Abstract
A bacterial cellulose (BC) based adsorbent was prepared by using polyethyleneimine (PEI) using epichlorohydrin as a cross-linker. The physicochemical characterization of the of the developed adsorbent was carried out using FTIR, BET analysis and SEM, respectively. The dye removal efficiency of the adsorbent was assessed towards two anionic model dyes viz. congo red (CR) and reactive red 120(RR). The kinetic data for the removal of both the anionic dyes were fitted better using pseudo-second-order model. Langmuir model describes well the process of adsorption with predicted maximum adsorption capacity (qmax) 515.46 and 300.3 mg L−1 for CR (at pH 6.0) and RR (at pH 3.0) respectively. The adsorption–desorption study showed that PEI-BC adsorbent is effective with more than 90% dye removal efficiency even after four cycles. Furthermore, the bactericidal activity of the adsorbent was analysed using Escherichia coli and Staphylococcus aureus and adsorbent showed good inhibition against both test organisms. The study reveals that PEI-BC adsorbent can be a good candidate for water remediation purpose bearing multifunctional behaviour.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An alarming rise in water pollution due to increased urbanization and industrialization is the major cause of decreased surface water quality. Different forms of pollutants like dyes, pesticides, heavy metals, surfactants etc., are the significant contributors to water pollution [1, 2]. Huge quantities of the various dyes are being discharged through textile, paper, leather, paint industries [1, 3, 4]. Most of these dyes are non-biodegradable, a potent carcinogen and mutagen [4, 5]. Besides, various anthropic activities also add different hazardous microbes to water bodies [6]. All these pollutants directly influence human, animals, and aquatic life [7, 8].
Various treatment methods such as precipitation, flocculation, ion exchange, adsorption, ultrafiltration or combinations of these two or more methods are generally used for water treatment [1, 9,10,11]. However, among them, adsorption is the most efficient method since it is convenient to use, and low cost method [3, 10, 12, 13]. The ideal adsorbent should possess a high surface area with plenty of adsorption sites, should be environmentally friendly and biodegradable [9, 14, 15]. Several different adsorbents like activated carbons [16], clays [17, 18] and carbon nanotubes[19, 20] have demonstrated to be used for the removing dyes from liquids. The activated carbon is the most frequently used adsorbent for the treatment of industrial wastewater because of its avilable surface area. Considering the high cost of the activated carbon, further use of this adsorbent material become limited. Research has to be continued for finding less expensive alternate new adsorbents which is having good adsorption efficiency [17].
Cellulose is the most attractive and ideal material for its non-toxic and biodegradable nature [10, 21]. Its structure provides vast range of possibilities for its modification [10]. Nowadays, nanocellulose has been attracted much attention due to its fascinating properties like high surface area, aspect ratio and high mechanical strength [15]. The dense distribution of surface hydroxyl groups provides an excellent opportunity for surface functionalization with different functional groups [9, 21]. By imparting additional functionality, it is possible to design the cellulose with enhanced properties by which its performance can be improved. The modified nanocellulose is thereby become more suitable for varieties of applications [15, 22,23,24]. For instance, various attempts have been done to develop nano-cellulose based adsorbent for wastewater treatment by tailoring the surface charge by carboxylation, amine functionalization, sulfonation etc. [25].
Bacterial nanocellulose has emerged as a versatile polymer, and its exceptional properties have been exploited in multifaceted applications. It is a naturally produced nanofibrillated form of cellulose, and its production is environmentally friendly than plant-based nanocellulose [26]. Besides, a unique three-dimensional network structure of the cellulosic fibrils makes it a more porous material. All these properties are suitable for the preparation of adsorbent. But, since native BC lacks adsorption sites, it needs to be modified by implanting surface functionality.
Polyethyleneimine (PEI) is a cationic polymer with many primary, secondary, and tertiary amine side groups [12, 22, 27, 28]. Chemically it contains polar amine groups separated by hydrophobic –CH2-CH2–spacer forming polyamine polymer [29]. PEI exhibit a strong affinity towards anionic compounds as well as heavy metals. However, due to the water-soluble nature of PEI, there are limitations in its direct applications, and it needs to be cross-linked or grafted on the solid support [12, 22].
In this present study, we have attemted to develop a BC-based adsorbent with surface modification by using polyethyleneimine. There are very sparse reports for the utilization of this unique biopolymer for the development of adsorbent. With a high number of surface amine groups, the given adsorbent is expected to remove anionic dyes effectively from the solution. Dye removal study was carried out using congo red and reactive red 120 as model anionic dyes. The morphological and structural characetrization of PEI-BC adsorbent were analysed by Fourier Transform Infrared (FTIR) Spectroscopy, Scanning Electron Microscopy (SEM) and Brunauer–Emmett–Teller (BET) analysis. Developed adsorbent was further evaluated for its bactericidal property and its applicability as a multifunctional material for the sustainable environment.
Materials and methods
Production of bacterial cellulose
The strain used for BC production was previously isolated in our lab. Bacterial cellulose membrane was produced and processed as described earlier [30]. Briefly, Komagataeibacter strain PG2 was inoculated in HS media using crude glycerol as best and economical carbon source. After 15 days of incubation membranes were harvested and treated with 2% NaOH at 80 °C and washed with distilled water repeatedly till neutral pH.
Derivatization of bacterial cellulose nanofiber by Polyethyleneimine (PEI)
The PEI-BC was prepared based on previously reported method [31]. First the epoxidised BC (EP-BC) was prepared. The lyophilised BC powder was soaked in 1.4 M NaOH and epichlorohydrin solution and the resultant mixture was refluxed at 65° C for 2 h. The epoxidised BC membarne was washed with distilled water to remove unbounded chemicals. The functionalised PEI-BC was then prepared by soaking further the epoxidised BC in solution of branched PEI (Mw = 25,000, Sigma-Aldrich) and NaHCO3 and reaction was carried out at 65° C for 5 h. The resultant PEI functionalised BC was further washed with deionised water. PEI was used in different proportion with BC and 7 different samples were generated (BC:PEI-1:0.5, 1:1, 1:2, 1:3, 1:4, 1:5, 1:6).
Characterization of BC, EP-BC and PEI-BC
The functional group analysis of BC, EP-BC and PEI-BC adsorbents were analysed using Fourier-transform infrared spectrometer (ATR-FTIR). The samples were analyzed using the ATR mode on a diamond ATR accessory-using spectrum two Perkin Elmer instrument. The FTIR spectra were recorded at the transmission mode in from 4000–400 cm−1 with a resolution of 4 cm−1 and an accumulation of 32 scans.
Surface morphology analysis of all the samples were carried out using FEI Quanta FEG 450 scanning electron microscope equipped with EDAX facility. Prior to SEM analysis the lyophilised samples were sputter coated with gold. The fibrilar morphology was determined at random positions. The nitrogen mapping of synthesised PEI-BC adsorbent was done using EDAX method. The specific surface area measurement was done using Brunauer–Emmett–Teller (BET) analysis. Prior to BET analysis the samples were degassed properly and N2 adsorption and desorption isotherms were carried out at 77 K.
Dye adsorption studies
The stock solutions of dyes with a concentration of 1000 mg L−1 were prepared by dissolving in DI water and diluted as per the experimental requirement. All the adsorption studies were carried out in a room temperature (28 °C). Initially, the different adsorbent samples were prepared by varying PEI proportion and tested for their ability to adsorb congo red as a model anionic dye. Based on the dye removal efficiency, the appropriate adsorbent sample was selected for further studies. The effect of pH on adsorption of CR and RR were studied in the pH range from 3–9 (adsorbent dosage: 250mgL−1, vol. of the dye solution: 30 ml). The batch adsorption study was performed to observe the effect of contact time on the removal of the dye. The adsorbent (400 mg L−1) was added in 250 ml flask containing 30 ml of dye solution with pH 6.0 for CR and pH 3.0 for RR with an concentration of 50 mg L−1. The flasks were further incubated on an rotary shaker at 150 rpm to ensure the proper mixing of the solution. Different aliquots of the samples were removed in the defined time intervals. The effect of the different dye concentration on the adsorption were studied from 25-300 mg L−1. The adsorption equilibrium, adsorption behaviour and maximum adsorption capacity of the given adsorbent was evaluated (adsorbent dosage: 400 mg L−1, pH 6.0 for CR and pH 3.0 for RR). UV–visible spectra of the solutions were determined at 500 nm and 510 nm for CR and RR respectively. The unknown concentration of dye was determined based on the calibration curve. The dye removal percentage and dye adsorption capacity was calculated using the following equations [3]
where, C0 and Ct are the initial concentration and concentration of dye (mg L−1) at time t respectively, V is the volume of the solution (L) and m is the mass of the adsorbent.
Determination of point zero charge of adsorbent
The pHPZC of adsorbent was determined by using previously reported solid titration method [32]. Briefly, 0.12 mg of adsorbent was added in 250 ml flasks containing 30 ml 0.01 mol L−1 of NaCl solution with pH in the range of 3.0–12.0. All the flasks were kept for shaking overnight and after equilibrium is achieved, the final pH of the NaCl solutions were measured. The graph of initial pH was plotted against difference in initial pH (3.0–12.0) value and final pH (ΔpH).
Reusability of adsorbent
For the reusalbility of the PEI-BC adsorbents, were evaluated by separating from the solution and treating with 0.5 M NaOH solution for desorption of the dye. The adsorbents were thoroughly washed with miliQ water till the neutral pH attains and material was lyophilised. The regenerated adsorbents were reused for next cycle of dye adsorption experiment.
Disinfection assay
The disinfection experiment was carried out as previously described. Briefly, the bacterial cell pellet obtained from overnight grown culture was re-suspended in sterile saline and diluted to 106 CFU/ml. Different amounts of PEI-BC adsorbent (0.25, 0.50, 0.75 and 1 g L−1) was then added into the diluted bacterial suspensions and kept for shaking at 37° C for 3 h. After that the suspensions were serially diluted and 100 µl was used for spreading on plates of LB agar. The plates were incubated further at 37 °C for overnight and CFU was determined. Bactericidal efficency of the adsorbent was evaluated against Gram-negative Escherichia coli (E.coli) and Gram-positive Staphylococcus aureus (S.aureus) organisms. The change in cell morphology after treatment was determined using FE-SEM analysis.
Results and discussion
Bacterial cellulose used in this study was produced using Komagataeibacter strain PG2 by utilizing cheap carbon source in the form of crude glycerol. Bacterial cellulose with its exceptional properties especially its nanofibrillated form, three dimensional structure and high crystallinity have been exploited in diverse sectors [30, 33,34,35]. Taking into account all these things, we envisaged that three dimensional and nanofibrillated BC could be used as an ideal candidate for development of adsorbent.
Fabrication of PEI-BC adsorbent
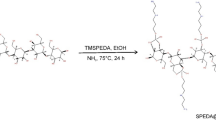
In this study, the adsorbent was produced by crosslinking the PEI on the surface of three dimensional BC nanofibers by using epichlorohydrin. Detailed synthesis of BC/PEI adsorbent is represented in scheme (Fig. 1). PEI with positively charged amino groups has been used in various environmental applications. Different compositions of PEI-BC were produced by varying the proportion of PEI. The effect of PEI concentration on the adsorption effociency was evaluated by using a anionic dye congo red at 50 mg L−1 concentration. With the increase in the concentration of PEI in the adsorbent the adsorption capacity was also increased. The BC:PEI at 1:6 ratio has shown better adsorption and more than 95% dye removal efficiency (Fig. 2). This composition of BC: PEI was used for further adsorption studies.
Removal of CR using different adsorbent prepared by varying PEI proportion(BC:PEI- 1:0.5, 1:1, 1:2, 1:3, 1:4,, 1:5, 1:6), (A) optical image of adsorption of congo red dye (B) UV–Visible spectra representing absorbance of CR after treatment with different adsorbents. BC and congo red solution (50 mg L−1) were used as control. (Dye conc: 50 mg L−1, adsorbent dose: 200 mg/L and time: 5 h)
The FTIR spectra of BC, EP-BC and PEI-BC is shown in Fig. 3A. There is no significant difference between spectra of EP-BC in comparison with BC. However, the appearance of new peak at 1564 cm−1 wavenumbers was observed in case of PEI-BC ascribed for N–H bending vibration of amines [36]. Besides, the broad band in the range of 3150–3470 cm−1 could be related to the overlapping of N–H and O–H stretching vibrations [36,37,38]. The specific surface area of the PEI-BC adsorbent was analysed using BET analysis. The N2 adsorption and desorption isotherm of the adsorbent followed Type IV isotherm and the surface area was found to be 35.34 m2/g (single point surface area at P/Po = 0.2488) (Fig. 3B).
The morphological features were analysed with FE-SEM. The PEI crosslinking does not affected the fibrilar morphology of bacterial cellulose. However, PEI coated fibrils showed the smoother surface in the appearance when compared to pristine (unmodified) BC fibrils. The distribution of nitrogen atom which belongs to PEI along the BC network was determined by the elemental mapping using EDAX. The dark green spots were representing nitrogen atom and was distributed uniformly in PEI-BC sponge surface (Fig. 4A−D). These results confirms the successful functionalisation of BC by crosslinking with PEI.
Effect of pH on adsorption
The pH refers as one of the crucial parameters that governs the process of adsorption. The pH of the dye solution affects the ionization of dye as well as adsorbent. Herein, we studied the effect of pH on the adsorption over a wide range pH 3.0–10.0. The effective removal of dye was observed at acidic pH. In the case of RR the highest adsorption was observed at pH 3.0. The adsorption of CR was almost the same at pH range 3–6. The decrease in adsorption capacity for both the anionic dyes was observed at pH 10 (Fig. 5A). The pHpzc was determined by titrimetric method and was found to be 7.4, which means at pH < pHpzc the surface of the adsorbent is positively charged and favours adsorption of anionic dyes. This proves the decrease in the adsorption at pH 10.0 due to deprotonation of adsorbent favours at alkaline pH (pH > pHpzc) (Fig. 5B). Considering these results the remaining batch adsorption studies were carried out at pH 6.0 and 3.0 for CR and RR, respectively.
Adsorption kinetic study
The kinetic analysis of process of adsorption of CR and RR was done using pseudo first order and pseudo second order kinetic models (Fig. 6). The equation for these models is expressed as below [5, 17].
Effect of contact time on adsorption of anionic dyes using PEI-BC adsorbent: (A) CR, (D) RR (initial dye conc.: 50mg L-1, adsorbent dose: 400mg L-1, pH of the solution: 6.0 for CR and 3.0 for RR), (B) and (C) pseudofirst-order kinetics, (E) and (F) pseudo-second-order kinetics for adsorption of CR and RR
where qt (mg g−1) and qe (mg g−1) is the amount of dye adsorbed at a time t and at a equilibrium time: k1 (min−1) and k2 (g mg−1 min−1) defines the rate constant of pseudo-first order and second-order models respectively.
The fitting of the experimental data in the above models showed that the adsorption of both CR and RR on PEI-BC surface follows a pseudo-second-order kinetic model with correlation coefficients 0.996 and 0.994, respectively. Also, the values of calculated qm values are in good agreement with experimental values (Table 1). In the literature, similar kinetic model fitting were reported for different pollutant-adsorbent systems [39,40,41].
Adsorption isotherm study
The adsorption isotherm analysis was carried out to study mechanism of dye adsorption by fitting of the experimental data using Langmuir model and Freundlich model (Fig. 7). Langmuir model assumes that the adsorbent is made up of homogenous surface with energetically equivalent adsorption sites which allows monolayer adsorption. Freundlich adsorption describes the multi-layered adsorption on heterogenous adsorbent surface [42]. The straight line equation for the models is expressed as given below.
Where, Ce (mg L−1) is the concentration of dye in a equilibrium solution, qe (mg g−1) equilibrium amount of dye on the adsorbent, kL (L mg−1) represents the Langmuir constant related to an adsorption energy, and qmax (mg g−1) is a maximum adsorption capacity of the PEI-BC corresponding to complete monolayer coverage, kf and 1/n are Freundlich constants related to adsorption capacity and adsorption intensity respectively.
The adsorption isotherm analysis for CR and RR was done by fitting of adsorption data in the above models. The different parameters evaluated in adsorption isotherm analysis are enlisted in Table 2. The adsorption data for both the dyes showed better fit with Langmuir adsorption isotherm model. With this model the correlation coefficients obtained were 0.99 and 0.993 for CR and RR respectively whereas predicted qmax values for CR and RR were 515.46 and 300.3 mg g−1 of adsorbent. The obtained adsorption isotherm results are consistent with the previous stuidies [43, 44]. A quick comparison among the different adsorbents for the adsorption of CR and RR is given in Table 3. These results indicated that PEI-BC based adsorbent is efficient for the removal of anionic dyes including high molecular weight bulky dyes such as reactive red 120.
Desorption and reusability
The ease of separation and recyclability of the adsorbent are the important properties that affects the operational cost of water treatment process. For the analysis of reusability of the adsorbent developed, PEI-BC was evaluated for its dye removal efficiency for four subsequent cycles. After completion of each cycle, the adsorbent was treated with 0.5 M NaOH and washed with deionised water. The regenerated adsorbent was reevaluated for its dye removal efficiency in the next cycle of experiment. The results obtained indicated that, the dye removal efficiency of the given adsorbent remain more than 90% even after 4 cycles of adsorption and desorption (Fig. 8). The slight decrease in the dye removal efficiency was observed that may be due to irreversible interaction of dye molecule with adsorbent. These results indicated that the PEI-BC adsorbent can be easily regenerated and had shown good reusability efficiency.
Bactericidal potential of PEI-BC adsorbent
The bactericidal potential of PEI-BC adsorbent was evaluated by plate count method in which the organisms were incubated with different adsorbent dosage for 3 h and the cell suspension was plated on LB agar. The study was performed using gram-negetive E. coli and gram-positive S. aureus organism. Bacterial cellulose (BC) was used as a control for the experiment. The results are depicted in the (Fig. 9A and B), represents inhibition of colony growth in PEI-BC treated plates compared to bacterial cellulose control. The graph of % non-viable cells using different adsorbent dosage highlights the strong bactericidal potential of PEI-BC based adsorbent towards both the organisms (Fig. 10A). The lowest adsorbent dosage i.e. 0.25 g/L could eliminate S. aureus and E.coli more than 99.9% and 94.5% respectively. However, the effective dosage of adsorbent was found to be 0.75 g/L that can eliminate both the organisms with more than 99.9% efficiency. Thus, the overall results indicates the excellent bactericidal potential of the adsorbent. The cationic PEI establish the electrostatic interaction between the bacteria and adsorbent that result into loss of membrane potential that further leads to damage of the membrane and leakage of the cell constituents [50, 51]. The SEM analysis of the bacterial cells treated with PEI-BC clearly indicated the damaged morphology of the cells as compared to control (Fig. 10B and C).
Conclusion
In conclusion, we have sucessfully engineered the BC material with cationic surface by grafting the polyethyleneimine (PEI) via chemical crosslinking. The developed material has been used as an adsorbent for removal of anionic dyes including congo red and high molecular weight azo dye reactive red 120. The adsorption process of both the dyes fitted well in pseudo-second order kinetic model. Langmuir adsorption isotherm best describes the adsorption data of both the dyes and the maximum adsorption capacity (qmax) obtained was 515.46 and 300.3 mg L−1 for CR (pH 6.0) and RR (pH 3.0) respectively. The bactericidal potential of PEI-BC adsorbent was also evaluated against E.coli and S.aureus. The given adsorbent demonstrated the efficient bactericidal activity against selected strains. Thereby, the BC based adsorbent with aim to remove contaminant from water was produced successfully. Future efforts should be done in direction of engineering versatile bacaterial nanocellulose to remove other contaminants such as cationic dyes as well.
References
Das SK, Khan MMR, Parandhaman T et al (2013) Nano-silica fabricated with silver nanoparticles: antifouling adsorbent for efficient dye removal, effective water disinfection and biofouling control. Nanoscale 5:5549–5560
Mallampati R, Xuanjun L, Adin A, Valiyaveettil S (2015) Fruit peels as efficient renewable adsorbents for removal of dissolved heavy metals and dyes from water. ACS Sustain Chem Eng 3:1117–1124
Ramalingam B, Khan MMR, Mondal B et al (2015) Facile synthesis of silver nanoparticles decorated magnetic-chitosan microsphere for efficient removal of dyes and microbial contaminants. ACS Sustain Chem Eng 3:2291–2302
Chowdhury A, Khan AA, Kumari S, Hussain S (2019) Superadsorbent ni-co-S/SDS nanocomposites for ultrahigh removal of cationic, anionic organic dyes and toxic metal ions: Kinetics, isotherm and adsorption mechanism. ACS Sustain Chem Eng 7:4165–4176
Ghorai S, Kumar A, Panda AB, Pal S (2013) Bioresource Technology Effective removal of Congo red dye from aqueous solution using modified xanthan gum / silica hybrid nanocomposite as adsorbent. Bioresour Technol 144:485–491
Kamal T, Anwar Y, Khan SB, Saeed Cahni MT, Asiri AM (2016) Dye adsorption and bactericidal properties of TiO2/Chitosan coating layer. Carbohydr Polymers 148:153–160
Tkaczyk A, Mitrowska K, Posyniak A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci Total Environ 717:137222
Rathi BS, Kumar PS, Show P-L (2020) A review on effective removal of emerging contaminants from aquatic systems: current trends and scope for further research. J Hazard Mater 409:124413
Wang L, Mao C, Sui N, Liu M, Yu WW (2017) Graphene oxide/ferroferric oxide/polyethylenimine nanocomposites for Congo red adsorption from water. Environ Technol 38:996–1004
Laureano-Anzaldo CM, Haro-Mares NB, Meza-Contreras JC et al (2019) Chemical modification of cellulose with zwitterion moieties used in the uptake of red Congo dye from aqueous media. Cellulose 26:9207–9227
Duan C, Meng X, Liu C, Lu W, Liu J, Wang W, Zhao W, Xiong C, Ni Y (2019) Carbohydrates-rich corncobs supported metal-organic frameworks as versatile biosorbents for dye removal and microbial inactivation. Carbohydr Polym 222:115042
Zhang S, Chen H, Zhang S, Kai C, Jiang M, Wany Q, Zhou Z (2019) Polyethylenimine grafted H 2 O 2 -oxidized cellulose membrane as a novel biosorbent for Cr (VI) adsorption and detoxification from aqueous solution. Cellulose 26:3437–3453
Salzano MD, Castaldo R, Altobelli R, Gioiella L, Filippone G, Gentile G, Ambrogi V (2017) Chitosan hydrogels embedding hyper-crosslinked polymer particles as reusable broad-spectrum adsorbents for dye removal. Carbohydr Polym 177:347–354
Zambare R, Song X, Bhuvana S, Antony Prince JS, Nemade P (2017) Ultrafast dye removal using ionic liquid-graphene oxide sponge. ACS Sustain Chem Eng 5:6026–6035
Mahfoudhi N, Boufi S (2017) Nanocellulose as a novel nanostructured adsorbent for environmental remediation: a review. Cellulose 24:1171–1197
Ayranci E, Duman O (2009) In-situ UV-visible spectroscopic study on the adsorption of some dyes onto activated carbon cloth. Sep Sci Technol 44:3735–3752
Duman O, Tunc S, Polat TG (2015) Adsorptive removal of triarylmethane dye (Basic Red 9) from aqueous solution by sepiolite as effective and low-cost adsorbent. Microporous Mesoporous Mater 210:176–184
Duman O, Tunç S, Polat TG (2015) Determination of adsorptive properties of expanded vermiculite for the removal of CI Basic Red 9 from aqueous solution: kinetic, isotherm and thermodynamic studies. Appl Clay Sci 109:22–32
Duman O, Tunç S, Polat TG, Bozoglan BK (2016) Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr Polym 147:79–88
Duman O, Tunç S, Bozoglan BK, Polat TG (2016) Removal of triphenylmethane and reactive azo dyes from aqueous solution by magnetic carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite. J Alloys Compd 687:370–383
Mohammed N, Grishkewich N, Tam KC (2018) Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ Sci Nano 5:623–658
Tang Y, Li M, Mu C, Zhou J, Shi B (2019) Ultrafast and efficient removal of anionic dyes from wastewater by polyethyleneimine-modified silica nanoparticles. Chemosphere 229:570–579
Trache D, Tarchoun AF, Derradji M, Hamidon TS, Masruchin N, Brosse N, Hussin MH (2020) Nanocellulose: From Fundamentals to Advanced Applications. Front Chem 8:392
Köse K, Mavlan M, Youngblood JP (2020) Applications and impact of nanocellulose based adsorbents. Cellulose 27:2967–2990
Ge H, Huang H, Xu M, Chen Q (2016) Cellulose / poly (ethylene imine) composites as efficient and reusable adsorbents for heavy metal ions. Cellulose 23:2527–2537
Urbina L, Guaresti O, Requies J, Gabilondo N, Eceiza A, Corcuera MA, Retegi A (2018) Design of reusable novel membranes based on bacterial cellulose and chitosan for the filtration of copper in wastewaters. Carbohydr Polym 193:362–372
Lungu CN, Diudea MV, Putz MV, Grudziński IP (2016) Linear and branched PEIs (Polyethylenimines) and their property space. Int J Mol Sci 17:555
Quan X, Sun Z, Meng H, Han Y, Wu J, Xu J, Xu Y, Zhang X (2019) Polyethyleneimine (PEI) incorporated Cu-BTC composites: Extended applications in ultra-high efficient removal of congo red. J Solid State Chem 270:231–241
Foo NHYKY, Hameed LDWBH, Hussin MH (2019) Microwave - assisted synthesis of polyethyleneimine grafted chitosan beads for the adsorption of acid red 27. J Polym Environ 28:542–552
Thorat MN, Dastager SG (2018) High yield production of cellulose by a: Komagataeibacter rhaeticus PG2 strain isolated from pomegranate as a new host. RSC Adv 8:29797–29805
Ma J, Wang C, Wei Y (2016) RSC Advances for the adsorption and enrichment of. RSC Adv 6:43648–43655
Wong S, Abd Ghafar N, Ngadi N, Razmi FA, Inuwa IM, Mat R, Amin NAS (2020) Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci Rep 10:1–13
Castro C, Zuluaga R, Putaux JL, Caro G, Mondragon I, Ganan P (2011) Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr Polym 84:96–102
Keshk SMAS, Haija MA (2011) A new method for producing microcrystalline cellulose from Gluconacetobacter xylinus and kenaf. Carbohydr Polym 84:1301–1305
Feng X, Ullah N, Wang X, Sun X, Li C, Chen L, Li Z (2015) Characterization of Bacterial Cellulose by Gluconacetobacter hansenii CGMCC 3917. J Food Sci 80:E2217–E2227
Chen X, Xu X, Li W, Sun B, Yan J, Chen C, Liu J, Qian J, Sun D (2018) Effective drug carrier based on polyethylenimine-functionalized bacterial cellulose with controllable release properties. ACS Appl Bio Mater 1:42–50
Zhao J, Li Q, Zhang X, Xioa M, Zhang W, Lu C (2017) Grafting of polyethylenimine onto cellulose nanofibers for interfacial enhancement in their epoxy nanocomposites. Carbohydr Polym 157:1419–1425
Long Y, Xiao L, Cao Q (2017) Co-polymerization of catechol and polyethylenimine on magnetic nanoparticles for efficient selective removal of anionic dyes from water. Powder Technol 310:24–34
Duman O, Ayranci E (2010) Adsorptive removal of cationic surfactants from aqueous solutions onto high-area activated carbon cloth monitored by in situ UV spectroscopy. J Hazard Mater 174:359–367
Ayranci E, Duman O (2010) Structural effects on the interactions of benzene and naphthalene sulfonates with activated carbon cloth during adsorption from aqueous solutions. Chem Eng J 156:70–76
Ayranci E, Duman O (2007) Removal of anionic surfactants from aqueous solutions by adsorption onto high area activated carbon cloth studied by in situ UV spectroscopy. J Hazard Mater 148:75–82
Litefti K, Freire MS, Stitou M, González-Álvarez J (2019) Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci Rep 9:1–11
Duman O, Ozcan C, Polat TG, Tunc S (2019) Carbon nanotube-based magnetic and non-magnetic adsorbents for the high-efficiency removal of diquat dibromide herbicide from water: OMWCNT, OMWCNT-Fe3O4 and OMWCNT-κ-carrageenan-Fe3O4 nanocomposites. Environ Pollut 244:723–732
Duman O, Polat TG, Diker CO, Tunc S (2020) Agar/κ-carrageenan composite hydrogel adsorbent for the removal of Methylene Blue from water. Int J Biol Macromol 160:823–835
Giano MC, Ibrahim Z, Medina SH, Sarhane KA, Christensen JM, Yamada Y, Brandacher G, Schneider JP (2014) Injectable bioadhesive hydrogels with innate antibacterial properties. Nat Commun 5:4095
Qiu WZ, Zhao ZS, Du Y, Hu M-X, Xu ZK (2017) Antimicrobial membrane surfaces via efficient polyethyleneimine immobilization and cationization. Appl Surf Sci 426:972–979
Etemadinia T, Barikbin B, Allahresani A (2019) Removal of Congo red dye from aqueous solutions using znfe2o4/sio2/Tragacanth gum magnetic nanocomposite as a novel adsorbent. Surfaces and Interfaces 14:117–126
Madan S, Shaw R, Tiwari S, Tiwari SK (2019) Adsorption dynamics of Congo red dye removal using ZnO functionalized high silica zeolitic particles. Appl Surf Sci 487:907–917
Jawad AH, Mubarak NSA, Abdulhameed AS (2020) Hybrid crosslinked chitosan-epichlorohydrin/TiO 2 nanocomposite for reactive red 120 dye adsorption: kinetic, isotherm, thermodynamic, and mechanism study. J Polym Environ 28:624–637
Mubarak NSA, Jawad AH, Nawawi WI (2017) Equilibrium, kinetic and thermodynamic studies of Reactive Red 120 dye adsorption by chitosan beads from aqueous solution. Energy Ecol Environ 2:85–93
Jawad AH, Abdulhameed AS, Reghioua A, Yaseen ZM (2020) Zwitterion composite chitosan-epichlorohydrin/zeolite for adsorption of methylene blue and reactive red 120 dyes. Int J Biol Macromol 163:756–765
Acknowledgements
Meghana Thorat is thankful to the University Grant Commission (UGC) for a Senior Research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thorat, M.N., Jagtap, A. & Dastager, S.G. Fabrication of bacterial nanocellulose/polyethyleneimine (PEI-BC) based cationic adsorbent for efficient removal of anionic dyes. J Polym Res 28, 354 (2021). https://doi.org/10.1007/s10965-021-02702-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02702-y