqueous solution with a maximum adsorption capacity of 178 mg/g because of the abundance of amide groups on its external surface. Likewise, regeneration tendency was reported for then adsorption–desorption cycles.
Digital images and SEM micrographs of cellulose hydrogel before (a, c) and after (b, d) calcium chloride loading [125]
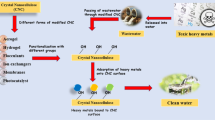
Goswami et al. [131] reported the removal of chromium ions from wastewater using nanocellulose/ sodium alginate/ charcoal composite beads. Cellulose nanocrystal was extracted from sugarcane bagasse fibers through acid hydrolysis using sulfuric acid, mixed with sodium alginate and charcoal, and then crosslinked to highly porous beads using calcium chloride. The resulting nanocellulose hydrogel with high crystallinity and abundant hydroxyl groups was found to be effective in removing chromium ions compared to pristine alginate/charcoal beads, suggesting the important role of nanocellulose in improving the adsorption behaviors of beads toward chromium ions. Moreover, it was observed that the elimination rate of the developed nanocellulosic beads increased with increasing the amount of CNC in the composite. This can be attributed to highly reactive functional groups such as hydroxyl and carboxyl on the outer surface of beads. Gelatin-cellulose nanocrystals hydrogel have successfully prepared to uptake copper and cobalt ions from mining process wastewater [132]. High elimination rates of 70.5 and 72.5% were noted for Cu (II) and Co (II) at pH 5 and 7, respectively. Nanocellulose/MOF composite with super magnetic features has been successfully prepared to uptake lead ions from aqueous environments [133]. Adsorption experiments revealed the excellent adsorption behaviors of the resulting nanocellulose composite toward Pb (II) ions, with a maximum adsorption capacity of 558.66 mg/g under well-optimized conditions of temperature pH, contact time, and so on. This high adsorption capacity was attributed to the dual effect of CNC and MOF in improving the surface area of the composite and the abundant hydroxyl and carboxyl groups possessing free electron pairs that react with Pb (II) through coordinative bonding. Moreover, regeneration tests confirmed the high stability of the composite after five adsorption–desorption cycles. On the other hand, Liang et al. [134] reported the conception of chemically cross-linked cellulose nanocrystal aerogels by directional freeze-drying of cellulose nanocrystals (CNCs) poly(methyl vinyl ether-co-maleic acid) (PMVEMA) and poly(ethylene glycol) (PEG) for methylene blue dye removal. From the advantages of the extremely porous structure, enhanced thermal stability, and improved dye adsorption capacity, CNC aerogels are attractive options for removing cationic dye from aqueous solutions. Aerogel with 25% cellulose nanocrystals displays the highest adsorption capacity, with a maximum of 116.2 mg/g, which can be conserved after five cycles of regeneration and reuse. Recently, Thorat et al. [135] have investigated the modification of bacterial nanocellulose with different amounts of cationic polyethyleneimine-rich amino groups for the removal of anionic dyes from wastewater, namely, congo red and reactive red 120. Bacterial nanocellulose was firstly generated from Komagataeibacter strain PG2 followed by the crosslinking of the PEI on its three-dimensional surface using epichlorohydrin. It was observed that the adsorption capacity increased as the PEI content in the adsorbent was increased. The ratio of BC:PEI at 1:6 displayed higher adsorption and dye removal efficiency, with maximum adsorption capacities of 515.46 and 300.3 mg/L for congo red and reactive red 120, respectively, which may be ascribed to its high surface area compared to other composites and pure bacterial nanocellulose. In addition, the adsorption capacity of the composite was found to be dependent on the pH of the dye solution. The bacterial nanocellulose composite has a high adsorption capacity for both dyes at acidic pH, as it is positively charged in this pH range, which promotes electrostatic interactions between it and the negatively charged surface of dyes. Adsorption isotherms and kinetics show that the adsorption process of anionic dyes fitted with the Langmuir and pseudo-second-order kinetic models, suggesting that the adsorption is monolayer and occurs through chemisorption. The composite shows also high bactericidal behaviors against Escherichia coli and Staphylococcus aureus with more than 99.9% efficiency. The presence of cationic PEI on the outer surface of the bacterial nanocellulose initiates electrostatic interactions between the bacteria and the adsorbent, causing damage to the membrane and consequently leading to leakage of cellular constituents. The mechanism of death of bacteria by adsorption or photocatalysis has been well described in our previous review [34]. Nanocellulose-based composites were also used for the removal of persistent pharmaceutical compounds from sewage, a vacuum freeze dryer has prepared bilayer amino-functionalized cellulose nanocrystals/chitosan composite for diclofenac sodium (DS) adsorption from aquatic media [136]. The resulting CNC-ED@CS-ED composite with more wrinkles and a rougher surface (Fig. 9) displays high adsorptive performances toward anti-inflammatory drug (DS), with a maximum adsorption capacity of 444.44 mg/g, confirming the most relevant role of the amino groups in improving the adsorption behaviors of the composite, providing additional active sites on its external surface for more beneficial capturing of DS molecules. Moreover, the authors suggested that DS adsorption can occur through acid–base or hydrogen bond interactions. Chen et al. [137] investigated the synergistic effect of adsorption and photocatalysis of cellulose/GO/TiO2 for methylene blue removal. A highly 3D porous network structure was obtained through a simple one spot method, resulting in a high degradation rate and reproducible performances upon photodegradation of methylene blue dye under UV light irradiation. About 93% of the dye was removed after 120 min of irradiation. The authors ascribed the outstanding removal efficiency of MB to the porous structure of the cellulosic hydrogel, which supplies additional active sites and enhances the surface adsorption of reactive species onto the surface of the composite photocatalysts, and to the synergistic effects between the GO sheets and TiO2 that promotes interfacial charge transfer and prevents electron–hole recombination phenomenon.
SEM micrographs of the bilayer amino-functionalized cellulose nanocrystals/chitosan composite [136]
5 Hemicellulose-based composites for wastewater purification
5.1 Hemicellulose extraction from lignocellulosic biomass
Hemicellulose term was initially used to refer to the alkali-soluble polysaccharides of the wall. Its principal function is the crosslinking of cellulose fibrils with the lignin matrix. Both cellulose and hemicellulose are commonly known as holocellulose. Hemicellulose consists of short chains of different heteropolysaccharides such as pentoses (xylose, arabinose), hexoses (galactose, glucose, rhamnose), and sugar acids (glucuronic acid, 4-O-methyl-d-glucuronic acid) that are held together by β-(1,4)- and/or β-(1,3)-glycosidic bonds [105, 138, 139]. Compared to cellulose, hemicellulose is a short-chained matrix with a lower degree of polymerization, amorphous structure, and high degradation, especially in acidic media (Fig. 10). Typically, the hemicellulose content depends on the lignocellulosic biomass sources, and herbaceous plants are always rich in hemicellulose [140]. The principal hemicellulose constituents in hardwood are O-acetyl-4-O-methylglucurono-b-d-xylan, also known as glucuronoxylans. While in softwood, the main hemicelluloses are O-acetyl-galactoglucomannans [141]. Hemicellulose may be separated from lignocellulosic biomass through various approaches, namely, acid or alkaline pretreatment, ionic liquid extraction, liquid hot water extraction, precipitation through supercritical CO2, etc., while the ionic liquid method permits to obtain of hemicellulose with high purity [120, 142, 143].
5.2 Hemicellulose-based composites for wastewater purification
Due to its high hydrophilicity resulting from the availability of a large number of hydroxyl groups on its outer surface and the structural versatility, several varieties of hemicellulose derived from different plants are used for the preparation of hydrogel, for the elimination of pollutants from wastewater [141]. In this context, Sun et al. [144] have successfully prepared a hemicellulose-g-poly(sodium acrylate) porous hydrogel to remove methylene blue dye from an aqueous solution. The hydrogel was synthesized using wheat straw hemicellulose and calcium carbonate as porogen by a free radical polymerization approach. Hemicellulose with 80.4% of xylose and 12.5% of arabinose was first isolated from wheat straw fibers through alkaline treatment using 10% KOH at room temperature and then undergoes a free radical polymerization in the presence of CaCO3, N,N-methylenebisacrylamide crosslinker, sodium acrylate solution in alkaline medium. After hydrogel formation, CaCO3 washed the hydrogels with diluted acid, resulting in a highly porous interconnected structure. Indeed, it was observed that the number of interconnected pore channels in the hydrogel increased with increasing calcium carbonate content. Adsorption results revealed that the developed hemicellulose-g-poly(sodium acrylate) porous hydrogel showed high adsorption capacity under well-defined conditions of initial dye concentration, contact time, and temperature. The hydrogel shows a maximum adsorption capacity of 1280 mg/g at only 4 h when the temperature reaches 70 °C. The adsorption behaviors of the hydrogel also increased by increasing the amount of calcium carbonate, which is expected since a high amount of CaCO3 leads to high porosity and, therefore, a large surface area promoting the diffusion of dye molecules into the hydrogel pores. Moreover, adsorption kinetics show that the adsorption of methylene blue on the hydrogels occurs through a chemisorption process involving the exchange or sharing of electrons between methylene blue and the functional groups on the HC-g-PSA hydrogels. Similarly, a magnetic nanocomposite hydrogel adsorbent was also synthesized to uptake methylene blue dye from aqueous environments [145]. Fe3O4-modified xylan/poly(acrylic acid) hydrogel with macroporous structure and interconnected porous channels displays high affinity towards the dye molecules, leading to the elimination of 96% of methylene blue with an initial concentration of 400 mg/L at an alkaline medium due to the high electrostatic interactions between the MB molecules charged positively and carboxyl groups in the external surface of the hydrogel charged negatively. Adsorption isotherm and kinetics studies revealed that the removal of MB molecules using the resulting hydrogel is monolayer adsorption controlled by chemisorption. Moreover, due to its high paramagnetic properties, the hemicellulose-based hydrogel can separate easily from solutions without being lossless. Hu et al. [146] successfully synthesized a hemicellulose-graft-ploy acrylamide (hemi-g-pAAm) hydrogel for the adsorption of dye molecules from wastewater (Fig. 11). The macro-and microstructure of the resulting hydrogel promotes the diffusion of dye molecules onto the porous structure, leading to high adsorption capacity. More than 90% of methylene blue dyes, with an initial dye concentration of 500 mg/L, were removed in only 40 min, suggesting the excellent adsorptive performances of the composite. The authors attributed the high adsorption capacity of the hydrogel also to the abundant carboxyl groups on its external surface, which promotes the sorption of dye molecules through electrostatic interactions. Huang et al. [147] investigated the removal of copper ions from aqueous solutions using a three-dimensional β-cyclodextrin adsorbent. It was observed that the macropores and the three-dimensional crosslink network structure of the resulting composite are responsible for its high adsorption capacity (107.37 mg/g). Adsorption isotherms and kinetics revealed that the adsorption of copper ions onto the hydrogel might result from ion-exchange or chemical interactions between hydroxyl groups on the outer surface of the sorbent, already confirmed through FTIR analysis and Cu2+ ions. Hemicellulose − chitosan adsorbents from coastal bermudagrass (CBG), pinewood (PW), and switchgrass (SG) have been successfully prepared for the removal of salts and heavy metals for water [148]. Hemicellulose was firstly isolated from different lignocellulosic biomasses through alkaline treatment at 75 °C, grafted with genetic acids, and then cross-linked to chitosan biopolymer. The resulting hydrogels endowed with high porosity and excellent mechanical features were found to eliminate sodium chloride salts and heavy metals, including lead, copper, and nickel ions from water. Although hemicellulose-based composites have proven effective in removing organic and inorganic pollutants from polluted waters, their applications are usually restricted by their weak thermostability, high swelling ability, brittle aspect, and weak mechanical features which require more attention and improvements to obtain highly efficient materials with large-scale applications.
A, C The macrostructure of the hydrogel before and after swelling. B, D The SEM micrographs of the hemicellulose-graft-ploy acrylamide (hemi-g-pAAm) hydrogel [146]
6 Lignin-based composites from wastewater purification
6.1 Lignin extraction from lignocellulosic biomass
After cellulose, lignin is the second most abundant organic polymeric material in the cell walls of lignocellulosic biomass. It represents 20 to 30% of the carbon of the plant biomass and is responsible for the plant’s rigidity and hydrophobicity. From a definition point of view, lignin is a complex, amorphous, and non-linear phenolic polymer with a three-dimensional structural network of phenylpropane units linked by ether bonds. These three phenylpropanoid units are p-coumaryl alcohol (H), guaiacyl, also known as monolignols coniferyl alcohol (G), and sinapyl alcohol (S) (Fig. 12) [149, 150]. The structural units of lignin are connected randomly through ether bonds and C–C bonds to produce a high molecular weight natural polymer with a 3D structure. The content and composition of lignin also vary across lignocellulosic biomass; softwoods are known to be the richest source of lignin, followed by hardwood and agricultural wastes [151]. Likewise, lignin is a low reactive, hydrophobic, and completely amorphous polymer. Its properties are close to plastic materials. The versatility of hydroxyl functional groups (aliphatic, phenolic, and carboxylic) makes lignin more attractive as a macromonomer for the synthesis of polymers and materials for various applications, including wastewater treatment. The hydroxyl groups and free positions in the aromatic ring are the most characteristic functions of lignin, which determine its reactivity. Hence, lignin insulation from lignocellulosic biomass is achieved in various experimental conditions whereby lignin polymer is broken down chemically into small molecular weight fragments, each with different physico-chemical properties. The Kraft process, sulfite process, and soda process are the most used in the literature [152].
6.2 Lignin-based composites for wastewater purification
The presence of abundant active functional groups such as methoxy, carbonyl, and hydroxyl groups and huge aromatic rings in lignin materials facilitates their interaction with different types of pollutants in sewage through a hydrogen bond, hydrophobic, π–π, electrostatic, and many other interactions, making it more useful in wastewater treatment applications [153, 154]. Recently, Naseer et al. [155] examined the adsorption capacity of novel composites based on lignin, alginate, and hydroxyapatite towards heavy metals, namely, copper and nickel ions. The three compounds were designed in the form of beads for easy separation after the adsorption process through a very simple approach (mixing) followed by crosslinking by calcium chloride. The resulting beads, with different lignin ratios, showed high porosity and large surface area, which increased by increasing the lignin amount in the composite, indicating the role of lignin in improving the physicochemical of the composite. Likewise, adsorption experiments revealed the high efficiency of the composites toward both heavy metal ions. The adsorption capacity of the composite increased with increasing the amount of lignin in beads due to the abundant functional groups of lignin that provide more active sites, favoring, therefore, the uptake of the ions. The adsorption capacity of the composite was also dependent on different experimental parameters such as the sorbent dose, initial concentration, pH of the polluted solution, and contact time. Maximum adsorption capacities of 79.67 and 71.18 mg/g with the initial concentration of 100 ppm at pH 5 at only 30 min and pH 7 after 60 min of contact time for copper and nickel ions, respectively. Kinetic and isotherm data confirmed that the adsorption of both ions onto the composite is monolayer occurred by chemisorption. In contrast, the reusability test confirmed the high stability of the adsorbent after four adsorption–desorption cycles, suggesting its high adsorptive performance. Nair et al. [156] also used lignin to modify the physicochemical of chitosan to eliminate chromium metal ions and dyes (Remazol Brilliant Blue R and anthraquinone dyes) from aqueous environments. The authors used the same preparation method previously described, except that in this case of chitosan hydrogel, the crosslinking is usually made by sodium hydroxide solution. Indeed, different composites with different lignin ratios were prepared to evaluate the role of lignin in the physicochemical properties and the adsorptive behaviors of the composite. FTIR analysis revealed high interactions between aromatic rings and hydrogen bonds of lignin and amine groups and β-1,4-glycosidic linkage of chitosan, respectively, and during the composite preparation, which was also confirmed by SEM micrographs (Fig. 13). In addition, the presence of lignin increases the pore volume, surface area, and thermal stability of the composite compared to pure chitosan hydrogel. These improvements play an important role in improving the adsorption capacity of the resulting composites. Indeed, chitosan-alkali lignin composite with a high amount of lignin (50%) exhibited excellent adsorption capacity toward all pollutants due to the strong electrostatic interactions of large, protonated amine hydroxyl aromatic rings of the composite with the anion of dyes and HCrO4– ions. Li et al. [157] also investigated the removal of lead ions using a three-dimensional highly porous lignosulfonate-based graphene hydrogel. The synthesis process occurred through electrostatic interaction, hydrogen bonds, and π–π interaction between graphene oxide nanosheets and lignin functional groups. Results show that the resulting composite exhibited high lightness, large surface area, high porosity, abundant active sites, and, therefore, excellent adsorption capacity for lead ions with a maximum of 1308 mg/g within only 40 min. Moreover, it can be regenerated and reused for many adsorption–desorption cycles. The authors attributed this high efficacity to the dual effect of both materials. On the other hand, Gassara et al. [158] have reported using a hydrogel composite composed of a mixture of lignin peroxidase and laccase, pectin, manganese peroxidase, and polyacrylamide for the biodegradation of bisphenol A. High degradation of bisphenol A was observed using the composite in contrast with neat materials. 90% of bisphenol A was degraded after a contact time of 8 h. The authors suggest that pectin is responsible for this high biodegradation. The greatest polymer protecting the ligninolytic enzymes from inactivity is pectin, covering the enzymes' catalytic sites. Lignin was also used as support for photocatalysts to reduce their agglomeration and improve their affinity toward different contaminants. In this context, Srisasiwimon et al. [159] reported the synthesis of a TiO2/lignin-based carbon composite to convert lignin to high-value products under UV light irradiations. The photocatalyst was immobilized into the lignin support through a sol–gel microwave approach. SEM micrographs show that a TiO2/lignin ratio of 1:1 is sufficient to prevent the agglomeration of nanoparticles after their preparation. The crystallite size of the composite decreased from 18 to 6 nm after lignin was loaded. The surface area of the resulting composite increased with increasing the lignin content until it reached a maximum at the ratio of 1:0.5 and then decreased, but in general, the results remain satisfactory. Photocatalytic tests revealed that the composite with 0.5 lignin exhibited high photocatalytic activity due to its high porosity, large surface area, and reduction of charge recombination. Lignin was successfully converted into butylated hydroxytoluene, vanillin, 4-hydroxybenzaldehyde, acetovanillone, and benzoic acid with a high yield. Khan et al. [160] investigated the photodegradation of benzyl alcohol under both UV and visible light irradiations using a series of nanocomposites with different weight ratios based on titania, chitosan, and lignin. The photocatalysts were prepared through two successive steps: impregnation and hydrothermal. Typically, homogeneous solutions of chitosan and lignin were first prepared, to which different amounts of titanium were added. The resulting solid was then hydrothermally treated and then recovered for use in the degradation of benzyl alcohol. Results reveal that the thermal stability as well as the surface area of the nanocomposites improved compared to neat chitosan, neat lignin, and composites without titanium dioxide. In fact, the surface area of the photocatalysts increased with increasing the amount of TiO2, until reaching a maximum and then slightly decreased. This enhancement in the surface area of composites after TiO2 impregnation was beneficial to enhance its adsorption and photocatalysis capacities either under UV and visible light irradiations since the chitosan/lignin composite might act as a radical scavenger and thus inhibit undesirable overoxidation to take place. However, the titanium dioxide used in this study was a mixture of anatase and brookite phases; the results could be more important if the titanium dioxide was a mixture of anatase and rutile phases known as the most recommended forms of titanium for photocatalysis applications due to their higher photocatalytic activity [161].
SEM micrographs of chitosan-lignin composite with different ratios [156]
7 Biochar derived from lignocellulosic biomass
Lignocellulosic biomass is an ideal raw material as a carbon precursor due to its high carbon content. It can be easily exploited to produce carbonaceous materials such as biochar, hydrochar, or activated carbon [20]. First, it was found more interesting to highlight the difference between these three types of carbon materials. True, they are made initially from lignocellulosic biomass and almost have the same composition, but the way they are produced is different [162, 163]. Biochar and activated carbon were made using pyrolysis (e.g., slow, fast, or flash pyrolysis), while hydrochar, as its name implies, is produced through hydrothermal carbonization. Indeed, biochar and activated carbon are carbon-rich solids derived from different lignocellulosic biomasses and produced in a limited oxygen environment. The difference between them is that activated carbon is biochar modified with acid, alkaline, or other treatment to increase its surface area porosity for effective contaminants removal. In the rest of this paper, we will focus on biochar and activated carbon, as they have proven their effectiveness in purification and remediation applications.
7.1 Biochar preparation, and physicochemical properties
Biochar is a carbon-rich product consisting of carbon black, inorganic compounds (Mg, Ca, Zn, Si, etc.), and other carbon-based products obtained through biomass pyrolysis. The conditions of its formation are similar to those of charcoal production in a forest fire or during the carbonization process in traditional charcoal kilns. More specifically, biochar is obtained by the thermal degradation of biomass residues in the absence of oxygen and at high temperatures generally ranging between 350 and 1000 °C [164]. The structure of biochar typically reflects the original structure of the used biomass with only minor changes in hydrogen and oxygen. It consists of a honeycomb structure resulting from the conversion of cellulose, hemicellulose, and lignin into aromatic carbon (Fig. 14). More particularly, biochar is made up of aromatic compounds comprising organized zones in the form of graphitic planes linked by Van der Waals bonds [165]. Pyrolysis is the most common and promising way to convert lignocellulosic biomass into biochar. By operating parameters, including temperature, heating rate, and residence time, pyrolysis can be classed as slow, fast, and flash pyrolysis. The pyrolysis process consists of biochar, liquid, and volatile fractions. Slow pyrolysis resulted in a high biochar yield with high porosity compared to other pyrolysis processes [166]. The physicochemical properties of biochar are also dependent on the processing parameters and the kind of raw biomass, but generally, biochar derived from different lignocellulosic biomasses shows high porosity, excellent surface area, and a high degree of surface reactivity, which promotes the pollutant's adsorption [167, 168]. Thus, to achieve better biochar adsorption performance, many physical and chemical activation methods have been proposed in the literature, namely, alkaline activation, acid activation, oxidation, ball-milling, aromatic condensation, and so on [169,170,171,172,173]. However, the excess of such treatments may cause damage to the honeycomb structure of the biochar and the destruction of its crystalline lattice. Therefore, moderate concentrations of activators and optimal working conditions are more advised to achieve the required characteristics.
Biochar structure [162]
7.2 Biochar-based composites for wastewater purification
Biochar from different lignocellulosic biomasses has already been used to uptake various types of contaminants from wastewater due to its fascinating features, including high porosity, large surface area, abundant functional groups, low cost, and high affinity toward pollutants as a result of many mechanisms. Thus, new biochar-based composites can be made by modifying the biochar or loading it with high-effective nanoparticles known for their high efficiency towards pollutants [174, 175]. In this regard, Leichtweis et al. [176] developed a new biochar-ZnO composite for acid red 97 dye removal through the synergistic effect of adsorption and photocatalysis processes. Biochar derived from pecan nutshells via slow pyrolysis was loaded with different amounts of zinc oxide ranging between 5 and 20% to prevent the aggregation of ZnO nanoparticles during the photocatalysis process. Textural results revealed an increase in the surface area of biochar composite compared to pristine ZnO. It is proportional to the increase of the ZnO amount on the surface of the biochar. This enhancement improves the adsorption behaviors of the composite and, therefore, enhances its photocatalytic activity. Complete dye removal was achieved with the newly developed photocatalyst within only 67 min of reaction time and at neutral conditions, suggesting the success of the preparation method and the treatment process. Furthermore, the composite exhibited great reuse features; it remains stable after 8 cycles, with a slight decrease (10%) in the ninth cycle, which implies the saturation of some active sites by intermediate components resulting from the photodegradation of the dye. Eltaweil et al. [177] investigated the preparation of a mesoporous magnetic biochar composite for malachite green dye adsorption from polluted water. The magnetic biochar composite was prepared through the reduction method. Typically, biochar derived from corn straw fibers was obtained using the pyrolysis process under an N2 atmosphere. Before pyrolysis, the raw corn straw fibers underwent alkaline treatment with sodium hydroxide to enhance the physicochemical properties of the resulting biochar for higher adsorption capacity. The biochar was loaded with zero-valent iron nanoparticles (nZVI) under alkaline conditions, resulting in a highly porous composite with improved surface area. The biochar composite also displays better hydrophilicity and great affinity towards dye molecules. Indeed, 99.9% of MG dye was eliminated after only 20 min of reaction time, confirming the high reactivity of the resulting biochar composite compared to neat biochar. The authors attributed this high elimination rate in a short time to both adsorption and oxidation mechanisms. Likewise, after seven runs, the biochar composite possessed good reusability and could be easily separated from an aqueous solution by an external magnetic field. Similarly, Iqbal et al. reported using nano-zerovalent manganese/biochar composite for anionic dye removal [178]. Results show that the resulting biochar composite was found able to eliminate 95% of persistent anionic in the presence of hydroxide oxygen due to the formation of highly reactive hydroxyl radical that attacks amine groups, N–N double bond, and SO3 group of congo red dye, leading to their mineralization into the water, carbon dioxide and some minerals. Abdul et al. [179] prepared biochar-graphene nanosheet composites for phthalic acid esters adsorption. The composites were successfully synthesized through a simple dip-coating method, followed by slow pyrolysis at different temperatures between 300 and 700 °C to evaluate the effect of temperature pyrolysis on the physicochemical feature of biochar. The resulting composites were found to exhibit greater porosity and higher surface area than biochar and increased phthalic acid ester adsorption capacity, thereby indicating the contribution of graphene nanosheets in the enhancement of biochar adsorption behaviors.
8 Conclusion and future perspectives
During the last decades, lignocellulosic biomass has raised a lot of attention as one of the most abundant and renewable sources on earth for use in environmental applications, especially in wastewater purification. Lignocellulosic biomass originated from plants and vegetable residues is a sustainable and plentiful resource that can be valorized and used as robust material for eliminating different pollutants from sewage, including organic pollutants, heavy metals, inorganic compounds, and microorganisms. This review represents the recent development in composites derived from lignocellulosic biomass for wastewater purification, including cellulosic-based composites, hemicellulose-based composites, lignin-based composites, and biochar-based composites. Specific characteristics for specific pollutants endow each type. But, in general, based on the fascinating data reported in this work, all composites exhibited high performance and improved physicochemical features compared to raw lignocellulosic biomass, making them more suitable for wastewater applications. Thus, it was necessary to highlight that there are more requirements that should be addressed in future research to get more relevant results as well as to scroll towards the large scale:
-
Most of the published work deals with single-pollutant laboratory wastewater treatments, rather than actual industrial wastewater that contains a mixture of organic, inorganic, and even biological contaminants. Consequently, there needs to be an effort to investigate the adsorption performance of composites derived from lignocellulosic biomass in real industrial effluents. A first attempt would be a pilot-scale treatment of industrial wastewater or at least simulated wastewater containing several pollutants that imitate existing industrial wastewater.
-
When regenerated, most cellulose-based hydrogels tend to lose sorption properties, therefore, it is important to explore the possibilities of improving the stability of the materials after several uses through chemical, physical, or other treatments. Likewise, biomass-derived hydrogels consistently suffer from swelling, poor thermal stability, and pH sensitivity (acid media), so many efforts are required to overcome these challenges.
-
Rare are the publications that provide clearance and concrete mechanisms during the removal of different contaminants using composites derived from lignocellulosic biomass. Hence, further investigations, both theoretical and experimental, are needed to understand the mechanisms of adsorption that may possibly help to identify the most appropriate and feasible one. Using artificial intelligence/ machine learning to predict the phenomenon involved during the uptake of pollutants from wastewater could be a very interesting approach.
References
Margot J, Rossi L, Barry DA, Holliger C (2015) A review of the fate of micropollutants in wastewater treatment plants. WIREs Water 2:457–487. https://doi.org/10.1002/wat2.1090
Korotta-Gamage SM, Sathasivan A (2017) A review: potential and challenges of biologically activated carbon to remove natural organic matter in drinking water purification process. Chemosphere 167:120–138. https://doi.org/10.1016/j.chemosphere.2016.09.097
Gedda G, Balakrishnan K, Devi RU, Shah KJ (2021) Introduction to conventional wastewater treatment technologies: limitations and recent advances. In: Advances in Wastewater Treatment I Ed RESEARCH FORUM LLC, USA.
Elgarahy AM, Elwakeel KZ, Mohammad SH, Elshoubaky GA (2021) A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean Eng Technol 4:100209. https://doi.org/10.1016/j.clet.2021.100209
Galal-Gorchev H (1993) WHO guidelines for drinking-water quality
Fan D, Lu Y, Zhang H et al (2021) Synergy of photocatalysis and photothermal effect in integrated 0D perovskite oxide/2D MXene heterostructures for simultaneous water purification and solar steam generation. Appl Catal B Environ 295.https://doi.org/10.1016/j.apcatb.2021.120285
Al AH, Kochkodan V, Hilal N (2013) Hybrid ion exchange - pressure driven membrane processes in water treatment: a review. Sep Purif Technol 116:253–264. https://doi.org/10.1016/j.seppur.2013.05.052
Khan ST, Malik A (2019) Engineered nanomaterials for water decontamination and purification: from lab to products. J Hazard Mater 363:295–308. https://doi.org/10.1016/j.jhazmat.2018.09.091
Sordello F, Berruti I, Gionco C et al (2019) Photocatalytic performances of rare earth element-doped zinc oxide toward pollutant abatement in water and wastewater. Appl Catal B Environ 245:159–166. https://doi.org/10.1016/j.apcatb.2018.12.053
Chkirida S, Zari N, Achour R et al (2021) Highly synergic adsorption/photocatalytic efficiency of alginate/bentonite impregnated TiO2 beads for wastewater treatment. J Photochem Photobiol A Chem 412:113215. https://doi.org/10.1016/j.jphotochem.2021.113215
Amoatey P, Bani R (2011) Wastewater management. In: Waste water - evaluation and management
El Allaoui B, Chakhtouna H, Zari N, Bouhfid R (2022) Recent developments in functionalized polymer NF membranes for biofouling control. Emergent Mater. https://doi.org/10.1007/s42247-022-00367-x
Singh NB, Nagpal G, Agrawal S, Rachna (2018) Water purification by using adsorbents: a review. Environ Technol Innov 11:187–240. https://doi.org/10.1016/j.eti.2018.05.006
Kyzas GZ, Siafaka PI, Kostoglou M, Bikiaris DN (2016) Adsorption of As(III) and As(V) onto colloidal microparticles of commercial cross-linked polyallylamine (Sevelamer) from single and binary ion solutions. J Colloid Interface Sci 474:137–145. https://doi.org/10.1016/j.jcis.2016.04.027
Saxena A, Bhardwaj M, Allen T et al (2017) Adsorption of heavy metals from wastewater using agricultural–industrial wastes as biosorbents. Water Sci 31:189–197. https://doi.org/10.1016/j.wsj.2017.09.002
Igwe JC, Abia AA (2006) A bioseparation process for removing heavy metals from waste water using biosorbents. Afr J Biotechnol 5:1167–1179. https://doi.org/10.4314/ajb.v5i11.43005
Asemave K, Thaddeus L, Tarhemba PT (2021) Lignocellulosic-based sorbents: a review. Sustain Chem 2:271–285. https://doi.org/10.3390/suschem2020016
Chakhtouna H, Zari N, Bouhfid R et al (2021) Novel photocatalyst based on date palm fibers for efficient dyes removal. J Water Process Eng 43:102167. https://doi.org/10.1016/j.jwpe.2021.102167
Liu Y, Nie Y, Lu X et al (2019) Cascade utilization of lignocellulosic biomass to high-value products. Green Chem 21:3499–3535. https://doi.org/10.1039/c9gc00473d
Qureshi UA, Hameed BH, Ahmed MJ (2020) Adsorption of endocrine disrupting compounds and other emerging contaminants using lignocellulosic biomass-derived porous carbons: a review. J Water Process Eng 38:101380. https://doi.org/10.1016/j.jwpe.2020.101380
Loow YL, Wu TY, Jahim JM et al (2016) Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 23:1491–1520. https://doi.org/10.1007/s10570-016-0936-8
Karimah A, Ridho MR, Munawar SS et al (2021) A review on natural fibers for development of eco-friendly bio-composite: characteristics, and utilizations. J Mater Res Technol 13:2442–2458. https://doi.org/10.1016/j.jmrt.2021.06.014
Kucharska K, Rybarczyk P, Hołowacz I et al (2018) Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 23:1–32. https://doi.org/10.3390/molecules23112937
Peng B, Yao Z, Wang X et al (2020) Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ 5:37–49. https://doi.org/10.1016/j.gee.2019.09.003
Syazwani N, Rahman A, Firdaus M, Baharin Y (2018) Utilisation of natural cellulose fibres in wastewater treatment. Cellulose 25:4887–4903. https://doi.org/10.1007/s10570-018-1935-8
Kyzas GZ, Christodoulou E, Bikiaris DN (2018) Basic dye removal with sorption onto low-cost natural textile fibers. Processes 6.https://doi.org/10.3390/pr6090166
Khatri N, Tyagi S (2015) Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front Life Sci 8:23–39. https://doi.org/10.1080/21553769.2014.933716
Yadav D, Singh S, Sinha R (2021) Microbial degradation of organic contaminants in water bodies. In: Pollutants and water management. John Wiley & Sons Ltd, pp 172–209
Rajasulochana P, Preethy V (2016) Comparison on efficiency of various techniques in treatment of waste and sewage water – a comprehensive review. Resour Technol 2:175–184. https://doi.org/10.1016/j.reffit.2016.09.004
Bolisetty S, Peydayesh M, Mezzenga R (2019) Sustainable technologies for water purification from heavy metals: review and analysis. Chem Soc Rev 48:463–487. https://doi.org/10.1039/c8cs00493e
Naidoo S, Olaniran AO (2013) Treated wastewater effluent as a source of microbial pollution of surface water resources. Int J Environ Res Public Health 11:249–270. https://doi.org/10.3390/ijerph110100249
Breida M, Alami Younssi S, Ouammou M et al (2020) Pollution of water sources from agricultural and industrial effluents: special attention to NO3ˉ, Cr(VI), and Cu(II). In: Water Chemistry. IntechOpen, London
Al-Gheethi AA, Efaq AN, Bala JD et al (2018) Removal of pathogenic bacteria from sewage-treated effluent and biosolids for agricultural purposes. Appl Water Sci 8:1–25. https://doi.org/10.1007/s13201-018-0698-6
Chakhtouna H, Benzeid H, Zari N et al (2021) Recent progress on Ag/TiO2 photocatalysts: photocatalytic and bactericidal behaviors. Environ Sci Pollut Res 28:44638–44666. https://doi.org/10.1007/s11356-021-14996-y
Aghalari Z, Dahms HU, Sillanpää M et al (2020) Effectiveness of wastewater treatment systems in removing microbial agents: a systematic review. Glob Health 16:1–11. https://doi.org/10.1186/s12992-020-0546-y
Akpor OB, Otohinoyi DA, Olaolu TD, Aderiye BI (2014) Pollutants in wastewater: impacts and remediation process. J Hell Vet Med Soc 65:115–120
Hussain S, Khan N, Gul S et al (2020) Contamination of water resources by food dyes and its removal technologies. Water Chem. https://doi.org/10.5772/intechopen.90331
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov 3:275–290. https://doi.org/10.1016/j.biori.2019.09.001
Chakhtouna H, Benzeid H, Zari N et al (2021) Functional CoFe2O4-modified biochar derived from banana pseudostem as an efficient adsorbent for the removal of amoxicillin from water. Sep Purif Technol:118592.https://doi.org/10.1016/j.seppur.2021.118592
Ivanković K, Kern M, Rožman M (2021) Modelling of the adsorption of pharmaceutically active compounds on carbon-based nanomaterials. J Hazard Mater 414.https://doi.org/10.1016/j.jhazmat.2021.125554
Patel AB, Shaikh S, Jain KR et al (2020) Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front Microbiol 11.https://doi.org/10.3389/fmicb.2020.562813
Jarjoui M, Geahchan A, Boutros E, Abou-Kaïs A (2000) Pollution des eaux souterraines par les hydrocarbures aromatiques polycycliques et évaluation du risque. La Houille Blanche 86:89–92. https://doi.org/10.1051/lhb/2000080
Huang L, Zhu X, Zhou S et al (2021) Phthalic acid esters: natural sources and biological activities. Toxins (Basel) 13.https://doi.org/10.3390/toxins13070495
He L, Gielen G, Bolan NS et al (2015) Contamination and remediation of phthalic acid esters in agricultural soils in China: a review. Agron Sustain Dev 35:519–534. https://doi.org/10.1007/s13593-014-0270-1
Xiaoyan T, Suyu W, Yang Y et al (2015) Removal of six phthalic acid esters (PAEs) from domestic sewage by constructed wetlands. Chem Eng J 275:198–205. https://doi.org/10.1016/j.cej.2015.04.029
Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12. https://doi.org/10.2478/v10102-009-0001-7
Sharma N, Singhvi R (2017) Effects of chemical fertilizers and pesticides on human health and environment: a review. Int J Agric Environ Biotechnol 10:675. https://doi.org/10.5958/2230-732x.2017.00083.3
Silva M, Azenha ME, Pereira MM et al (2010) Immobilization of halogenated porphyrins and their copper complexes in MCM-41: environmentally friendly photocatalysts for the degradation of pesticides. Appl Catal B Environ 100:1–9. https://doi.org/10.1016/j.apcatb.2010.07.033
Kroiss H, Rechberger H, Egle L (2011) Phosphorus in water quality and waste management. In: Integrated waste management - volume II. IntechOpen, London
Bunce JT, Ndam E, Ofiteru ID et al (2018) A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front Environ Sci 6:1–15. https://doi.org/10.3389/fenvs.2018.00008
Seruga P, Krzywonos M, Pyzanowska J et al (2019) Removal of ammonia from the municipal waste treatment effuents using natural minerals. Molecules 24.https://doi.org/10.3390/molecules24203633
Hamdan R, Ibrahim II, Haron SZ (2015) Ammonia nitrogen removal from domestic wastewater via nitrification process using aerated rock filter. Appl Mech Mater 773–774:1350–1354. https://doi.org/10.4028/www.scientific.net/amm.773-774.1350
Fisher RM, Alvarez-Gaitan JP, Stuetz RM, Moore SJ (2017) Sulfur flows and biosolids processing: using material flux analysis (MFA) principles at wastewater treatment plants. J Environ Manage 198:153–162. https://doi.org/10.1016/j.jenvman.2017.04.056
Agoro MA, Adeniji AO, Adefisoye MA, Okoh OO (2020) Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in eastern cape province, south africa. Water (Switzerland) 12.https://doi.org/10.3390/w12102746
Renu AM, Singh K (2017) Heavy metal removal from wastewater using various adsorbents: a review. J Water Reuse Desalin 7:387–419. https://doi.org/10.2166/wrd.2016.104
Akpor OB (2014) Heavy metal pollutants in wastewater effluents: sources, effects and remediation. Adv Biosci Bioeng 2:37. https://doi.org/10.11648/j.abb.20140204.11
Biosci IJ, Alfarra RS, Ali NE et al (2014) Removal of heavy metals by natural adsorbent: review. Int J Biosci 6655:130–139. https://doi.org/10.12692/ijb/4.7.130-139
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418
Dutta S, Gupta B, Srivastava SK, Gupta AK (2021) Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Mater Adv 2:4497–4531. https://doi.org/10.1039/d1ma00354b
Elbasiouny H, Darwesh M, Elbeltagy H et al (2021) Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: a review. Environ Monit Assess 193.https://doi.org/10.1007/s10661-021-09236-2
Jun BM, Lee HK, Park S, Kim TJ (2022) Purification of uranium-contaminated radioactive water by adsorption: a review on adsorbent materials. Sep Purif Technol 278:119675. https://doi.org/10.1016/j.seppur.2021.119675
Rashid R, Shafiq I, Akhter P et al (2021) A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. Environ Sci Pollut Res 28:9050–9066. https://doi.org/10.1007/s11356-021-12395-x
Berger AH, Bhown AS (2011) Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Procedia 4:562–567. https://doi.org/10.1016/j.egypro.2011.01.089
Rathi BS, Kumar PS (2021) Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ Pollut 280:116995. https://doi.org/10.1016/j.envpol.2021.116995
Zheng Y, Li Q, Yuan C et al (2018) Thermodynamic analysis of high-pressure methane adsorption on coal-based activated carbon. Fuel 230:172–184. https://doi.org/10.1016/j.fuel.2018.05.056
Ashraf MT, AlHammadi AA, El-Sherbeeny AM et al (2022) Synthesis of cellulose fibers/zeolite-a nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; Characterization and advanced equilibrium studies. J Mol Liq 360:119573. https://doi.org/10.1016/j.molliq.2022.119573
Girish CR, Murty VR (2016) Mass transfer studies on adsorption of phenol from wastewater using Lantana camara, forest waste. Int J Chem Eng 2016.https://doi.org/10.1155/2016/5809505
Alaqarbeh M (2021) Adsorption phenomena: definition, mechanisms, and adsorption types: short review. RHAZES Green Appl Chem 13:43–51
Patel H (2020) Batch and continuous fixed bed adsorption of heavy metals removal using activated charcoal from neem ( Azadirachta indica ) leaf powder. Sci Rep 10:16895. https://doi.org/10.1038/s41598-020-72583-6
Mariana M, Abdul AK, Mistar EM et al (2021) Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J Water Process Eng 43:102221. https://doi.org/10.1016/j.jwpe.2021.102221
Crini G, Lichtfouse E, Wilson LD, Morin-Crini N (2019) Conventional and non-conventional adsorbents for wastewater treatment. Environ Chem Lett 17:195–213. https://doi.org/10.1007/s10311-018-0786-8
Liu B, Kim KH, Kumar V, Kim S (2020) A review of functional sorbents for adsorptive removal of arsenic ions in aqueous systems. J Hazard Mater 388:121815. https://doi.org/10.1016/j.jhazmat.2019.121815
Tsade H, Murthy HCA, Muniswamy D (2020) Bio-sorbents from agricultural wastes for eradication of heavy metals: a review. J Mater Environ Sci 11:1719–1735
Devanna N, Begum BA, Chari MA (2019) Low-cost adsorbents procedure by means of heavy metal elimination from wastewater. Preprints. https://doi.org/10.20944/preprints201902.0013.v1
Awad AM, Shaikh SMR, Jalab R et al (2019) Adsorption of organic pollutants by natural and modified clays: a comprehensive review. Sep Purif Technol 228:115719. https://doi.org/10.1016/j.seppur.2019.115719
Dicko M, Guilmont M, Lamari F (2018) Adsorption and biomass: current interconnections and future challenges. Curr Sustain Energy Rep 5:247–256. https://doi.org/10.1007/s40518-018-0116-6
Semlali Aouragh Hassani F, Ouarhim W, Bensalah MO et al (2019) Mechanical properties prediction of polypropylene/short coir fibers composites using a self-consistent approach. Polym Compos 40:1919–1929. https://doi.org/10.1002/pc.24967
Essabir H, Achaby ME, Hilali EM et al (2015) Morphological, structural, thermal and tensile properties of high density polyethylene composites reinforced with treated argan nut shell particles. J Bionic Eng 12:129–141. https://doi.org/10.1016/S1672-6529(14)60107-4
Adekomaya O, Adama K (2018) A review on application of natural fibre in structural reinforcement: challenges of properties adaptation. J Appl Sci Environ Manag 22:749. https://doi.org/10.4314/jasem.v22i5.27
Isikgor H, Remzi Becer C (2010) Lignocellulosic biomass: a sustainable platform for production of bio-based chemicals and polymers. Polym Chem 1:13. https://doi.org/10.1039/c000660m
Rangabhashiyam S, Balasubramanian P (2019) The potential of lignocellulosic biomass precursors for biochar production: performance, mechanism and wastewater application—a review. Ind Crops Prod 128:405–423. https://doi.org/10.1016/j.indcrop.2018.11.041
Silveira-Junior EG, Perez VH, Justo OR et al (2021) Valorization of guava (Psidium guajava L.) seeds for levoglucosan production by fast pyrolysis. Cellulose 28:71–79. https://doi.org/10.1007/s10570-020-03506-x
Baruah J, Nath BK, Sharma R et al (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6.https://doi.org/10.3389/fenrg.2018.00141
Mansor AM, Lim JS, Ani FN et al (2019) Characteristics of cellulose, hemicellulose and lignin of MD2 pineapple biomass. Chem Eng Trans 72:79–84. https://doi.org/10.3303/CET1972014
Zhang L, Dou X, Yang Z et al (2021) Advance in hydrothermal bio-oil preparation from lignocellulose: effect of raw materials and their tissue structures. Biomass 1:74–93. https://doi.org/10.3390/biomass1020006
Bai YY, Xiao LP, Shi ZJ, Sun RC (2013) Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int J Mol Sci 14:21394–21413. https://doi.org/10.3390/ijms141121394
Muktham R, Bhargava SK, Bankupalli S, Ball AS (2016) A review on 1st and 2nd generation bioethanol production-recent progress. J Sustain Bioenergy Syst 06:72–92. https://doi.org/10.4236/jsbs.2016.63008
Komuraiah A, Kumar NS, Prasad BD (2014) Chemical composition of natural fibers and its influence on their mechanical properties. Mech Compos Mater 50:359–376. https://doi.org/10.1007/s11029-014-9422-2
Pirayesh H, Khazaeian A, Tabarsa T (2012) The potential for using walnut (Juglans regia L.) shell as a raw material for wood-based particleboard manufacturing. Compos Part B Eng 43:3276–3280. https://doi.org/10.1016/j.compositesb.2012.02.016
Li X, Liu Y, Hao J, Wang W (2018) Study of almond shell characteristics. Materials (Basel) 11.https://doi.org/10.3390/ma11091782
Mokhena TC, John MJ (2020) Cellulose nanomaterials: new generation materials for solving global issues. Springer, Dordrecht
Sanjay MR, Arpitha GR, Naik LL et al (2016) Applications of natural fibers and its composites: an overview. Nat Resour 07:108–114. https://doi.org/10.4236/nr.2016.73011
Candido ICM, Pires ICB, de Oliveira HP (2021) Natural and synthetic fiber-based adsorbents for water remediation. Clean Soil Air Water 49:1–11. https://doi.org/10.1002/clen.202000189
Saman N, Johari K, Song ST et al (2017) High removal efficacy of Hg(II) and MeHg(II) ions from aqueous solution by organoalkoxysilane-grafted lignocellulosic waste biomass. Chemosphere 171:19–30. https://doi.org/10.1016/j.chemosphere.2016.12.049
Kartina S, Karim A, Lim SF et al (2016) Banana fibers as sorbent for removal of acid green dye from water. J Chem 9648312. https://doi.org/10.1155/2016/9648312
Pavan FA, Camacho ES, Lima EC et al (2014) Formosa papaya seed powder (FPSP): preparation, characterization and application as an alternative adsorbent for the removal of crystal violet from aqueous phase. J Environ Chem Eng 2:230–238. https://doi.org/10.1016/j.jece.2013.12.017
Idan IJ (2017) Adsorption of anionic dye using cationic surfactant-modified kenaf core fibers. OALib 04:1–18. https://doi.org/10.4236/oalib.1103747
Lee BG, Rowell RM (2004) Removal of heavy metal ions from aqueous solutions using lignocellulosic fibers. J Nat Fibers 1:97–108. https://doi.org/10.1300/J395v01n01_07
Asadi F, Shariatmadari H, Mirghaffari N (2008) Modification of rice hull and sawdust sorptive characteristics for remove heavy metals from synthetic solutions and wastewater. J Hazard Mater 154:451–458. https://doi.org/10.1016/j.jhazmat.2007.10.046
Lei M, Yang L, Shen Y et al (2021) Efficient adsorption of anionic dyes by ammoniated waste polyacrylonitrile fiber: mechanism and practicability. ACS Omega 6:19506–19516. https://doi.org/10.1021/acsomega.1c01780
Yue X, Huang J, Jiang F et al (2019) Synthesis and characterization of cellulose-based adsorbent for removal of anionic and cationic dyes. J Eng Fiber Fabr 14.https://doi.org/10.1177/1558925019828194
Akter M, Bhattacharjee M, Dhar AK et al (2021) Cellulose-based hydrogels for wastewater treatment: a concise review. Gels 7:1–28. https://doi.org/10.3390/gels7010030
Elumalai, S, Agarwal, B, Runge, TM, Sangwan, RS (2018). Advances in transformation of lignocellulosic biomass to carbohydrate-derived fuel precursors. In: Kumar, S, Sani, R (eds) Biorefining of biomass to biofuels. Biofuel and Biorefinery Technologies, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-67678-4_4
Hon DNS (1994) Cellulose: a random walk along its historical path. Cellulose 1:1–25. https://doi.org/10.1007/BF00818796
Okolie JA, Nanda S, Dalai AK, Kozinski JA (2021) Chemistry and specialty industrial applications of lignocellulosic biomass. Waste Biomass Valorization 12:2145–2169. https://doi.org/10.1007/s12649-020-01123-0
Cichosz S, Masek A (2020) IR study on cellulose with the varied moisture contents: insight into the supramolecular structure. Materials (Basel) 13:1–22. https://doi.org/10.3390/ma13204573
O’Dell WB, Baker DC, McLain SE (2012) Structural evidence for inter-residue hydrogen bonding observed for cellobiose in aqueous solution. PLoS ONE 7:25–27. https://doi.org/10.1371/journal.pone.0045311
Zoghlami A, Paës G (2019) Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis. Front Chem 7.https://doi.org/10.3389/fchem.2019.00874
Nasir M, Hashim R, Sulaiman O, Asim M (2017) Nanocellulose: preparation methods and applications. In: Jawaid M, Boufi S, Abdul Khalil HPS (eds) Cellulose-reinforced nanofibre composites: production, properties and applications. Elsevier Ltd, pp 261–276
Rongpipi S, Ye D, Gomez ED, Gomez EW (2019) Progress and opportunities in the characterization of cellulose – an important regulator of cell wall growth and mechanics. Front Plant Sci 9:1–28. https://doi.org/10.3389/fpls.2018.01894
Mittal A, Katahira R, Himmel ME, Johnson DK (2011) Effects of alkaline or liquid-ammonia treatment on crystalline cellulose: changes in crystalline structure and effects on enzymatic digestibility. Biotechnol Biofuels 4:1–16. https://doi.org/10.1186/1754-6834-4-41
Tian SQ, Zhao RY, Chen ZC (2018) Review of the pretreatment and bioconversion of lignocellulosic biomass from wheat straw materials. Renew Sustain Energy Rev 91:483–489. https://doi.org/10.1016/j.rser.2018.03.113
Tu WC, Hallett JP (2019) Recent advances in the pretreatment of lignocellulosic biomass. Curr Opin Green Sustain Chem 20:11–17. https://doi.org/10.1016/j.cogsc.2019.07.004
Chakhtouna H, Benzeid H, Zari N, Qaiss A, Bouhfid R, Hybrid materials from cellulose nanocrystals for wastewater treatment, In: Rodrigue D, Qaiss A, Bouhfid R (eds) Cellulose Nanocrystal/Nanoparticles Hybrid Nanocomposites. Woodhead Publishing. https://doi.org/10.1016/B978-0-12-822906-4.00001-3
Mishra RK, Sabu A, Tiwari SK (2018) Materials chemistry and the futurist eco-friendly applications of nanocellulose: status and prospect. J Saudi Chem Soc 22:949–978. https://doi.org/10.1016/j.jscs.2018.02.005
Huang YB, Fu Y (2013) Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem 15:1095–1111. https://doi.org/10.1039/c3gc40136g
Chau M, Sriskandha SE., Thérien-Aubin H, Kumacheva E (2015) Supramolecular nanofibrillar polymer hydrogels. In: Seiffert S (eds) Supramolecular polymer networks and gels. Advances in Polymer Science, vol 268. Springer, Cham. https://doi.org/10.1007/978-3-319-15404-6_5
Ullah MW, Manan S, Ul-Islam M et al (2021) Introduction to nanocellulose. In: Nanocellulose: synthesis, structure, properties and applications. pp 1–50
Trache D, Tarchoun AF, Derradji M et al (2020) Nanocellulose: from fundamentals to advanced applications. Front Chem 8.https://doi.org/10.3389/fchem.2020.00392
Mateo S, Peinado S, Morillas-Gutiérrez F et al (2021) Nanocellulose from agricultural wastes: products and applications—a review. Processes 9.https://doi.org/10.3390/pr9091594
Moon RJ, Martini A, Nairn J et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40(7):3941–3994. https://doi.org/10.1039/C0CS00108B
Jamshaid A, Hamid A, Muhammad N et al (2017) Cellulose-based materials for the removal of heavy metals from wastewater - an overview. ChemBioEng Rev 4:240–256. https://doi.org/10.1002/cben.201700002
Rathod M, Haldar S, Basha S (2015) Nanocrystalline cellulose for removal of tetracycline hydrochloride from water via biosorption: equilibrium, kinetic and thermodynamic studies. Ecol Eng 84:240–249
Santoso SP, Kurniawan A, Soetaredjo FE et al (2019) Eco-friendly cellulose–bentonite porous composite hydrogels for adsorptive removal of azo dye and soilless culture. Cellulose 26:3339–3358. https://doi.org/10.1007/s10570-019-02314-2
Chong KY, Chia CH, Zakaria S et al (2015) CaCO3-decorated cellulose aerogel for removal of Congo Red from aqueous solution. Cellulose 22:2683–2691. https://doi.org/10.1007/s10570-015-0675-2
Chen X, Liu L, Luo Z et al (2018) Facile preparation of a cellulose-based bioadsorbent modified by hPEI in heterogeneous system for high-efficiency removal of multiple types of dyes. React Funct Polym 125:77–83. https://doi.org/10.1016/j.reactfunctpolym.2018.02.009
Yu Z, Hu C, Dichiara AB et al (2020) Cellulose nanofibril/carbon nanomaterial hybrid aerogels for adsorption removal of cationic and anionic organic dyes. Nanomaterials 10:1–20. https://doi.org/10.3390/nano10010169
Wei X, Huang T, Nie J et al (2018) Bio-inspired functionalization of microcrystalline cellulose aerogel with high adsorption performance toward dyes. Carbohydr Polym 198:546–555. https://doi.org/10.1016/j.carbpol.2018.06.112
Sharma RK, Kumar R (2019) Functionalized cellulose with hydroxyethyl methacrylate and glycidyl methacrylate for metal ions and dye adsorption applications. Int J Biol Macromol 134:704–721. https://doi.org/10.1016/j.ijbiomac.2019.05.059
Sun N, Wen X, Yan C (2018) Adsorption of mercury ions from wastewater aqueous solution by amide functionalized cellulose from sugarcane bagasse. Int J Biol Macromol 108:1199–1206. https://doi.org/10.1016/j.ijbiomac.2017.11.027
Goswami R, Mishra A, Bhatt N, Naithani P (2020) Removal of chromium using nanocellulose based adsorbent. J Crit Rev 7:4148–4155
Kabuba J, Lukusa T (2021) Synthesis of Gelatin-Cellulose Nanocrystals Hydrogel Membrane For Removal of Cu (II) And Co (II) From Mining Processes Wastewater. Res Square. https://doi.org/10.21203/rs.3.rs-383692/v1
Wang N, Ouyang X, Yang L, Omer AM (2017) Fabrication of a magnetic cellulose nanocrystal/metal − organic framework composite for removal of Pb(II) from water. ACS Sustain Chem Eng 5:10447–10458. https://doi.org/10.1021/acssuschemeng.7b02472
Liang L, Zhang S, Goenaga GA et al (2020) Chemically cross-linked cellulose nanocrystal aerogels for effective removal of cation dye. Front Chem 8:1–9. https://doi.org/10.3389/fchem.2020.00570
Thorat MN, Jagtap A, Dastager SG (2021) Fabrication of bacterial nanocellulose/polyethyleneimine (PEI-BC) based cationic adsorbent for efficient removal of anionic dyes. J Polym Res 28:1–11. https://doi.org/10.1007/s10965-021-02702-y
Hu D, Jiang R, Wang N et al (2019) Adsorption of diclofenac sodium on bilayer amino-functionalized cellulose nanocrystals / chitosan composite. J Hazard Mater 369:483–493. https://doi.org/10.1016/j.jhazmat.2019.02.057
Chen Y, Xiang Z, Wang D et al (2020) Effective photocatalytic degradation and physical adsorption of methylene blue using cellulose/GO/TiO2 hydrogels. RSC Adv 10:23936–23943. https://doi.org/10.1039/d0ra04509h
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559. https://doi.org/10.1039/c5py00263j
Huang J-q, Qi R-t, Pang M-r et al (2017) Isolation, chemical characterization, and immunomodulatory activity of naturally acetylated hemicelluloses from bamboo shavings. J Zhejiang Univ Sci B 18:138–151. https://doi.org/10.1631/jzus.B1500274
Sorieul M, Dickson A, Hill SJ, Pearson H (2016) Plant fibre: molecular structure and biomechanical properties, of a complex living material, influencing its deconstruction towards a biobased composite. Materials 9(8):618. https://doi.org/10.3390/ma9080618
Hu L, Du M, Zhang J (2018) Hemicellulose-based hydrogels present status and application prospects: a brief review. Open J For 08:15–28. https://doi.org/10.4236/ojf.2018.81002
Yao S, Nie S, Zhu H et al (2017) Extraction of hemicellulose by hot water to reduce adsorbable organic halogen formation in chlorine dioxide bleaching of bagasse pulp. Ind Crops Prod 96:178–185. https://doi.org/10.1016/j.indcrop.2016.11.046
Bokhary A, Maleki E, Liao B (2018) Ultrafiltration for hemicelluloses recovery and purification thermomechanical pulp mill process waters. Desalin Water Treat 118:103–112. https://doi.org/10.5004/dwt.2018.22641
Sun XF, Gan Z, Jing Z et al (2015) Adsorption of methylene blue on hemicellulose-based stimuli-responsive porous hydrogel. J Appl Polym Sci 132:19–22. https://doi.org/10.1002/app.41606
Sun XF, Liu B, Jing Z, Wang H (2015) Preparation and adsorption property of xylan/poly(acrylic acid) magnetic nanocomposite hydrogel adsorbent. Carbohydr Polym 118:16–23. https://doi.org/10.1016/j.carbpol.2014.11.013
Hu N, Chen D, Guan QQ et al (2020) Preparation of hemicellulose-based hydrogels from biomass refining industrial effluent for effective removal of methylene blue dye. Environ Technol 0:1–22. https://doi.org/10.1080/09593330.2020.1795930
Huang Z, Liu S, Zhang B et al (2012) Equilibrium and kinetics studies on the absorption of Cu(II) from the aqueous phase using a β-cyclodextrin-based adsorbent. Carbohydr Polym 88:609–617. https://doi.org/10.1016/j.carbpol.2012.01.009
Ayoub A, Venditti RA, Pawlak JJ et al (2013) Novel hemicellulose-chitosan biosorbent for water desalination and heavy metal removal. ACS Sustain Chem Eng 1:1102–1109. https://doi.org/10.1021/sc300166m
Del Río JC, Rencoret J, Gutiérrez A et al (2020) Lignin monomers from beyond the canonical monolignol biosynthetic pathway: another brick in the wall. ACS Sustain Chem Eng 8:4997–5012. https://doi.org/10.1021/acssuschemeng.0c01109
Becker J, Wittmann C (2019) A field of dreams: lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol Adv 37:107360. https://doi.org/10.1016/j.biotechadv.2019.02.016
Abo BO, Gao M, Wang Y et al (2019) Lignocellulosic biomass for bioethanol: an overview on pretreatment, hydrolysis and fermentation processes. Rev Environ Health 34:57–68. https://doi.org/10.1515/reveh-2018-0054
Khan A, Nair V, Colmenares JC, Gläser R (2018) Lignin-based composite materials for photocatalysis and photovoltaics. Top Curr Chem 376:1–31. https://doi.org/10.1007/s41061-018-0198-z
Erfani Jazi M, Narayanan G, Aghabozorgi F et al (2019) Structure, chemistry and physicochemistry of lignin for material functionalization. SN Appl Sci 1:1–19. https://doi.org/10.1007/s42452-019-1126-8
Li Y, Li F, Yang Y et al (2021) Research and application progress of lignin-based composite membrane. J Polym Eng 41:245–258. https://doi.org/10.1515/polyeng-2020-0268
Naseer A, Hamid A, Ghauri M et al (2020) Lignin/alginate/hydroxyapatite composite beads for the efficient removal of copper and nickel ions from aqueous solutions. Desalin Water Treat 184:199–213. https://doi.org/10.5004/dwt.2020.25356
Nair V, Panigrahy A, Vinu R (2014) Development of novel chitosan-lignin composites for adsorption of dyes and metal ions from wastewater. Chem Eng J 254:491–502. https://doi.org/10.1016/j.cej.2014.05.045
Li F, Wang X, Yuan T, Sun R (2016) A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb(II) removal. J Mater Chem A 4:11888–11896. https://doi.org/10.1039/c6ta03779h
Gassara F, Brar SK, Verma M, Tyagi RD (2013) Bisphenol A degradation in water by ligninolytic enzymes. Chemosphere 92:1356–1360. https://doi.org/10.1016/j.chemosphere.2013.02.071
Srisasiwimon N, Chuangchote S, Laosiripojana N, Sagawa T (2018) TiO2/lignin-based carbon composited photocatalysts for enhanced photocatalytic conversion of lignin to high value chemicals. ACS Sustain Chem Eng 6:13968–13976. https://doi.org/10.1021/acssuschemeng.8b02353
Khan A, Goepel M, Lisowski W et al (2021) Titania/chitosan–lignin nanocomposite as an efficient photocatalyst for the selective oxidation of benzyl alcohol under UV and visible light. RSC Adv 11:34996–35010. https://doi.org/10.1039/d1ra06500a
Chakhtouna H, Zari N, Benzeid H et al (2021) Hybrid nanocomposites based on graphene and titanium dioxide for wastewater treatment. In: Qaiss A, Bouhfid R, Jawaid M (eds) Graphene and Nanoparticles Hybrid Nanocomposites. Composites Science and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-33-4988-9_8
Chakhtouna H, Mekhzoum MEM, Zari N, Benzeid H, Qaiss, A, Bouhfid R (2021) Biochar‐Supported materials for wastewater treatment. In: Ahamed R (eds) Applied Water Science, vol 1. Inamuddin. https://doi.org/10.1002/9781119725237.ch7
Hagemann N, Spokas K, Schmidt HP et al (2018) Activated carbon, biochar and charcoal: linkages and synergies across pyrogenic carbon’s ABCs. Water (Switzerland) 10:1–19. https://doi.org/10.3390/w10020182
Oni BA, Oziegbe O, Olawole OO (2019) Significance of biochar application to the environment and economy. Ann Agric Sci 64:222–236. https://doi.org/10.1016/j.aoas.2019.12.006
Pusceddu E, Santilli SF, Fioravanti G et al (2019) Chemical-physical analysis and exfoliation of biochar-carbon matter: from agriculture soil improver to starting material for advanced nanotechnologies. Mater Res Express 6.https://doi.org/10.1088/2053-1591/ab4ba8
Naeem MA, Khalid M, Arshad M, Ahmad R (2014) Yield and nutrient composition of biochar produced from different feedstocks at varying pyrolytic temperatures. Pak J Agric Sci 51:75–82
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Leng L, Xiong Q, Yang L et al (2021) An overview on engineering the surface area and porosity of biochar. Sci Total Environ 763:144204. https://doi.org/10.1016/j.scitotenv.2020.144204
Gao Y, Yue Q, Gao B, Li A (2020) Insight into activated carbon from different kinds of chemical activating agents: a review. Sci Total Environ 746:141094. https://doi.org/10.1016/j.scitotenv.2020.141094
Arslanoğlu H (2019) Direct and facile synthesis of highly porous low cost carbon from potassium-rich wine stone and their application for high-performance removal. J Hazard Mater 374:238–247. https://doi.org/10.1016/j.jhazmat.2019.04.042
Fu Y, Shen Y, Zhang Z et al (2019) Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci Total Environ 646:1567–1577. https://doi.org/10.1016/j.scitotenv.2018.07.423
Liew RK, Azwar E, Yek PNY et al (2018) Microwave pyrolysis with KOH/NaOH mixture activation: a new approach to produce micro-mesoporous activated carbon for textile dye adsorption. Bioresour Technol 266:1–10. https://doi.org/10.1016/j.biortech.2018.06.051
El Bakouri H, Usero J, Morillo J, Ouassini A (2009) Adsorptive features of acid-treated olive stones for drin pesticides: equilibrium, kinetic and thermodynamic modeling studies. Bioresour Technol 100:4147–4155. https://doi.org/10.1016/j.biortech.2009.04.003
Li L, Zou D, Xiao Z et al (2019) Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J Cleaner Production 210:1324–1342. https://doi.org/10.1016/j.jclepro.2018.11.087
Tan X-F, Liu Y-G, Gu Y-L et al (2016) Biochar-based nano-composites for the decontamination of wastewater: a review. Bioresour Technol 212:318–333. https://doi.org/10.1016/j.biortech.2016.04.093
Leichtweis J, Silvestri S, Carissimi E (2020) New composite of pecan nutshells biochar-ZnO for sequential removal of acid red 97 by adsorption and photocatalysis. Biomass Bioenergy 140:105648. https://doi.org/10.1016/j.biombioe.2020.105648
Eltaweil AS, Ali Mohamed H, Abd El-Monaem EM, El-Subruiti GM (2020) Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: characterization, adsorption kinetics, thermodynamics and isotherms. Adv Powder Technol 31:1253–1263. https://doi.org/10.1016/j.apt.2020.01.005
Iqbal J, Shah NS, Sayed M et al (2021) Nano-zerovalent manganese/biochar composite for the adsorptive and oxidative removal of Congo-red dye from aqueous solutions. J Hazard Mater 403:123854. https://doi.org/10.1016/j.jhazmat.2020.123854
Abdul G, Zhu X, Chen B (2017) Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem Eng J. https://doi.org/10.1016/j.cej.2017.02.074
Funding
This work was supported by the MAScIR Foundation.
Author information
Authors and Affiliations
Contributions
Hanane Chakhtouna:writing—original draft preparation, Hanane Benzeid: writing—review and editing, Nadia Zari: writing—review and editing, Abou el kacem Qaiss: supervision and review, Rachid Bouhfid: supervision and review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chakhtouna, H., Benzeid, H., Zari, N. et al. Recent advances in eco-friendly composites derived from lignocellulosic biomass for wastewater treatment. Biomass Conv. Bioref. 14, 12085–12111 (2024). https://doi.org/10.1007/s13399-022-03159-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03159-9