Abstract
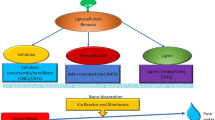
The present review describes the application of lignocellulosic biomass-derived nanocellulose for wastewater remediation with a focus on the removal of heavy metals. Nanocellulose and its nanocomposite are among the emerging materials of this century, with an abundance of application in the versatile field of composites, medicines, functional additives, and water treatment. Water treatment has received attention from the commercial and academic sector, with a large emphasis on one of the biggest problems faced by humans in the 21st century i.e., clean potable water. There are various sources of water pollution including heavy metal toxifications. The applications of cellulose and its various composites for heavy metal removal for wastewater treatment have been elaborated on in this review. Several biosorbent based on nanocellulose such as aerogels, hydrogels, ion-exchange beds, flocculants, and photocatalyst to remove heavy metal toxicity have been discussed furthermore. Research work on the effect of composites of carbon nanotube, functionalized cellulose has been covered too.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing Water Pollution by Industrial Pollutants
Water pollution has become a burning problem for advanced and emerging economies in the 21st century. The worldwide water consumption is increasing day by day for the growing demands of human habitation, agricultural irrigation, and other necessities. Recent studies reported that the world's water consumption has risen 8-fold over the last 100 years. Since several agricultural, residential, and industrial activities, wastewater effluent is released into the environment. These activities lead to an escape of a wide range of contaminants into the water resources as per their origin. Chemical pollution, notably from many heavy metals such as arsenic, lead, cadmium, etc., is the most conscientious part of water pollution because it is intended to affect population wellbeing [1]. Groundwater is one of India’s most important water resources accounting for about 63% of irrigation water and around 80% of domestic and urban water supplies. Over the past seven years, 54% of India’s groundwater wells have been deteriorated and 21 major cities are being projected to run out of groundwater by 2020. So, our goal is to review the application of lignocellulosic biomass-derived nanocellulose in water treatment applications [2].
Analogously, industrial activities have been producing the greatest volume and the maximum variety of pollutants day by day. Thus, the generated effluents are the utmost contributors to various water pollution problems. Over 50,000 synthetic materials are produced or processed for multiple applications across the globe and approximately 2,000 new compounds are launched onto the marketplace annually. These industrial wastes encompass a series of chemicals that can be classified as organic and inorganic, synthetic or compostable, polar or non-polar, hydrophilic or hydrophobic, inert or reactive, poisonous or non-toxic, etc. These wastes contaminated the water sources harming human and environmental health. Pollutants can reach water resources present on the surface or underground through the emission of gaseous, liquid, and solid components from industries, or during its transportation and disposing of utilized products, from households and agricultural production. Runoffs, wastewater can also hold numerous pollutants that cause negative environmental impacts. Thus, wastewater effluent should be treated well and systematically to lower the adverse health effect of the user of surface water resources as well as the aquatic ecosystem [3].
There are innumerable conventional methods for removing toxic metal ions like chemical precipitation, electrochemical treatment, membrane filtration, adsorption, reverse osmosis, ion exchange, flotation, coagulation/flocculation, photocatalysis, and separation membrane technologies. Although metal ion concentrations in aqueous solutions are low, the chemical precipitation and electrochemical treatment are proved to be useless and because of their low efficiency and expensive setup. They generate a large quantity of waste sludge and require further treatment of the sludge. Both membrane technologies and ion exchange techniques are much expensive due to their high efficiency in the selectivity of ions, but the ion exchange technique is in persistent use due to its reusability. Adsorption technology is also found as efficient and prevalent, due to the exceedingly small concentrations of metal accumulation and its economically practical characteristics. Therefore, if low-cost adsorbents or biosorption technology can perform well in removing heavy metals, they can be adopted and widely used to minimize operational cost and for improving profitability [4].
The use of nanomaterials has taken an advantage to adsorb heavy metals in water because of their exceptional properties. Previously, these nanomaterials were mainly based on carbon, metal oxide materials, zero-valent metal nanomaterials, and nanocomposites. They showed efficient adsorption of pollutants; however, they are highly unstable, thus reducing their removal efficiency. Furthermore, it is quite difficult to separate these nanomaterials from the aqueous medium at full tilt due to their nanoscale size. Since, many studies confirmed the adsorption by these nanomaterials, hence its utilization should be taken into consideration. Nanostructured materials deliver greater adsorption abilities and stronger binding than macroscale counterparts [5]. Recently, the naturally produced nanomaterial- nanocellulose from lignocellulosic biomass have been used for metal detoxifications in wastewater treatment. The use of nanocellulose for water remediation has emerged as an attractive choice among the diverse application of nanocellulose [6, 7].
In this review, comprehensive information on cellulose-derived nanocellulose, and its applications in the aspect of water remediation have been discussed. The role of composites based on nanocellulose in reducing cytotoxic effects from metal ions, such as lead, arsenic, cadmium, mercury, and so forth, are reviewed here. Besides this, the effect of heavy metals on the environment and the human population has been studied, and the use of bio adsorbent has been discussed to mitigate the problem of heavy metals contaminants. Moreover, the potential of cellulosic adsorbent materials for various heavy metal uptake abilities has been reported here.
Lignocellulosic Biomass
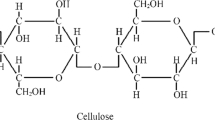
The dry matter of plants (biomass) is referred to as lignocellulosic biomass. Lignocellulosic biomass is the most bountiful raw material on the earth. The plant cells are composed of two carbohydrate polymers (cellulose, hemicellulose), and an aromatic polymer (lignin) (Fig. 1). However, depending on the type of plant, the composition and concentration of these plant cell components vary. Lignin and hemicellulose represent 10–25% and 20–35% of the total dry weight of dry lignocellulosic biomass respectively [2]). Whereas cellulose is the major element in lignocellulosic biomass which is primarily found in the plant cell wall with its total concentration of about 35–50% of dry lignocellulosic biomass. It comprises of the linear homopolysaccharide of β-1, 4- linked anhydro-d-glucose units with the repeating unit of cellobiose (2, 8).

Adapted from the permission with Zhang et al. [18]
Schematic representation of lignocellulosic biomass with the structure of cellulose, hemicellulose, and lignin.
The cellobiose monomer is comprised of three hydroxyl groups forming a strong hydrogen bond with the adjoining glucose unit by intramolecular and intermolecular hydrogen bonding networks (Fig. 2). This hydrogen bonding leads to strong and tight packing in the crystalline parts of cellulose fibrils. This enhances the tensile and mechanical strength of the polymeric fibre and makes it insoluble in water, many acids, alkalis. In cellulose, the alignment of glucose molecules and hydrogen bonding networks is broadly oriented, leading to various cellulose allomorphs (cellulose I and II). The variation of cellulose allomorphs (cellulose I and II) depends on the origin of lignocellulosic biomass and the processing method. By its abundant carbon, hydroxyl groups, glucose monomeric units, cellulose has been found as the most naturally occurring substance of carbon properties, with different hydroxyl groups and solid hydrogen-bonding networks. Nevertheless, cellulose fibrils aggregate to form crystalline and amorphous structures for greater rigidity and strength. Wood and crop wastes have immense potential as energy or animal feed for the production of high-value-added materials without vying with a man and livestock [8].

Adapted from the permission with Phanthong et al. [8]
Networks of intramolecular (−) and intermolecular (−) hydrogen bonding in cellulose structure.
Nanocellulose and its Applications
The nanocellulose is the naturally occurring biomaterial derived from cellulose. In general, nanocellulose fibre comprises polymeric fibres of less than 100 nm in diameter and several micrometers in length. The nanocellulosic fibres are comprised of 3–100 µm in diameter with a length of 1–4 µm in size. They are comprised of desirable properties such as high tensile, mechanical, and thermal strength. They are biologically degradable nanofibre with a low density of 1.6 gm/ cc and with exceptional strength properties and has high mechanical strength of up to 220 GPA which is significantly higher than Kevlar fibre. Nanocellulose also owns other physical properties such as high tensile strength of up to 10 GPA, higher thermal stability with lightweight and, transparency. Nanocellulose fibre is transparent and because of the presence of reactive surface of hydroxyl groups, it becomes functional by various alterations for surface functionalization and surface modification [2, 8, 9]
Presently, nanocellulose has been explored as a good biosorbent. Before nanocellulose, various biomaterials have been studied including non-living biomass (such as chitosan, xylan-rich hemicellulose, longan shell, soy protein, potato peels, tea wastes, almond shell, lignin, coconut husk fibres, sawdust), algal biomass, and microbial biomass (such as bacteria, fungi, and yeast) for their potential biosorbent properties for the extraction of metal ions from wastewater. But nanocellulose, its derivatives, and composites have shown magnificent results in the case of biosorption properties for metal removal from effluent. This is due to its nano-dimension, large surface area, higher mechanical strength, thermal stability, and biocompatibility as compared to other biomaterials. Another advantage of nanocellulose is that it is a green biomaterial, which is abundant in nature as compared to animal-derived chitosan [10]. For these reasons, nanocellulose is found better than all biomaterial available [11]. The mechanical and physical properties of synthetic materials have been evaluated by incorporating soybean-derived nanocellulose to three sorts of new polymers and found that the flexural modulus and stiffness of the nanocellulose-reinforced polymer were substantially improved compared to the pure polymer matrices [12].
Scientific investigation proved that the use of nanocellulose strengthened the contact between matrix fillers when reinforced with poly (lactic acid) fibre to structure the new polymer composites. The thermal properties, as well as crystallization, were also strengthened [8, 13]. With the highly charged property of dicarboxylate chains, when electrosterically stabilized nanocrystalline cellulose (ENCC) was developed, it eliminated copper approximately 185 mg/g, which would be beneficial in wastewater elimination of toxic metals [14]. Nanocellulose may also be applied in other areas, also with the above uses. For reference, it can be used as a thickening agent for textured natural food colorant preparation as a specific textile filler, as a compostable kit, as a CO2 adsorbent, and as an oil recovery [14, 15].
Toxicity of Heavy Metals
Heavy metals exist naturally on the earth that have approximately five times higher atomic weight and density than water. There are numerous industrial, household, agrarian, pharmaceutical, and economic implementations that have been resulted in various range distribution of heavy metals, citing fears about their possible impact on public health and the environment. There are some water pollutants, their sources, and their harmful effects are described in Table 1. Most heavy metals including arsenic, mercury, lead, copper, cadmium, chromium, zinc, and nickel are generally associated with water pollution and various toxic issues, especially when they available in dissolved form. The presence of any of these heavy metals at an extreme level is dangerous to people, the environment, and other life forms.
The potency of heavy metals relies on various factors. These factors include the dosage, exposure path, and chemical species and age of the affected individual, gender, genomes, and dietary status. Heavy metals are highly soluble in aquatic environments and get easily absorbed by living organisms. Arsenic, cadmium, chromium, lead, and mercury are highly toxic and hazardous heavy metals. Such metallic elements are considered to be cumulative toxicants and cause health issues even at lower occupational exposure. As per the US Environmental Protection, and International Cancer Research Agency, these heavy metals are also listed as carcinogenic to human beings [16]. Non-biodegradability and bioaccumulation of these hazardous metal ions can cause various adverse health and environmental issues. The source of heavy metals varies as per the geographical region of the globe- depending upon the industrial development, respective government policies on pollution control of that region. The major sources of heavy metal pollutants are mining industries, metal alloy industries, paint and dye industries, leather industries, industries involved in electro-plating activities, fertilizer manufacturing industries, cement industries, and so on. These heavy metals even at low concentrations inversely affect highly to aquatic environments and also lead to natural degradation that is dangerous to all of us. Henceforth, nowadays researchers are highly focused on the heavy metal removal from the waste effluent [17].
Biosorbents for Heavy Metal Removal from Contaminated Water
To effectively extract heavy metals, several appropriate recovery methods have been applied, which have already been discussed above. Amid them, biosorption draws keen interest for eliminating minor quantities of hazardous heavy metal contamination. Biosorption is a natural physiochemical phenomenon that refers to the ability of biological materials to accumulate heavy metal ions from wastewater and the biomaterial used for biosorption is known as biosorbent. The binding of a chemical compound to a sorbent is a fundamental concept of adsorption [18]. In terms of equipment and substances used, biosorption is flexible and responsive. The components may be, pristine or mixed, non-renewable or renewable, organic or inorganic, etc. however, in many other countries, biomass and biomaterials make up a major chunk of trash. They have an immense application for electricity generation, manufacturing products, and treating waste. Hence, there is a need for many studies to investigate the feasibility of such biomaterials for specific applications [19].
A major issue is the identification of a suitable biosorbent that can bind and uptake the metal ions with greater affinities. Mostly natural materials that are abundant in nature may be used for the removal of contaminants from water resources. Besides other plants, microbial and animal biomass and industrial by-products can be developed as biosorbents [20]. Hence selection of these biosorbents from a large broad spectrum of available materials is critical. High affinity for metals (biosorption capacity), easy desorption of the absorbed metal ions, availability in large quantities, and low economic values (low cost) along with multiple uses of biosorbent are some of the desired characteristics of an ideal adsorbent. Biomaterials hold a high capacity for environmentally sustainable wastewater treatment methods including such adsorption, according to several research studies reviewed here [21]. Agricultural by-products are commonly utilized both with and without modification for the adsorption. Their natural adsorption capabilities can sometimes be indeed very dependable without alteration. Moreover, biosorption shows many advantages over traditional methods as follows:
-
Biosorption is a highly lucrative and feasible process.
-
It presents a great opportunity in the recycling of biosorbent and metal recovery from effluent.
-
It shows a coherent recovery of metals even at low concentrations.
-
It does not produce secondary sludge.
-
The low-cost agricultural and industrial waste biosorbent shows greater efficiency in the removal of heavy metals from an aqueous medium [22].
Innovative biosorbents that can deliver heavy metal recovery are still being studied. It is relatively inexpensive as well as requires minimum waste management resulting from the absorption mechanism. As compared to other iron oxide particles, starch and carboxymethyl cellulose have been assessed as excellent stabilizers for the preparation of magnetic particles and hence absorbed arsenic more effectively [23].
Nanocellulose, which provides a combination of biosorption, nano dimensions, and unique cellulosic nature, has enormous potential to solve the current problems of heavy metal pollution. It is expected that a high specific area of nanocellulose will provide a large number of active sites on the surface of the biosorbent to immobilize metal ions. Regardless of microscale and nanoscale, surface functional groups acting as metal-binding sites on the biomass are known to be responsible for the immobilization of heavy metal ions. Moreover, the abundance of a hydroxyl group on the surface of nanocellulose offers a unique medium for surface modification onto the surface of the cellulosic structure. Nanocellulose has strong mechanical strength and rigidity that make them useful in actual water treatment applications, especially in a high-pressure environment. In an aqueous environment, hydrophilicity, and stability of nanocellulose are beneficial to reduce biofouling [24]. Besides, nanocellulose generally has high crystallinity, particularly for CNC, which makes them resistant to chemical and biological corrosion in an aqueous environment. However, some problems like agglomeration, immobilization, cost-effectiveness, and long-term performance create difficulties in nanocellulose water purification. Some operational difficulties arose if adsorbents are being used with agricultural by-products. These involve low adsorption capacity in the original form of the adsorbent. These could be surmounting by reconfiguring the adsorbent mechanically or chemically [21].
Nanocellulose Biosorbent
Polluted wastewater often consists of a variety of toxic elements, namely heavy metal ions, such as chromium, lead and arsenic, dyes, organic solvents, pesticides, herbicides, and so forth. Unless the wastewater is not well processed before release, such polluted chemical compounds can spill into the groundwater to develop serious diseases. Treating wastewater techniques like sand, precipitation, reverse osmosis, adsorptive filtration by ion exchange resins, active alumina, and iron oxide can only eliminate some of the heavy metals. There are different methods for water remediation such as membrane separation, chemical oxidation, electro-precipitation, electro-dialysis, liquid extraction, electrochemical treatment, membrane technologies, and adsorption on activated carbons.
However, a major problem with these methods is the incomplete precipitation and the formation of large volumes of sludge that can be difficult to filter. Another restriction of their applications is the prohibitive cost especially for activated carbons that suffer from material losses during regeneration. The key issue with some of these approaches production of significant amounts of effluent, which can be hard to sort. The use of biomass in water treatment and pollution control is currently gaining a great deal of attention. Nevertheless, untreated biomass is usually not usable, and the adsorption potential differs based on the composition of biomass. Consequently, surface modification is a vital step in nanocellulose-based adsorbent materials to facilitate the adsorption of a particular class of contaminants and improve adsorption ability [25].
Bio-based nanoparticles have become the focus of increasing interest due to the characteristics of these nanoparticles, particularly their non-hazardous quality, degradability, and substrate reactivity. Nanocellulose is by far the most studied of such materials, and therefore its processing has achieved a massive level [26]. Even though the utility of nanocellulose as a modern category of nanostructured adsorbent has recently obtained a great deal of interest. Nanocellulose incorporates essential cellulose properties, like hydrophilicity, crystalline character, broad chemical modification potential, and high surface area [27, 28].
Aerogels Adsorbents from Nanocellulose
Aerogels are emergence of a new type of nanoscale mesoporous materials with such an open structure with high porosity (between 95 and 99%), low density as well as a high surface area. Aerogel materials are formulated by replacing the liquid solvent in the gel with air without significantly changing the composition of the network or the volume of the gel. This form provides the benefit of fast recovery of the adsorbent by quick filtration with the probability of regeneration with no chance of agglomeration [25]. In particular, the advancing nanocellulose-based aerogels produced by the aqueous solution are flexible and less fragile compared to inorganic aerogels. Such nanocellulose aerogels have potential application in oil adsorption since they are low-cost, reusable, environmentally friendly, and have a high adsorption ability [28].
Aerogels could cross-specific surface areas from 60 to 350 m2g−1 acquired based on the heating process being used and thickness of the cellulose fibrils used. Cellulose aerogels are incredibly versatile and have excellent mechanical strength. They show ductility and can be compressed to large strains of even more than 80%. Their compression strength is between 100 and 300 kPa and 40–200 kPa, relying on their density. The aerogel density and porosity depend on the initial nanocellulose concentration and can be theoretically predicted [25].
Hydrogels Adsorbents from Nanocellulose
Hydrogels are heterogeneous mixtures of two or more phases. The dispersed phase is water, and the solid phase is a solid three-dimensional network. They consist of a 3-D polymer network filled with water. The formation of hydrogels mainly depends on hydrogen bonds, electrostatic interactions, covalent and, van der Waals forces between a cross-linking agent (multifunctional monomer) and the polymer chains. Hydrogels can be synthesized from synthetic polymers or natural polymers such as cellulose, chitosan, and starch. Biodegradable polymers have gained much attention in the last decades due to the increase of public pressure on environmental issues [29].
To make these polymers' hydrogels more strengthened as compared with other materials, the incorporation of reinforcing agents such as nanocellulose has been studied to improve their properties. For instance, Fiorati et al. [30], synthesized a stable tunable hydrogel with TEMPO-oxidized CNF by incorporating calcium ions. These hydrogels were proved to be suitable controlled release systems by measuring the diffusion coefficient of a drug model (ibuprofen, IB). Cellulose hydrogels can be classified as "green" nanocomposite materials because of their renewable and biodegradable design. The production of these "green" materials combines and creates new and attractive properties that can be explored in the fields of biomedicine, food, agriculture, etc. [29]. Maestri et al. [31] developed a cellulose-chitosan hydrogel for oral delivery of macromolecular therapeutics to the intestinal tract. The CNC provided mechanical support to the drug delivery vehicle.
Ion Exchange Mechanism
An ion-exchange mechanism is one of the ways to reduce heavy metal toxicity where the adsorbing metal ions compete with other metal ions already associated with the sorbent surface. However, the maximum adsorption capacity is limited by stoichiometry rules and cannot exceed half the content of the surface ionic site. For this reason, surface modification is necessary to increase or introduce ionizable or complexing sites such as carboxylic sulfate and amine groups on the surface of the nanocellulose on which the metal is adsorbed to enhance the adsorption capacity. The surface modification of CNC with phosphate groups leads to enhanced adsorption of silver, copper, and ferric ions as compared with the native one. The removal efficiency was mainly because of specific surface area, nature, and density of functional groups on the nanocellulose surface [25].
Nanocellulose-Based Flocculant
Biopolymer-based flocculants such as chitosan, cellulose, and alginate are nowadays becoming attractive due to a high specific surface area. An effective flocculant generally contains the right amount of (–OH) groups on its surface [32]. Usually, modification of nanocellulose to create effective flocculants is accomplished by adding anionic, cationic, or hydrophobic functional groups to the nanocellulose surfaces. The (–OH) groups on the cellulose surface facilitate the facile fabrication of nanocellulose to incorporate the necessary functions and develop high-performance flocculants. The association of cellulose with different chemicals may be improved by the establishment of innovative functional groups to maximize surface polarity as well as hydrophilicity [32, 33].
Nanocellulose-Based Photocatalysts
Cellulose individually possesses minimal photocatalytic behaviour under Ultraviolet or visible light irradiation, but many semiconductor materials being applied to intensify photocatalytic activity. Many other scientific researchers have proved photocatalytic water purification using cellulose-based metal oxide nanostructures in the shape of a thin layer, membrane, fibre as well as composite materials under solar illumination. Nanocellulose composites with a metal oxide such as titanium oxide, zinc oxide, and graphene oxide can be used as photocatalysts to accelerate the amount of oxidation of organic compounds individual's specific products.
Cellulose derivatives would effectively capture metal oxide substrate surface and have additional hydroxyl present on the surface of metal oxides. Here as consequence, the surface area of such a hybrid model is anticipated to accelerate and expand the wavelength sensitivity to the visible spectrum. Bacterial cellulose demonstrates the potential to function as a support for nanoparticle metal oxide. It does have a high specific surface area with a fibre diameter of 40–70 nm, high strength, and wettability characteristics. Due to the well-separated nano and microfibrils, the incredibly 3-D network provides bacterial cellulose with such a highly precise surface area. Besides, the existence of hydroxyl binding sites, as well as a fibrous framework, can promote the biosorption of metal oxide to its surface. An et al. reported that nano-fibrillated cellulose/magnetite/titanium dioxide nanocomposites have a better generation rate of photocatalytic hydrogen than the nano-fibrillated cellulose/titanium dioxide specimen [32, 33].
Nanocellulose-Based Membranes
Membranes and filters may be used to separate various chemicals and metal ions. The selectivity of a membrane is linked to the membrane material's microstructure and interfacial chemistry (Fig. 3) [34]. Cellulose nanofibres and nanocrystals have been studied by many researchers for wastewater treatment and removal of heavy metals [10, 32]. The membranes can be engineered with some well-defined pores for molecular diffusion and designed surface chemistry to adsorb different solutes selectively focused on the size of pores. Membrane adsorption as a substitute is based on a porous membrane filtration method that enables the convective flow of solution through the pores with maximum outturn. Several multiple types of membranes have been used to build adsorptive membrane beds such as cellulose acetate, thin polyamide composites, and sulfonated polysulfone [35].

Adapted from the permission with Voisin et al. [34]
Schematic diagram to represent the schematic diagram for different types of membrane filter.
Another type is a nonwoven membrane bed made by a wet-laid procedure which is usually mostly used for filter paper production. Such a bed consists of non-woven fibres that are arranged arbitrarily to give the shape of entwined pores. Besides adsorption, that very framework enables a large flow rate as well as a rapid mass transfer due to the increased surface area and permeability. In terms of thickness, porosity as well as pore size, proper assessment of the wet-laid procedure, would also facilitate the creation of a consistent membrane bed. Nevertheless, hardly a few earlier types of research have revealed heavy metal filter-bed adsorption using certain non-woven membranes to date. The challenge must have been to implement appropriate fibres for adsorption and usage of bed effectively for high loading, let alone the single-pass flow which can often be needed in the treatment of polluted water [35].
On the other hand, filter papers integrated with adsorbents including activated carbon aren't exceptional in air filtration of volatile organic compounds (VOC) inside a room, or the odor removal dependent on circulating multi-pass flow of air that is much easier to remove the contaminants. After incorporating Ca2+, an increasing coexisting ion, the breakthrough gradient of Pb2+ was investigated. It was ended up finding that Ca2+ quickly dropped via the bed with no adsorption and with no spectacular impact on the formation of the Pb2+ breakthrough curve, signifying the high selectivity of the bed for Pb2+ over Ca2+. Earlier research also revealed that in situ TiO2/CF fibre has not supported the adsorption of several other co-ions including certain copper, nickel, zinc, magnesium, barium and, silver [36]. However, the literature documented heavy metal adsorption in batch as well as the fixed-bed reactor using NPs, and the constant process fixed-bed column is sometimes preferred. A fixed-bed configuration has still two major disadvantages: a massive drop in pressure and a sluggish transfer of mass. Further, the operational flow rate is restricted by the diminutive diameter of NPs that need to be overcome [37].
Surface Modification of Nanocellulose for Wastewater Remediation
Nanoparticles (NPs) have drawn extensive attention in multiple areas such as wastewater treatment besides their potential applications. Compared to conventional particles, NPs possess a high surface area enhanced sites of surface activation, and a great affinity to detach target species. Metal oxide NPs do have a remarkably promising possibility for adsorption both for organic and inorganic contaminants, proven to be effective adsorbents for eliminating toxic contaminants from the marine environment. Besides real application areas, NPs self-agglomerate and then its significant fraction of the anticipated surface area get lost. Regardless of their eventual loss/training in static or dynamic adsorption processes, these may also induce severe environmental contamination. The toxicity of NPs and their harm to the ecosystem have also been mentioned elsewhere, so right means of using them are extremely desirable [38]. The combination of two or more polymers has now become an important and on-going approach for analysing metal contaminants in wastewater, which often exhibit properties which an individual polymer could not achieve.
In this review, biopolymer-based NPs, their derivatives, and composites for biosorption of toxic metals from wastewater have been emphasized. It is well known that the adsorption efficiency of these biopolymers-based NPs is not satisfactory in pure form; therefore, to improve the chemical and mechanical properties of these biosorbents, they are to be chemically modified. Modification of these biopolymers is necessary to increase the adsorption capacities and their applicability in different mediums.
The mechanism of biosorption is a complex process that involves the binding of sorbate onto the biosorbent. Unmodified nanocellulose has a low heavy metal adsorption capacity as well as variable physical stability. Therefore, chemical modification of nanocellulose can be carried out to achieve adequate structural durability and efficient adsorption capacity for heavy metal ions (as shown in Table 1) [39].
Modified nanocellulose can be used as biosorbents which involve the binding of metal ions by physical (electrostatic interaction or van der Waals forces) or chemical (displacement of either bound metal cations (ion exchange) or protons) binding, chelation, reduction, precipitation, and complexation. Biosorbents contain chemical/functional groups like amine, amide, imidazole, thioether, sulfonate, carboxyl, sulfhydryl, carboxyl, phosphodiester, phenolic, imine, and phosphate groups that can attract and sequester metal ions. The key factors that control the formation of metal-binding biocomposites include: -
-
The chemical, stereochemical, and coordination characteristics of metal ions like molecular weight, ionic radius, and oxidation state of the targeted metal species.
-
Properties of the biosorbent, such as the structure and nature (in case of microorganism—living/non-living);
-
type of the binding site (biological ligand)
-
the process parameters like pH, temperature, the concentration of sorbate and sorbent, and other competing metal ions; and
-
availability of the binding sites.
The presence of a large number of hydroxyl groups on the surface of cellulose provides a platform to get modified with different functional groups such as carboxyl, sulfate, aldehyde, amino, phosphate, and thiol groups for different features. There are many methods for surface modification such as mechanical refining, enzymatic hydrolysis, hydrolysis using hydrochloric acid, and TEMPO oxidation (Fig. 4) [32]. The additional groups that are added can be traced by elemental analysis and by X-Ray photoelectron spectroscopy and can be characterized by FTIR and NMR [53].
Cellulose-Based Adsorption Materials
As discussed earlier various biopolymers have been used as effectual biosorbents in the removal of metal contaminants from wastewater. Among them, cellulose is an efficient natural and ample material that has been played a pivotal role in sewage treatment from a multitude of sources. Cellulose-based products are workable in industrialized nations for treating wastewater since they originate from waste sources. Finding has also shown that cellulose can be chemically altered or modified with various materials for the achievement of better results to disposal of waste materials [54].
Much as hydroxyl groups (−OH) on cellulose are less reactive, and the chemical modification takes place first at the primary hydroxyl found on carbon 6 (C6), which may occur through different methods. Moreover, modifications can also occur on the secondary hydroxyl groups (−OH) present on carbons 2 and 3. The significant modifications of cellulose occur through halogenation, oxidation, etherification, and esterification, etc. These modifications can offer improvement to the cellulose biopolymer's capacity for adsorption. For this reason, cellulose has excellent binding properties to heavy metals. Yet more research is needed to set up strategies to use cellulose-based materials for the removal of heavy metals from sewage on a large scale [55, 56].
Numerous plant and animal-based biomaterials that constitute cellulose are being used to adsorb heavy metals. Many of these are leftover materials. Bagasse becomes the very compound that provides cellulose of 50%. It could be used to adsorb metal ions from industrial effluents in native and immobilized forms. Zhou et al. [57], utilized Pb (II) adsorption with such a cellulose-based fibre. Despite its cellulosic structure (45–50%), other waste products via wood processing, wood sawdust, can also be used for adsorption. A simple and reliable pathway studied for the processing of altered cellulose fibre (Cell MW-HPEI) hyperbranched polyethyleneimine (HPEI) as an efficient adsorbent for removing inorganic arsenic. The findings showed that the experimental results well matched the Langmuir and pseudo-second-order models [58].
In one of the studies, waste cellulosic materials like orange juice residues were chemically modified and iron-loaded to form gels. The iron loading capacity of orange waste was higher than that of pure cellulose, leading to higher adsorption and hence the removal of heavy metal ions [12]. These are all often overlooked biomaterials and though can be used to increase the adsorption process. A cellulosic adsorbent prepared by halogenation of microcrystalline cellulose, which is followed by the functionalization with pyridone diacid for the removal of Pb (II) and Co (II) from aqueous solutions. The content of carboxyl groups in this cellulosic adsorbent was responsible for the high adsorption toward metal ions [45].
In a study, carboxy cellulose nanofibre was found to be an effective medium to remove Cd2+ ions from water, which was prepared from untreated Australian spinifex grass by the nitro-oxidation method. A large concentration of Cd2+ ions was removed by a low concentration of nanofibre suspension in less than 5 min [43]. Recently Bhabha Atomic Research Centre developed a unique cellulose-based water purifier via radiation-induced graft polymerization process, which is simple and efficient for removal of arsenic, chromium, fluorides, dyes, etc. from drinking water both at domestic and community scale. It also regenerates the cartridge by eluting the adsorbed contaminant to reuse the system. The removal of Hg (II) ions was conducted by green cellulose adsorbent (Cell-DMA) bearing N, N—dimethyl benzalaniline chelating group. Thermodynamic studies of cell-DMA showed that the adsorption process is workable and exothermic [59].
From literature, it was found that most of the adsorption studies are limited to only batch-scale and are not fully developed at the pilot and industrial scales for the treatment of industrial effluents that require more investigation for the selectivity of adsorbents. Upon chemical modification, cellulose-based adsorbents can lead to the high adsorption capacity of these adsorbents. The increase in active binding sites upon modifications of cellulose-based adsorbents may result in the addition of new functional groups that can be considered in the higher uptake of pollutants [35, 54]. Here are some modified and unmodified nanocellulose adsorbents are listed in Table 2 [60], which have been used for the removal of the heavy metals and dyes.
Cellulose-Based Composites
Cellulose may be blended with several components of wastewater treatment. Even then, in a membrane, advanced technologies polymer composites mainly of cellulose and chitosan are growing in importance [2]. Cellulose also may be coupled with chitosan (CTS) to shape composite materials of chitosan/cellulose to remove heavy metals from contaminated water [80]. The organic solvents have been utilized to adsorb ions like copper, zinc, chromium, nickel, and lead, to develop cellulose and chitin composite materials [81].
Several other substances can be used to pump up adsorbent adsorption capacity to remove metal ions from wastewater. Polymer composites have expanded adsorption capability, particularly in comparison also with polymer members' exclusive potential. In Fig. 5 the combined cellulose fibres (CF) with TiO2 to develop with their nanocomposites [36]. The subsequent nanocomposites of cellulose/TiO2 showed a considerable spike in adsorption capacity in experimental analysis with lead. The concentration of CF/TiO2 nanocomposites in Pb removal was 371.0 mg/gm and was significantly greater than those for TiO2 only which was 20.0 mg/gm. The nanocomposite filtration bed has shown 12 times larger ability than just a simple CF bed, further highlighting the significance of polymer products. These investigations have been extended while using CF/TiO2 nanocomposites for Pb adsorption with such a fibrous membrane bed, in such a single-stage fluid flowing. It demonstrated an adsorption capacity 9 times greater than that of the self-assembled filtration process, as well as 13 times larger than that of the purely CF bed [36].
In situ diagrammatic representation of TiO2 fabricated with cellulose microfibres. Adapted with the permission of Li et al. [36]
A simple, universal method has been applied in the preparation of cellulose-based magnetic iron oxide nanoparticles through a green pathway. The prepared cellulose@Fe2O3 composites exhibited excellent adsorption efficiency of arsenic compared with other magnetic materials reported [50]. Cellulose can also be used for adsorption once it has been modified to build beads. In one research, Zhou et al. [45] adsorbed Cu2+, Pb2+, and Cd2+ via an aqueous medium. Chitin/cellulose beads have been established using an aqueous solution of sodium hydroxide as well as thiourea (Fig. 6).
Different mechanisms to prepare a magnetite nanoparticles b CCNFs c hydrogels. Adapted with the permission of Zhou et al. [57]
The high adsorption capacity, fast adsorption rate, and quick magnetic separation has been shown from treated water of nanoscale zero-valent iron and cellulose composites, (cellulose@nZVI). They are expected to be an efficient magnetic adsorbent for arsenic removal from aqueous solutions [12, 23, 57].
Coagulation is the concept underneath bead formation, including the use of sulphuric acid as a coagulant. The maximum adsorption was seen for lead accompanied cadmium and copper ions. Also, in the presence of such an additive poly (ethylene glycol) 600 (PEG600), cellulose acetate could be used to produce a combination of sulfonated poly (ether imide) (SPEI). The composite material significantly showed higher carry-up capacity in the order of cadmium, zinc, nickel and, copper ions [57].
In a study, nanofibre composite fabricated for phenol adsorption from aqueous solution via hybridization of nano-magnetite zinc oxide and cellulose acetate (CA). Their results showed an increase in phenol adsorption percentage as contact time and dosage increased. The removal of arsenic [As (V)] impurity has been examined from water using zinc oxide (ZnO) nanocrystals incorporated micro fibrillated cellulose (R-MFC) scaffolds. ZnO-R-MFC showed as an effective adsorbent for the removal of arsenic ions with great adsorption capacity at pH 7 [3]. The cellulose acetate (CA)‑iron oxide nanocomposites (NC1 and NC2) have been experimented with for the detection and removal of fluorine by solid-phase extraction technique (SPE). NC2 thin film showed maximum selectivity to bind with fluorine as compared to other compounds and higher adsorption capacity [11].
Nanocellulose Iron Oxide Nanobiocomposites (NIONs) have been synthesized from rice husk and sugarcane bagasse-derived nano cellulose for removal of arsenic and associated contaminants present in groundwater samples by adsorption. These NIONSs were magnetically recoverable due to their superparamagnetic properties and exhibit promising recyclability [40]. A novel aerogel has been synthesized from a hydrogel precursor, which is formed by incorporating nano bentonite into the dialdehyde nanocellulose and carboxymethyl chitosan mesh to examine the removal of harmful components from wastewater. This showed accountable adsorption capacity against many dyes, organic solvents, and oils [82]. A novel method of self-assembly of nanocellulose and nanochitin has been developed to produce high-efficiency and versatile biohybrid hydrogel (BHH) and aerogel (BHA) for water purification. Thus, this versatile BHA offers simple and green alternatives to the conventional adsorbent from synthetic polymers [28].
Cellulose-Based Derivatives
Cellulose derivatives are frequently often used to extract toxic heavy metals from wastewater together with chitosan as well as other ingredients. As discussed in the research findings referenced earlier in this thread, nanocellulose with modifications has enhanced the amount of adsorption based on the modifying agents. Nanocelluloses can be enzymatically transformed to develop variants of their phosphorylate.
Cellulose can be used as a substitute,—for example, carboxymethyl cellulose (CMC), to extract heavy metals by the formation of complexes from sewage. Singha et al. [83] developed another methodology also for the formulation of such a successful adsorbent for heavy metals by incorporating graft copolymers via cellulose biomaterials. An and Zhao [23] stated that As (III) laden soil treated with CMC stabilized Fe–Mn could reduce the water leachable arsenic by 91–96%.
For one research, the potential of PAMAM den-dimer (G4) modified CNT to eliminate copper and lead ions from municipal effluents for single or two-part systems was assessed and it's been shown that PAMA/CNT functions as an extremely-adsorbent. The adsorption performance was progressively enhanced by increasing nanocomposite concentration. Specifications of the adsorption velocity were perceived to strengthen with the growing dosage of PAMAM/CNT. The results revealed that PAMAM/CNT can be positively related as a super-adsorbent for multi-component adsorption of heavy metals [38].
Functionalized Cellulose
The waste from biomass residues is yet another source of cellulose-based biosorbents, which can be used efficiently. The product is available in abundance, possesses versatile surface morphology, large specific surface area, and valuable mechanical characteristics. The original natural resources have been chemically synthesized utilizing TEMPO oxidation. Nano-reductions and adjustments expanded cellulose adsorption capacity for copper, chromium, zinc, and nickel ions. To remove chromium ions from aqueous solutions, cellulose from cellulose acetate can be functionalized [37].
To create a composite material, Cellulose acetate is combined with tetraethoxysilane; the composite is being created in nanofibrous membranes by surface functionalization. Nano-structured cellulose could also be functionalized in many other ways for the removal of heavy metals. Functionalization of cellulose could also be achieved utilizing different polymers. In a study, polyethyleneimine was supplemented to exclude mercury from sewage. At lower adsorbent quantities the adsorbent displayed a high adsorption efficiency of approximately 288 mg/gm in batch mode [84]. Cellulose may also be altered to adsorb ions of lead, copper, nickel, cadmium, and zinc with an elevated efficiency improvement utilizing p-aminobenzoic groups in the immobilized form on the cellulose surface. Cellulose has been efficiently used to modified to develop iron-coordinated amino-functionalized poly (glycidyl methacrylate)-grafted TiO2- cellulose for the removal of arsenic ions from polluted water through adsorption. The latter functionalized adsorbents have been proven to be efficient [85].
Cellulose, in the form of nanocrystals, has also been proven to perform very effectively. It has several industrial applications that are increasingly growing. Among such approaches is the development in treating wastewater. There are so many outlets for gleaning cellulose nanocrystal. Nanoporous cellulose can also be used in sewage for the processing of dyes. The sulfur-bearing adsorbent groups induced strong heavy metal adsorption capacity. In another research, cotton was utilized as raw material to get cellulose nanocrystals. Those cellulose-nanocrystals were then chemically modified through an aqueous phase to adsorb Pb2+ and cadmium ions. They were altered to develop succinic anhydride cellulose nanocrystals (SCNCs) as well as sodium bicarbonate (NaHCO3) for NaSCNCs, the sodium nanoadsorbent, was accomplished through succinic anhydride [50].
Oxolane-2, 5-dione-functionalized cellulose nanofibres were being used for adsorption of Pb and Cd from model sewage water. Cellulose has been chosen for its relatively high flexibility, large surface area, and ease of access to chemicals. However, cellulose could not be used efficiently as an adsorbent without eventually incorporating some sort of physical or chemical alteration [48]. For another research, magnetic chitosan hydrogel beads have been synthesized for the adsorption of lead ions via the aqueous medium. That is composed of carboxylated nanofibrils of cellulose and the analysis reveals that perhaps the presence of carboxylate groups triggered the adsorbent to boost its adsorption capacity [57].
Because of the strong reinforcing effect of nanosized cellulose, chitosan when blended with nanofibrils (CNFs) and cellulose nanowhiskers (CNWs) has indeed been reported to have strengthened the tensile and thermal properties. Strong acid hydrolysis eliminates most of the amorphous regions of native cellulose but also produces CNFs or CNWs. Besides, PVA-mixed chitosan may establish better a three-dimensional structure with no chemical cross-linking, which would be advantageous for the eventual removal of heavy metals. The use of the magnetic adsorption technique is now one of the effective ways of addressing environmental issues. Effective segregation of adsorbents from sewage until the recycling of both sorbates and adsorbents is indeed a significant step, specifically when the adsorbent generates excessively small particles [37]. In one analysis, magnetic chitosan hydrogel beads were loaded with CCNFs and formulated using an immediate gelation method. The basic behaviours of adsorption of lead ions on an aqueous solution by the magnetic hydrogels have been studied in detail [57].
Two novels designed nano and micro cellulose supported adsorbents with organic support (amino-functionalized) and inorganic nanohybrid precipitated adsorptive material (magnetite) exhibited favourable properties towards arsenate [86]. Maleic anhydride modified nanocellulose showed 5-times higher adsorption capacity showing the effect of an increased number of amino surface bonding sites on adsorption. In recent research led by Chen et al. [3], cellulose-based nanostructured materials have been prepared from wood pulp for the extraction of heavy metals from the water. For removal of mercury from wastewater, Anirudhan et al. [41], reported a nanocellulose based novel adsorbent,2-mercaptobenzamide modified and itaconic acid-grafted-magnetite nanocellulose composite [P(MB-IA)-g-MNCC]. Complete removal of Hg (II) from aqueous solution was possible with an adsorbent dosage of 2.0 g/L.
In another research, TiO2/CF nanocomposites were synthesized in the presence of cellulose fibres via microwave-assisted hydrolysis of TiOSO4. On the surface of cellulose fibres have been well dispersed mesoporous TiO2 particles with an average size of 100 nm. Those very particles are principal nanoparticles of 10 nm TiO2. Through the chemical reaction between the functional hydroxyl groups of cellulose as well as the Ti crystal nucleus, the particles get attached to the cellulose material to generate the TiAO bond. Those nanocomposites exhibited a mesoporous structure and a large surface area, enabling fast adsorption rate and high lead ions adsorption efficiency in synthetic wastewater. Adsorption upon these nanoengineered composite materials accompanied Freundlich isotherm and showed tremendous regeneration potential, enabling functional use of the nanosorbents as just accessible renewable energy for the prompt and efficient elimination of toxic metals from polluted water [18, 87].
A diethylenetriaminepentaacetic acid (DTPA)-modified cellulose-based adsorbent has been synthesized using N [3 (trimethoxysilyl) propyl] ethylenediamine as a crosslinking reagent to remove mercuric ions from an aqueous solution. The DTPA effectively fabricated cellulose and coordinated DTPA moiety significantly enhanced the adhesive affinity towards heavy metal ions [36]. Figure 7 depicts the schematic mechanism of adsorption of mercuric ions on DTPA. Diethylenetriaminepentaacetic acid (DTPA) is a probable ligand with five groups of carboxylates that would generate adsorbents with exceptional complex formation to heavy metal ions after being grafted onto cellulose.
Schematic illustration of adsorption of mercuric ions on DTMC. Adapted with permission from Li et al. [36]
Conclusion
Heavy metal poisoning is quite prevalent amongst all the sources of water pollutions. The use of heavy metals has been illustrated from earlier civilizations for different applications, and its findings of toxic effects are well-validated. It has been used widely because of its significant physicochemical properties. Hence safer ways are required to avoid unnecessary contamination and mitigate ramifications.
Cellulose is a nanoparticle that has several industrial applications. Cellulose is identified as the most found naturally carbon-property material, with various hydroxyl groups and strong hydrogen-bonding networks forming crystalline and amorphous structures for greater rigidity and resistance. Nanocellulose has appeared to be a viable strategy for an adsorbent and filter membrane for removing contaminants. This capability is due to the increased aspect ratio, high geographical areas, high retention ability, and environmental inertia. Besides, the involvement of binding sites enables the integration of chemical functional groups which may increase the binding efficiency with the pollutants.
Despite the noteworthy developments in the preparation of heavy metal binding biocomposites from cellulose and other material, there are many issues such as higher binding efficiencies and low costs that remain vague which require further research and improvements.
References
Literathy P (1996) Industrial wastes and water pollution. In Regional Approaches to Water Pollution in the Environment (pp. 21–32). Springer, Dordrecht.
Sharma A, Thakur M, Bhattacharya M, Mandal T, Goswami S (2019) Commercial application of cellulose nano-composites: a review. Biotechnol Rep 21:e00316
Chen H, Sharma SK, Sharma PR, Yeh H, Johnson K, Hsiao BS (2019) Arsenic (iii) removal by nanostructured dialdehyde cellulose–cysteine microscale and nanoscale fibers. ACS Omega 4(26):22008–22020
Grenni P, Caracciolo AB, Mariani L, Cardoni M, Riccucci C, Elhaes H, Ibrahim MA (2019) Effectiveness of a new green technology for metal removal from contaminated water. Microchem J 147:1010–1020
Yang J, Hou B, Wang J, Tian B, Bi J, Wang N, Li X, Huang X (2019) Nanomaterials for the removal of heavy metals from wastewater. Nanomater. https://doi.org/10.3390/nano9030424
Mok CF, Ching YC, Osman NAA, Muhamad F, Dai Hai N, Choo JH, Hassan CR (2020) Adsorbents for removal of cationic dye: nanocellulose reinforced biopolymer composites. J Polym Res 27(12):1–15
Tasrin S, Fazil SMM, Senthilmurugan S, Selvaraju N (2021) Facile preparation of nanocellulose embedded polypyrrole for dye removal: unary and binary process optimization and seed toxicity. Int J Environ Sci Technol 18(2):365–378
Phanthong P, Reubroycharoen P, Hao X, Xu G, Abudula A, Guan G (2018) Nanocellulose: extraction and application. Carbon Res Convers 1(1):32–43
Malik DS, Jain CK, Yadav AK (2017) Removal of heavy metals from emerging cellulosic low-cost adsorbents: a review. Appl Water Sci 7(5):2113–2136
Sharma A, Mandal T, Goswami S (2017) Cellulose nanofibers from rice straw: Process development for improved delignification and better crystallinity index. Trends Carbohydrate Res 9
Tshikovhi A, Mishra SB, Mishra AK (2020) Nanocellulose-based composites for the removal of contaminants from wastewater. Int J Biol Macromol 152:616–632. https://doi.org/10.1016/j.ijbiomac.2020.02.221
Wang B, Sain M (2007) Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos Sci Technol - Compos Sci Technol 67:2521–2527. https://doi.org/10.1016/j.compscitech.2006.12.015
Robles E, Urruzola I, Labidi J, Serrano L (2015) Surface-modified nano-cellulose as reinforcement in poly (lactic acid) to conform new composites. Ind Crops Prod 71:44–53
Yang H, Sheikhi A, van de Ven TGM (2016) Reusable green aerogels from cross-linked hairy nanocrystalline cellulose and modified chitosan for dye removal. Langmuir 32:11771–11779. https://doi.org/10.1021/acs.langmuir.6b03084
Langan P, Petridis L, O’Neill HM, Pingali SV, Foston M, Nishiyama Y, Schulz R, Lindner B, Hanson BL, Harton S, Heller WT, Urban V, Evans BR, Gnanakaran S, Ragauskas AJ, Smith JC, Davison BH (2014) Common processes drive the thermochemical pretreatment of lignocellulosic biomass. Green Chem 16:63–68. https://doi.org/10.1039/C3GC41962B
Deng S, zhang G, Chen S, Xue Y, Du Z, Wang P (2016) Rapid and effective preparation of a HPEI modified biosorbent based on cellulose fiber with a microwave irradiation method for enhanced arsenic removal in water. J Mater Chem A 4:15851–15860. https://doi.org/10.1039/C6TA06051J
Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5(3):2782–2799
Zhang X, Lei H, Chen S, Wu J (2016) Catalytic co-pyrolysis of lignocellulosic biomass with polymers: a critical review. Green Chem 18:4145–4169. https://doi.org/10.1039/C6GC00911E
Sag Y, Kutsal T (2001) Recent trends in the biosorption of heavy metals: a review. Biotechnol Bioprocess Eng 6(6):376
Bilal M, Rasheed T, Sosa-Hernández JE, Raza A, Nabeel F, Iqbal H (2018) Biosorption: an interplay between marine algae and potentially toxic elements—a review. Mar Drugs 16(2):65
Park JB, Lakes RS (1992) Introduction to biomaterials. In Biomaterials (pp. 1–6). Springer, Boston, MA.
Bashir A, Manzoor T, Malik LA, Qureashi A, Pandith AH (2020) Enhanced and selective adsorption of Zn(II), Pb(II), Cd(II), and Hg(II) ions by a dumbbell- and flower-shaped potato starch phosphate polymer: a combined experimental and DFT calculation study. ACS Omega 5:4853–4867
An B, Zhao D (2012) Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe-Mn oxide nanoparticles. J Hazard Mater 211–212:332–341. https://doi.org/10.1016/j.jhazmat.2011.10.062
Mansouri J, Harrisson S, Chen V (2010) Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities. J Mater Chem 20:4567–4586. https://doi.org/10.1039/B926440J
Mahfoudhi N, Boufi S (2017) Nanocellulose as a novel nanostructured adsorbent for environmental remediation: a review. Cellulose 24:1171–1197. https://doi.org/10.1007/s10570-017-1194-0
Thakur M, Sharma A, Ahlawat V, Bhattacharya M, Goswami S (2020) Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater Sci Energy Technol 3:328–334
Sharma A, Mandal T, Goswami S (2020) Dispersibility and stability studies of cellulose nanofibers: implications for nanocomposite preparation. J Polymers Environ 1–10
Zhang X, Elsayed I, Navarathna C, Schueneman GT, Hassan EIB (2019) Biohybrid Hydrogel And Aerogel From Self-Assembled Nanocellulose And Nanochitin As A High-Efficiency Adsorbent For Water Purification. ACS Appl Mater Interfaces 11:46714–46725. https://doi.org/10.1021/acsami.9b15139
Nascimento DM, Nunes YL, Figueirêdo MC, de Azeredo HM, Aouada FA, Feitosa JP, Dufresne A (2018) Nanocellulose nanocomposite hydrogels: technological and environmental issues. Green Chem 20(11):2428–2448
Fiorati A, Negrini NC, Baschenis E, Altomare L, Faré S, Schieroni AG, Piovani D, Mendichi R, Ferro M, Castiglione F, Mele A, Punta C, Melone L (2020) TEMPO-nanocellulose/Ca2+ hydrogels: ibuprofen drug diffusion and in vitro cytocompatibility. Materials (Basel) 13:183. https://doi.org/10.3390/ma13010183
Maestri CA, Motta A, Moschini L, Bernkop-Schnürch A, Baus RA, Lecca P, Scarpa M (2020) Composite nanocellulose-based hydrogels with spatially oriented degradation and retarded release of macromolecules. J Biomed Mater Res, Part A 108(7):1509–1519
Shak KPY, Pang YL, Mah SK (2018) Nanocellulose: recent advances and its prospects in environmental remediation. Beilstein J Nanotechnol 9:2479–2498. https://doi.org/10.3762/bjnano.9.232
Koshani R, Tavakolian M, van de Ven TGM (2020) Cellulose-based dispersants and flocculants. J Mater Chem B 8:10502–10526. https://doi.org/10.1039/D0TB02021D
Voisin H, Bergström L, Liu P, Mathew AP (2017) Nanocellulose-based materials for water purification. Nanomater (Basel Switzerland). https://doi.org/10.3390/nano7030057
Khulbe KC, Matsuura T (2021) Membrane applications. In Nanotechnology in Membrane Processes (pp. 199–343). Springer, Cham
Li Y, Cao L, Li L, Yang C (2015) In situ growing directional spindle TiO2 nanocrystals on cellulose fibers for enhanced Pb(2+) adsorption from water. J Hazard Mater 289:140–148. https://doi.org/10.1016/j.jhazmat.2015.02.051
Jamshaid A, Hamid A, Muhammad N, Naseer A, Ghauri M, Iqbal J, Shah NS (2017) Cellulose-based materials for the removal of heavy metals from wastewater–an overview. ChemBioEng Rev 4(4):240–256
Hayati B, Maleki A, Najafi F, Daraei H, Gharibi F, McKay G (2017) Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J Hazard Mater 336, 146–157. https://doi.org/10.1016/j.jhazmat.2017.02.059
Abouzeid RE, Khiari R, El-Wakil N, Dufresne A (2019) Current state and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromol 20:573–597. https://doi.org/10.1021/acs.biomac.8b00839
Baruah J, Chaliha C, Kalita E, Nath BK, Field RA, Deb P (2020) Modelling and optimization of factors influencing adsorptive performance of agrowaste-derived Nanocellulose Iron Oxide Nanobiocomposites during remediation of Arsenic contaminated groundwater. Int J Biol Macromol 164:53–65. https://doi.org/10.1016/j.ijbiomac.2020.07.113
Anirudhan TS, Shainy F, Deepa JR (2019) Effective removal of Cobalt(II) ions from aqueous solutions and nuclear industry wastewater using sulfhydryl and carboxyl functionalised magnetite nanocellulose composite: batch adsorption studies. Chem Ecol 35:235–255. https://doi.org/10.1080/02757540.2018.1532999
Sharma PR, Sharma SK, Antoine R, Hsiao BS (2019) Efficient removal of arsenic using zinc oxide nanocrystal-decorated regenerated microfibrillated cellulose scaffolds. ACS Sustain Chem Eng 7(6):6140–6151
Sharma PR, Chattopadhyay A, Sharma SK, Geng L, Amiralian N, Martin D, Hsiao BS (2018) Nanocellulose from spinifex as an effective adsorbent to remove cadmium(II) from water. ACS Sustain Chem Eng 6:3279–3290. https://doi.org/10.1021/acssuschemeng.7b03473
Sharma PR, Chattopadhyay A, Zhan C, Sharma SK, Geng L, Hsiao BS (2018) Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 25(3):1961–1973
Sun C, Ni J, Zhao C, Du J, Zhou C-E, Wang S, Xu C (2017) Preparation of a cellulosic adsorbent by functionalization with pyridone diacid for removal of Pb(II) and Co(II) from aqueous solutions. Cellulose. https://doi.org/10.1007/s10570-017-1519-z
Liu P, Borrell PF, Božič M, Kokol V, Oksman K, Mathew AP (2015) Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J Hazard Mater 294:177–185. https://doi.org/10.1016/j.jhazmat.2015.04.001
Hokkanen S, Repo E, Suopajärvi T, Liimatainen H, Niinimaa J, Sillanpää M (2014) Adsorption of Ni (II), Cu (II) and Cd (II) from aqueous solutions by amino modified nanostructured microfibrillated cellulose. Cellulose 21(3):1471–1487
Liu P, Sehaqui H, Tingaut P, Wichser A, Oksman K, Mathew P, A. (2014) Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose. https://doi.org/10.1007/s10570-013-0139-5
Pillai SS, Deepa B, Abraham E, Girija N, Geetha P, Jacob L, Koshy M (2013) Biosorption of Cd(II) from aqueous solution using xanthated nano banana cellulose: Equilibrium and kinetic studies. Ecotoxicol Environ Saf 98:352–360. https://doi.org/10.1016/j.ecoenv.2013.09.003
Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, Song W (2013) Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J Environ Sci 25:933–943. https://doi.org/10.1016/S1001-0742(12)60145-4
Ma H, Hsiao BS, Chu B (2012) Ultrafine Cellulose Nanofibers as Efficient Adsorbents for Removal of UO22+ in Water. ACS Macro Lett 1:213–216. https://doi.org/10.1021/mz200047q
Shen W, Chen S, Shi S, Li X, Zhang X, Hu W, Wang H (2009) Adsorption of Cu (II) and Pb (II) onto diethylenetriamine-bacterial cellulose. Carbohyd Polym 75(1):110–114
Tao H, Lavoine N, Jiang F, Tang J, Lin N (2020) Reducing end modification on cellulose nanocrystals: strategy, characterization, applications and challenges. Nanoscale horizons 5(4):607–627
Olivera S, Muralidhara HB, Venkatesh K, Guna VK, Gopalakrishna K, Kumar Y (2016) Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: a review. Carbohyd Polym 153:600–618
Guo X, Xu D, Yuan H, Luo Q, Tang S, Liu L, Wu Y (2019) A novel fluorescent nanocellulosic hydrogel based on carbon dots for efficient adsorption and sensitive sensing in heavy metals. J Mater Chem A 7(47):27081–27088
Ahmad M, Ahmed S, Swami B, Ikram S (2015) Adsorption of heavy metal ions: role of chitosan and cellulose for water treatment. Int J Pharmacogn 2: 280–289. https://doi.org/10.13040/IJPSR.0975-8232.IJP.2(6).280-89
Zhou Y, Wang X, Zhang M, Jin Q, Gao B, Ma T (2014) Removal of Pb (II) and malachite green from aqueous solution by modified cellulose. Cellulose 21(4):2797–2809
Vlotman DE, Ngila CJ, Ndlovu T, Malinga SP (2018) Hyperbranched polymer integrated membrane for the removal of arsenic (III) in water. J Membrane Sci Res 4(2):53–62
Mahalakshmi R, Ravikumar L, Rathina K (2017) A study on the removal of mercury (II) ions from aqueous solution by chemically modified cellulose green adsorbent: Kinetic and equilibrium studies. Rasayan J Chem 10:286–297. https://doi.org/10.7324/RJC.2017.1011633
Varghese AG, Paul SA, Latha MS (2019) Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ Chem Lett 17:867–877. https://doi.org/10.1007/s10311-018-00843-z
Wang C, Zhang Y, Liang J, Shan G, Wang Y, Shi Q (2006) Impacts of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in testis. Clin Chim Acta 370(1–2):82–88
Vaziri ND (2008) Mechanisms of lead-induced hypertension and cardiovascular disease. Am J Physiol-Heart Circul Physiol 295(2):H454–H465
Mason LH, Harp JP, Han DY (2014) Pb neurotoxicity: neuropsychological effects of lead toxicity. BioMed Res Int 2014
Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK (2014) Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ Sci Pollut Res 21(18):11066–11074
Mancuso F, Arato I, Lilli C, Bellucci C, Bodo M, Calvitti M, Aglietti MC, dell’Omo M, Nastruzzi C, Calafiore R, Luca G (2018) Acute effects of lead on porcine neonatal Sertoli cells in vitro. Toxicol In Vitro 48:45–52
Lee MY, Bae ON, Chung SM, Kang KT, Lee JY, Chung JH (2002) Enhancement of platelet aggregation and thrombus formation by arsenic in drinking water: a contributing factor to cardiovascular disease. Toxicol Appl Pharmacol 179(2):83–88
Kapaj S, Peterson H, Liber K, Bhattacharya P (2006) Human health effects from chronic arsenic poisoning–a review. J Environ Sci Health, Part A 41(10):2399–2428
States JC, Barchowsky A, Cartwright IL, Reichard JF, Futscher BW, Lantz RC (2011) Arsenic toxicology: translating between experimental models and human pathology. Environ Health Perspect 119(10):1356–1363
Argos M, Ahsan H, Graziano JH (2012) Arsenic and human health: epidemiologic progress and public health implications. Rev Environ Health 27(4):191–195
Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121(3):295–302
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2(5):112–118
Martin S, Griswold W (2009) Human health effects of heavy metals. Environ Sci Technol Briefs Citizens 15:1–6
Bernard A (2008) Cadmium & its adverse effects on human health. Indian J Med Res 128(4):557
Karri V, Kumar V, Ramos D, Oliveira E, Schuhmacher M (2018) Comparative in vitro toxicity evaluation of heavy metals (lead, cadmium, arsenic, and methylmercury) on HT-22 hippocampal cell line. Biol Trace Elem Res 184(1):226–239
Costa M, Klein CB (2006) Toxicity and carcinogenicity of chromium compounds in humans. Crit Rev Toxicol 36(2):155–163
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189(1–2):147–163
Uriu-Adams JY, Keen CL (2005) Copper, oxidative stress, and human health. Mol Aspects Med 26(4–5):268–298
Gosens I, Cassee FR, Zanella M, Manodori L, Brunelli A, Costa AL, Bokkers BG, De Jong WH, Brown D, Hristozov D, Stone V (2016) Organ burden and pulmonary toxicity of nano-sized copper (II) oxide particles after short-term inhalation exposure. Nanotoxicology 10(8):1084–1095
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16(12):29592–29630
Thakur VK, Voicu SI (2016) Recent advances in cellulose and chitosan based membranes for water purification: a concise review. Carbohydr Polym 146:148–165. https://doi.org/10.1016/j.carbpol.2016.03.030
Sun X, Peng B, Ji Y, Chen J, Li D (2009) Chitosan(chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J 55:2062–2069. https://doi.org/10.1002/aic.11797
Sharma V, Shahnaz T, Subbiah S, Narayanasamy S (2020) New insights into the remediation of water pollutants using nanobentonite incorporated nanocellulose chitosan based aerogel. J Polym Environ 28:2008–2019. https://doi.org/10.1007/s10924-020-01740-9
Singha AS, Guleria A (2014) Chemical modification of cellulosic biopolymer and its use in removal of heavy metal ions from wastewater. Int J Biol Macromol 67:409–417. https://doi.org/10.1016/j.ijbiomac.2014.03.046
Navarro RR, Sumi K, Fujii N, Matsumura M (1996) Mercury removal from wastewater using porous cellulose carrier modified with polyethyleneimine. Water Res. 30:2488–2494. https://doi.org/10.1016/0043-1354(96)00143-1
Anirudhan TS, Divya L, Parvathy J (2013) Arsenic adsorption from contaminated water on Fe(III)-coordinated amino-functionalized poly(glycidylmethacrylate)-grafted TiO2-densified cellulose. J Chem Technol Biotechnol 88:878–886. https://doi.org/10.1002/jctb.3916
Taleb K, Markovski J, Veličković Z, Rusmirović J, Rančić M, Pavlović V, Marinković A (2019) Arsenic removal by magnetite-loaded amino modified nano/microcellulose adsorbents: Effect of functionalization and media size. Arab J Chem 12(8):4675–4693
Xiong R, Wang Y, Zhang X, Lu C (2014) Facile synthesis of magnetic nanocomposites of cellulose@ultrasmall iron oxide nanoparticles for water treatment. RSC Adv 4:22632–22641. https://doi.org/10.1039/C4RA01397B
Acknowledgements
The authors would like to acknowledge Center of Innovative and Applied Bioprocessing (CIAB), Mohali for equipment and infrastructural facilities. They would like to acknowledge Department of Biotechnology (DBT), India for source of funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, A., Anjana, Rana, H. et al. A Comprehensive Review on the Heavy Metal Removal for Water Remediation by the Application of Lignocellulosic Biomass-Derived Nanocellulose. J Polym Environ 30, 1–18 (2022). https://doi.org/10.1007/s10924-021-02185-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02185-4