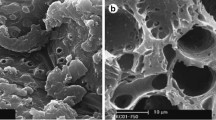
Abstract
In the last decade, the amount of contaminated water resources has increased dramatically with the rapid growth in industrial sectors. Additionally, the growth in world population and the effects of climate changes have also increased the water contamination levels in several areas. Thus, there is a crucial need for effective and eco-friendly water treatment materials. The current available water treatment methods and materials have multiples drawbacks that limit their usability. Materials such as metal oxide nanoparticles, carbon nanotubes, and polymer membranes are used widely in the water treatment field. However, the efficiency of these materials is limited by the complexity of the water contaminants. Therefore, highly efficient activated carbon is introduced as a proper approach to treat contaminated water. Typically, activated carbon is produced from different types of biomass. Hence, activated carbon can be produced almost everywhere. Currently, Lignocellulosic biomass is provided as a reliable renewable resource that can be used to produce activated carbon. Indeed, Lignocellulosic biomass can be utilized to produce several materials such as biogases, biofuels, and biochar. Activated carbon is produced from biomass using different thermal conversion technologies such as pyrolysis, anaerobic digestion, torrefaction hydrothermal processing, and gasification. Historically, pyrolysis technology is used for hundreds of years to produce biofuel and char from woody biomass. This chapter focuses on the different reaction phases during pyrolysis and the effect of the reaction conditions on biomass to produce activated carbon. Moreover, the impact of technological development on the energy density of the lignocellulosic residues is covered in the chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
The inevitability of seeking reliable wastewater treatment techniques is evident. Presently, the global supply of clean water relies on accessible natural resources such as rivers, lakes, and groundwater. Moreover, recent studies show a massive decrement in the amount of natural water resources. The vast demand for new water and wastewater treatment approaches is associated with the huge growth in the human population, climate changes, and industrial revolutions. The industrial revolutions have the most significant impact on the water treatment sector. In fact, the dramatic growth in the industrial fields is associated with the growth in the world population and climate change. Hence, the three factors are connected like a circle. Nonetheless, the impact of the industrial revolution on the environment is not comparable with population growth or climate change. In the last years, industrial fields have introduced new types of complex contaminants into the environment in the form of wastes known as industrial wastes. The danger of industrial wastes rises with industrial developments. Generally, industrial wastes are discharged in the natural water resources such as rivers and lakes. Consequently, the industrial waste leak from surface water to the surrounding area and groundwater. The industrial waste stored inform of wastewater causes other challenges in urban areas. Apart from the environmental effects, wastewater is associated with severe health issues in urban areas.
Researchers seek effective, inexpensive, and environmentally friendly techniques and materials for water treatment to eliminate the adverse health and environmental effects of wastewater. Lignocellulosic biomass is proposed as a promising precursor to produce highly efficient activated carbon from natural resources. The special characteristics of biomass products have drawn the attention of several researchers. Also, the inorganic component of biomass contains a few sulphur, ash, and nitrogen quantities that can be neglected or utilized for other applications. Thus, the combustion of biomass is preferable because it produces fewer toxic gases such as carbon dioxide (\({\text{CO}}_{2}\)), sulphur dioxide (\({\text{SO}}_{2}\)) and nitrogen oxides (\({\text{NO}}_{\text{X}}\)). Nevertheless, photosynthesis can be used to recycle and control carbon dioxide emissions (CO2) [1]. At present, biomass products require more practical applications and demonstrations with an appropriate calculation of materials and energy. Indeed, there are multiple methods used to produce biomass products, such as biofuel, biogases, and char. Nonetheless, the thermotical base of the methods requires more sophisticated studies. Thermal conversion technologies such as pyrolysis, anaerobic digestion, hydrothermal processing, and gasification are used widely to produce biofuel and char from different biomass types. Typically, thermochemical production of liquids and chars is conducted via flash or fast pyrolysis on an industrial scale. However, these products have not been implemented for commercialization applications.
Several biological and thermochemical processes have been adopted to convert biomass into value-added products. In general, pyrolysis is considered the most convenient conversion process among the other processes. Indeed, the pyrolysis process is considered inexpensive, flexible, efficient, requires simple transportation methods, easy to operate, and requires simple storing of products. Despite the unique features of the pyrolysis process, some challenges face the process, such as processing solid waste and biomass. Furthermore, the pyrolysis process is considered at the initial stage; several practical challenges are facing the expansion of the process to compete with the conventional thermochemical technologies [2, 3]. The pyrolysis of different lignocellulosic residues to produce biomass products such as solid char, gas, or liquid biofuel has been intensively studied earlier. Examples of these biomass species include woody biomass [6, 7], straws [8], seedcakes [9], bagasse [5], municipal solid waste (MSW) [10, 11], and beechwood [4]. Figures 7.1, 7.2, and 7.3 illustrate the different types of biomass conversion processes with their outputs and the decomposition temperature.
Pyrolysis is a process where a thermal decomposition of lignocellulosic derivatives occurs under an inert atmosphere in the absence of oxygen. The word is derived from two Greek words: ‘pyro’, meaning fire, and ‘lysis’ which means separation into different parts. Historically, pyrolysis is used by people in Southern Europe and the Middle East to prepare charcoal as a source of energy [12]. Additionally, ancient Egyptians made tar for sealing boats using pyrolysis technology [13]. The recent widespread applications of pyrolysis technologies can be seen in the energy field in the form of char and biofuel production. The pyrolysis products are also used in multiple industries such as materials fabrication, electronics, and fertilizers. Typically, high temperature obtained from burning charcoal is used to melt copper with tin to produce bronze. Throughout the modern era, pyrolysis processes gained the attention of many researchers as an effective technology for transforming waste and biomass into bio-oil [14]. Most of the present studies revolve around eventual objectives to enhance pyrolysis processes and produce reliable high-value products that can compete and replace the non-renewable fossil fuels and conventional materials to treat wastewater. Biowaste to produce value-added products via pyrolysis shadows several global challenges such as waste management, energy, and water and wastewater treatment. However, gaining more knowledge about pyrolysis technology is the true challenge. Several ideas proposed the use of biofuel in the airplane, trains, ships, and vehicles to replace fuels such as petrol and diesel [15, 16]. Nonetheless, few studies focused on the use of biomass-based carbon materials to remediate wastewater. Pyrolysis technology is under continuous enhancements to increase the quality of biomass products. Commonly, equipment such as pyrolysis reactor, lignocellulosic residues pre-treatment unit, and subsequent unit for downstream processing are parts of the pyrolysis system unit. The units are classified further into slow pyrolysis units to produce only heat and biochar or units that use fast pyrolysis to produce biochar and bio-oils.
Researchers have been working on the thermal conversion of biomass into biomass-based carbon products using advanced pyrolysis processes in the last year. The optimization of process parameters offers many advantages to pyrolysis technology over other thermochemical conversion technologies. Nevertheless, pyrolysis technology needs more improvements for commercial applications. Hence, this chapter investigates the current status of pyrolysis technology and the potential for practical application for biochar to produce highly efficient activated carbon. Additionally, the pyrolysis products, types of pyrolysis and principles, biomass compositions and characteristics, physiochemical properties of pyrolysis products, and the economic aspects of amorphous carbon for wastewater treatment are discussed in this chapter. Moreover, the production and properties of activated carbon are stated in this chapter to study the limitations in wastewater treatment. Eventually, the future of activated carbon production from biomass is discussed to enhance the wastewater treatment process.
7.2 Classification of Thermochemical Processes to Produce Activated Carbon
Thermochemical processes are the conversion of carbonaceous materials such as biomass into value-added products like gaseous and liquid fuels, solid fuel, and chemical feedstock. Indeed, thermal conversion techniques are used in ancient India and China before 4000 BCE to produce charcoal from biomass. At present, gaseous and liquid biomass products are treated and used in several fields. Besides that, charcoal is still used by several countries as a primary source of energy. Mainly, thermochemical conversion processes are classified into pyrolisation and gasification. The significant difference between the techniques relies on the final product. Pyrolisation is primarily used to produce char and liquid fuel, whereas gasification is used to convert biomass into a conventional gaseous product. The process of producing liquid fuel from biomass is known as liquefaction. Pyrolisation is further classified into multiple methods according to the preparation temperature and final production. In general, pyrolisation is classified into pyrolysis, carbonization, and torrefaction methods. Pyrolysis is the most adopted approach to convert biomass into carbon-rich materials. Pyrolysis, carbonization, and torrefaction processes are conducted in an oxygen-starved atmosphere. Therefore, carbonaceous materials are mainly formed from biomass via thermochemical conversion processes in the absence of oxygen. The presence of oxygen during the thermochemical convention will burn the biomass precursors and produce a large amount of ashes. The thermochemical process which involves oxidation is known as combustion. As discussed before, studying and improving the thermochemical processes will produce a high quality of carbon materials from biomass. Using high-quality activated carbon will improve the quality of wastewater treatment. In several publications, thermochemical conversion processes are stated to be independent due to the different temperature values. Nevertheless, the thermochemical convention processes used to produce solid carbon substances are interconnected. In fact, carbonization and torrefaction are part of the pyrolysis process. Figure 7.4 illustrates the connection between the thermochemical processes.
7.2.1 Pyrolysis
The thermal decomposition of pyrolysis using lignocellulosic biomass requires a special environment. Initially, the pyrolysis of lignocellulosic biomass occurs in an oxygen-deficient environment in an inert atmosphere. Followingly, nitrogen (N) or argon (Ar) gases are diffused into an inert atmosphere to complete the process. Generally, several steps are involved in the chemical reaction of the process, which makes it very complicated. The outputs of biomass pyrolysis are products such as biochar, gases, and bio-oil. The pyrolysis process emits materials that can be either discharged or used in further applications, such as hydrogen (H), carbon dioxide (CO2), carbon monoxide (CO), and methane (CH4). The decomposition of organic materials based on biomass substrate starts around 350–550 ℃. However, the decomposition can proceed until 700–800 ℃ in the absence of oxygen/air [17, 18].
The chemical structure of biomass consists of lignin, a long polymer chain of cellulose, pectin, hemicellulose, and others. The small molecules formed from the bulky molecules of organic materials. These small molecules are then released from the pyrolysis process as solid char, steam of gases, tar, and oil as condensable vapors. Multiple factors affect the proportion of the final products, such as heat rate, temperature, and types of precursors, pressure, reactor design, and system configuration. The effect of temperature on the decomposition process of prime lignocellulosic residues is shown in Fig. 7.3 and, the effects of pyrolysis on the lignocellulose components is illustrated in Fig. 7.5. Likewise, the moisture percentage of the biomass has a vital impact on the pyrolysis processes. The raw biomass moisture percentage must be around 10% during the fast pyrolysis process [18]. The high percentage of biomass moisture will turn the major final products into liquids.
On the other hand, the low water level will cause a high risk because the process will produce an enormous amount of dust instead of oil. Hence, it is necessary to dry the sludge produced from meat-processing wastes and waste streams before involving it in the pyrolysis process. To demonstrate, the required condition to produce biochar is a temperature less than 450 ℃ when the heating rate is slow. In contrast, when the heating rate is high and the temperature is higher than 800 ℃, the obtained products are a large amount of gases and ash. In like manner, medium temperature when the heating rate is high, the main yield is bio-oil. When the temperature is between 250 and 300 ℃ at the beginning of the process, a large amount of volatile materials is disposed of almost ten times faster than the subsequent step [20].
As mentioned before, charcoal is mainly produced using woody biomass. The charcoal produced from wood has many advantages, like the production of a limited amount of smoke. Historically, charcoal is used to melt ore rocks to yield iron. Nevertheless, this process has many disadvantages, like it produces a huge amount of air pollutants, less production percentage, and less energy. Consequently, advanced technology has been adopted and developed to extract the extreme amount of energy from biomass via exothermic and endothermic processes such as combustion, gasification, and pyrolysis [21]. Combustion is used to produce heat by burning biomass in the presence of oxygen. The efficiency of combustion is not sufficient [22, 23]. Gasification may occur in an oxygen-starved environment to produce gaseous fuels. However, the pyrolysis process is more advantageous compared to combustion and gasification [24, 25]. Indeed, pyrolysis can be present as part of the combustion and gasification processes [26]. Figure 7.6 shows the products of biomass substrate during the pyrolysis process [27].
In general, the pyrolysis process is classified into slow and fast processes. The significant difference between the two types is the heating rate involved in the process. The required time of heating precursors to reach pyrolysis temperature in the slow pyrolysis process is longer than the time used to keep the substrate at the characteristic pyrolysis reaction temperature. On the other hand, the initial heating time of the biomass substrate in the fast pyrolysis process is less than the final retention time of the substrate at the maximum pyrolysis temperature. There are two other types of pyrolysis based on the ambiance of the process, hydro-pyrolysis and hydrous pyrolysis. As mentioned before, usually fast and slow pyrolysis process takes place in an inert atmosphere, while hydro-pyrolysis occurs in the presence of hydrogen and hydrous pyrolysis occurs in the presence of water. In the slow pyrolysis process, vapors present in the pyrolysis medium for a longer time. Hence, this process is utilized to produce more char and activated carbon. In fact, slow pyrolysis is usually classified into conventional and carbonization processes. Conversely, in fast pyrolysis vapors exist in the medium for seconds or milliseconds. Thus, fast pyrolysis is mainly utilized to produce gas and bio-oil. Fast pyrolysis is further classified into a flash and ultra-rapid pyrolysis process. Table 7.1 shows different types of pyrolysis processes with basic characteristics.
-
(i)
Fast Pyrolysis
The fast pyrolysis process involves using a high heating rate to burn the precursors at high temperatures in the absence of oxygen. The quantity of the products produced via fast pyrolysis is related to the initial weight of the biomass. Using 15–25% of biochar precursors in the fast pyrolysis process can produce 60–75% of liquid biofuels [54]. Besides that, it is possible to obtain 10–20% of gaseous products, depending on the biomass type [54]. Fast pyrolysis involves short vapors retention time. Nevertheless, the produced vapors and aerosol can be chilled quickly during the process to increase the percentage of obtained bio-oil [54]. Typically, the obtained liquid biofuels are used to operate engines, turbines, boilers, and industrial applications. Fast pyrolysis technology is receiving global attention as a liquid fuel producing process due to its practical advantages, such as the inexpensive development cost, simple storability and transportation of obtained products, and the utilization of second-generation bio-oil as industrial wastes, and municipal to produce. In addition, fast pyrolysis can derive secondary fuels and chemicals from motor fuels. Lastly, the process can assure a pre-disintegration of lignin portions and simple oligomers from lignocellulosic biomass with consecutive improvement [55,56,57].
-
(ii)
Slow Pyrolysis
The slow pyrolysis technology uses a low heating rate and low temperature to produce a high quality charcoal. In this process, the vapor residence time is longer than flash pyrolysis, around 5–30 min. To obtain char and liquid in slow pyrolysis, a reaction occurs between the volatile organic fragments [61]. Nonetheless, the quality of the obtained bio-oil in this process is very poor. Longer vapor residence time creates further cracking, which lowers bio-oil production. Slow pyrolysis has some drawbacks, such as the higher consumption of energy due to the low heat transfer rates with longer retention time [62, 63]. The charcoal produced via slow pyrolysis is the core of activated carbon production. Basically, high-quality charcoal means the high efficiency of activated carbon. The following equation is the stoichiometric Eq. (7.1) of charcoal production by [11].
$$ {\text{C}}_{6} {\text{H}}_{{10}} {\text{O}}_{5} \to {3}{\text{.74C + 2}}{\text{.65H}}_{2} {\text{O + 1}}{\text{.17CO}}_{2} { + 1}{\text{.08CH}}_4 $$(7.1)
7.2.2 Carbonization
Thermochemical carbonization is a process where the pyrolysis of biomass precursors is conducted in a deficient oxygen atmosphere. Carbonization is conducted at a low temperature between 300 and 700 ℃. Primarily, the carbonization process is used to produce carbon-rich char from organic substances. The carbonization of biomass is widely known in rural areas due to the simplicity of the process. Moreover, several industries are using carbonization to produce high-quality commercial char for energy sectors. Usually, carbonization is preferred to produce activated carbon as it is an inexpensive process and consumes low energy. In addition, the carbonization process produces a low amount of wastes compared to other thermochemical processes. Carbonization produces a char with a high volume of narrow pores on the surface. Frequently, a large amount of surface pores on the char are blocked by liquid tar and other substances. The tarry substances are attached to the pores of the char during the diffusion of volatile components out of carbon structure into the gas stream. Indeed, the diffusion process also forms cracks on the char structure. Thus, the structure of the char contains a high volume of pores and tunnels. The char produced by carbonization can be used for water and wastewater treatment as adsorbents. However, this char will have a poor adsorption capacity due to the existence of blocked pores. Carbonization involves four main phases at different temperatures (Table 7.2). Carbonisation of biomass is further subdivided into a process known as hydrothermal carbonization. Hydrothermal carbonization is utilized to reduce energy consumption and increase the porosity of produced char.
-
(i)
Hydrothermal Carbonization
Hydrothermal carbonization (HTC) is a widely used process to convert industrial and agricultural wastes into green products. It produces solids, liquids, and gaseous products [44]. The solids formed are known as hydrochar (HC), while liquids and gases are known as biofuels and biogases, respectively [44, 45]. Hydrochar is a carbon-rich product that is further treated to produce activated carbon. Meanwhile, the liquids are usually used in the agricultural field as fertilizer or converted into effective biofuels. Normally, HTC is used as a pre-treatment process to optimize the surface chemistry of activated carbon [45]. After HTC, hydrochar yields are further treated with a pyrolysis process to produce high-quality activated carbon with low moisture percentage [44,45,46].
The major feature of HTC is the presence of oxidants in the form of deionized water. Indeed, deionized water is used as a green solvent. During the HTC, deionized water is added to the reactor along with the biomass feedstock. Subsequently, the biomass is carbonized at a low-temperature range between 180 and 250 ℃. The deionized water evaporates and creates high inner pressure [44]. Followingly, the water molecules bounce at high speed inside the reactor and break down the bulky biomass into small particles. Simultaneously, water function as an oxidation agent that optimizes the surface chemistry of the hydrochar, hence enhancing the pores volume of the material [45].
HTC is used on an industrial scale as it consumes low energy, produces a low amount of toxic gases or tars, inexpensive, easy to operate, and eco-friendly [45, 47]. Besides, the process produces high-quality hydrochar, which is further treated through the pyrolysis process to obtain highly effective activated carbon. The activated carbon is subsequently treated with physical, chemical, or physiochemical activation to increase the surface area via open blocked pores and tunnels.
-
(ii)
Torrefaction
Torrefaction is a new thermal conversion process used to produce carbon-rich solids from biomass. The torrefaction process is adopted in energy sectors as it is inexpensive and requires lower operational energy compared to carbonization. The principle of torrefaction is similar to carbonization but at a lower temperature. Typically, torrefaction occurs in the absence of oxygen. However, oxygen may introduce into the reaction but in a deficient percentage to prevent combustion.
Briefly, torrefaction converts biomass precursors into solids in slow heat under temperatures between 200 and 300 ℃. During the heating process, the carbon content and energy density increase while the oxygen and moisture content decrease [46]. Thus, the obtained is a charcoal-like product with a high percentage of carbon content. Torrefaction is used to reduce storability and simplify the transportation process. Therefore, torrefaction is used to enhance the commercial value of wood by-products for energy production.
7.3 Preparation of Activated Carbon
The preparation of activated carbon using biomass precursors involves two stages, thermochemical conversion, and activation. The two stages may conduct simultaneously as one-step processes or sequentially as dual steps processes. At the first stage, a thermochemical conversion such as pyrolysis, carbonization, or torrefaction is used to produce char with high carbon content. As discussed before, the obtained char may have a very poor adsorption capacity due to the existence of a high volume of blocked pores on its structure. Thus, different activation types are used to eliminate the presence of blocked pores and increase the adsorption capacity. The activation process may include chemical activation, physical activation, or physiochemical activation. Basically, the primary objective of the activation stage is to convert biomass-based char into highly efficient activated carbon.
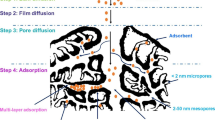
Briefly, the activation process is used to enhance the porosity and create a fine solid cavity on the surface of the char. Generally, the created pores are classified into three types, macropores (>50 nm), mesopores (2–50 nm), and micropores (<2 nm) [47]. Adjusting the percentage of pores type relies on the type of biomass precursor and activation process. Usually, activated carbon with a high percentage of micropores is used for gas filtration or as a base substance for other nanocarbon materials. On the other hand, macropores and mesopores are used in water and wastewater treatment to trap and hold different types of water contaminants such as heavy metals, pharmaceutical wastes, dyes, and pesticides. Chemical and physical activation processes are the most widely adopted activation methods to produce activated carbon. Physiochemical activation is a combination of physical and chemical activations which usually used to enhance the adsorption performance of activated carbon.
7.3.1 Physical Activation
Physical activation involves the oxidation of char using oxidizing agents such as air/oxygen, water steam (H2O), diluted oxygen gas, carbon dioxide (CO2), or their mixture [47]. Usually, physical activation conducts at an elevated temperature around 800–1,100 ℃ [48]. During the physical activation, the volatile chemicals on the char structure tend to skip in the form of gases. Simultaneously, existed narrow pores are widening, and new pores are formed, thence, the porosity and surface area of the char. At this stage, the modified char is known as activated carbon. Physical activation can be conducted in a single or dual stages process. There are no significant differences between the two processes on the efficiency of produced activated carbon. However, a single-stage process requires a shorter preparation time and lower operational energy.
The most remarkable oxidizing agents are water stream and carbon dioxide. Carbon dioxide has an endothermic nature and low reactivity at high temperatures [49]. Therefore, carbon dioxide is typically used to ensure high controllability of the activation process. In addition, carbon dioxide is simple to handle and provide a slow reaction rate at low temperature. Indeed, increasing the activation time and temperature will increase the CO2 reaction. However, increasing the activation time and temperature to a certain limit may form ash residues and destroy the existed pores. Carbon dioxide as an activation agent tends to form more micropores, whereas water steam tends to expand the existed micropores. Hence, water steam generates activated carbons with low micropore volume and high meso and macropore volumes [49]. Nonetheless, both carbon dioxide and steam can be very effective activating agents because the most important factor is the conditions of the activation process. Therefore, steam and carbon dioxide can be suitable for certain types of biomass precursors. The physical activation process is commercially favorable as it is inexpensive and requires no chemicals that will make it an environmentally friendly procedure. On the other hand, physical activation has drawbacks that limit its scalability, like it produces activated carbon with low adsorption capacity, consumes high energy, and usually, the process needs a long activation time [48, 49]. The precise selection of proper oxidizing agents for specific biomass precursors will help produce activated carbon with large pore size distribution and surface area. The detailed process for activated carbon production using physical activation is shown in Table 7.3.
7.3.2 Chemical Activation
The chemical activation method, also known as wet oxidation, uses chemical agents as dehydrating and oxidizing agents. The chemical activating agents can be acids, bases, or salt. Usually, the activation process is synchronized with the carbonization process at a temperature between 400 and 900 ℃ according to the used precursor and activating agents [48]. The chemical activation process is divided into three main steps. First, impregnation in which the carbonaceous materials are oxidized and dehydrated with chemicals. Subsequently, the mixture is dried and then heated for a given time at a specific temperature. Lastly, the mixture is washed repeatedly to remove the excess activating agent and obtain activated carbon [48]. Although several chemicals are studied as activating agents, only a few produce activated carbon with high efficiency. The activating agents with high potential are either alkaline or acidic groups. Examples of commonly used alkaline groups are sodium hydroxide (NaOH), potassium carbonate (K2CO3), potassium hydroxide (KOH), and calcium chloride (CaCl2). On the other hand, the most used acidic groups are sulphuric acid (H2SO4) and phosphoric acid (H3PO4). In addition to acidic and alkaline groups, some metal salts exhibited high potential, such as zinc chloride (ZnCl2) [48].
Generally, potassium hydroxide produces activated carbon with a high surface area. However, the surface area of the activated carbon is also related to the type of used precursors [47, 48]. Indeed, recent studies are focusing on enhancing the activation performance of potassium species to form different types of pores on the surface of activated carbons [50]. To obtain activated carbon with high pores size and volume, some variables need to be monitored, such as activation method, time, temperature and atmosphere, carbonization temperature and time, and impregnation ratio [45]. Although chemical activation produces activated carbons with more pores size distribution and high pores volume, the process has drawbacks that limit its applicability. For instance, the produced activated carbon by chemical activation requires repeated and long washing steps to remove the excess activating agent. Moreover, the process produces toxic wastewater, which contains some chemical agents that can be harmful to the environment upon discharging inappropriately [48]. Nonetheless, chemical activation has several advantages that make it desirable over physical activation. For instance, chemical activation produces activated carbon with a high adsorption capacity. Additionally, the process is economically viable because it requires a lower temperature and less activation time [48]. The detailed process for activated carbon production using chemical activation is shown in Table 7.4.
7.3.3 Physiochemical Activation
Physiochemical activation is a process where physical and chemical activations are performed simultaneously. The physiochemical activation is mainly used to enhance the pore volume and surface area of activated carbon. Typically, physiochemical activation is conducted via two approaches, a chemical treatment before the carbonization following by physical activation or a chemical treatment after the carbonization following by physical activation. Studies revealed that the sequence of chemical activation has negligible effects on the properties of produced activated carbon. Generally, activated carbon produced via physiochemical activation has a better pore structure and higher surface area compared to physical or chemical activations. Physiochemical activation can be used to increase the volume of mesopores on the surface of activated carbon. Thus, producing activated carbon is suitable for wastewater treatment. Apart from the advantages of physiochemical activation, the process is considered costly and time-consuming in comparison to physical activation and chemical activation. The detailed process for activated carbon production using physicochemical activation is shown in Table 7.5.
7.4 Types of Lignocellulosic Biomass Precursors for Activated Carbon
Lignocellulosic biomass is a natural material that is formed from living species such as animals and plants. Recently, lignocellulosic biomass is proposed as an alternative source of energy. In compassion to fossil fuel, biomass is inexpensive and environmentally friendly. Moreover, biomass is used to produce value-added products such as activated carbon and fertilizers. The new adoption of lignocellulosic biomass will solve the environmental challenges caused by waste management and fossil fuels.
Lignocellulosic biomass has a very complicated chemical structure as it is composed of several types of organic materials. In general, lignocellulosic biomass is composed of three biomacromolecules, Lignin, cellulose, and hemicellulose. In addition, the chemical structure contains minerals and extractives. Each type of biomass has a different percentage of chemicals in the structure [11, 29, 30]. Pyrolysis of cellulose and hemicellulose produce chemicals in the form of liquids and gases or condensable steam. However, the pyrolysis of lignin produces solid char, liquid, and gas. Additionally, the simplicity of extractives to evaporates and decompose also helps in producing liquids and gases. The ashes in the char structure are mainly composed of minerals. To summarise, Table 7.6 shows the components of the biomass with its related products.
The obtained vapors from the primary stage of reaction go through a second stage to produce soot. This soot varies according to the type of pyrolysis, either fast or slow. Catalysts like alkali metals are added to enhance char production. The ignition properties of biochar are directly related to the percentage of minerals [11]. Typically, the cellulosic substrate produces bio-oils when the temperature is around 500 ℃ [31]. Lignin is mainly used to extract biochar. Hence, biomass substrate with a higher percentage of lignin derivatives is used to obtain char. Table 7.6 contains a list of biomasses with the proportion of hemicellulose, cellulose, and lignin substrate [1, 32,33,34,35,36,37].
7.4.1 Cellulose
Cellulose is the most abundant organic compound on earth. The basic structure of cellulose is a linear polymer contain six-carbon glucose units. The glucose units are connected together via β-(1–4)-glycosides bonds. Typically, cellulose is insoluble in some solvents due to the connection between glucose units and cellulose polymers. Indeed, the cellulose polymers are arranged and connected parallel to each other. The cellulose connection is influenced by the presence of intermolecular and intramolecular hydrogen bonds among the hydroxide groups (OH) of the cellulose polymer. The cellulose structure has two different terminals, which allow for different reactions at the same structure. One of the cellulose terminals is known as the reducing end group as it consists of the reducing hemiacetal group in position C1. The second terminal is known as a non-reducing group and consists of an extra secondary hydroxyl group in position C4. Biomass with high content of cellulose is typically used to produce biofuel through a thermochemical convention. Therefore, the new approach of studying cellulosic materials involves the modification of chemical structure to produce a high quality biofuel and replace fossil fuel.
7.4.2 Lignin
Lignin is a complex form of organic polymers found in lignocellulosic biomass. Lignin polymers are the core of biomass structure. Lignin consists of phenyl propane building blocks and has a form similar to resin. The structure of lignin consists of hydroxyl group and methoxyl group in para and meta positions, respectively. Moreover, the aromatic rings in the structure are connected via carbon-to-carbon or carbon-to-oxygen bonds. Lignin has a complex structure that contains multiple groups such as carbonyl, hydroxyl, and methoxyl groups. Therefore, lignin has a strong structure and insoluble. The lignin present in biomass at different percentages. On average, biomass may contain 10% to 30% of lignin. Nonetheless, materials such as coconut fibers and husk may consist of 45% of lignin. Additionally, hardwood contains 16–24% of lignin, and softwood composed of 25–31%. Lignin in hardwood is connected with xylans via a covalent bond, whereas softwood is connected to galactoglucommannans. Lignocellulosic biomass with a high percentage of lignin is used to produce carbonaceous solids such as char and activated carbon. Thus, the quality and structure of biomass have a significant impact on the properties of produced activated carbon used for wastewater treatment.
7.4.3 Hemicellulose
Hemicellulose (also known as polyose) is a heteropolymer that has a random amorphous structure. Typically, hemicellulose exists along with cellulose in the biomass structure. Hemicellulose structure consists of several monosaccharide units joined together. The primary units of hemicellulose are pentose sugar. In comparison to cellulose, the hemicellulose chain is shorter and unorganized. Thus, hemicellulose has better solubility compared to cellulose. The hemicellulose polymer has two forms, either a homopolymer or a heteropolymer. Homopolymer structure composes of units from a single type of sugar, whereas heteropolymer structure composes of different kinds of sugar units. Hemicellulose acts as an adhesive where it holds the fiber and cellulose in biomass structure.
7.4.4 The Physiochemical Properties of Lignocellulosic Biomass
The final obtained products of the biomass conversion process are significantly influenced by the percentage of moisture in biomass which is further affected by the type of biomass feedstock, the design of the reactor, and process parameters [11]. As discussed before, charcoal production involves two steps: the drying and pyrolysis processes. In the primer phase of the drying step, the water contained in the pores structure as free water tends to evaporate and diffuse at a temperature around 110 ℃. The higher is the percentage of water, and the more energy is required to evaporate water. Additionally, when the temperature is between 150 and 200 ℃, an obvious reduction occurs for the combined water inside the cellulose chain of wood. White smoke of water vaporizes from the charcoal oven chamber during the initial stage of carbonization. Generally, the rate of temperature rise does not rely on the evaporated water because the fast pyrolysis process is effective in drying the feedstock [38]. The existence of moisture in the wood is usually around 15–20% [11]. Moisture content can significantly affect the properties of the production of activated carbon [39]. The differences between the obtained char or liquid are directly connected to the particle size of the biomass matrix. When the particle sizes of the biomass are large, char is mostly formed. However, a secondary char forming reaction is usually required due to the low rate of disintegration caused by the size of the large particles [11]. Hence, to produce more carbon, it is better to use larger particle sizes, while smaller particle sizes are used to increase the percentage of gained liquid during the fast pyrolysis process. Better biochar substrate production is related to the temperature of the pyrolysis and lignin and carbon quantity. Thus, to obtain a better biochar substrate, a higher percentage of lignin and fixed carbon is used while the pyrolysis process occurs at a medium temperature of 500 ℃. On the other hand, to obtain more bio-oils and syngas, the proportion of the volatile materials must be high [29]. Precursors such as olive stone, walnut shell, and hazel nutshell are highly selected to produce biochar because it contains a higher percentage of lignin. Furthermore, materials such as dead wood, cereal straw, and grasses are suitable to produce syngas and bio-oils [40]. Briefly, the physicochemical properties, transformation mechanisms, and composition of lignocellulosic biomass directly impact the type of final products as shown in Fig. 7.6.
7.4.5 The Influential Factors on Activated Carbon Production
-
(i)
Temperature
Controlling the temperature profile is crucial for biomass product optimization because the heating rate, pressure, contact time, and peak temperature between solid and gaseous phases are partially affected by the temperature profile. During the fast pyrolysis, rapid heating and cooling rate are utilized to reduce the duration of secondary reactions. As a result, the product quality will be downgraded, whereas the liquid yield is reduced. Additionally, the obtained product will contain a mixture with high complexity and viscosity [38]. On the contrary, to increase the char yields, the pyrolysis process involves slow heating rates, though; this is not consistent [11]. Increasing the temperature can assure the release of more evaporative fractions, which increase the content of carbon in the biochar. At the same time, hosting the biomass for a long time under high temperatures will significantly reduce the biochar yield. In regard to liquid and gaseous products, the effect of temperature is very complicated. To obtain a higher portion of liquid, the pyrolysis temperature must reach up to 400–550 ℃. Moreover, when the temperature is higher than 550 ℃, the final products contain fewer liquid percentages due to the decomposition of condensable vapor via secondary reactions. A higher percentage of liquid can be obtained during the fast pyrolysis when the temperature is around 500 ℃. Around 28–4% of liquid can be gained during the slow pyrolysis when the temperature is between 377 and 577 ℃, and this depending on the type of biomass feedstock [13]. On the other hand, 4.93–45% of the liquid can be obtained at a temperature around 385–450 ℃ using different cellulose-rich feedstock [19].
-
(ii)
Gas Flow Rate
The influence of the gas flow rate during the pyrolysis process appears on the degree of secondary char formation. Applying a low gas flow rate is preferable during the slow pyrolysis process to form char. Conversely, higher gas flows are applied during the fast pyrolysis to adequately and quickly remove formed vapors. High pressure will increase the formation of secondary char because it increases the activity of vapors at the surface of char particles and within the reactor. On the other hand, conducting pyrolysis under vacuum produces a higher proportion of liquid and less char. Moisture that exists in the form of vapors can increase carbon production systematically when pyrolysis takes place under pressure. This occurs due to water, which acts as a catalyst and lowers the activation time of pyrolysis reactions [46]. Conspicuously, the gas flow rate affects the thermodynamics of the pyrolysis process. Using a low gas flow rate, the reaction is more exothermic when the pressure is high. The exothermic pyrolysis process assures more char yield. Additionally, these conditions are favorable because the total energy balance of the processes will seek carbon and char as prime products. Generally, to form more char and gain less liquid, it is important to increase the contact between the main generated vapors and hot char surface during the pyrolysis process. This can happen using particles with large size, high pressure, slow heating rate, or low gas flow rate.
7.5 Applications of Activated Carbon for Water Treatment
Activated carbon is used in several industrial and agricultural applications. Primarily, activated carbon is used for air and water filtration. Indeed, the use of activated carbon in water treatment to remove contaminants and pollutants has increased dramatically [89]. It is stated that water purification and filtration consume around 80% of activated carbon worldwide [90]. Activated carbon is widely adopted in the water treatment field due to its unique physical and chemical characteristics. Activated carbon is used mainly as highly efficient adsorbents for several types of contaminants. The high adsorption capacity of activated carbon is directly related to its high surface area and porous structure [91]. Moreover, the surface of activated carbon contains several types of functional groups that interact efficiently with contamination particles in water.
The recent approaches of utilizing activated carbon in water treatments revolve around enhancing the adsorption performance via nanoparticles [92, 93]. Basically, activated carbons are loaded with nanoparticles to produce nanocomposites with high adsorption capacity and high chemical and mechanical stability. Popularly adapted nanoparticles include iron nanoparticles [94], tungsten oxide nanoparticles [95], silver nanoparticles [96], and zero-valent iron nanoparticles [97]. Typically, activated carbon modified with nanoparticles is used to remove metal ions and dyes from contaminated water. However, removing these types of contaminants relies on other factors such as the solution temperature and pH level. Indeed, pH level is considered as a key factor. pH level control both the anionic and cationic nature of the surfaces and the surface charge density. Therefore, a high pH level solution will attract cations, whereas a low pH level will attract anions. In general, the pH level is considered during the synthesis of modified activated carbon. Acidic carbons are preferred to catch cations, while basic carbons are used to remove anions from polluted water [90].
7.5.1 Elimination of Metallic Contaminants
Metallic contaminants exist in a high percentage of wastewater. Typically, metallic contaminants present as heavy metals and/or metalloids. Generally, metallic contaminants are extremely dangerous to human health. Moreover, metallic contaminants are subject to biomagnification in plants and animals. Above all, they are non-degradable materials that make the water purification and treatment process difficult and costly. Consuming food or water contaminated with metallic pollutants for the short term may cause several diseases such as diarrhea, fever, and damage to the liver and kidney. However, long-term exposure to metallic contamination via water or food can cause liver cancer, skin cancer and may lead to death. The impact of heavy metals on human health is associated with the long-term accumulation of these materials in the human body [98]. In general, metallic contaminants are introduced to the environment from different agricultural and industrial sources. These sources include activities such as mining, the metal industry, usage of pesticides and fertilizers, and semiconductors manufacturing [99].
In the last decade, removal processes of metallic contaminants from water have developed significantly. New technologies are explicitly designed to eliminate heavy metals and loids from water. However, most of the developed technologies are expensive and have low efficiency compared to activated carbon. Thus, several studies reported the use and development of activated carbon for metallic pollutant removal. Activated carbon is considered a low-cost and highly efficient solution for water contamination [98]. Activated carbon is used to remove metals such as lead (Pb(II)), cadmium (Cd(II)), copper (Cu(II)), chromium (Cr(II)), iron (Fe), and arsenic (As) [100]. Indeed, the elimination process of metallic contaminants from the water via activated carbon is considered a simple process due to the size of metallic contaminants and the properties of activated carbon. Usually, metallic contaminants present in the water as small charged particles. Hence, the interaction between the activated carbon and the metal ions occurs directly (electrostatic interaction) [101]. Similar to other types of contaminants, the adsorption process of metallic pollutants is affected by the pH level of the solution. Moreover, there are other factors that influence the adsorption behavior, such as the structure and size of metal ions, the surface area and porosity of activated carbon, and the functional groups present on the surface of activated carbon [84].
As mentioned before, the type of biomass used to produce activated carbon greatly influences the adsorption of metallic pollutants. Min et al. has conducted a study on the removal of Cd(II) from contaminated water using oil palm shell-activated carbon [57]. Their study revealed that oil palm shell-activated carbon has 99.5% removal efficiency for Cd(II). A similar study is conducted by Ahmed, where he used chemically activated date seeds activated carbon to remove Cd(II) ions from water [58]. His results exhibited a great tendency of date seed activated carbon to adsorb cadmium ions. The reported adsorption capacities were in the range of 118.1–127 mg·g−1. Sajjadi et al. have published a study on the removal of mercury (Hg) from polluted water using chemically activated pistachio wood wastes activated carbon [77]. The study used ammonium nitrate (NH4NO3) as an activation agent for the carbon. The produced activated carbon has a high surface area of about 1,448 m2·g−1. The maximum adsorption capacity was 201.095 mg·g−1.
Heavy metals are highly toxic metallic elements that have a higher density than other common metals. Indeed, heavy metals exist naturally in many areas and contaminate soil, air, and water. The danger of heavy metals is related to their toxicity, wide distribution, and extensive usage in industrial applications. There are several types of heavy metals such as arsenic (As), lead (Pb), cadmium (Cd), nickel (Ni), mercury (Hg), chromium (Cr), selenium (Se), and cobalt (Co). These heavy metals contaminate the environmental resources at different levels. Generally, the dramatic increase in the concentration of heavy metals is associated with anthropogenic activities such as semiconductor manufacturing, mining, combustion of fossil fuel, and material fabrication.
Heavy metals can be found in natural water resources such as lakes, rivers, and groundwater. In fact, heavy metals such as arsenic can be found at high concentrations in groundwater as it leaks from surrounding soils and rocks. Such a high concentration of heavy metal above the standard level poses health risks. Moreover, in urban areas, the concentration of heavy metals increases in surface water resources, especially rivers. In general, urban areas discharge highly toxic wastes into rivers and coastal regions. The discharged wastes contain several contaminants such as heavy metals, polyaromatic hydrocarbons (PAHs), and polyfluoroalkyl substances (PFAS).
Primarily, heavy metals are removed from water using different types of adsorbents. Nonetheless, other methods such as membrane filtration and reverse osmosis are used to remove certain types of heavy metals. Generally, removing heavy metals from contaminated water using adsorbents is the most preferred treatment approach. Among the different types of adsorbents, activated carbon has exhibited an outstanding performance in removing highly toxic heavy metals such as arsenic, lead, and mercury. Activated carbon has special physical and chemical properties that make it suitable for wastewater treatment. Briefly, activated carbon is inexpensive, environmentally friendly, easy to regenerate, and has high adsorption capacity. Typically, activated carbon is used to eliminate arsenic and lead from water and wastewater. Arsenic and lead are used intensively in manufacturing and can spread quickly in the environment.
7.5.2 Elimination of Non-Metallic Contaminants
The threat of non-metallic contaminants on human health has been observed in the last few years. Similar to metal ions, anions as non-metallic contaminants are considered essential elements to human bodies. Nonetheless, excessive consumption of non-metallic pollutants can cause diseases and lead to death. Molybdate, phosphate, and fluoride are the most popular types of anions consumed by humans. These anions are detrimental not only to humans but also to other living species. Globally, millions of people have consumed excessive fluorosis and suffered from fluorosis [102]. Typically, people consume fluoride through contaminated water, especially in developing countries. Indeed, fluoride is used in some water treatment plants as part of the process in controllable percentages. However, in some developing countries, fluorides are used in excessive amount due to the lack of monitoring and regulations. Viswanathan et al. have published a study on the impact of fluoride ions on human health [103]. In the study, they measured the level of fluoride ions in drinking water. The level of fluoride ion was 3.24 ppm, and children are the most affected with fluorosis. Like fluorides, molybdate ions can accumulate in humans and animals and cause severe diseases [104]. Anions can be removed easily via activated carbon. The removal of anions is influenced by the type of activated carbons and the pH value of the solution.
7.5.3 Elimination of Various Dyes from Water
In the last decades, the rapid development in the industrial sectors caused severe damage to the environment via introducing new types of complex contaminants. Dyes are one of the most extensively used materials in several industries such as textiles, papers, cosmetics, and paint production. Indeed, the textile industry is considered the major source of water contamination in several areas [105]. It is estimated that there are around 3,600 types of dyes in the industries, where 2–20% are typically discharged directly into the environment [106, 107]. Dyes discharged into the natural water resources are highly toxic and deadly. Regarding the environmental effects, the presence of dyes can reduce the photosynthesis process of the aquatic flora and fauna [89].
Several studies have proposed activated carbon as a great solution to remove different types of dyes from water. The adsorption of dyes is directly influenced by the types and properties of activated carbon, such as the porosity and high surface area, the functional groups. Besides that, the solubility and molecular size of dyes and the pH level of the aquatic medium affect the adsorption behavior [108]. Katheresan et al. stated that pretreating and activating carbon via steam could produce activated carbon with a high tendency to adsorb dye [109]. Further studies have revealed the great performance of activated carbon prepared from Acacia Mangium and Acacia Nilotica in removing different types of dyes such as Methyl orange (90.5%) and Methylene blue (250 mg·g−1) [66, 110].
Methylene Blue (MB) is considered the most popular among the different types of dyes. In fact, MB present in wastewater at high percentages. Wang et al. have published a study on the removal of MB using activated carbon [111]. The study used potassium hydroxide (KOH) as an activation agent. The analytical data showed that activated carbon has a surface area of 1,430 m2·g−1. Moreover, the obtained adsorption capacity was 934.579 mg·g−1 at pH 5.8. Further study has been published by Ahmed on the removal of MB via rice straw and Ramulus mori-based activated carbon [112]. The study involved the use of diammonium phosphate (N2H9PO4) as an activation agent. The adsorption capacities of both rice straw-based and Ramulus mori-based activated carbon was 129.5 and 1,061 mg·g−1, respectively.
7.5.4 Elimination of Phenolic Compounds
Phenolic compounds are highly toxic chemicals that can affect the ecosystem for decades. Usually, phenolic compounds are discharged into the environmental system through chemicals and petrochemical industries [113]. The impact of phenolic compounds is monitored by the environmental protection agencies. Phenolic compounds are carcinogenic and can destroy the aquatic ecosystem [114]. Different standards are made to limit the amount of phenolic compounds in water bodies [115]. For instance, the Environmental Protection Agency (EPA) has limited the amount of phenolic pollutants to 0.1 mg·L−1 at maximum. However, the World health organization (WHO) has made the acceptable standard lower to 0.001 mg·L−1 for drinkable water [115]. Activated carbon has shown an outstanding performance in removing phenolic compounds from contaminated water. The removal of phenol via activated carbon relies mainly on surface functionality. Therefore, the presence of functional groups such as carboxyl and hydroxyl on the activated carbon surface will increase the adsorption of the phenolic compounds. The interaction between the activated carbon and phenol is directed by pi-pi bond [116]. Besides the surface functionality, the pH level of the solution has a significant impact on the removal of phenolic compounds from water. Indeed, the adsorption performance decrease for both high and low pH values [117].
7.5.5 Elimination of Pesticides
Recently, pesticides are used intensively in agricultural fields to eliminate pests and several plant diseases. However, the intensive use of pesticides has formed global environmental threats. Pesticides can leak from the agricultural fields to the surrounding water resources. As a result, the contamination level of the natural water resources increases and destroys the ecosystem. The most commonly used pesticides include. 2,4-dichlorophenoxyacetic acid, carbofuran, and bentazon. Generally, the environmental protection agencies have set a permissible concentration of these pesticides to 0.1, 0.05, and 0.09 mg·L−1 for 2,4-dichlorophenoxyacetic acid, bentazon, and carbofuran, respectively [118]. Typically, pesticides are removed from water via highly efficient activated carbon. The removal process relies on the presence of organic matter and the flow rate in the adsorption [108].
7.5.6 Elimination of Pharmaceutical Contaminants
Recently, pharmaceutical contaminants have been introduced in the ecosystem due to the increase in the usage of medical products. Pharmaceutical contaminates involve the waste and the byproducts of pharmaceutical products discharged into the environment. The pharmaceutical compounds are difficult to remove due to their high stability and hydrophilicity. Thus, they can present in water bodies for a long time. Although the concentration of pharmaceutical contaminants is considered low, the longtime exposure to contaminated water via drinking can cause severe diseases [119,120,121]. Activated carbon is used as an adsorbent for different types of pharmaceutical compounds [119]. Indeed, the adsorption of pharmaceutical compounds via activated carbon relies on operational conditions, type of precursor, activation method, and the properties of a pharmaceutical compound. The operational conditions involve the pH level of the solution, temperature, organic structure, adsorbent (activated carbon) dose, and ionic capability [122].
7.6 Conclusions
In summary, the production of value-added products using proper biomass feedstock requires more studies and experiments to enhance the overall technology. There is a crucial need for more experiments on the consistency of pyrolysis reactions to be used in large-scale applications and become more sustainable. Moreover, the relation between the general operation of the pyrolysis plant and the applied feedstock or precursors must be more evident. Nevertheless, the fundamentals of pyrolysis technology have been covered elaborately in this chapter to specify the influential factors on the process performance. This includes different factors to design the process and obtain a preferred product, such as an activated carbon with high adsorption performance for wastewater. The major influential factors involve the selection of conditional parameters for the pyrolysis process, types of reactors, and the type of lignocellulosic biomass.
The suggestions for future studies on the thermochemical convention of biomass include the improvement of the biochar separation process to enhance the quality of biofuels and activated carbon production. Moreover, the type of selected biomass is considered a vital factor in the process. Lignocellulosic biomass with a high portion of cellulose is preferred to obtain a maximum bio-oil yield. On the other hand, lignin-based biomass can be used to obtain biochar. Apart from activated carbon production, there are few studies on the enhancement of gas production from biomass precursors. Hence, there is a crucial need for sophisticated studies on the development of gaseous products via metal catalysts. Several methods can be utilized for the kinetics of the pyrolysis of biomass. Initially, to obtain char and gas, lower activation energy is used by applying a low temperature. On the other hand, applying higher temperature results in higher activation energy and produces condensable fumes, liquid aerosols, and oils. However, heating biomass quickly at a proper high temperature will lead to obtaining a high percentage of liquid fuels. For fast biomass heating, it is preferable to use smaller particle sizes of precursors to initiate constant particle heating. The pyrolysis processes require more development from the environmental and economic aspects. As a result, the cost of biomass products will become competitive with conventional products. In addition, maximizing the activated carbon production is also related to the quality of pyrolysis reactors. Attention must be drawn towards the pyrolysis efficiency, physicochemical properties of the biochar, carbon and slag deposition, and emission of microparticles during the pyrolysis process. Presently, activated carbon exhibited a great performance in treating wastewater at an industrial scale as an alternative to metal oxides and membrane polymers. However, the need for development in activated carbon production is continuous as novel contaminants become more challenging to remove from wastewater.
References
Xu L, Xiu Y, Liu F, Liang Y, Wang S (2020) Research progress in conversion of CO2 to valuable fuels. Molecules 25(16):3653. https://doi.org/10.3390/molecules25163653
Xia C, Cai L, Zhang H, Zuo L, Shi SQ, Lam SS (2021) A review on the modeling and validation of biomass pyrolysis with a focus on product yield and composition. Biofuel Res J 8(1):1296–1315. https://doi.org/10.18331/brj2021.8.1.2
Stąsiek J, Szkodo M (2020) Thermochemical conversion of biomass and municipal waste into useful energy using advanced HiTAG/HiTSG technology. Energies 13(16):4218. https://doi.org/10.3390/en13164218
Idriss IM, Ahmed MM, Grema AS, Baba D (2017) Modeling and simulation of pyrolysis process for a beech wood material. Arid Zone J Eng 13(6):710–717
Saif AGH, Wahid SS, Ali MR (2020) Pyrolysis of sugarcane bagasse: the effects of process parameters on the product yields. Mater Sci Forum 1008:159–167. https://doi.org/10.4028/www.scientific.net/msf.1008.159
Papari S, Hawboldt K (2015) A review on the pyrolysis of woody biomass to bio-oil: focus on kinetic models. Renew Sustain Energy Rev 52:1580–1595. https://doi.org/10.1016/j.rser.2015.07.191
Januszewicz K, Kazimierski P, Klein M, Kardaś D, Łuczak J (2020) Activated carbon produced by pyrolysis of waste wood and straw for potential wastewater adsorption. Materials 13(9):2047. https://doi.org/10.3390/ma13092047
Aqsha A, Tijani MM, Moghtaderi B, Mahinpey N (2017) Catalytic pyrolysis of straw biomasses (wheat, flax, oat and barley) and the comparison of their product yields. J Anal Appl Pyrol 125:201–208. https://doi.org/10.1016/j.jaap.2017.03.022
Mulimani HV (2017) Production of solid fuel biochar from de-oiled seed cake by pyrolysis. IOSR J Mech Civ Eng 14(02):57–61. https://doi.org/10.9790/1684-1402035761
Sipra AT, Gao N, Sarwar H (2018) Municipal solid waste (MSW) pyrolysis for bio-fuel production: a review of effects of MSW components and catalysts. Fuel Process Technol 175:131–147. https://doi.org/10.1016/j.fuproc.2018.02.012
Świechowski K, Syguła E, Koziel JA, Stępień P, Kugler S, Manczarski P, Białowiec A (2020) Low-temperature pyrolysis of municipal solid waste components and refuse-derived fuel—process efficiency and fuel properties of carbonized solid fuel. Data 5(2):48. https://doi.org/10.3390/data5020048
Lofrano G, Brown J (2010) Wastewater management through the ages: a history of mankind. Sci Total Environ 408(22):5254–5264. https://doi.org/10.1016/j.scitotenv.2010.07.062
Parkash V, Singh S (2020) A review on potential plant-based water stress indicators for vegetable crops. Sustainability 12(10):3945. https://doi.org/10.3390/su12103945
Panwar NL, Paul AS (2020) An overview of recent development in bio-oil upgrading and separation techniques. Environ Eng Res 26(5):200382–0. https://doi.org/10.4491/eer.2020.382
Mizik T, Gyarmati G (2021) Economic and sustainability of biodiesel production—a systematic literature review. Clean Technol 3(1):19–36. https://doi.org/10.3390/cleantechnol3010002
Popp J, Kot S, Lakner Z, Oláh J (2018) Biofuel use: peculiarities and implications. J Secur Sustain Issues 7(3). https://doi.org/10.9770/jssi.2018.7.3(9)
Sarkar JK, Wang Q (2020) Different pyrolysis process conditions of south asian waste coconut shell and characterization of gas, bio-char, and bio-oil. Energies 13(8):1970. https://doi.org/10.3390/en13081970
Pawar A, Panwar NL, Salvi BL (2020) Comprehensive review on pyrolytic oil production, upgrading and its utilization. J Mater Cycles Waste Manage 22(6):1712–1722. https://doi.org/10.1007/s10163-020-01063-w
Jahirul M, Rasul M, Chowdhury A, Ashwath N (2012) Biofuels production through biomass pyrolysis —a technological review. Energies 5(12):4952–5001. https://doi.org/10.3390/en5124952
Xu Z, Xiao X, Fang P, Ye L, Huang J, Wu H, Tang Z, Chen D (2020) Comparison of combustion and pyrolysis behavior of the peanut shells in air and N2: kinetics thermodynamics and gas emissions. Sustainability 12(2):464. https://doi.org/10.3390/su12020464
Tanneberger T, Schimek S, Paschereit CO, Stathopoulos P (2019) Combustion efficiency measurements and burner characterization in a hydrogen-oxyfuel combustor. Int J Hydrogen Energy 44(56):29752–29764. https://doi.org/10.1016/j.ijhydene.2019.05.055
Chunbao X, Baoqiang L, Shushen P, Madhumita BR, Mohammad ST (2018) 1.19 Biomass energy, vol 19, 1st edn. Elsevier Inc., pp 770–794. https://doi.org/10.1016/B978-0-12-809597-3.00121-8
Gvero P, Papuga S, Mujanic I, Vaskovic S (2016) Pyrolysis as a key process in biomass combustion and thermochemical conversion. Therm Sci 20(4):1209–1222. https://doi.org/10.2298/tsci151129154g
Gądek W, Mlonka-Mędrala M, Prestipino M, Evangelopoulos P, Kalisz S, Yang W (2016) Gasification and pyrolysis of different biomasses in lab scale system: a comparative study. In: E3S Web of Conferences, vol 10, 00024. https://doi.org/10.1051/e3sconf/20161000024
Jayaraman K, Gökalp I (2015) Pyrolysis, combustion and gasification characteristics of miscanthus and sewage sludge. Energy Convers Manage 89:83–91. https://doi.org/10.1016/j.enconman.2014.09.058
Chowdhury Z, Krishnan B, Sagadevan S, Rafique R, Hamizi N, Abdul Wahab Y, Khan A, Johan R, Al-douri Y, Kazi S, Tawab Shah S (2018) Effect of temperature on the physical, electro-chemical and adsorption properties of carbon micro-spheres using hydrothermal carbonization process. Nanomaterials 8(8):597. https://doi.org/10.3390/nano8080597
Montoya Arbeláez JI, Chejne Janna F, Garcia-Pérez M (2015) Fast pyrolysis of biomass: a review of relevant aspects. Part I: parametric study. DYNA 82(192):239–248. https://doi.org/10.15446/dyna.v82n192.44701
Panchasara H, Ashwath N (2021) Effects of pyrolysis bio-oils on fuel atomisation—a review. Energies 14(4):794. https://doi.org/10.3390/en14040794
Fogarassy C, Toth L, Czikkely M, Finger DC (2019) Improving the efficiency of pyrolysis and increasing the quality of gas production through optimization of prototype systems. Resources 8(4):182. https://doi.org/10.3390/resources8040182
Álvarez-Chávez BJ, Godbout S, Le Roux T, Palacios JH, Raghavan V (2019) Bio-oil yield and quality enhancement through fast pyrolysis and fractional condensation concepts. Biofuel Res J 6(4):1054–1064. https://doi.org/10.18331/brj2019.6.4.2
Dellarose Boer F, Valette J, Commandre JM, Thevenon MF (2021) Slow pyrolysis of sugarcane bagasse for the production of char and the potential of its by-product for wood protection. J Renew Mater 9(1):97–117. https://doi.org/10.32604/jrm.2021.013147
Varma AK, Shankar R, Mondal P (2012) A review on pyrolysis of biomass and the impacts of operating conditions on product yield, quality, and upgradation. Springer, pp 227–259. https://doi.org/10.1007/978-981-13-1307-3_10
David E (2020) Evaluation of hydrogen yield evolution in gaseous fraction and biochar structure resulting from walnut shells pyrolysis. Energies 13(23):6359. https://doi.org/10.3390/en13236359
Williams CL, Emerson RM, Tumuluru JK (2017) Biomass compositional analysis for conversion to renewable fuels and chemicals. Energy Convers Manage. https://doi.org/10.5772/65777
Clauser NM, González G, Mendieta CM, Kruyeniski J, Area MC, Vallejos ME (2021) Biomass waste as sustainable raw material for energy and fuels. Sustainability 13(2):794. https://doi.org/10.3390/su13020794
Zhang S, Yang X, Zhang H, Chu C, Zheng K, Ju M, Liu L (2019) Liquefaction of biomass and upgrading of bio-oil: a review. Molecules 24(12):2250. https://doi.org/10.3390/molecules24122250
Fahmi R, Bridgwater A, Donnison I, Yates N, Jones J (2008) The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 87(7):1230–1240. https://doi.org/10.1016/j.fuel.2007.07.026
Demirbas A (2004) Current technologies for the thermo-conversion of biomass into fuels and chemicals. Energy Sources 26(8):715–730. https://doi.org/10.1080/00908310490445562
Demirbaş A (1997) Calculation of higher heating values of biomass fuels. Fuel 76(5):431–434. https://doi.org/10.1016/s0016-2361(97)85520-2
Bridgwater A (2000) Fast pyrolysis processes for biomass. Renew Sustain Energy Rev 4(1):1–73. https://doi.org/10.1016/s1364-0321(99)00007-6
Zanzi R, Bai X, Capdevila P, Bjornbom E (2001) Pyrolysis of biomass in the presence of steam for preparation of activated carbon, liquid and gaseous products. In: 6th World Congress of Chemical Engineering, pp 23–27
Friedl A, Padouvas E, Rotter H, Varmuza K (2005) Prediction of heating values of biomass fuel from elemental composition. Anal Chim Acta 544(1–2):191–198. https://doi.org/10.1016/j.aca.2005.01.041
Chen X, Zhang R, Zhao B, Fan G, Li H, Xu X, Zhang M (2020) Preparation of porous biochars by the co-pyrolysis of municipal sewage sludge and hazelnut shells and the mechanism of the nano-zinc oxide composite and Cu(II) adsorption kinetics. Sustainability 12(20):8668. https://doi.org/10.3390/su12208668
Román S, Libra J, Berge N, Sabio E, Ro K, Li L, Ledesma B, Álvarez A, Bae S (2018) Hydrothermal carbonization: modeling, final properties design and applications: a review. Energies 11(1):216. https://doi.org/10.3390/en11010216
Hagemann N, Spokas K, Schmidt HP, Kägi R, Böhler M, Bucheli T (2018) Activated carbon, biochar and charcoal: linkages and synergies across pyrogenic carbon’s ABCs. Water 10(2):182. https://doi.org/10.3390/w10020182
Basu P (2013) Biomass gasification, pyrolysis, ad torrefaction practical design and theory, 2nd edn. Elsevier Inc. https://doi.org/10.1017/CBO9781107415324.004
Inagaki M, Kang F (2014) Materials science and engineering of carbon fundamentals, 2nd edn. Elsevier Ltd. https://doi.org/10.16309/j.cnki.issn.1007-1776.2003.03.004
Heidarinejad Z, Dehghani MH, Heidari M, Javedan G, Ali I, Sillanpää M (2020) Methods for preparation and activation of activated carbon: a review. Environ Chem Lett 18(2). https://doi.org/10.1007/s10311-019-00955-0
Pallarés J, González-Cencerrado A, Arauzo I (2018) Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenerg 115:64–73. https://doi.org/10.1016/j.biombioe.2018.04.015
Wang B, Zhu C, Zhang Z, Zhang W, Chen X, Sun N, Wei W, Sun Y, Ji H (2016) Facile, low-cost, and sustainable preparation of hierarchical porous carbons from ion exchange resin: An improved potassium activation strategy. Fuel 179:274–280. https://doi.org/10.1016/j.fuel.2016.03.088
Largitte L, Brudey T, Tant T, Dumesnil PC, Lodewyckx P (2016) Comparison of the adsorption of lead by activated carbons from three lignocellulosic precursors. Microporous Mesoporous Mater 219:265–275. https://doi.org/10.1016/j.micromeso.2015.07.005
Dong L, Liu W, Yu Y, Hou L, Gu P, Chen G (2019) Preparation, characterization, and application of macroporous activated carbon (MAC) suitable for the BAC water treatment process. Sci Total Environ 647:1359–1367. https://doi.org/10.1016/j.scitotenv.2018.07.280
Tchikuala EF, Mourao PAM, Nabais JMV (2017) Removal of phenol by adsorption on activated carbon from aqueous solution. Wastes: Solutions, Treatments and Opportunities. Faculty of Engineering of the University of Porto, Porto, Portugal, pp 1–3
Tsoncheva T, Mileva A, Tsyntsarski B, Paneva D, Spassova I, Kovacheva D, Petrov N (2018) Activated carbon from Bulgarian peach stones as a support of catalysts for methanol decomposition. Biomass Bioenerg 109:135–146. https://doi.org/10.1016/j.biombioe.2017.12.022
Zhang H, Gao Z, Liu Y, Ran C, Mao X, Kang Q, Ao W, Fu J, Li J, Liu G, Dai J (2018) Microwave-assisted pyrolysis of textile dyeing sludge, and migration and distribution of heavy metals. J Hazard Mater 355:128–135. https://doi.org/10.1016/j.jhazmat.2018.04.080
Li L, Sato Y, Shimizu T (2015) Promoting effect of PKS ash on activated carbon preparation from cypress Sawdust Liuyun. Int Proc Chem, Biol Environ Eng 51:139–142. https://doi.org/10.7763/IPC
Min HS, Abbas M, Kanthasamy R, Abdul Aziz H, Tay CC (2017) Activated Carbon: Prepared From Various Precursors. Ideal International E - Publication Pvt Ltd
Ahmed MJ (2016) Preparation of activated carbons from date (Phoenix dactylifera L.) palm stones and application for wastewater treatments: review. Process Saf Environ Prot 102:168–182. https://doi.org/10.1016/j.psep.2016.03.010
Menya E, Olupot P, Storz H, Lubwama M, Kiros Y (2018) Production and performance of activated carbon from rice husks for removal of natural organic matter from water: a review. Chem Eng Res Des 129:271–296. https://doi.org/10.1016/j.cherd.2017.11.008
Kecira Z, Benturki A, Daoud M, Benturki O (2018) Effect of chemical activation on the surface properties of apricot stones based activated carbons and its adsorptive properties toward aniline. In: Proceedings of the third international symposium on materials and sustainable development. Springer International Publishing, Springer Nature Switzerland AG, pp 228–240. https://doi.org/10.1007/978-3-319-89707-3_27
Rashidi NA, Yusup S (2017) A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem Eng J 314:277–290. https://doi.org/10.1016/j.cej.2016.11.059
Niksiar A, Nasernejad B (2017) Activated carbon preparation from pistachio shell pyrolysis and gasification in a spouted bed reactor. Biomass Bioenerg 106:43–50. https://doi.org/10.1016/j.biombioe.2017.08.017
Jeguirim M, Belhachemi M, Limousy L, Bennici S (2018) Adsorption/reduction of nitrogen dioxide on activated carbons: textural properties versus surface chemistry—a review. Chem Eng J Biochem Eng J 347:493–504. https://doi.org/10.1016/j.cej.2018.04.063
Ogungbenro AE, Quang DV, Al-Ali K, Abu-Zahra MRM (2017) Activated carbon from date seeds for CO2 capture applications. Energy Procedia 114:2313–2321. https://doi.org/10.1016/j.egypro.2017.03.1370
Gupta TB, Lataye DH (2017) Adsorption of indigo carmine dye onto Acacia nilotica (babool) sawdust activated carbon. J Hazard Toxic Radioact Waste 21(4):04017013. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000365
Dass B, Jha P (2015) Batch adsorption of phenol by improved activated Acacia nilotica branches char: equilibrium, kinetic and thermodynamic studies. Int J ChemTech Res 8:269–279
Zbair M, Ainassaari K, Drif A, Ojala S, Bottlinger M, Pirilä M, Keiski RL, Bensitel M, Brahmi R (2017) Toward new benchmark adsorbents: preparation and characterization of activated carbon from argan nut shell for bisphenol A removal. Environ Sci Pollut Res 25(2):1869–1882. https://doi.org/10.1007/s11356-017-0634-6
Ahmed S, Parvaz M, Johari R, Rafat M (2018) Studies on activated carbon derived from neem (Azadirachta indica) bio-waste, and its application as supercapacitor electrode. Mater Res Express 5(4):045601. https://doi.org/10.1088/2053-1591/aab924
Ahmed MJ (2016) Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption. J Environ Chem Eng 4(1):89–99. https://doi.org/10.1016/j.jece.2015.10.027
Din MI, Ashraf S, Intisar A (2017) Comparative study of different activation treatments for the preparation of activated carbon: a mini-review. Sci Prog 100(3):299–312. https://doi.org/10.3184/003685017x14967570531606
Saygılı H, Guzel F (2018) Novel and sustainable precursor for high-quality activated carbon preparation by conventional pyrolysis: optimization of produce conditions and feasibility in adsorption studies. Adv Powder Technol 29(3):726–736. https://doi.org/10.1016/j.apt.2017.12.014
Niazi L, Lashanizadegan A, Sharififard H (2018) Chestnut oak shells activated carbon: Preparation, characterization and application for Cr (VI) removal from dilute aqueous solutions. J Clean Prod 185:554–561. https://doi.org/10.1016/j.jclepro.2018.03.026
Altintig E, Onaran M, Sarı A, Altundag H, Tuzen M (2018) Preparation, characterization and evaluation of bio-based magnetic activated carbon for effective adsorption of malachite green from aqueous solution. Mater Chem Phys 220:313–321. https://doi.org/10.1016/j.matchemphys.2018.05.077
Islam MA, Tan IAW, Benhouria A, Asif M, Hameed BH (2015) Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem Eng J Biochem Eng J 270:187–195
Wang H, Xie R, Zhang J, Zhao J (2018) Preparation and characterization of distillers’ grain based activated carbon as low cost methylene blue adsorbent: mass transfer and equilibrium modelling. Adv Powder Technol 29(1):27–35. https://doi.org/10.1016/j.apt.2017.09.027
Kumar A, Jena HM (2016) Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys 6:651–658. https://doi.org/10.1016/j.rinp.2016.09.012
Sajjadi SA, Mohammadzadeh A, Tran HN, Anastopoulos I, Dotto GL, Lopičić ZR, Sivamani S, Rahmani-Sani A, Ivanets A, Hosseini-Bandegharaei A (2018) Efficient mercury removal from wastewater by pistachio wood wastes-derived activated carbon prepared by chemical activation using a novel activating agent. J Environ Manage 223:1001–1009. https://doi.org/10.1016/j.jenvman.2018.06.077
Beltrame KK, Cazetta AL, de Souza PSC, Spessato L, Silva TL, Almeida VC (2018) Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol Environ Saf 147:64–71. https://doi.org/10.1016/j.ecoenv.2017.08.034
Jaria G, Silva CP, Oliveira JA, Santos SM, Gil MV, Otero M, Calisto V, Esteves VI (2019) Production of highly efficient activated carbons from industrial wastes for the removal of pharmaceuticals from water—a full factorial design. J Hazard Mater 370:212–218. https://doi.org/10.1016/j.jhazmat.2018.02.053
Shrestha D, Gyawali G, Rajbhandari AR (2009) Preparation and characterization of activated carbon from waste sawdust from saw mill. J Hazard Mater 165:481–485. https://doi.org/10.1016/j.jhazmat.2008.10.011
Tsoncheva T, Mileva A, Tsyntsarski B, Paneva D, Spassova I, Kovacheva D, Velinov N, Karashanova D, Georgieva B, Petrov N (2018) Activated carbon from Bulgarian peach stones as a support of catalysts for methanol decomposition. Biomass Bioenerg 109:135–146. https://doi.org/10.1016/j.biombioe.2017.12.022
Ozbay N, Yargic AS (2016) Comparison of surface and structural properties of carbonaceous materials prepared by chemical activation of tomato paste waste: the effects of activator type and impregnation ratio. J Appl Chem 2016:1–10. https://doi.org/10.1155/2016/8236238
Li K, Ruan H, Ning P, Wang C, Sun X, Song X, Han S (2018) Preparation of walnut shell-based activated carbon and its properties for simultaneous removal of H2S, COS and CS2 from yellow phosphorus tail gas at low temperature. Res Chem Intermed 44(2):1209–1233. https://doi.org/10.1007/s11164-017-3162-6
Bian Y, Yuan Q, Zhu G, Ren B, Hursthouse A, Zhang P (2018) Recycling of waste sludge: preparation and application of sludge-based activated carbon. Int J Polym Sci 2018:1–17. https://doi.org/10.1155/2018/8320609
Maguana Y, El E, N., Bouchdoug, M., & Benchanaa, M. (2018) Study of the influence of some factors on the preparation of activated carbon from walnut cake using the fractional factorial design. J Environ Chem Eng 6(1):1093–1099. https://doi.org/10.1016/j.jece.2018.01.023
Amin MT, Alazba AA (2017) Comparative study of the absorptive potential of raw and activated carbon Acacia nilotica for Reactive Black 5 dye. Environ Earth Sci 76:581. https://doi.org/10.1007/s12665-017-6927-8
Rashidi NA, Yusup S (2017) A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem Eng J Biochem Eng J 314:277–290. https://doi.org/10.1016/j.cej.2016.11.059
Shen Y, Fu Y (2018) KOH-activated rice husk char via CO2 pyrolysis for phenol adsorption. Materials Today Energy 9:397–405. https://doi.org/10.1016/j.mtener.2018.07.005
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J Clean Prod 175:361–375. https://doi.org/10.1016/j.jclepro.2017.12.059
Rivera-Utrilla J, Sánchez-Polo M, Ferro-García MN, Prados-Joya G, Ocampo-Pérez R (2013) Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 93(7):1268–1287. https://doi.org/10.1016/j.chemosphere.2013.07.059
Briones R, Serrano L, Younes RB, Mondragon I, Labidi J (2011) Polyol production by chemical modification of date seeds. Ind Crops Prod 34(1):1035–1040. https://doi.org/10.1016/j.indcrop.2011.03.012
Fu X, Yang H, Lu G, Tu Y, Wu J (2015) Improved performance of surface functionalized TiO2/activated carbon for adsorption–photocatalytic reduction of Cr(VI) in aqueous solution. Mater Sci Semicond Process 39:362–370. https://doi.org/10.1016/j.mssp.2015.05.034
Jamshidi M, Ghaedi M, Dashtian K, Ghaedi A, Hajati S, Goudarzi A, Alipanahpour E (2016) Highly efficient simultaneous ultrasonic assisted adsorption of brilliant green and eosin B onto ZnS nanoparticles loaded activated carbon: artificial neural network modeling and central composite design optimization. Spectrochim Acta Part A Mol Biomol Spectrosc 153:257–267. https://doi.org/10.1016/j.saa.2015.08.024
Sahu UK, Sahu S, Mahapatra SS, Patel RK (2017) Cigarette soot activated carbon modified with Fe3O4 nanoparticles as an effective adsorbent for As(III) and As(V): material preparation, characterization and adsorption mechanism study. J Mol Liq 243:395–405. https://doi.org/10.1016/j.molliq.2017.08.055
Anfar Z, Zbair M, Ahsaine HA, Ezahri M, Alem NE (2018) Well-designed WO3/Activated carbon composite for Rhodamine B Removal: synthesis, characterization, and modeling using response surface methodology. Fullers Nanotub Carbon Nanostruct 26(6):389–397. https://doi.org/10.1080/1536383x.2018.1440386
Tuan TQ, Son NV, Dung HTK, Luong NH, Thuy BT, Anh NTV, Hoa ND, Hai NH (2011) Preparation and properties of silver nanoparticles loaded in activated carbon for biological and environmental applications. J Hazard Mater 192(3):1321–1329. https://doi.org/10.1016/j.jhazmat.2011.06.044
Khosravi R, Moussavi G, Ghaneian MT, Ehrampoush MH, Barikbin B, Ebrahimi AA, Sharifzadeh G (2018) Chromium adsorption from aqueous solution using novel green nanocomposite: Adsorbent characterization, isotherm, kinetic and thermodynamic investigation. J Mol Liq 256:163–174. https://doi.org/10.1016/j.molliq.2018.02.033
Burakov AE, Galunin EV, Burakova IV, Kucherova AE, Agarwal S, Tkachev AG, Gupta VK (2018) Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol Environ Saf 148:702–712. https://doi.org/10.1016/j.ecoenv.2017.11.034
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Reviews 4(1):37–59. https://doi.org/10.1002/cben.201600010
Yusuff, A. S. (2018). Optimization of adsorption of Cr(VI) from aqueous solution by Leucaena leucocephala seed shell activated carbon using design of experiment. Appl Water Sci 8(8). https://doi.org/10.1007/s13201-018-0850-3
Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, Wang H, Ok YS, Jiang Y, Gao B (2019) Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem Eng J 366:608–621. https://doi.org/10.1016/j.cej.2019.02.119
Alagumuthu G, Rajan M (2010) Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon. Chem Eng J 158(3):451–457. https://doi.org/10.1016/j.cej.2010.01.017
Viswanathan G, Jaswanth A, Gopalakrishnan S, Siva Ilango S (2009) Mapping of fluoride endemic areas and assessment of fluoride exposure. Sci Total Environ 407(5):1579–1587. https://doi.org/10.1016/j.scitotenv.2008.10.020
Namasivayam C, Sangeetha D (2006) Removal of molybdate from water by adsorption onto ZnCl2 activated coir pith carbon. Biores Technol 97(10):1194–1200. https://doi.org/10.1016/j.biortech.2005.05.008
Oladipo AA, Ifebajo AO (2018) Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: Two-stage adsorber analysis. J Environ Manage 209:9–16. https://doi.org/10.1016/j.jenvman.2017.12.030
Bernhardt A, Gysi N (2016) World’s worst pollution problems: the toxics beneath our feet. Fabrikstrasse 17:8005. Zurich, Switzerland
Ahmed TF, Sushil M, Krishna M (2012) Impact of dye industrial effluent on physicochemical characteristics of Kshipra River, Ujjain City, India. Int Res J Environ Sci 1:41–45
Sharma P, Kaur H, Sharma M, Sahore V (2011) A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ Monit Assess 183(1–4):151–195. https://doi.org/10.1007/s10661-011-1914-0
Katheresan V, Kansedo J, Lau SY (2018) Efficiency of various recent wastewater dye removal methods: a review. J Environ Chem Eng 6(4):4676–4697. https://doi.org/10.1016/j.jece.2018.06.060
Danish M, Hashim R, Ibrahim MNM, Sulaiman O (2013) Characterization of physically activated Acacia mangium wood-based carbon for the removal of methyl orange dye. BioResources 8(3). https://doi.org/10.15376/biores.8.3.4323-4339
Wang H, Xie R, Zhang J, Zhao J (2018) Preparation and characterization of distillers’ grain based activated carbon as low cost methylene blue adsorbent: mass transfer and equilibrium modeling. Adv Powder Technol 29(1):27–35. https://doi.org/10.1016/j.apt.2017.09.027
Ahmed MJ (2016) Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption: review. J Environ Chem Eng 4(1):89–99. https://doi.org/10.1016/j.jece.2015.10.027
Hammani H, Boumya W, Laghrib F, Farahi A, Lahrich S, Aboulkas A, El Mhammedi M (2017) Electrocatalytic effect of NiO supported onto activated carbon in oxidizing phenol at graphite electrode: application in tap water and olive oil samples. J Assoc Arab Univ Basic Appl Sci 24(1):26–33. https://doi.org/10.1016/j.jaubas.2017.06.006
Theydan SK, Ahmed MJ (2012) Adsorption of methylene blue onto biomass-based activated carbon by FeCl3 activation: equilibrium, kinetics, and thermodynamic studies. J Anal Appl Pyrol 97:116–122. https://doi.org/10.1016/j.jaap.2012.05.008
Chowdhury ZZ, Abd Hamid, SB, Das R, Hasan MR, Zain SM, Khalid K, Uddin, MN (2013) Preparation of carbonaceous adsorbents from lignocellulosic biomass and their use in removal of contaminants from aqueous solution. BioResources 8(4). https://doi.org/10.15376/biores.8.4.6523-6555
González-García P (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sustain Energy Rev 82:1393–1414. https://doi.org/10.1016/j.rser.2017.04.117
El Gamal M, Mousa HA, El-Naas MH, Zacharia R, Judd S (2018) Bio-regeneration of activated carbon: a comprehensive review. Sep Purif Technol 197:345–359. https://doi.org/10.1016/j.seppur.2018.01.015
Salman JM, Hussein FH (2014) Batch adsorber design for different solution volume/adsorbate mass ratios of bentazon, carbofuran and 2,4-D adsorption on to date seeds activated carbon. J Environ Anal Chem 02(01). https://doi.org/10.4172/2380-2391.1000120
Ahmed MJ (2017) Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons: review. J Environ Manage 190:274–282. https://doi.org/10.1016/j.jenvman.2016.12.073
Boudrahem N, Delpeux-Ouldriane S, Khenniche L, Boudrahem F, Aissani-Benissad F, Gineys M (2017) Single and mixture adsorption of clofibric acid, tetracycline and paracetamol onto activated carbon developed from cotton cloth residue. Process Saf Environ Prot 111:544–559. https://doi.org/10.1016/j.psep.2017.08.025
Mansour F, Al-Hindi M, Yahfoufi R, Ayoub GM, Ahmad MN (2017) The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: a review. Rev Environ Sci Bio/Technol 17(1):109–145. https://doi.org/10.1007/s11157-017-9456-8
Chayid MA, Ahmed MJ (2015) Amoxicillin adsorption on microwave prepared activated carbon from Arundo donax Linn: isotherms, kinetics, and thermodynamics studies. J Environ Chem Eng 3(3):1592–1601. https://doi.org/10.1016/j.jece.2015.05.021
Acknowledgements
Authors are grateful to the University Malaya, Malaysia for the financial support (Grant IF 065-2021 and IF 073-2019).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ali, A.E., Chowdhury, Z.Z., Faisal, A.N., Das, R., Wahab, Y.A., Ramakrishnan, S. (2022). Thermochemical Conversion of Lignocellulosic Waste to Activated Carbon: A Potential Resource for Industrial Wastewater Treatment. In: Das, R., Saha, B.B. (eds) Rapid Refrigeration and Water Protection. Springer Water. Springer, Cham. https://doi.org/10.1007/978-3-030-93845-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-93845-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93844-4
Online ISBN: 978-3-030-93845-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)