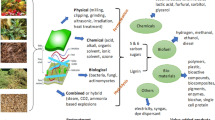
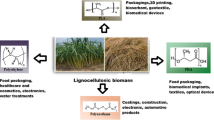
Abstract
Lignocellulosic feedstocks are gaining increased popularity for novel industrial applications because of their availability and bio-renewability. Using lignocellulosic materials, especially from agricultural and forestry sectors could help reduce the over-dependence on petrochemical resources while providing a sustainable waste management alternative. This review aims to describe the chemistry of different components of lignocellulosic biomass (cellulose, hemicellulose, lignin, extractives and ash). Besides, many novel industrial applications of lignocellulosic biomass have been comprehensively described, which includes biorefining for biofuel and biochemical production, biomedical, cosmeceuticals and pharmaceuticals, bioplastics, multifunctional carbon materials and other eco-friendly specialty products. The production and applications of lignocellulose-derived carbon materials such as activated carbon, carbon nanotubes, carbon nanohorns, etc. have been highlighted. The potential industrial utility of cellulose and lignin-based specialty materials such as cellulose fiber, bacterial cellulose, epoxides, polyolefins, phenolic resins, bioplastics are discussed in this review. The cutting-edge industrial utilization of lignocellulosic biomass described in this review suggests its major role in establishing a circular bioeconomy that consists of innovative design and advanced production methods to facilitate industrial recovery and reuse of waste materials beyond biofuel and biochemical production.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Biomass-based economy seems most practical to complement the existing fossil fuel-based energy systems and products (e.g. petrochemicals). Lignocellulosic biomass such as agricultural and forestry residues seems to be promising for the production of eco-friendly biofuels and biochemicals as well as carbon-neutral products for utility in biomedical, pharmaceutical, cosmeceutical and other specialty material industries. We present advanced literature that reveals the chemistry of lignocelluloses, conversion processes, product characteristics and cutting-edge applications. This article reviews novel concepts in which lignocellulosic biomass is converted to a variety of useful products (e.g. biofuels, biochemicals, specialty materials and multifunctional carbon materials) through environmentally benign processes. For a successful practical option, biomass conversion must be accomplished in a cost-effective and energy-efficient manner without creating ecological risks. This is precisely what this review article has to offer..
Introduction
The increasing energy demand due to the rise in world population has rekindled the interest in the search for renewable alternatives such as biofuels, biochemicals and biomaterials. Fossil fuel resources are used predominantly to quench the elevating energy demands and to produce a wide range of synthetic polymers, cosmetic products and platform chemicals used to improve the quality of life [1, 2]. In addition, the diminishing fossil fuel resources together with their alarming environmental challenges such as greenhouse gas emissions have stimulated interest in the utilization of renewable materials [3, 4]. Although the use of petroleum products have increased the global industrialization by quenching the energy demands, their adverse effects cannot be neglected [5, 6].
Solar, wind and geothermal sources are few examples of renewable energy resources that can complement heat and power (electricity) generation. However, they are characterized by unpredictable profiles largely influenced by seasonal or environmental variations and, most importantly, they cannot produce synthetic transportation fuels. On the other hand, waste organic biomass is inexpensive, easily available and has proven to be a good source to generate eco-friendly fuels, chemicals and materials [7]. It is known that biomass may help reduce detrimental environmental impacts caused by the usage of fossil fuels and their derivatives.
About 84 million barrels of crude oil is utilized per day in the energy and transportation sector, which is projected to increase up to 116 million barrels by 2030 [8]. Currently, the global consumption of fossil fuels as a primary energy source is nearly 13,865 million tons of oil equivalent with a growth rate of 2.9% per annum [9]. Nevertheless, the consumption of renewable energy (accounting from solid biomass, biofuels, landfill gas, solar, wind, tidal and geothermal) has grown considerably by 14% in 2018 comprising 9% of the global electricity supply [10]. Presently, in the plastic manufacturing industries, fossil fuels account for 99% of the raw materials base [11] but only 4% of the global fossil fuel resources are used for plastic production [8]. However, the application of waste biomass for the manufacturing of bioplastics has been growing rapidly over the years, which had reached 2.1 million tons in 2018 [11]. Additionally, waste biomass is estimated to supplement nearly 30% of the raw materials used in the chemical industry by 2025 [12]. Using fuels, chemicals and materials derived from waste renewable biomass, especially lignocellulosic residues could reduce the dependency on petrochemical resources and help mitigate climate change while addressing the issues of the clean environment, energy security and rural development [5]. It is projected that the research and development in biomass resources could result in more than 30% of fossil fuel replacement in the near future for biofuels, biochemicals and biomaterials.
Lignocellulosic feedstocks refer to the non-edible plant materials that consist of three main building blocks (i.e. cellulose, hemicellulose and lignin) along with extractives [13]. Extractives consist of fats, lipids, resins, steroids, tannins, terpenes, terpenoids, phenolic and flavonoid compounds [14]. Approximately 90% of dry matter found in lignocellulosic biomass comprises of cellulose (35–55 wt%), hemicellulose (20–40 wt%) and lignin (10–25 wt%) as shown in Table 1. Lignocellulosic biomass can be primarily classified into crop residues (e.g. rice straw, wheat straw, cotton stalk, oat hall, rice hall, corn stover, etc.); forestry biomass (e.g. wood chips, wood logs, bark, sawdust, etc.), dedicated energy crops (e.g. switchgrass, timothy grass, elephant grass, poplar, willow, etc.) [32]. Lignocellulosic biomass is a promising feedstock for the sustainable production of fuels, chemicals and materials due to their non-seasonal availability and socioeconomic advantages. Moreover, lignocellulosic feedstocks are non-edible and do not pose any competition to food crops and arable lands, thus being considered as next-generation feedstocks [33]. However, the supply of lignocellulosic biomass for biorefining and biomanufacturing could also be limited due to several factors such as geography, seasonal availability, scattered nature of residue generation and differences in local situations [34, 35].
Lignocellulosic biomass can be converted to biofuels through several thermochemical conversion technologies (e.g. pyrolysis, liquefaction, gasification, torrefaction and carbonization) and biochemical conversion technologies (e.g. anaerobic digestion and fermentation) into biofuels and biochemicals [13, 36]. Significant literature is available on the pros and cons of replacing fossil fuels with biofuels and with co-processing both the fuels together with different biofuel upgrading processes [37]. Besides, lignocellulosic biomass has the potential to substitute plastic and other petrochemical polymeric materials. Lignocellulosic feedstocks are heterogeneous materials comprising of organic and inorganic (mineral) matter, and their interactions can considerably affect their conversion to biofuels, biochemicals and biomaterials. The literature on lignocellulosic biomass conversion to biofuels and biochemicals is accessible, whereas there is negligible information available on their material applications. With this objective, this article makes a comprehensive review of the chemistry of different components present in lignocellulosic biomass (i.e. cellulose, hemicellulose, lignin, extractives, ash and minerals) in addition to their novel industrial applications in the production of biofuels, biochemicals, biomaterials and multifunctional specialty products with high industrial relevance. The information provided in this article aims to fill the gaps in the literature on the value-added industrial applications of lignocellulosic biomass.
Composition of Lignocellulosic Biomass
Cellulose
Cellulose is often regarded as the most abundant renewable organic resource available on earth with an annual production of approximately 7.5 × 1010 tons [38]. It is widely distributed in terrestrial plants, marine algae and bacteria. Cellulose is a polymer of glucose comprising of numerous β-1,4-linked d-glucose subunits joined together by glycosidic linkages, van der Waals forces and hydrogen bonds [39] (Fig. 1). Celluloses are hexose sugars represented by the chemical formula (C6H10O5)n where ‘n’ is the number of glucose groups present in a molecule. The degree of polymerization of cellulose is in the range of 1510–5500 [40]. Cellulose is a fibrous substance with the molecules held together by strong hydrogen bonding, which helps the plant cell walls to maintain their structure [41]. Cellulose is a long-chain polysaccharide typically consisting of 7000–15,000 units of glucose monomers [42]. Cellulose has a molecular weight of 342.3 g/mol [43].
The biosynthesis of cellulose produces individual molecules, which then undergo spinning in an ordered manner at the site of synthesis. The aggregation of multiple cellulose chains to produce fibrils is enhanced by intermolecular hydrogen bonds between hydroxyl groups and oxygen of neighboring molecules [41]. The fibrils are further arranged into large units known as microfibrils (diameter of 5–50 nm and length of several microns), which are in turn packed into the cellulose fibers [38]. Cellulose microfibrils are composed of crystalline linear assemblies of β-(1 → 4)-d-glucan chains attached by hydrogen bonds. The arrangement of each glucan chain starts and ends at different positions within the plant cell walls. Hence, their length could extend to several hundred micrometers. The degree of hydrogen bonding between the glucan chains indicates the degree of crystallinity within the fibrils. A highly structured three-dimensional lattice is produced when all the available sites for hydrogen bonding between the chains are filled. On the other hand, when the glucans are cross-linked, they are structured in a para-crystalline form with a less ordered structure.
Cellulose microfibrils are seen as the basic building unit of plant cell walls, although their structure is largely dependent on the biosynthesis process, which differs based on the plant species. For example, the cellulose microfibrils obtained from plant cell walls reveal an average diameter range of 2–4 nm [44, 45]. On the other hand, cellulose microfibrils found in bacteria have a relatively large diameter of 4–8 nm [45]. Some regions exist within the cellulose fibrils where the chains are highly ordered (crystalline) or disordered (amorphous) [41]. In the crystalline regions, cellulose chains are strongly packed together in the form of crystallites stabilized by strong intramolecular and intermolecular hydrogen-bonding networks [38]. The variation in the molecular arrangement and hydrogen-bonding network in cellulose leads to the formation of cellulose polymorphs.
Six interconvertible polymorphs of crystalline cellulose have been reported such as cellulose I, cellulose II, cellulose III1, cellulose III2, cellulose IV1 and cellulose IV2 [46]. Cellulose I, also known as the native or natural cellulose, is the polymorph found naturally in bacteria, algae and plants. It has a thermodynamically metastable structure that can be transformed into either cellulose II or III [41, 45, 46]. Cellulose II is known to exhibit a monoclinic structure with applications in the production of cellophane and synthetic textile fibers [47]. Cellulose II can be produced from cellulose I by regeneration (i.e. solubilization in a solvent followed by re-crystallization) or mercerization (i.e. treatment with aqueous sodium hydroxide) [46]. Liquid ammonia or amine treatments of celluloses I and II can lead to the formation of cellulose III1 and III2, respectively while subsequent heating of cellulose III1 and III2 up to 206 °C in glycerol could produce cellulose IV1 and IV2. Cellulose I is further divided into two crystalline forms known as cellulose Iα and cellulose Iβ. A para-crystalline form of cellulose has also been reported, which has greater mobility and less ordered arrangement compared to cellulose Iα and Iβ [48, 49].
Hemicellulose
Hemicellulose is another biopolymer in lignocellulosic biomass that comprises of polysaccharide mixtures such as pentose sugars (e.g. xylose and arabinose), hexose sugars (e.g. glucose, mannose and galactose) and sugar acids (e.g. glucuronic acid and galacturonic acid). Compared to cellulose, hemicellulose can be degraded easily in the acidic or hot aqueous medium due to its lower degree of polymerization (50–200) and amorphous structure [50]. Hemicellulose is a relatively short-chained matrix polysaccharide typically made up of 500–3000 sugar monomers with acidic groups [42]. One of the predominantly found hemicellulosic sugars, i.e. xylose (C5H10O5) and has a molecular weight of 150.1 g/mol [51].
Xylans are known to be the most abundant polysaccharides of hemicelluloses with polymeric chains comprising of 1,4-linked β-d-xylose units. The composition of xylan differs based on its source. For example, xylan produced from birch wood contains 89.3 wt% xylose, 1.4 wt% glucose, 1 wt% arabinose and 8.3 wt% anhydrouronic acid [52]. While xylan obtained from corn fiber contains 48–54 wt% xylose, 33–35 wt% arabinose, 5–11 wt% galactose and 3–6 wt% glucuronic acid [53]. Arabinoxylan from wheat contains 65.8 wt% xylose, 33.5 wt% arabinose, 0.3 wt% glucose, 0.1 wt% mannose and 0.1 wt% galactose [54]. Xyloglucans is another component of hemicellulose that contains β-1,4 linked d-glucopyranose units, which is similar to that of cellulose. Unlike cellulose, about 75% of the glucosyl residues of xyloglucan are substituted with α-D-xylopyranose on their main chains [50]. In general, xylose, glucose and galactose are the main components of xyloglucans in the ratio 3:4:1 respectively [55].
Mannan compounds present in hemicellulose include glucomannan, galactomannan, glucuronic acid and galacturonic acid [55]. The residues of mannose are linked together by β-1,4 bonds while galactomannan is formed when mannose residues are connected to galactose residues by α-1,6 bonds. Glucomannan comprises of connected chains of glucose and mannose with a residual ratio of 1:3 [55]. Since it contains a single galactose as the branched-chain, it is often referred to as galactoglucomannan. Arabinoglucuronoxylans (also known as arabino-4-O-metylglucuronoxylans) are mostly present as the major components of non-woody biomass such as agricultural crop residues and also present as minor quantities in softwoods [56]. In general, they also contain linear β-1,4-d-xylopyranose as the backbone chains with α-l-arabinofuranosyl and glucopyranosyl uronic acid, which are connected by α-1,2 and α-1,3 glycosidic bonds [56]. The chemical structures of the different building blocks of hemicellulose are shown in Fig. 2.
The content of hemicellulose and other polysaccharides are highly dependent on the sources of lignocellulosic biomass. Softwoods and hardwoods typically have hemicellulose contents of 18–23 wt% and 10–15 wt%, respectively, while herbaceous plants contain 20–25 wt% of hemicellulose [57]. In terms of the polysaccharides present in lignocellulosic biomass, hemicellulose from softwoods consists mainly of xyloglucan, galactoglucomannan and arabinoglucuronoxylan while the hardwood hemicellulose primarily comprises of glucuronoxylan, glucomannan and xyloglucan [56]. On the other hand, hemicellulose from herbaceous plants mostly consists of xyloglucan and glucuronoarabinoxylan [58].
Lignin
Lignin is a polymer of phenylpropane monomeric units, which are randomly and non-linearly linked together by ester bonds. A few ester bonds can also be found in the end units of the monomers within the ferulic acid and p-coumaric units [59, 60]. The strength and rigidity are provided to the plants by the cross-linking of lignin molecules with cellulose and hemicellulose polymers [61, 62]. The three main phenyl propane monomers in lignin are p-coumaryl alcohol (4-hydroxycinnamyl alcohol), coniferyl alcohol (3-methoxy-4-hydroxycinnamyl alcohol) and sinapyl alcohol (3,5-dimethoxy-4-hydroxycinnamyl alcohol) [63]. They are also known as the p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units because they are polymerized by hydroxyphenyl propane, guaiacyl propane and syringyl propane, respectively [50, 56]. The basic structural units of the three monomers of lignin are illustrated in Fig. 3. Lignin composition and amount also vary among different lignocellulosic biomasses. For instance, the lignin content in grasses, hardwood and softwood is in the order: grasses < hardwood < softwood [63]. Besides, hardwood lignin contains a significant amount of methoxyl components due to the presence of almost the same proportions of syringyl and guaiacyl units. On the other hand, softwood lignin contains about 90 wt% of guaiacyl units.
The macromolecules of lignin consist of branched chains of phenolic compounds with the phenyl propane molecules connected by the C–C and C–O–C linkages. The compounds such as phenolic, methoxyl, hydroxyl and terminal aldehyde groups are present in their side chains [13]. The highly branched, cross-linked and polymeric nature of lignin contributes to its higher molecular weight. It is noteworthy that the molecular weight of lignin is dependent on several factors such as the inherent properties (e.g. source and origin) and biochemical composition of the biomass as well as the isolation and purification methods for lignin. For example, the molecular weight for organosolv lignin (C81H92O28) is 1513.6 g/mol [64]. Lignin also has a calorific value in the range of 23.3–25.6 MJ/kg, which is nearly 30% higher than that of cellulose and hemicellulose together [65]. Since cellulose and hemicellulose sugars have a high degree of oxidation than lignin, they tend to have a lower calorific value and energy density than lignin [66].
The presence of lignin in the biomass makes it very difficult to extract the cellulose and hemicellulose to produce fermentable sugars. Therefore, it is important to remove the lignin to expose the cellulose microfibrils and hemicellulose sugars for hydrolysis and fermentation to alcohols using enzymes and microorganisms [67]. Due to its hydrophobic nature, lignin is insoluble in water under ambient conditions. However, it is only soluble in alkaline solutions, ionic liquids and appropriate extraction solvents when using organosolv technologies. Alkaline solutions cleave the ferulic acid cross-link between lignin and hemicelluloses and degrade the lignin. Alkaline solutions also alter the polyelectrolyte properties of lignin induced by free carboxy1 groups of phenolic acids ethers [68].
The exact structure of the proto-lignin (i.e. untreated lignin naturally found in the plants) remains unclear. In most cases, the structure of lignin is modified during isolation, which is different from that of the proto-lignin [69]. Furthermore, several newer methods have been developed for the elucidation of structural structures of native and modified lignins, which include Fourier-transform infrared (FTIR) spectroscopy [39], near-infrared (NIR) spectroscopy [70], mid-infrared spectroscopy [71], ultraviolet (UV) spectrophotometry [72], Raman spectroscopy [39], nuclear magnetic resonance (NMR) spectroscopy [73], X-ray photoelectron spectroscopy (XPS) [74] and mass spectrometry (MS) methods (e.g. MS with electrospray ionization, atmospheric pressure photoionization and matrix‐assisted laser desorption/ionization and tandem MS) [69, 75].
The functional groups present in lignin molecules as well as the chemical structure of lignin are highly dependent on the isolation method. For example, lignin produced from the Kraft process (i.e. Kraft lignin) has relatively lower sulfur impurities while the lignin obtained during the lignosulfonate process contains a substantial amount of sulfonate groups [63]. Pure and unaltered lignins are produced from the organosolv lignin production process due to the absence of harsh reaction conditions. Furthermore, the organosolv process does not use sulfur-based chemicals as compared to the lignosulfonate or Kraft process [62, 76]. The organosolv process involves the use of organic solvents such as aliphatic alcohols (e.g. ethanol, butanol, methanol, ethylene glycol) and aromatic alcohols (e.g. phenol) to solubilize lignin typically at 170–220 °C [62]. Aromatic alcohols are found to be more effective in lignin solubilization when compared to aliphatic alcohols [76]. Lewis acid catalysts (e.g. FeCl3, FeCl2 and CuCl2) are often used to elevate delignification rates or catalyze the reactions at relatively lower temperatures [77]. The Alcell® process is often regarded as one of the most popular organosolv processes. The process developed by GE Corporation in the 1970s using ethanol–water mixtures to delignify wood [78]. The Alcell® process separates cellulose, hemicelluloses and lignin in different streams with relatively high purity. The molecular weight and chemical formula of lignin produced from different sources are shown in Table 2.
Steam explosion is one of the widely used biomass pretreatment processes [80], which involves rupturing the lignocellulosic matrix within the biomass cell wall under pressurized hydrothermal conditions (180–240 °C and 1–3 MPa), leading to subsequent extraction of lignin through alkali washing or other appropriate methods [81]. A few examples of large-scale industrial processes that use steam explosion treatment include the Beta Renewables plant in Crescentino Italy, Poet DSM plant in Iowa, U.S. and the GranBio plant in Alagoas, Brazil [82].
Klason lignin refers to the insoluble portion of the lignocellulosic biomass after the removal of ash [83]. It involves a series of key steps such as: (i) ethanol or benzene extraction, (ii) addition of 72% H2SO4 at 30 °C for 4 h, (iii) dilution of the acidic reaction medium up to 3% with reflux for 2 h, and (iv) recovery of acid-insoluble lignin [83, 84]. During the alkaline oxidation process, oxygen and hydrogen peroxide are used to oxidize and solubilize lignin [79]. However, in the alkaline oxidation process, relatively lower delignification rates have been reported [62].
Extractives
Extractives are the non-structural components of lignocellulosic biomass, which are soluble in water or neutral organic solvents such as ethanol and hexane. Extractives are sources of diverse biopolymers such as terpenes, terpenoids, steroids, fats, lipids, proteins, waxes and phenolic compounds such as lignans, tannins and stilbenes [85]. The amounts of extractives vary between different lignocellulosic biomass as illustrated in Table 1.
Terpenoids (also known as isoprenoids due to their isoprene-derived structures) are popular commercially because of their utilization in food flavors and fragrance (in cosmetics and perfumes) and medicinal properties (e.g. cannabinoids). With more than 40,000 molecules, terpenoids are regarded as the largest and the most diverse group secondary metabolites produced by plants [86]. They can be classified based on the number of isoprene structures as monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30) and tetraterpenes (C40) [87]. The phenolic compounds help in protecting the biomass from microbial and fungal attacks, although they are usually eroded as resins from live trees. They have chemical structures identical to that of lignin with a larger amount of syringol and guaiacol moieties.
Pectin can be found between the microfilaments of cellulose in the cell wall [50]. Pectin can be classified into pectic acid, protopectin and pectin. Pectic acid is soluble in water and can form a gel of pectate calcium when reacted with calcium. It comprises of a straight-chain connected by an α-1,4 bond by nearly 100 galacturonic acids. On the other hand, protopectin can be found mostly in the primary wall and are insoluble in water. However, under the effect of protopectinase or dilute acids, protopectin can be converted into pectin [50]. Pectin molecules can form a cross-linked network with each other due to the formation of the calcium bridge between its molecules. The network structure plays a very important role in allowing free movement of water in the cells.
Ash and minerals
Ash is the inorganic non-combustible solid residue left behind after the complete burning of biomass residues, which includes elements and minerals such as silicates, carbonates, phosphates, calcium, magnesium, sodium, potassium, etc.[14, 88]. Silica, a major component of biomass ash, is present as silicates in the plants (e.g. chlorite, feldspars, plagioclase, kaolinite, quartz, opal, etc.) [88]. In larger plants like trees, silicates help in providing strength to the supportive tissues such as bark [89]. Ash content varies from different lignocellulosic biomass as shown in Table 1. Typically, woody biomass has a very low ash content compared to that of agricultural residues [14, 50]. Ash could also contain some unburnt carbon produced as a result of incomplete combustion and inefficient fuel usage. Demirbas [90] noted that the ash produced from the boiler contains more than 50% unburnt carbon, which makes it unsuitable for forest recycling. The presence of unburnt carbon in the ash could reduce chemical hardening and increase ash volume significantly [91].
A good understanding of the physical and chemical properties of lignocellulosic ashes is important for the prediction of deposits formation in the boiler during gasification or incineration. Bottom and fly ash are the two types of ash produced during the thermochemical degradation of biomass. They have varying physicochemical properties due to different operating conditions, type of biomass and the biomass conversion systems used [91]. Volatile heavy metals present in the ash could pose severe environmental effects if they are not managed and disposed of properly. The potential utilization of ash is restricted by its heavy metal composition, which depends on the origin of the biomass precursor and the biomass conversion process conditions [92, 93]. Heavy metals are mostly concentrated in the fly ash with a low concentration in bottom ash [94].
Vamvuka [95] studied the thermal behavior of ashes produced from olive kernel in fixed and fluidized bed combustor along with the environmental impacts upon their disposal to local soil. The fluidized bed was loaded with the olive kernel containing 1 wt% moisture at a feed rate of 480 kg/h at 900 °C operating temperature. On the other hand, 0.5 kg of fuel was loaded for the fixed bed with 6 m3/h of air provided in excess to ensure that the combustion is complete after which the fly ash and bottom ash were collected from each reactor for further analysis. The authors observed that the ash produced from the fixed bed olive kernel had high concentrations of Cr, Cu, Zn, Ni and Mn with low concentrations of toxic metals such as Se and Pb. The low concentrations of toxic heavy metals in the ash were attributed to the soil chemistry and mineralogy. On the other hand, the increased extraction rates for Mn, Zr and Cr were associated with the presence of sulfidic organic matter and carbonates in the ash samples.
Novel Industrial Applications of Lignocellulosic Biomass
Biofuels
Biofuels produced from lignocellulosic biomass are considered as carbon–neutral alternatives to conventional petroleum resources in the transportation and energy sector. Biofuels can exist in liquid (e.g. bio-oil, bioethanol, biobutanol, biodiesel, jetfuels, etc.) and gaseous forms (e.g. syngas, biogas, biomethane and biohydrogen). The production of these fuels can be achieved through product-specific biochemical and thermochemical conversion pathways. Pyrolysis, liquefaction, torrefaction, gasification, Fischer–Tropsch synthesis and transesterification are a few examples of thermochemical conversion technologies, whereas biochemical conversion technologies mostly include enzymatic hydrolysis, fermentation and anaerobic digestion [7, 13, 96]. Figure 4 illustrates the thermochemical and biological conversion pathways and several industrially relevant bioproducts.
Pyrolysis and liquefaction are two main thermochemical biomass-to-liquid conversion methods used to obtain bio-oils from biomass. The quantity and quality of bio-oil largely depend on the operating conditions such as temperature, heating rate, pressure, residence time, catalysts, feedstock concentration, reactor type and biomass properties. Compared to slow pyrolysis that yields higher amounts of biochar, fast pyrolysis results in maximum yields of bio-oils due to faster heating rate, shorter vapor residence time and rapid quenching of hydrocarbon vapors [97]. In terms of oxygen content, liquefaction produces an oil with lower oxygen content compared to pyrolysis, thereby demonstrating greater heating value and improved fuel properties.
Bio-oils produced from lignocellulosic biomass contain different proportions of cellulose, hemicellulose and lignin degradation products. Bio-oils are oxygenated liquid products containing varied mixtures of aldehydes, ketones, acids, alcohols, phenols, carbonyls, vanillin, esters, ethers, catechols, pyranones, etc. [98]. Compounds such as alcohols, aldehydes, esters, sugars, ketones and furans are produced during the decomposition of cellulose and hemicellulose [99]. On the contrary, lignin degrades into catechols, guaiacols, syringols and phenolics. Rutkowski [100] studied the chemical structure of bio-oils obtained from the pyrolysis of cellulose, xylan and lignin in a vertical Pyrex reactor at 450 °C with and without ZnCl2 or K2CO3 as catalysts. Bio-oil yield without catalysts was in the order of cellulose (44 wt%) > xylan (32 wt%) > lignin (28.5 wt%). The addition of 10 wt% K2CO3 and ZnCl2 elevated the bio-oil yield with more deoxygenated oils produced from all the three model compounds. The authors concluded that the type of biopolymer and the presence of catalysts have an impact on bio-oil yield. Carboxylic acids, alcohols and phenols were largely present in the bio-oils [101].
Ethanol produced from biomass through enzymatic hydrolysis and microbial fermentation can be blended with gasoline for use as a transportation fuel. Compared to conventional fuels, bioethanol has numerous advantages. Firstly, it contains an oxygen fraction of about 35%, which improves its combustion and minimizes the emission of CO, NO, NOx, particulate matters and hydrocarbons [102]. Secondly, it is produced from renewable biomass materials that are abundant in nature. Moreover, the use of bioethanol as transportation fuels could reduce greenhouse emissions from fossil fuels. It should also be noted that the greater oxygen fraction in bioethanol could lead to a lower energy density, thus demonstrating a lower mileage than gasoline, which requires frequent refueling [103]. The presence of oxygen in bioethanol can become corrosive to the engine if used at higher blending ratios [104].
Bioethanol production from sugar or starch-based feedstocks has adverse effects on the economics, food security and environment because of the diversion of food crops as fuel crops raising food versus fuel debate. In contrast, bioethanol production from lignocellulosic biomass (i.e. non-food crops) has attracted immense attention both from environmental and economic perspective due to no competition to food supply and arable lands. Moreover, bioethanol production from lignocellulosic biomass can boost the rural economy, optimize supply chain and logistics cost, reduce dependency on fossil fuels and enhance energy security [5].
Biobutanol is widely seen as an emerging alternative to bioethanol due to its superior fuel properties and high-energy content (30% more than that of bioethanol) [105, 106]. The fuel properties of bioethanol and butanol are shown in comparison with gasoline in Table 3. Compared to bioethanol, biobutanol can be used in its pure form or blended with gasoline in flexible ratios for use in existing vehicle engines without any mechanical modification. This is in contrast to bioethanol, which requires engine modification for use in pure form. Furthermore, biobutanol is safe to handle due to its non-corrosiveness and low vapor pressure [109]. On the other hand, the non-hygroscopic nature of biobutanol also makes it easier to blend with gasoline at the refinery before storage and distribution. Moreover, the lower solubility of biobutanol with water prevents it from mixing with water during transportation and freezing inside the pipeline or carburetor ducts during cold weather.
Although bioalcohols such as bioethanol and biobutanol are promising biofuels due to their potential to minimize the overdependence on fossil fuels, the overall feasibility of their production process is largely dependent on the separation processes employed [106]. Since the separation process is energy-intensive, it could influence the process economics. While bioethanol can be separated from the fermentation medium through fractional distillation which is commercially used, the separation of biobutanol is more challenging, time-consuming, energy-intensive and costly due to lower product yields. Some of the biobutanol separation technologies include adsorption, pervaporation, perstraction, liquid–liquid extraction, gas stripping and supercritical fluid extraction [110].
Biomethane has emerged as a promising fuel in heavy vehicles due to its low density, higher calorific value and greater thermal efficiency [111]. Biomethane can be produced from different categories of lignocellulosic biomass through anaerobic digestion or biomethanation. Biomethane is employed as a substitute for fossil-derived natural gas for combined heat and power generation or as a source of energy in rural areas for cooking, heating and electricity generation. Compressed biomethane (bio-CNG) exhibits similar properties as compressed natural gas (CNG) in terms of gas consumption, the performance of engine and efficiency when used as a vehicular fuel [111].
It should be noted that when biomethane is used in heavy motor vehicles there is about a 63% reduction in greenhouse gas emission compared to CNG (Table 4) [112, 113]. Nevertheless, the emission level of NOx, hydrocarbons and CO is slightly higher compared to the emissions from CNG. The reason for greater levels of NOx is that biomethane has higher N2 content compared to CNG [111]. In vehicular engines operating with biomethane, low warm-up temperatures reduce the rate at which CO is oxidized to CO2 resulting in high CO emission [114]. Similarly, superior hydrocarbon emissions result from low warm-up temperatures, which cause incomplete combustion because of poor oxidation.
Similar to biomethane, syngas can be used for electricity generation at high-efficiency levels (approximately 85% overall thermal efficiency can be achieved for steam production and close to 40% for electrical efficiency) or as a clean fuel for integrated gasification combined cycle (IGCC) [115]. Syngas consists of mostly H2, CO, CO2 and CH4 with traces of C2+ gases. Syngas can be used to produce liquid hydrocarbon fuels through Fischer–Tropsch synthesis over the transition metal catalysts, which are represented as the d-block elements in the periodic table. The Fischer–Tropsch process is also regarded as one of the most important gas-to-liquid conversion technologies together with syngas fermentation [116]. Compared to the Fischer–Tropsch process, syngas fermentation is a biological conversion technology that uses anaerobic bacteria (acetogens). A few examples of such bacteria are Clostridium spp., Eubacterium limosum and Butyribacterium methylotrophicum. These bacteria use H2 and CO from the syngas as the respective sources of hydrogen and carbon for their metabolism to produce ethanol, butanol and other short-chain alcohols. Syngas fermentation has high selectivity to alcohols and the process does not depend on the H2/CO ratio [117]. Besides, the process is considered more economical due to its operation under ambient temperature without the involvement of heat and metal catalysts as the prerequisites for the Fischer–Tropsch synthesis.
Hydrogen is a clean energy carrier with an extremely high-energy content (142 kJ/kg), which is almost three times that of gasoline (47.5 kJ/kg) [118]. Compared to methane, propane and gasoline, the autoignition temperature of hydrogen is relatively high (585 °C). Nevertheless, its ignition energy at 1.9 × 10–8 Btu is about an order of magnitude lower leading to its easy ignition [119]. Several approaches have been applied for the biological production of hydrogen from lignocellulosic biomass through photo-fermentation and dark fermentation [120]. Biohydrogen production from photo-fermentation occurs in the presence of light and microorganisms, which acts as biological converters catalyzed by nitrogenase enzymes [121, 122]. On the other hand, dark fermentation is carried out without light with the help of anaerobic bacteria. Depending on the type of microorganism used, the reaction could occur under mesophilic (25–40 °C), thermophilic (40–65 °C), extremely thermophilic (65–80 °C) or hyperthermophilic (> 80 °C) temperatures. Hydrogen can also be produced through subcritical and supercritical water gasification of lignocellulosic biomasses [123,124,125,126,127].
Transesterification is one of the most common routes for the production of biodiesel from lignocellulosic residues [128]. The transesterification reaction is a reversible reaction similar to hydrolysis except that water is replaced with alcohol. The process involves the chemical reaction of vegetable oil with an alcohol to produce fatty acid alkyl esters (biodiesel) and glycerol [129]. Biodiesel production from lignocellulosic residues presents significant environmental importance that is more than the economic benefits [130]. Biodiesel is a renewable, non-toxic and environmentally friendly fuel that can be used as a drop-in fuel in diesel engines without engine modification or blended with conventional petro-diesel at suitable proportions [131, 132]. Several researchers have investigated the production of biodiesel from lignocellulosic residues. Shiu et al. [133] explored the use of crude rice bran oil for biodiesel production via transesterification. The authors employed an in-situ process whereby esterification was followed by the transesterification reaction without intermittent product separation [133]. Another study by Deeba et al. [134] used paper mill sludge as a feedstock for biodiesel production via transesterification reaction.
Biochemicals
Lignocellulosic biomass is emerging as a useful substrate for the production of value-added chemicals due to the chemistry of its major biocomponents, i.e. cellulose, hemicellulose and lignin. At present, nearly 75% of the platform chemicals commercially used comprise of propylene, benzene, ethylene, xylene and toluene [29]. These chemicals are used for the synthesis of organic compounds that are processed into useful chemicals such as polymers and resins. Most of the aromatic compounds result from the thermochemical degradation of lignin due to its aromatic nature, while cellulose and hemicellulose can be thermally upgraded to low molecular weight aliphatic compounds.
Enzymatic or chemical hydrolysis of cellulose can be used to produce glucose, which is further upgraded to platform chemicals such as levulinic acid and 5-hydroxymethylfurfural (5-HMF) [135]. Levulinic acid and 5-HMF are identified as promising chemicals for the production of different high-value organic chemicals, flavoring compounds, polymers and fuel additives due to their varied functionalities [136]. The conversion of lignocellulosic biomass into 5-HMF and levulinic acid has been reported by several researchers [29, 135,136,137,138]. Wang et al. [138] studied the hydrolysis of cellulose to levulinic acid in the presence of sulfated TiO2 as a solid acid catalyst. A maximum yield of levulinic acid (27.2%) was obtained at 240 °C in 15 min reaction time using 0.7 g of sulfated TiO2 catalyst.
Zhao et al. [139] investigated the conversion of sugars into 5-HMF using metal halides in 1-alkyl-3-methylimidazolium chloride as a catalyst. Although the negligible amount of levulinic acid was formed in the reaction, nearly 70% yield of 5-HMF was obtained with CrCl2. In another study by Efremov et al. [140], cellulose and different wood species (i.e. pine, fir, aspen and beech) were converted into levulinic acid in water using Al2(SO4)3, CoSO4 and Fe2(SO4)3 as catalysts at 150–250 °C. The yields of levulinic acid from cellulose and woody biomass were about 35 wt% and 15.6–17.8 wt%, respectively. Maximum yields for the woody biomass was in the order of beech (17.8 wt%) > aspen and pine (16 wt%) > spruce (15.6 wt%). Asghari and Yoshida [141] reported the kinetics of fructose dehydration to 5-HMF and the rehydration of 5-HMF to form levulinic acid and formic acids using HCl catalyst. The reaction was carried out at temperatures of 210–270 °C, 4–15 MPa and 0.5–300 s. The authors found that pressure change does not affect the decomposition reactions, although the main reaction products were 5-HMF, levulinic acid and formic acid.
Xylitol (C5 sugar alcohol) is an important chemical produced from the hydrolysis of hemicellulose and its derivatives compounds such as xylose, xylan, arabinose and other sugars, which are soluble in dilute acids. Xylitol can be used as an alternative to sucrose in food sweeteners for diabetic patients and chewing gums, in pharmaceutical and thin coating applications [142]. Xylitol also has a tremendous prospect in odontological application such as toothpaste formulations, teeth hardening as an antimicrobial agent, mouthwash and chewing vitamin tablets [143]. The production of xylitol from hemicellulose and its derivative compounds has been a subject of immense interest among researchers. At present, xylitol is produced by the chemical reduction of xylose and its derivatives compounds in alkaline conditions.
The chemical reduction process is restricted by some challenges such as high-pressure requirements (up to 50 atm), use of expensive catalysts, separation and purification methods [54]. For this reason, the fermentation process is emerging as an attractive alternative for the production of xylitol [144]. Saha and Bothast [144] studied the fermentation of xylose to xylitol using Candida peltata NRRL Y-6888. The optimal xylitol yield of 0.56 g/g xylose was obtained at pH 6, 28 °C and 200 rpm on 50 g/L xylose. Recently, López-Linares et al. [145] evaluated the use of rapeseed straw for the production of xylitol using two yeast strains such as Debaryomyces hansenii and Candida guilliermondii. They obtained the maximum conversion of xylose (YPE/S) as 0.55 g/g and 0.45 g/g for C. guilliermondii and D. hansenii, respectively. YPE/S in the study is defined as the ratio between the amount of xylitol produced and the xylose consumed.
Vanillin (4-hydroxy-3-methoxybenzaldehyde) and gallic acid are two valuable chemicals that have attracted interest recently as starting monomeric materials for the production of new polymers such as polyesters, epoxy and polycarbonates [143, 146]. Vanillin can be produced through the depolymerization of lignin. The process involves the treatment of an aqueous solution of lignin with oxidants at high temperatures and pressures and a pH within the alkaline range to depolymerize the lignin into vanillin [147]. More than 20,000 tons of vanillin is produced annually of which 15% is generated from lignin precursor [147]. It is widely regarded as one of the pure molecular phenolic compounds produced commercially from biomass.
Vanillin is used in food industries due to its vanilla-like flavor, in the pharmaceutical industries for the production of herbicides, drugs and anti-foaming agents and as an intermediate for the synthesis of fine chemicals [148]. Gallic acid has many phytochemical benefits and is used as an antioxidant in tea formulations, paper manufacturing, ink dyes and tanning industries [149]. Furfural is another valuable platform chemical with an annual production of about 250,000 tons from the concentrated sulfuric acid hydrolysis of forestry or agricultural waste [150]. It is often employed in the refining of lubricating oil and as an intermediate for the production of important chemicals such as furan, furfuryl alcohol and tetrahydrofuran [151]. Several other valuable platform chemicals can be obtained from lignocellulosic biomass through biological or chemical routes as shown in Fig. 5. To effectively utilize the available lignocellulosic biomass, new conversion methods are being developed to produce green chemicals while considering the economics of the process.

Adapted from Ge et al. [152] and recreated with copyright permission from Elsevier
Conversion routes for the platform chemicals produced from lignocellulosic biomass. HMF 5-hydroxymethylfurfural, FDCA furandicarboxylic acid, EMP pathway Embden–Meyerhof–Parnas, TCA tricarboxylic acid cycle.
Bioplastics and Biocomposites
Bioplastics are sustainable alternatives to plastic products for use in everyday lives, textile industry, health care, electronics and packaging applications [153]. In recent years, bioplastics materials are emerging as possible alternatives to complement and gradually replace petroleum-based plastics. Compared to petrochemical-based plastics, bioplastics are synthetically produced from lignocellulosic biomass, which is biodegradable and reduces the adverse environmental impacts. Petrochemical plastics contain dioxins and polycyclic aromatic hydrocarbons (PAHs) that pose severe health issues when released to the environment [153, 154]. Additionally, for every 1 kg of plastic burnt, 2.8 kg of CO2 is released into the atmosphere [155]. Mekennen et al. [154] reported that 34 million tons of plastic wastes are produced annually of which 93% is disposed of in landfills and oceans. Some petrochemical plastics are non-biodegradable and pose effective disposal issues as they accumulate in landfills and oceans for hundreds to thousands of years hampering many natural ecosystems. These environmental constraints have stimulated interest in bioplastics, which could help in overcoming the sustainability and environmental challenges caused during the production, usage and disposal of synthetic plastics.
Bioplastics can fulfill the criteria of bio-based and biodegradable products, which are compostable and prone to decay under natural or stimulated conditions. The precursors of bioplastics can be produced from microorganisms (e.g. polyhydroxyalkanoates or PHAs), cellulose (e.g. regenerated cellulose and pectin), proteins (e.g. wool, gelatin and casein) and lipids (e.g. plants oils or animal fats) [155]. Furthermore, bio-based plastics such as polybutylene succinate (PBS) and polylactic acid (PLA) can be synthesized chemically from bio-derived products [156]. Furthermore, some biodegradable plastics such as polycaprolactone (PCL), poly(butylene succinate-co-terephthalate) (PBST) and polyglycolic acid (PGA) show some degree of biodegradability also have promising applications [157]. One of the biodegradable thermoplastics, polylactic acid, is used for biomedical implants, sutures and other surgical applications [158].
There are other forms of biomass-based plastics known as drop-in bioplastics or (bio-based) drop-in chemicals such as bio-propylene (bio-PP), bio-polyethylene terephthalate (bio-PET) and bio-polyethylene (bio-PE). The term “drop-in” appears for these bioplastics because their manufacturing uses the majority of the pathways, infrastructure and machinery as the petrochemical plastics except for their precursor raw material being plant-based biomass. However, due to lack of technological constraints and economy of scale, the production cost of drop-in bioplastics are comparatively more expensive than petrochemical plastics in the current market. Different routes for bioplastics production from lignocellulosic biomass are illustrated in Fig. 6.

Adapted from Brodin et al. [159] and recreated with copyright permission from Elsevier
Production routes of bioplastics from lignocellulosic biomass. PF Phenol–formaldehyde resin, PUR polyurethane, PLA polylactic acid, PE polyethylene.
The biocomposites produced from lignocellulosic biomass are also gaining equal popularity as alternatives to natural fiber-reinforced composites due to their environmental friendliness, durability and biodegradability [160]. As the name suggests, biocomposite materials are a blend of petrochemical resources (i.e. conventional plastics) and renewable biomass (i.e. lignocellulosic residues). Biocomposites refer to composite materials formed by the reinforcement of natural fibers with organic matrix or biopolymers. The matrix phase is made from organic materials or biopolymers, while the fibers could comprise of lignocellulosic biomass or other biogenic wastes. The fibers help to improve the mechanical properties of the polymers and protects them against environmental degradation. The reinforcements (e.g. hemp, flax, wood, paper, etc.) and biopolymers (e.g. natural biopolymers, polylactic acid, polyvinyl alcohol, etc.) are biodegradable and renewable, thereby contributing positively towards environmental sustainability. However, there are some technological challenges in component separation at the end of the life of biocomposite materials [161].
Specialty Materials from Cellulose
As stated earlier, cellulose is one of the most abundant renewable biopolymer resources on earth. In recent years, there has been increasing attention towards the use of cellulose to produce specialty materials due to its low cost, availability, non-toxicity, renewability and environmental friendliness [162]. However, due to the complex nature of its molecular arrangement, crystalline and amorphous structures, biopolymeric networking with hemicellulose and lignin as well as close packing over several hydrogen bonds, van der Waals forces it is difficult to process it chemically [163]. Therefore, the important applications of cellulose are based on its dissolution. However, the dissolution of cellulose is rather challenging as it does not dissolve or melt in the popular organic and aqueous solvents due to its polymeric structures and degree of crystallinity [164]. Thus, it is important to develop inexpensive and environmentally friendly solvents to dissolve cellulose for useful applications.
The dissolution of cellulose in a solvent is usually preceded by polymer swelling where the physical properties and volume of the biopolymer are altered significantly while the semi-solid or solid fractions remain unaltered [163]. Cellulose dissolution helps to facilitate the preparation of innovative materials such as cellulose films and cellulose fibers. Some examples of solvents used include N-methylmorpholine-N-oxide (NMMO), ionic liquids [e.g. 1-allyl-3-methylimidazolium chloride (AMIMCl), alkylimidazolium salts and tetrabutylphosphonium] and NaOH aqueous solution. Certain cellulolytic enzymes such as cellulases and β-glycosidases help degrade the glycosidic bonds between the sugars monomers and degrading cellulose to fermentable glucose monomers [101].
Cellulose regeneration provides a simple route to transform natural cellulose into different forms of useful materials. In the first step, raw cellulose is dissolved through chemical or physical methods after which it is regenerated through a spinning process (e.g. dry, dry jet or wet spinning methods) [165]. The process is environmentally benign as most of the solvents used can be recycled and reused with no or negligible hazardous wastes generated [162]. The structure and properties of the produced cellulose can be controlled by changing the regeneration parameters (e.g. temperature, time and coagulants). Consequently, regenerated cellulose exists in different shapes such as fibers, powders, hydrogels, aerogels, microspheres, beads, films, etc.[162, 166,167,168]. It should be noted that during cellulose regeneration, physical or chemical treatments could be applied to produce some cross-functional materials such as hydrogels/aerogels, films/membranes, bioplastics, inorganic/cellulose hybrids, etc.
Regenerated cellulose fiber (also known as rayon) is one of the first artificial fibers used in the textile and clothing industries currently accounting for more than 5% of the overall annual production of artificial fibers [165]. Regenerated cellulose fibers appear to be smooth and shiny with good water absorption ability, which is similar to that of cotton. These properties enhance its suitability for the production of fabrics and apparels such as skirts, lining jackets and suits. Based on the method of production, regenerated cellulose fibers can be grouped into three different types such as viscose, lyocell and cupro [165]. Viscose rayon fiber is the most dominant regenerated cellulose with the largest market share of about 93% of the cellulose-based fiber market [162]. Viscose rayon can be manufactured from the reaction of alkali cellulose (produced by soaking pure cellulose in NaOH) with carbon disulfide to produce sodium cellulose xanthogenate [169].
The manufacturing process of lyocell is considered eco-friendly because the solvent used can be purified and recovered. Lyocell can be manufactured by dissolving cellulose directly in the solvent N-methylmorpholine-N-oxide (NNMO). On the contrary, cupro rayon fiber can be manufactured by dissolving cellulose in a cuprammonium solution followed by wet spinning for cellulose regeneration and the removal of copper and ammonia. Due to the high price of cotton cellulose and copper salts, cupro rayon fiber has a small market share compared to viscose rayon fiber. The fabric is currently being produced globally and finds useful applications in the clothing and textile industries.
Cellulose hydrogels and aerogels are gaining attention because of their unique features such as biodegradability, low cost, hydrophilic nature and biocompatibility [162]. Hydrogels are chemically or physically cross-linked three-dimensional network of polymers, hydrophilic and could swell with adsorption of water or biological fluids [170]. Hydrogels can be fabricated from native cellulose by dissolution in a suitable solvent followed by cross-linking (gelation) of the cellulose chains. Due to the poor solubility of cellulose in most solvents, only a few specific solvents such as lithium chloride/dimethylacetamide (LiCl/DMAc), alkali/urea and ionic liquids are effective for dissolution [171]. Overall, hydrogels can be divided into two different types based on the method of cross-linking physical and chemical gels [172]. The latter are formed via covalent bonding while the physical gels are produced by the self-assembly of molecules through hydrogen or ionic bonds.
Hydrogels can also be classified based on their origin and nature of constituent polymers as natural, synthetic or hybrid hydrogels [173]. The polymers of natural hydrogels are of natural origins such as gelatin, starch, cellulose and collagen, whereas synthetic hydrogels are manufactured from synthetic polymers such as polyethylene glycol, polyamides, polyacrylic acid and their copolymers [174]. Hydrogels can be applied in various fields of biotechnology, biomedical science (e.g. breast implants, contact lenses and cell culture), tissue engineering (e.g. scaffolds), pharmaceuticals (e.g. drug delivery), sanitation apparel (e.g. disposable diapers and napkins), agriculture (e.g. water retention and pesticide control) [171, 174, 175].
Aerogels are produced from hydrogels through freeze-drying or supercritical drying [176]. They possess some special characteristics such as high porosity, refractive index, specific surface area, dielectric constant, thermal conductivity and low density, which are optimal for a wide variety of applications [177]. Aerogels are widely used in thermal insulation, gas sensing and photocatalytic degradation [178].
Specialty Materials from Lignin
Each year enormous amounts of lignin are generated worldwide as byproducts of cellulose production. However, most of the lignin extracted from lignocellulosic biomass for use in the pulp and paper industry are burnt as a low-value fuel in pulping boilers for energy recovery [179, 180]. Over the last few years, there has been significant interest in research areas related to the non-energetic use of lignin because of the need to develop an eco-friendly pulping process. Only a small fraction of lignin is isolated from spent pulping liquor and used for the production of specialty products [181]. Despite the unique properties of lignin and its derivatives such as biodegradability, adhesive properties, high thermal stability and antioxidant properties, lignin is vastly underutilized [182]. Some applications of lignin beyond the pulp and paper industry include its use as a binding and dispersing agent in various industries and as a substitute for phenolics in resin systems [179], binder for biomass pellets [183, 184], epoxides, adhesives, pesticide, insecticides, polyolefins, antioxidants, antimicrobials [185] and batteries [186].
Lignin has been documented as an important binder and a flow enhancer (plastifier) in mortar and concrete, especially in construction systems [187]. The use of lignin as a binding agent is based on a simple solid–solid dispersion where lignin helps in maintaining fluidity due to the lignin–lignin and lignin–water interactions [180]. Nadif et al. [187] investigated the use of sulfur-free lignin produced from the acid precipitation of black liquor during the soda pulping process as a flow enhancer of concrete and mortar. They also compared the results with that of commercially available additives such as naphthalene sulfonates and lignosulfonates. The authors noted that lignin improved the flow properties of mortar, although flax lignin showed better performance than lignosulfonates due to the presence of sulfur. Their study opened up new frontiers in the use of sulfur-free lignin as a flow enhancer in mortar. Sulfur-free lignin also exhibits good binding properties with soil particles, thereby improving their stability in road construction.
Fadele and Ata [188] investigated the water absorption ability of sawdust lignin extract-stabilized laterite bricks. The water absorption is an important parameter used to ascertain the durability of the bricks as well as their interactions with the binding agents. The water absorption of lignin samples was reported in the ranges of 2–6%. Compared to lignin-stabilized samples, cement-stabilized samples showed superior water absorption of 6% to 15%. The author suggested that further improvement in sawdust additives is required to improve water absorption.
Lignin produces a broad range of phenolic products as degradation intermediates in its pyrolysis and liquefaction derived bio-oil and biocrude [98]. However, the phenolic market is price competitive with a surplus amount of phenol being produced from the cumene oxidation process [189]. A significant portion of the phenols is used to generate phenol–formaldehyde resins, which are commercial synthetic resins (i.e. plastics) used in coating, adhesives and molded products. With the increasing demand for biomaterials, the demand for phenol–formaldehyde resins and its precursor lignin is expected to increase in recent years.
During the manufacture of friction enhancing products (e.g. brake and clutch materials), phenolic powder resins are widely used as binders [181]. Nehez [190] initially proposed and patented the use of organosolv lignin as a partial substitute for phenolic resins in manufacturing frictional materials (e.g. automotive brake pads) with lignin replacing up to 30 wt% of the phenolic resin in the frictional material. It has been reported that brake pads formulation containing 80% of phenolic resin and 20% lignin showed superior advantages in terms of wear behavior and stability of the co-efficient of friction to temperature changes compared to a formulation with 10% phenolic resins [181]. Construction wood panels such as oriented strand boards can partially replace phenolic powders with lignin as binders. The use of lignin can provide additional cost benefits while improving the shelf life of the wooden construction panels due to its water repellant (hydrophobicity), antimicrobial and good curing properties [183]. Çetin and Özmen [191] also showed that organosolv lignin could directly replace phenols in phenol–formaldehyde resins for use as adhesives in particleboard production.
The use of lignin in epoxy resins has been a subject of immense interest with a lot of research developments focused on different lignin-epoxy formulations. The use of lignin in resin formulations requires that the lignin is free from impurities such as moisture, sugars and free salts. This can be achieved by replacing waste lignin such as Kraft and soda lignin with the ones produced from high-purity processes such as organosolv lignin or by post-isolation purification methods (e.g. deionization and precipitation) [180]. Regardless, it should be noted that almost all-waste lignin poses enormous challenges with organic solvent solubility, thereby requiring special solvent mixtures for their formulation [181]. Lignin-epoxy resins with 50% lignin formulation have been reported to fabricate printed wiring boards for use in the electronics industry while exhibiting similar properties to those of common laminate resins [192]. To incorporate lignin as a polyol precursor in epoxy resins many strategies have been adopted as mentioned below. One approach involves blending lignin for reaction with epoxy polymer [193]. Another approach involves reacting lignin with epichlorohydrin followed by cross-linking while other methods involve chemical modification of lignin (e.g. hydroxypropylation and phenolation), which address the challenges of poor reactivity and solubility of lignin [194].
Technical lignins, obtained as byproducts from the industrial processing of lignocellulosic biomass such as soda lignin, lignosulfonates, organosolv lignin, etc., contain several functional groups (e.g. hydroxyl, carbonyl, methoxyl and phenolic), which are reported to be biologically active [195, 196]. Hence, technical lignins are considered as antioxidants because of their phenolic groups that can behave as stabilizers in oxygen and its reactive species induced reactions [197]. Antioxidants are substances that could inhibit free radicals present in living systems, thereby preventing oxidation [198]. Therefore, they can be used as natural additives to substitute synthetic compounds in pharmaceutical, food, cosmetic and plastic industries. Their application in these commercial sectors is a result of their ability to prevent the degradation of natural ingredients such as sugars, lipids and proteins. Besides, technical lignins can slow down the aging process and protect the skin cells from being damaged owing to their antioxidant, antimicrobial and antimutagenic properties [199].
The chemical structure of lignin is dependent mostly on the precursor properties (i.e. herbaceous or woody biomass) and the extraction process. Furthermore, different experimental conditions for delignification (e.g. use of alkali or acid, temperature, time, etc.) could affect the chemical structure and bioactivities of lignin [61]. Several researchers have reported the influence of experimental conditions on the antioxidant potential of lignin [197, 198, 200, 201]. Pan et al. [200] studied the antioxidant potential of twenty-one organosolv ethanol lignin samples obtained from hybrid poplar (Populus nigra × P. maximowiczii) under different extraction conditions. Their findings indicated that the antioxidant activity of lignin is favored at elevated temperature, longer reaction times, dilute ethanol and increased catalyst concentration. El Hage et al. [202] obtained similar results by using lignin derived from Miscanthus giganteus.
Multifunctional Carbon Materials
One of the promising technologies, which utilizes lignocellulosic biomass, is in the production of activated carbon. This approach is important in mitigating environmental challenges such as air and water pollution and the accretion of agricultural wastes. Activated carbon can be prepared from lignocellulosic biomass in two main steps. In the first step, biomass is converted to chars through thermochemical conversion technologies such as carbonization, pyrolysis or gasification by heating under controlled levels of oxygen at high temperatures [203,204,205,206]. The moisture, volatiles and hydrocarbon vapors are removed from lignocellulosic biomass during this process, which can be quenched to produce bio-oil. In the next step, the produced char undergoes physical or chemical activation to produce activated carbon [97].
Chemical activation involves carbonization and activation occurring simultaneously using acids or alkali metals such as NaOH, KOH, H3PO4 and ZnCl2 [207]. In contrast, physical activation uses activating agents such as O2, CO2 and steam. Among the activating agents used during physical activation, CO2 is mostly preferred because it is relatively inexpensive and contributes to the production of ultra-micropores [207]. Activated carbon can be produced from a wide variety of lignocellulosic biomass such as rice husk [208], coffee grounds [209], cotton stalks [210], coconut shell [211], pinecone [212], pinewood sawdust [213], wheat straw [213], flax straw [213] and poultry filter [213] to name a few.
Activated carbon typically has a surface area of nearly 1500 m2/g, which is approximately 50 times higher than that of the surface area of biochar [214]. Activated carbon is widely used as an adsorbent due to its versatile properties such as favorable pore size, high surface area and improved microporous structure [97]. These features enhance the surface reactivity and accessibility of gas/liquid reactants into the internal pore surface of activated carbon with desirable surface chemistry [207]. The applications of activated carbon can be found in the following sectors:
-
(i)
adsorption of contaminants or pollutants from flue gas and wastewater [215],
-
(ii)
chemical industries as a catalyst or catalyst support [216],
-
(iii)
electrochemical industries as battery electrodes and capacitors [217],
-
(iv)
cosmetics and apparel industries (face masks) [218],
-
(v)
pharmaceutical industries (drug delivery and antidote for adsorption of toxins and drug overdose) [219, 220] and several other commercial sectors.
Biochar is a solid carbonaceous product obtained from pyrolysis or gasification of lignocellulosic biomass and other biogenic wastes [221]. Biochar consists of mostly aromatic carbon and other inorganic components such as alkali and alkaline earth metals [222]. Biochar has unique properties concerning its structure, reactivity, functionality and composition. Some of the emerging biochar applications are realized in agriculture (for enhancing soil fertility, crop productivity and soil organic carbon) as well as adsorption of heavy metal and organic pollutants from wastewater and contaminated soils, catalyst support, carbon sequestration, biocomposite fillers and in the synthesis of activated carbon and other multifunctional carbon materials [223].
Carbon nanotubes (CNTs) are emerging as one of the most promising one-dimensional carbon materials because of their exclusive structures with superior optoelectronic, electronic, physicochemical and mechanical properties [224]. CNTs are long cylinders made of covalently bonded atoms of carbon. CNTs can be synthesized through high-temperature degradation of hydrocarbons (e.g. ethylene, methane, alcohols, benzene, xylene, etc.) or inorganic substrates (e.g. quartz, graphene, alumina, silica, etc.) in the presence of a catalyst (usually transition metals, e.g. Ni, Fe, Co, etc.). Three primary methods are used to prepare CNTs such as chemical vapor deposition, arc discharge and laser ablation [225, 226]. Although chemical vapor deposition is considered the most popular method concerning industrial scale-up due to the energy efficiency of the process, ease of operation, high yield and low purity and the raw materials used [227]. However, the synthesis of CNTs by chemical vapor deposition is dependent on the type of catalyst, the nature of the carbonaceous feedstock and the available power consumption. The increasing environmental challenges have stimulated interest in the synthesis of CNTs from renewable and environmentally friendly resources.
Lignocellulosic feedstock such as rice straw [224], rice husk [227], wood fiber [228], bamboo [228, 229], wood sawdust [230], cellulose [228] and purified lignin [228] have also been reported as good precursors for CNT synthesis. SWCNTs are the basic cylindrical structure made up of a single sheet wrapped into a cylindrical tube. In contrast, MWCNTs comprises of coaxial cylinders with an array of concentrically nested cylindrical nanotubes. Some potential applications of CNTs include energy storage and energy conversion devices, catalysis, hydrogen storage system, automotive, electromagnetic shields, building composite materials, sensors, textile functionalization, field emission displays and radiation sources [231, 232].
Carbon nanohorns (CNHs) represent another category of carbon nanostructures with a conical shape containing closed cages of sp2-bonded carbon atoms [233]. Therefore, they are sometimes referred to as carbon nanocones with 2–5 nm diameter and a length of 40–50 nm [234]. Analogous to CNTs, CNHs belong to the category of one-dimensional carbon materials. However, CNTs exhibit exceptional properties, which include high chemical stability, low toxicity, favorable specific surface area, excellent catalytic properties, high resistance to oxidation, good conductivity and porosity [235]. Furthermore, they can be synthesized in large quantities industrially at room temperature without the use of a potentially toxic metal catalyst and subsequent purification steps [234].
During the synthesis of CNTs, additional treatment with strong acids is required to eliminate the metal catalyst, which could potentially result in the loss of some carbon materials. CNHs are currently viewed as a replacement to CNTs because graphene has several applications in supercapacitors, energy conversion, gas storage, drug delivery, fuel cell electrodes, electrochemical sensing and biosensing [235, 236]. The production method of CNHs is similar to that of CNTs where a sufficient amount of energy is required (via arc discharge, laser ablation or Joule heating) to vaporize and restructure the target carbon material [236]. Afterwards, a rapid quenching usually in an inert gas occurs.
Bacterial Cellulose Applications in Cosmeceutical and Pharmaceutical Applications
Bacterial cellulose is a form of cellulose produced by certain microorganisms such as Gluconacetobacter, Rhizobium, Sarcina and Agrobacterium [237]. Bacterial cellulose can be synthesized using four main enzymatically catalyzed steps [238]. The first step involves phosphorylation of glucose to produce glucose-6-phosphate followed by the isomerization of glucose-6-phosphate to glucose-1-phosphate. The third step involves the subsequent conversion of glucose-1-phosphate to uridine diphosphate glucose. Lastly, the synthesis of glucan chains from uridine diphosphate glucose occurs. Glucan chains are aggregated and converted to crystalline form to microfibrils, which are further aggregated to cellulose fiber bundles [239].
Bacterial cellulose is odorless and colorless and exists in the form of gels. Compared to plant-derived cellulose, bacterial cellulose can be synthesized as a versatile biopolymer in its pure form without the need of a rigorous process to remove contaminants and impurities such as holocellulose (cellulose and hemicellulose), pectin, extractives and lignin [239]. Additionally, bacterial cellulose has superior mechanical, biological and physical properties such as relative high porosity and permeability to gases and liquids, tensile strength, ultrafine network and extremely high water uptake (> 90 wt%) [238,239,240]. These properties make it suitable for applications in pharmaceuticals (e.g. controlled drug delivery, macromolecular drug production, etc.), cosmetics (e.g. facial mask and scrubs, contact lenses, facial and body cleaning formulations, etc.) and biomedical science (e.g. tissue engineering, artificial blood vessels, bone regeneration, etc.) [238].
Cosmeceutical Applications
A cosmetic is any substance that can be applied to the human body to improve or change the appearance of the body without influencing the structure or normal body functions. Bacterial cellulose has been reported to be an excellent biopolymer for use in cosmetics because of its low toxicity, biodegradability and its skin hydration ability [241, 242]. Amnuaikit et al. [242] investigated the effect of bacterial cellulose facial mask on the human skin characteristics and user satisfaction. Thirty healthy volunteers aged between 21 and 40 years old participated in the study among which two groups were created (i.e. control and experimental group). The control group was asked to put moist towels on their face for 25 min while the experimental group applied bacterial cellulose facial mask within the same duration. In the subsequent week, the groups were interchanged to the alternative treatment. Their findings showed that the bacteria facemask improved the moisture content of the skin to a significant level compared to the moist towel upon a single treatment. Furthermore, the bacterial cellulose mask did not affect any other skin characteristics and received a rating of 4 on a scale of 5 for the satisfaction rating scale [242].
In another study by Almeida et al. [243], the skin irritation potential of bacterial cellulose in humans was evaluated with and without glycerin (acting as plasticizer). Regarding the transepidermal water loss, the authors observed no significant difference with the erythema recording a zero clinical score [243]. However, when glycerin was applied, the skin moisturizing effect elevated significantly. Lin et al. [244] patented a cosmetic substance containing fragments of bacterial cellulose with 0.05–1 wt%. The authors claimed that the fragments of bacterial cellulose improved the skin moisturizing ability and skin exfoliation; therefore could be applied in the design of lips, skin and nail care products.
Pharmaceutical Applications
The ability of bacterial cellulose to function as a drug carrier has been investigated by some researchers [245,246,247]. The most important properties of bacterial cellulose that endears its usage in drug delivery are its biocompatibility and bioavailability. In controlled or sustained drug delivery applications, bacterial cellulose can function as a modifier to adjust the gelling behavior of drugs, thereby resulting in favorable mechanisms for controlling drug release profiles [248].
Trovatti et al. [246] studied the use of bacterial cellulose membranes for controlled drug loading and release. The authors assessed the therapeutic ability by comparing the permeating ability of two model drugs (i.e. lidocaine hydrochloride and ibuprofen) in bacterial cellulose and another drug formulation system. A uniform distribution of both drugs in bacterial cellulose was observed with ibuprofen in bacterial cellulose showing three times permeating ability than that of the drug in a gel. Their findings indicate that bacterial cellulose could play a vital role in drug delivery. Weyell et al. [249] suggested that bacterial cellulose could be used for combined wound dressing and drug delivery systems. Tamahkar et al. [250] developed and tested a novel bacterial cellulose drug carrier system for antibiotic release. The composite molecularly imprinted nanofibers (MIP), which was prepared with gentamicin fabricated onto bacterial cellulose nanofibers via the in-situ graft polymerization method showed favorable drug delivery ability.
Conclusions and Perspectives
Lignocellulosic biomass known as the most abundant renewable biomass on earth is an important bioresource for energy and different applications in polymer, textile, composites, biomedical, cosmeceutical and pharmaceutical industries. Owing to the growing environmental concern and challenges of overdependence on fossil-based resources, lignocellulosic materials could have a major role to play in the future to ensure that the energy and societal demands of the elevating population are fulfilled adequately. Compared to crude oil, they are significantly less expensive, readily available from different sources such as agriculture and forestry.
Lignocellulosic biomass can be converted to biofuels via the thermochemical and biochemical routes. Biofuels are finding useful applications as substitutes for gasoline in the automotive industry. Additionally, a wide variety of synthetic polymers and chemicals such as ethanol, sorbitol, butanol, glycerol, etc. can be obtained from lignocellulosic materials. However, the structure of the constituents of lignocellulosic biomass (i.e. cellulose, hemicellulose, lignin, ash and extractives) are required to be thoroughly understood to postulate the potential applications in novel industrial areas of bioenergy, biomedical, cosmeceuticals, pharmaceuticals, biocomposites and bioplastics.
Bioplastics produced from lignocellulosic materials are slowly substituting petroleum-based plastics in the textile, electronic and health care industry. The sustainability and economic feasibility of bioplastics production process are dependent on different environmental and political drivers together with the development of appropriate business models. Analogous to bioplastics, biocomposites obtained from lignocellulosic materials are gaining equal popularity as substitutes to naturally reinforced composites because of their environmental benign nature. The biocomposites find useful applications in the automobile (vehicle interiors), marine industry and production of packaging materials.
Specialty materials such as cellulose fiber have proven effective in the textile and clothing industry. Cellulose hydrogels and aerogels are also gaining attention in metal removal, pesticide control, agriculture and biomedical applications due to their affordability, biodegradability and biocompatibility. Bacterial cellulose has also been established as a useful material in cosmeceutical and pharmaceutical industries. Analogous to cellulose, specialty materials from lignin (e.g. phenolic resins, epoxides, adhesives and polyolefins) are useful as antioxidants, binders, flow enhancers, dispersing agents, epoxides, pesticides and insecticides. To conclude, it is important to mention that all the novel applications described in this review suggest that lignocellulosic biomass has a major role to play in establishing a circular bioeconomy and sustainable market that consist of innovative design and advanced production methods to facilitate the reuse of waste materials for commercial recovery of value-added products.
References
Rana, R., Nanda, S., Kozinski, J.A., Dalai, A.K.: Investigating the applicability of Athabasca bitumen as a feedstock for hydrogen production through catalytic supercritical water gasification. J. Environ. Chem. Eng. 6, 182–189 (2018)
Rana, R., Nanda, S., Maclennan, A., Hu, Y., Kozinski, J.A., Dalai, A.K.: Comparative evaluation for catalytic gasification of petroleum coke and asphaltene in subcritical and supercritical water. J. Energ. Chem. 31, 107–118 (2019)
Adekunle, K.F., Okolie, J.A.: A Review of biochemical process of anaerobic digestion. Adv. Biosci. Biotechnol. 6, 205–212 (2015)
Nanda, S., Reddy, S.N., Mitra, S.K., Kozinski, J.A.: The progressive routes for carbon capture and sequestration. Energ. Sci. Eng. 4, 99–122 (2016)
Nanda, S., Azargohar, R., Dalai, A.K., Kozinski, J.A.: An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sust. Energ. Rev. 50, 925–941 (2015)
Rana, R., Nanda, S., Reddy, S.N., Dalai, A.K., Kozinski, J.A., Gökalp, I.: Catalytic gasification of light and heavy gas oils in supercritical water. J. Energy Inst. (2020). https://doi.org/10.1016/j.joei.2020.04.018
Parakh, P.D., Nanda, S., Kozinski, J.A.: Eco-friendly transformation of waste biomass to biofuels. Curr. Biochem. Eng. 6, 120–134 (2020)
Ahorsu, R., Medina, F., Constantí, M.: Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: a review. Energies 11, 3366 (2018)
British Petroleum. Statistical Review of World Energy. https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html (2020a) (Accessed 7 April 2020)
British Petroleum. Renewable Energy. https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy/renewable-energy.html (2020b) (Accessed 7 April 2020)
British Plastics Federation. Oil Consumption. https://www.bpf.co.uk/press/oil_consumption.aspx (2020) (Accessed 7 April 2020)
Kamm, B.: Production of platform chemicals and synthesis gas from biomass. Angew. Chemie Int. Ed. 46, 5056–5058 (2007)
Nanda, S., Mohammad, J., Reddy, S.N., Kozinski, J.A., Dalai, A.K.: Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Conv. Bioref. 4, 157–191 (2014)
Nanda, S., Mohanty, P., Pant, K.K., Naik, S., Kozinski, J.A., Dalai, A.K.: Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenerg. Res. 6, 663–677 (2013)
Konwer, D., Kataki, R., Deka, D.: Fuel-wood characteristics of some indigenous tree species of north-east India. Indian J. For. 24, 316–319 (2001)
Demirbaş, A.: Fuel characteristics of olive husk and walnut, hazelnut, sunflower, and almond shells. Energ. Sources 24, 215–221 (2002)
Shen, D., Gu, S., Luo, K., Bridgwater, A., Fang, M.X.: Kinetic study on thermal decomposition of woods in oxidative environment. Fuel 88, 1024–1030 (2009)
Naik, S., Goud, V.V., Rout, P.K., Jacobson, K., Dalai, A.K.: Characterization of Canadian biomass for alternative renewable biofuel. Renew. Energ. 35, 1624–1631 (2010)
Adapa, P., Tabil, L., Schoenau, G.: Compaction characteristics of barley, canola, oat and wheat straw. Biosyst. Eng. 104, 335–344 (2009)
Razvigorova, M., Goranova, M., Minkova, V., Cerny, J.: On the composition of volatiles evolved during the production of carbon adsorbents from vegetable wastes. Fuel 73, 1718–1722 (1994)
Sánchez, C.: Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 27, 185–194 (2009)
Prasad, S., Singh, A., Joshi, H.C.: Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl. 50, 1–39 (2007)
Kim, T.H., Kim, J.S., Sunwoo, C., Lee, Y.Y.: Pretreatment of corn stover by aqueous ammonia. Bioresour. Technol. 90, 39–47 (2003)
Stevulova, N., Cigasova, J., Estokova, A., Terpakova, E., Geffert, A., Kacik, F., Singovszka, E., Holub, M.: Properties characterization of chemically modified hemp hurds. Materials 7, 8131–8150 (2014)
Butler, E., Devlin, G., Meier, D., McDonnell, K.: Characterisation of spruce, salix, miscanthus and wheat straw for pyrolysis applications. Bioresour. Technol. 131, 202–209 (2013)
Aqsha, A., Tijani, M.M., Moghtaderi, B., Mahinpey, N.: Catalytic pyrolysis of straw biomasses (wheat, flax, oat and barley) and the comparison of their product yields. J. Anal. Appl. Pyrolysis 125, 201–208 (2017)
Brebu, M., Ucar, S., Vasile, C., Yanik, J.: Co-pyrolysis of pine cone with synthetic polymers. Fuel 89, 1911–1918 (2010)
Kumar, R., Wyman, C.E.: Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Prog. 25, 302–314 (2009)
Howard, R.L., Abotsi, E., van Jansen, R.E.L., Howard, S.: Lignocellulose biotechnology: issues of bioconversion and enzyme production. Af. J. Biotechnol. 2, 602–619 (2015)
Garca-Pèrez, M., Chaala, A., Roy, C.: Vacuum pyrolysis of sugarcane bagasse. J. Anal. Appl. Pyrolysis 65, 111–136 (2002)
McKendry, P.: Energy production from biomass (part 1): overview of biomass. Bioresour. Technol. 83, 37–46 (2002)
Singh, A., Nanda, S., Berruti, F.: A review of thermochemical and biochemical conversion of miscanthus to biofuels. In: Nanda, S., Vo, D.V.N., Sarangi, P.K. (eds.) Biorefinery of Alternative Resources: Targeting Green Fuels and Platform Chemicals, pp. 195–220. Springer Nature, Singapore (2020)
Nanda, S., Rana, R., Sarangi, P.K., Dalai, A.K., Kozinski, J.A.: A broad introduction to first, second and third generation biofuels. In: Sarangi, P.K., Nanda, S., Mohanty, P. (eds.) Recent Advancements in Biofuels and Bioenergy Utilization, pp. 1–25. Springer Nature, Singapore (2018)
Ullah, K., Sharma, V.K., Dhingra, S., Braccio, G., Ahmad, M., Sofia, S.: Assessing the lignocellulosic biomass resources potential in developing countries: a critical review. Renew. Sust. Energy Rev. 51, 682–698 (2015)
Achinas, S., Euverink, G.J.W.: Consolidated briefing of biochemical ethanol production from lignocellulosic biomass. Electron. J. Biotechnol. 23, 44–53 (2016)
Nanda, S., Rana, R., Zheng, Y., Kozinski, J.A., Dalai, A.K.: Insights on pathways for hydrogen generation from ethanol. Sustain. Energ. Fuels 1, 1232–1245 (2017)
Ahmad, E., Vani, A., Pant, K.K.: An overview of fossil fuel and biomass-based integrated energy systems: co-firing, co-combustion, co-pyrolysis, co-liquefaction, and co-gasification. In: Nanda, S., Sarangi, P.K., Vo, D.V.N. (eds.) Fuel Processing and Energy Utilization, pp. 15–30. CRC Press, New York (2019)
Habibi, Y., Lucia, L.A., Rojas, O.J.: Cellulose nanocrystals: CHEMISTRY, self-assembly, and applications. Chem. Rev. 110, 3479–3500 (2010)
Nanda, S., Maley, J., Kozinski, J.A., Dalai, A.K.: Physico-chemical evolution in lignocellulosic feedstocks during hydrothermal pretreatment and delignification. J. Biobased Mater. Bioenerg. 9, 295–308 (2015)
Hallac, B.B., Ragauskas, A.J.: Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuel Bioprod. Bioref. 5, 215–225 (2011)
Moon, R.J., Martini, A., Nairn, J., Simonsen, J., Youngblood, J.: Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 40, 3941–3994 (2011)
Gibson, L.J., Soc, J.R.: The hierarchical structure and mechanics of plant materials. Interface 9, 2749–2766 (2012)
Pubchem. Cellulose. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/CELLULOSE (Accessed 4 May 2020)
Newman, R.H., Hill, S.J., Harris, P.J.: Wide-angle X-ray scattering and solid-state nuclear magnetic resonance data combined to test models for cellulose microfibrils in Mung bean cell walls. Plant Physiol. 163, 1558–1567 (2013)
Martínez-Sanz, M., Gidley, M.J., Gilbert, E.P.: Application of X-ray and neutron small angle scattering techniques to study the hierarchical structure of plant cell walls: a review. Carbohydr. Polym. 125, 120–134 (2015)
O’sullvian, A.C.: Cellulose: the structure slowly unravels. Cellulose 4, 173–207 (1997)
Klemm, D., Heublein, B., Fink, H.P., Bohn, A.: Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chemie Int. Ed. 44, 3358–3393 (2005)
Larsson, P.T., Westermark, U., Iversen, T.: Determination of the cellulose Iα allomorph content in a tunicate cellulose by CP/MAS 13 C-NMR spectroscopy. Carbohydr. Res. 278, 339–343 (1995)
Larsson, P.T., Hult, E.L., Wickholm, K., Pettersson, E., Iversen, T.: CP/MAS 13C-NMR spectroscopy applied to structure and interaction studies on cellulose I. Solid State Nucl. Magn. Reson. 15, 31–40 (1999)
Chen, H.: Chemical composition and structure of natural lignocellulose. In: Chen, H. (ed.) Biotechnology of Lignocellulose, pp. 25–71. Springer, Dordrecht (2014)
Pubchem. D-Xylose. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/135191 (Accessed 4 May 2020)
Kormelink, F.J.M., Voragen, A.G.J.: Degradation of different [(glucurono)arabino]xylans by a combination of purified xylan-degrading enzymes. Appl. Microbiol. Biotechnol. 38, 688–695 (1993)
Doner, L.W., Hicks, K.B.: Isolation of hemicellulose from corn fiber by alkaline hydrogen peroxide extraction. Cereal Chem. 74, 176–181 (1997)
Saha, B.C.: Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30, 279–291 (2003)
Yang, S.H.: Plant fiber chemistry. Light Industry Press, Beijing (2008)
Gírio, F.M., Fonseca, C., Carvalheiro, F., Duarte, L.C., Marques, S., Bogel-Łukasik, R.: Hemicelluloses for fuel ethanol: a review. Bioresour. Technol. 101, 4775–4800 (2010)
Wang, S., Dai, G., Yang, H., Luo, Z.: Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog. Energy Combust. Sci. 62, 33–86 (2017)
Ragauskas, A.J., Nagy, M., Kim, D.H., Eckert, C.A., Hallett, J.P., Liotta, C.L.: From wood to fuels: integrating biofuels and pulp production. Ind. Biotechnol. 2, 55–65 (2006)
Buranov, A.U., Mazza, G.: Lignin in straw of herbaceous crops. Ind. Crops Prod. 28, 237–259 (2008)
Reinoso, F.A.M., Rencoret, J., Gutiérrez, A., Milagres, A.M.F., del Río, J.C., Ferraz, A.: Fate of p-hydroxycinnamates and structural characteristics of residual hemicelluloses and lignin during alkaline-sulfite chemithermomechanical pretreatment of sugarcane bagasse. Biotechnol. Biofuels 11, 153 (2018)
Fougere, D., Nanda, S., Clarke, K., Kozinski, J.A., Li, K.: Effect of acidic pretreatment on the chemistry and distribution of lignin in aspen wood and wheat straw substrates. Biomass Bioenerg. 91, 56–68 (2016)
Zakzeski, J., Bruijnincx, P.C.A., Jongerius, A.L., Weckhuysen, B.M.: The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010)
Pandey, M.P., Kim, C.S.: Lignin depolymerization and conversion: a review of thermochemical methods. Chem. Eng. Technol. 34, 29–41 (2011)
Pubchem. Lignin, organosolv. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/73555271 (Accessed 4 May 2020)
Novaes, E., Kirst, M., Chiang, V., Winter-Sederoff, H., Sederoff, R.: Lignin and biomass: a negative correlation for wood formation and lignin content in trees. Plant Physiol. 154, 555–561 (2010)
Demirbas, A.: Combustion characteristics of different biomass fuels. Prog. Energ. Combust. Sci. 30, 219–230 (2004)
Nanda, S., Dalai, A.K., Kozinski, J.A.: Butanol and ethanol production from lignocellulosic feedstock: biomass pretreatment and bioconversion. Energ. Sci. Eng. 2, 138–148 (2014)
Lawther, J.M., Sun, R., Banks, W.B.: Fractional characterization of alkali-labile lignin and alkali-insoluble lignin from wheat straw. Ind. Crop. Prod. 5, 291–300 (1996)
Lupoi, J.S., Singh, S., Parthasarathi, R., Simmons, B.A., Henry, R.J.: Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sust. Energy Rev. 49, 871–906 (2015)
Elle, O., Richter, R., Vohland, M., Weigelt, A.: Fine root lignin content is well predictable with near-infrared spectroscopy. Sci. Rep. 9, 6396 (2019)
Tamaki, Y., Mazza, G.: Rapid determination of lignin content of straw using Fourier transform mid-infrared spectroscopy. J. Agric. Food Chem. 59, 504–512 (2011)
Chen, Z., NaderiNasrabadi, M., Staser, J.A., Harrington, P.B.: Application of generalized standard addition method and ultraviolet spectroscopy to quantify electrolytic depolymerization of lignin. J. Anal. Test. 4, 35–44 (2020)
Lu, Y., Lu, Y.C., Hu, H.Q., Xie, F.J., Wei, X.Y., Fan, X.: Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectr. (2017). https://doi.org/10.1155/2017/8951658
Ju, X., Engelhard, M., Zhang, X.: An advanced understanding of the specific effects of xylan and surface lignin contents on enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 132, 137–145 (2013)
Banoub, J., Delmas Jr., G.H., Joly, N., Mackenzie, G., Cachet, N., Benjelloun-Mlayah, B., Delmas, M.: A critique on the structural analysis of lignins and application of novel tandem mass spectrometric strategies to determine lignin sequencing. J. Mass Spec. 50, 5–48 (2015)
Chundawat, S.P.S., Balan, V., Sousa, L.D.C., Dale, B.E.: Thermochemical pretreatment of lignocellulosic biomass. In: Waldron, K. (ed.) Bioalcohol Production, pp. 24–72. Woodhead Publishing, Sawston (2010)
Constant, S., Basset, C., Dumas, C., Di Renzo, F., Robitzer, M., Barakat, A., Quignard, F.: Reactive organosolv lignin extraction from wheat straw: Influence of Lewis acid catalysts on structural and chemical properties of lignins. Ind. Crops Prod. 65, 180–189 (2015)
Macfarlane, A.L., Mai, M., Kadla, J.F.: Bio-based chemicals from biorefining: Lignin conversion and utilisation. In: Waldron, K. (ed.) Advances in Biorefineries, pp. 659–692. Woodhead Publishing, Sawston (2014)
Holladay, J.E., White, J.F., Bozell, J.J., Johnson, D.: Top Value-Added Chemicals from Biomass—Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin. Pacific Northwest National Laboratory, Richland (2007)
Keskin, T., Abubackar, H.N., Arslan, K., Azbar, N.: Biohydrogen production from solid wastes. In: Pandey, A., Mohan, S.V., Chang, J.S., Hallenbeck, P.C., Larroche, C. (eds.) Biohydrogen (second edition), pp. 321–346. Elsevier, New York (2019)
Bandyopadhyay-Ghosh, S., Ghosh, S.B., Sain, M.: The use of biobased nanofibres in composites. In: Faruk, O., Sain, M. (eds.) Biofiber Reinforcements in Composite Materials, pp. 571–647. Woodhead Publishing, Sawston (2015)
Brethauer, S., Studer, M.H.: Biochemical conversion processes of lignocellulosic biomass to fuels and chemicals—a review. CHIMIA Int. J. Chem. 69, 572–581 (2015)
Chen, H.: Lignocellulose biorefinery feedstock engineering. In: Chen, H. (ed.) Lignocellulose Biorefinery Engineering: Principles and Applications, pp. 37–86. Woodhead Publishing, Sawston (2015)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D.: Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Technical report NREL/TP-510-42623. National Renewable Energy Laboratory (NREL), Colorado (2008)
Okolie, J.A., Rana, R., Nanda, S., Dalai, A.K., Kozinski, J.A.: Supercritical water gasification of biomass: a state-of-the-art review of process parameters, reaction mechanisms and catalysis. Sustain. Energy Fuel. 3, 578–598 (2019)
Aharoni, A., Jongsma, M.A., Bouwmeester, H.J.: Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 10, 594–602 (2005)
Wang, X., Ort, D.R., Yuan, J.S.: Photosynthetic terpene hydrocarbon production for fuels and chemicals. Plant Biotechnol. J. 13, 137–146 (2015)
Vassilev, S.V., Baxter, D., Andersen, L.K., Vassileva, C.G., Morgan, T.J.: An overview of the organic and inorganic phase composition of biomass. Fuel 94, 1–33 (2012)
Pettersson, A., Zevenhoven, M., Steenari, B.M., Amand, L.E.: Application of chemical fractionation methods for characterisation of biofuels, waste derived fuels and CFB co-combustion fly ashes. Fuel 87, 3183–3193 (2008)
Demirbas, A.: Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog. Energy Combust. Sci. 31, 171–192 (2005)
James, A.K., Thring, R.W., Helle, S., Ghuman, H.S.: Ash management review-applications of biomass bottom ash. Energies 5, 3856–3873 (2012)
Mohanty, P., Nanda, S., Pant, K.K., Naik, S., Kozinski, J.A., Dalai, A.K.: Evaluation of the physiochemical development of biochars obtained from pyrolysis of wheat straw, timothy grass and pinewood: effects of heating rate. J. Anal. Appl. Pyrolysis 104, 485–493 (2013)
Nanda, S., Abraham, J.: Remediation of heavy metal contaminated soil. Afr. J. Biotechnol. 12, 3099–3109 (2013)
Khan, A.A., de Jong, W., Jansens, P.J., Spliethoff, H.: Biomass combustion in fluidized bed boilers: potential problems and remedies. Fuel Process. Technol. 90, 21–50 (2009)
Vamvuka, D.: Comparative fixed/fluidized bed experiments for the thermal behaviour and environmental impact of olive kernel ash. Renew. Energy 34, 158–164 (2009)
Nanda, S., Kozinski, J.A., Dalai, A.K.: Lignocellulosic biomass: A review of conversion technologies and fuel products. Curr. Biochem. Eng. 3, 24–36 (2015)
Nanda, S., Dalai, A.K., Berruti, F., Kozinski, J.A.: Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valor. 7, 201–235 (2016)
Mohan, D., Pittman, C.U., Steele, P.H.: Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuel 20, 848–889 (2006)
Joshi, N., Lawal, A.: Hydrodeoxygenation of pyrolysis oil in a microreactor. Chem. Eng. Sci. 74, 1–8 (2012)
Rutkowski, P.: Pyrolysis of cellulose, xylan and lignin with the K2CO3 and ZnCl2 addition for bio-oil production. Fuel Process. Technol. 92, 517–522 (2011)
Nanda, S., Mohanty, P., Kozinski, J.A., Dalai, A.K.: Physico-chemical properties of bio-oils from pyrolysis of lignocellulosic biomass with high and slow heating rate. Energ. Environ. Res. 4, 21–32 (2014)
Kim, S., Dale, B.E.: Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenerg. 26, 361–375 (2004)
Morone, P., Cottoni, L.: Biofuels: technology, economics, and policy issues. In: Luque, R., Lin, C.S.K., Wilson, K., Clark, J. (eds.) Handbook of Biofuels Production: Processes and Technologies, pp. 61–83. Woodhead Publishing, Sawston (2016)
Khuong, L.S., Masjuki, H.H., Zulkifli, N.W.M., Mohamad, E.M., Kalam, M.A., Alabdulkarem, A., Arslan, A., Mosarof, M., Syahir, A.Z., Jamshaid, M.: Effect of gasoline–bioethanol blends on the properties and lubrication characteristics of commercial engine oil. RSC Adv. 7, 15005–15019 (2017)
Nanda, S., Golemi-Kotra, D., McDermott, J.C., Dalai, A.K., Gökalp, I., Kozinski, J.A.: Fermentative production of butanol: perspectives on synthetic biology. New Biotechnol. 37, 210–221 (2017)
Nanda, S., Rana, R., Vo, D.V.N., Sarangi, P.K., Nguyen, T.D., Dalai, A.K., Kozinski, J.A.: A spotlight on butanol and propanol as next-generation synthetic fuels. In: Nanda, S., Vo, D.V.N., Sarangi, P.K. (eds.) Biorefinery of Alternative Resources: Targeting Green Fuels and Platform Chemicals, pp. 105–126. Springer Nature, Singapore (2020)
Dürre, P.: Biobutanol: an attractive biofuel. Biotechnol. J. 2, 1525–1534 (2007)
Lee, S.Y., Park, J.H., Jang, S.H., Nielsen, L.K., Kim, J., Jung, K.S.: Fermentative butanol production by clostridia. Biotechnol. Bioeng. 101, 209–228 (2008)
Sarangi, P.K., Nanda, S.: Recent developments and challenges of acetone–butanol–ethanol fermentation. In: Sarangi, P.K., Nanda, S., Mohanty, P. (eds.) Recent Advancements in Biofuels and Bioenergy Utilization, pp. 111–123. Springer Nature, Singapore (2018)
Nanda, S., Dalai, A.K., Kozinski, J.A.: Butanol from renewable biomass: highlights on downstream processing and recovery techniques. In: Mondal, P., Dalai, A.K. (eds.) Sustainable Utilization of Natural Resources, pp. 187–211. CRC Press, Florida (2017)
Khan, I.U., Hafiz Dzarfan Othman, M., Hashim, H., Matsuura, T., Ismail, A.F., Rezaei-DashtArzhandi, M., Wan Azelee, I.: Biogas as a renewable energy fuel—a review of biogas upgrading, utilisation and storage. Energy Convers. Manage. 150, 277–294 (2017)
Makareviciene, V., Sendzikiene, E., Pukalskas, S., Rimkus, A., Vegneris, R.: Performance and emission characteristics of biogas used in diesel engine operation. Energy Convers. Manage. 75, 224–233 (2013)
Molino, A., Migliori, M., Ding, Y., Bikson, B., Giordano, G., Braccio, G.: Biogas upgrading via membrane process: modelling of pilot plant scale and the end uses for the grid injection. Fuel 107, 585–592 (2013)
Mathai, R., Malhotra, R.K., Subramanian, K.A., Das, L.M.: Comparative evaluation of performance, emission, lubricant and deposit characteristics of spark ignition engine fueled with CNG and 18% hydrogen-CNG. Int. J. Hydrogen Energy 37, 6893–6900 (2012)
Rapagnà, S., Jand, N., Foscolo, P.U.: Catalytic gasification of biomass to produce hydrogen rich gas. Int. J. Hydrogen Energy 23, 551–557 (2002)
Sonal Ahmad, E., Upadhyayula, S., Pant, K.K.: Biomass-derived CO2 rich syngas conversion to higher hydrocarbon via Fischer–Tropsch process over Fe–Co bimetallic catalyst. Int. J. Hydrogen Energy 44, 27741–27748 (2019)
Munasinghe, P.C., Khanal, S.K.: Biomass-derived syngas fermentation into biofuels. Biofuels 26, 79–98 (2011)
Okolie, J.A., Nanda, S., Dalai, A.K., Berruti, F., Kozinski, J.A.: A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sust. Energy Rev. 119, 109546 (2020)
Jamal, Y., Wyszynski, M.L.: On-board generation of hydrogen-rich gaseous fuels-a review. Int. J. Hydrogen Energy 19, 557–572 (1994)
Levin, D.B., Zhu, H., Beland, M., Cicek, N., Holbein, B.E.: Potential for hydrogen and methane production from biomass residues in Canada. Bioresour. Technol. 98, 654–660 (2007)
Nanda, S., Li, K., Abatzoglou, N., Dalai, A.K., Kozinski, J.A.: Advancements and confinements in hydrogen production technologies. In: Dalena, F., Basile, A., Rossi, C. (eds.) Bioenergy Systems for the Future, pp. 373–418. Woodhead Publishing, Sawston (2017)
Sarangi, P.K., Nanda, S.: Biohydrogen production through dark fermentation. Chem. Eng. Technol. 43, 601–612 (2020)
Nanda, S., Dalai, A.K., Kozinski, J.A.: Supercritical water gasification of timothy grass as an energy crop in the presence of alkali carbonate and hydroxide catalysts. Biomass Bioenergy 95, 378–387 (2016)
Nanda, S., Reddy, S.N., Vo, D.V.N., Sahoo, B.N., Kozinski, J.A.: Catalytic gasification of wheat straw in hot compressed (subcritical and supercritical) water for hydrogen production. Energy Sci. Eng. 6, 448–459 (2018)
Okolie, J.A., Nanda, S., Dalai, A.K., Kozinski, J.A.: Optimization and modeling of process parameters during hydrothermal gasification of biomass model compounds to generate hydrogen-rich gas products. Int. J. Hydrogen Energy (2019). https://doi.org/10.1016/j.ijhydene.2019.05.132
Okolie, J.A., Nanda, S., Dalai, A.K., Kozinski, J.A.: Hydrothermal gasification of soybean straw and flax straw for hydrogen-rich syngas production: experimental and thermodynamic modeling. Energy Convers. Manage. 208, 112545 (2020)
Sun, J., Xu, L., Dong, G.H., Nanda, S., Li, H., Fang, Z., Kozinski, J.A., Dalai, A.K.: Subcritical water gasification of lignocellulosic wastes for hydrogen production with Co modified Ni/Al2O3 catalyst. J. Supercrit. Fluids 162, 104863 (2020)
Meher, L.C., Sagar, D.V., Naik, S.N.: Technical aspects of biodiesel production by transesterification—a review. Renew. Sustain. Energy Rev. 10, 248–268 (2006)
Srivastava, A., Prasad, R.: Triglycerides-based diesel fuels. Renew. Sust. Energy Rev. 4, 111–133 (2000)
Reddy, S.N., Nanda, S., Kozinski, J.A.: Supercritical water gasification of glycerol and methanol mixtures as model waste residues from biodiesel refinery. Chem. Eng. Res. Des. 113, 17–27 (2016)
Reddy, S.N., Nanda, S., Sarangi, P.K.: Applications of supercritical fluids for biodiesel production. In: Sarangi, P.K., Nanda, S., Mohanty, P. (eds.) Recent Advancements in Biofuels and Bioenergy Utilization, pp. 261–284. Springer Nature, Singapore (2018)
Nayak, S.K., Nayak, B., Mishra, P.C., Noor, M.M., Nanda, S.: Effects of biodiesel blends and producer gas flow on overall performance of a turbocharged direct injection dual-fuel engine. Energy Sources A (2019). https://doi.org/10.1080/15567036.2019.1694101
Shiu, P.J., Gunawan, S., Hsieh, W.H., Kasim, N.S., Ju, Y.H.: Biodiesel production from rice bran by a two-step in-situ process. Bioresour. Technol. 101, 984–989 (2010)
Deeba, F., Pruthi, V., Negi, Y.S.: Converting paper mill sludge into neutral lipids by oleaginous yeast Cryptococcus vishniaccii for biodiesel production. Bioresour. Technol. 213, 96–102 (2015)
Alonso, D.M., Wettstein, S.G., Dumesic, J.A.: Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 15, 584–595 (2013)
Peng, L., Lin, L., Zhang, J., Zhuang, J., Zhang, B., Gong, Y.: Catalytic conversion of cellulose to levulinic acid by metal chlorides. Molecules 15, 5258–5272 (2010)
Román-Leshkov, Y., Chheda, J.N., Dumesic, J.A.: Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 312, 1933–1937 (2006)
Wang, P., Zhan, S.H., Yu, H.B.: Production of levulinic acid from cellulose catalyzed by environmental-friendly catalyst. Adv. Mater. Res. 96, 183–187 (2010)
Zhao, H., Holladay, J.E., Brown, H., Zhang, Z.C.: Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 316, 1597–1600 (2007)
Efremov, A.A., Pervyshina, G.G., Kuznetsov, B.N.: Production of levulinic acid from wood raw material in the presence of sulfuric acid and its salts. Chem. Nat. Comp. 34, 182–185 (1998)
Asghari, F.S., Yoshida, H.: Kinetics of the decomposition of fructose catalyzed by hydrochloric acid in subcritical water: Formation of 5-hydroxymethylfurfural, levulinic, and formic acids. Ind. Eng. Chem. Res. 46, 7703–7710 (2007)
Rahman, S.H.A., Choudhury, J.P., Ahmad, A.L., Kamaruddin, A.H.: Optimization studies on acid hydrolysis of oil palm empty fruit bunch fiber for production of xylose. Bioresour. Technol. 98, 554–559 (2007)
Kumar, R., Singh, S., Singh, O.V.: Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 35, 377–391 (2008)
Saha, B.C., Bothast, R.J.: Production of xylitol by Candida peltata. J. Ind. Microbiol. Biotechnol. 22, 633–636 (1999)
López-Linares, J.C., Romero, I., Cara, C., Castro, E., Mussatto, S.I.: Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour. Technol. 247, 736–743 (2018)
Cetera, P., D’Auria, M., Mecca, M., Todaro, L.: Gallic acid as main product in the water extractives of Quercus frainetto ten. Nat. Prod. Res. 14, 1–4 (2018)
Fache, M., Boutevin, B., Caillol, S.: Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 4, 35–46 (2016)
Kumar, N., Pruthi, V.: Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 4, 86–93 (2014)
Zanwar, A.A., Badole, S.L., Shende, P.S., Hegde, M.V., Bodhankar, S.L.: Role of gallic acid in cardiovascular disorders. Polyphenols Hum. Heal. Dis. 2, 1045–1047 (2013)
Gallezot, P.: Conversion of biomass to selected chemical products. Chem. Soc. Rev. 41, 1538–1558 (2012)
Lichtenthaler, F.W.: Unsaturated O- and N-heterocycles from carbohydrate feedstocks. Acc. Chem. Res. 35, 728–737 (2002)
Ge, X., Chang, C., Zhang, L., Cui, S., Luo, X., Hu, S., Qin, Y., Li, Y.: Conversion of lignocellulosic biomass into platform chemicals for biobased polyurethane application. Adv. Bioenerg. 3, 161–213 (2018)
Thakur, S., Chaudhary, J., Sharma, B., Verma, A., Tamulevicius, S., Thakur, V.K.: Sustainability of bioplastics: opportunities and challenges. Curr. Opin. Green Sustain. Chem. 13, 68–75 (2018)
Mekonnen, T., Mussone, P., Khalil, H., Bressler, D.: Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 1, 13379–13398 (2013)
Burgos, N., Valdés, A., Jiménez, A.: Valorization of agricultural wastes for the production of protein-based biopolymers. J. Renew. Mater. 4, 165–177 (2016)
Song, J.H., Murphy, R.J., Narayan, R., Davies, G.B.H.: Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B. Biol. Sci. 364, 2127–2139 (2009)
Bátori, V., Åkesson, D., Zamani, A., Taherzadeh, M.J., Sárvári Horváth, I.: Anaerobic degradation of bioplastics: a review. Waste Manag. 80, 406–413 (2018)
Pawar, R.P., Tekale, S.U., Shisodia, S.U., Totre, J.T., Domb, A.J.: Biomedical applications of poly(lactic acid). Rec. Patents Regen. Med. 4, 40–51 (2014)
Brodin, M., Vallejos, M., Opedal, M.T., Area, M.C., Chinga-Carrasco, G.: Lignocellulosics as sustainable resources for production of bioplastics—a review. J. Clean. Prod. 162, 646–664 (2017)
Trache, D., Hussin, M.H., HuiChuin, C.T., Sabar, S., Fazita, M.R.N., Taiwo, O.F.A., Hassan, T.M., Haafiz, M.K.M.: Microcrystalline cellulose: Isolation, characterization and bio-composites application—a review. Int. J. Biol. Macromol. 93, 789–804 (2016)
Ramamoorthy, S.K., Skrifvars, M., Persson, A.: A review of natural fibers used in biocomposites: plant, animal and regenerated cellulose fibers. Polym. Rev. 55, 107–162 (2015)
Wang, S., Lu, A., Zhang, L.: Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 53, 169–206 (2016)
Medronho, B., Lindman, B.: Competing forces during cellulose dissolution: from solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 19, 32–40 (2014)
Medronho, B., Lindman, B.: Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 222, 502–508 (2015)
Babu, K.M.: Natural textile fibres: animal and silk fibres. In: Sinclair, R. (ed.) Textiles and Fashion: Materials, Design and Technology, pp. 57–78. Woodhead Publishing, Sawston (2014)
Fink, H.P., Weigel, P., Purz, H.J., Ganster, J.: Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 26, 1473–1524 (2001)
Qi, H., Chang, C., Zhang, L.: Properties and applications of biodegradable transparent and photoluminescent cellulose films prepared via a green process. Green Chem. 11, 177–184 (2009)
Chen, J.: Synthetic textile fibers: Regenerated cellulose fibers. In: Sinclair, R. (ed.) Textiles and Fashion: Materials, Design and Technology, pp. 79–95. Woodhead Publishing, Sawston (2014)
Wilkes, A.G.: The viscose process. In: Woodings, C. (ed.) Regenerated Cellulose Fibres, pp. 37–61. Woodhead Publishing, Sawston (2001)
Kayra, N., Aytekin, A.Ö.: Synthesis of cellulose-based hydrogels: preparation, formation, mixture, and modification. In: Mondal, M.I.H. (ed.) Cellulose-Based Superabsorbent Hydrogels, pp. 1–28. Springer, Cham (2019)
Shen, X., Shamshina, J.L., Berton, P., Gurau, G., Rogers, R.D.: Hydrogels based on cellulose and chitin: fabrication, properties, and applications. Green Chem. 18, 53–75 (2015)
Kulkarni, A.R., Soppimath, K.S., Aminabhavi, T.M., Dave, A.M., Mehta, M.H.: Glutaraldehyde crosslinked sodium alginate beads containing liquid pesticide for soil application. J. Control. Release 63, 97–105 (2000)
Mogoşanu, G.D., Grumezescu, A.M.: Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 463, 127–136 (2014)
Arbona, V., Iglesias, D.J., Jacas, J., Primo-Millo, E., Talon, M., Gómez-Cadenas, A.: Hydrogel substrate amendment alleviates drought effects on young citrus plants. Plant Soil 270, 73–82 (2005)
Qiu, Y., Park, K.: Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 53, 321–339 (2001)
Job, N., Théry, A., Pirard, R., Marien, J., Kocon, L., Rouzaud, J.N., Béguin, F., Pirard, J.P.: Carbon aerogels, cryogels and xerogels: influence of the drying method on the textural properties of porous carbon materials. Carbon 43, 2481–2494 (2005)
Nayak, A.K., Das, B.: Introduction to polymeric gels. In: Pal, K., Banerjee, I. (eds.) Polymeric Gels, pp. 3–27. Woodhead Publishing, Sawston (2018)
Ansari, M.O., Kumar, R., Pervez Ansari, S., Abdel-wahab Hassan, M.S., Alshahrie, A., Barakat, M.A.E.-F.: Nanocarbon aerogel composites. In: Khan, A., Jawaid, M., Inamuddin, A., Asiri, A.M. (eds.) Nanocarbon and its Composites, pp. 1–26. Woodhead Publishing, Sawston (2019)
Domínguez, J.C., Oliet, M., Alonso, M.V., Gilarranz, M.A., Rodríguez, F.: Thermal stability and pyrolysis kinetics of organosolv lignins obtained from Eucalyptus globulus. Ind. Crops Prod. 27, 150–156 (2008)
Stewart, D.: Lignin as a base material for materials applications: chemistry, application and economics. Ind. Crops Prod. 27, 202–207 (2008)
Lora, J.H., Glasser, W.G.: Recent industrial applications of lignin: a sustainable alternative to nonrenewable materials. J. Polym. Environ. 10, 39–48 (2002)
Naseem, A., Tabasum, S., Zia, K.M., Zuber, M., Ali, M., Noreen, A.: Lignin-derivatives based polymers, blends and composites: a review. Int. J. Biol. Macromol. 93, 296–313 (2016)
Azargohar, R., Nanda, S., Dalai, A.K.: Densification of agricultural wastes and forest residues: a review on influential parameters and treatments. In: Sarangi, P.K., Nanda, S., Mohanty, P. (eds.) Recent Advancements in Biofuels and Bioenergy Utilization, pp. 27–51. Springer Nature, Singapore (2018)
Azargohar, R., Nanda, S., Kang, K., Bond, T., Karunakaran, C., Dalai, A.K., Kozinski, J.A.: Effects of bio-additives on the physicochemical properties and mechanical behavior of canola hull fuel pellets. Renew. Energy 132, 296–307 (2019)
Wang, J., Vermerris, W.: Antimicrobial nanomaterials derived from natural products—a review. Materials 9, E255 (2016)
Espinoza-Acosta, J.L., Torres-Chávez, P.I., Olmedo-Martínez, J.L., Vega-Rios, A., Flores-Gallardo, S., Zaragoza-Contreras, E.A.: Lignin in storage and renewable energy applications: a review. J. Energy Chem. 27, 1422–1438 (2018)
Nadif, A., Hunkeler, D., Käuper, P.: Sulfur-free lignins from alkaline pulping tested in mortar for use as mortar additives. Bioresour. Technol. 84, 49–55 (2002)
Fadele, O.A., Ata, O.: Water absorption properties of sawdust lignin stabilised compressed laterite bricks. Case Stud. Constr. Mater. 9, e00187 (2018)
Weber, M., Weber, M., Weber, V.: Phenol. In: Ullmann’s Encyclopedia of Industrial Chemistry. https://doi.org/10.1002/14356007.a19_299.pub3 (2020)
Nehez, N.: Lignin-based friction material. European Patent Office: EP0856030A4, pp. 242–554 (1997)
Çetin, N.S., Özmen, N.: Use of organosolv lignin in phenol-formaldehyde resins for particleboard production: I. Organosolv lignin modified resins. Int. J. Adhes. Adhes. 22, 477–480 (2002)
Kosbar, L.L., Gelorme, J.D., Japp, R.M., Fotorny, W.T.: Introducing biobased materials into the electronics industry: developing a lignin-based resin for printed wiring boards. J. Ind. Ecol. 4, 93–105 (2001)
Upton, B.M., Kasko, A.M.: Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem. Rev. 116, 2275–2306 (2016)
Koike, T.: Progress in development of epoxy resin systems based on wood biomass in Japan. Polym. Eng. Sci. 52, 701–717 (2012)
Lora, J.: Industrial commercial lignins: sources, properties and applications. In: Belgacem, M.N., Gandini, A. (eds.) Monomers, Polymers and Composites, pp. 225–271. Elsevier, New York (2008)
Belgacem, M.N., Gandini, A.: Materials from vegetable oils: major sources, properties and applications. In: Belgacem, M.N., Gandini, A. (eds.) Monomers, pp. 39–66. Elsevier, New York (2008)
Arshanitsa, A., Ponomarenko, J., Dizhbite, T., Andersone, A., Gosselink, R.J.A., Van Der Putten, J., Lauberts, M., Telysheva, G.: Fractionation of technical lignins as a tool for improvement of their antioxidant properties. J. Anal. Appl. Pyrolysis 103, 78–85 (2013)
Okolie, J.A., Henry, O.E., Epelle, E.I.: Determination of the antioxidant potentials of two different varieties of banana peels in two different solvents. Food Nutr. Sci. 7, 1253–1261 (2016)
Espinoza-Acosta, J.L., Torres-Chávez, P.I., Ramírez-Wong, B., López-Saiz, C.M., Montaño-Leyva, B.: Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. BioResources 11, 5452–5481 (2016)
Pan, X., Kadla, J.F., Ehara, K., Gilkes, N., Saddler, J.N.: Organosolv ethanol lignin from hybrid poplar as a radical scavenger: relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 54, 5806–5813 (2006)
Dong, X., Dong, M., Lu, Y., Turley, A., Jin, T., Wu, C.: Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crops Prod. 34, 1629–1634 (2011)
El Hage, R., Perrin, D., Brosse, N.: Effect of the pre-treatment severity on the antioxidant properties of ethanol organosolv Miscanthus x giganteus lignin. Nat. Resourc 3, 29 (2012)
Azargohar, R., Nanda, S., Kozinski, J.A., Dalai, A.K., Sutarto, R.: Effects of temperature on the physicochemical characteristics of fast pyrolysis bio-chars derived from Canadian waste biomass. Fuel 125, 90–100 (2014)
Azargohar, R., Nanda, S., Dalai, A.K., Kozinski, J.A.: Physico-chemistry of biochars produced through steam gasification and hydro-thermal gasification of canola hull and canola meal pellets. Biomass Bioenergy 120, 458–470 (2019)
Kang, K., Nanda, S., Sun, G., Qiu, L., Gu, Y., Zhang, T., Zhu, M., Sun, R.: Microwave-assisted hydrothermal carbonization of corn stalk for solid biofuel production: Optimization of process parameters and characterization of hydrochar. Energy 186, 115795 (2019)
Kang, K., Nanda, S., Lam, S.S., Zhang, T., Huo, L., Zhao, L.: Enhanced fuel characteristics and physical chemistry of microwave hydrochar for sustainable fuel pellet production via co-densification. Environ. Res. 109480 (2020)
Mukherjee, A., Okolie, J.A., Abdelrasoul, A., Niu, C., Dalai, A.K.: Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 83, 46–63 (2019)
Boonpoke, A., Chiarakorn, S., Laosiripojana, N., Towprayoon, S., Chidthaisong, A.: Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption capacity. J. Sustain. Environ. 2, 77–81 (2011)
Plaza, M.G., González, A.S., Pevida, C., Pis, J.J., Rubiera, F.: Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl. Energy 99, 272–279 (2012)
Zhang, X., Zhang, S.H., Yang, H.P., Shi, T., Chen, Y.Q., Chen, H.P.: Influence of NH3/CO2 modification on the characteristic of biochar and the CO2 capture. Bioenergy Res. 6, 1147–1153 (2013)
Yi, H., Zuo, Y., Liu, H., Tang, X., Zhao, S., Wang, Z., Gao, F., Zhang, B.: Simultaneous removal of SO2, NO, and CO2 on metal-modified coconut. Water. Air. Soil Pollut. 225, 1965 (2014)
Jain, S., Kumar, P., Vyas, R.K., Pandit, P., Dalai, A.K.: Adsorption optimization of acyclovir on prepared activated carbon. Can. J. Chem. Eng. 92, 1627–1635 (2014)
Shahkarami, S., Dalai, A.K., Soltan, J., Hu, Y., Wang, D.: Selective CO2 capture by activated carbons: Evaluation of the effects of precursors and pyrolysis process. Energy Fuels 29, 7433–7440 (2015)
Azargohar, R., Dalai, A.K.: Biochar as a precursor of activated carbon. Appl. Biochem. Biotechnol. 129–132, 762–773 (2006)
De, M., Azargohar, R., Dalai, A.K., Shewchuk, S.R.: Mercury removal by bio-char based modified activated carbons. Fuel 103, 570–578 (2013)
Bu, Q., Lei, H., Wang, L., Yadavalli, G., Wei, Y., Zhang, X., Zhu, L., Liu, Y.: Biofuel production from catalytic microwave pyrolysis of Douglas fir pellets over ferrum-modified activated carbon catalyst. J. Anal. Appl. Pyrolysis 112, 74–79 (2015)
Portet, C., Yushin, G., Gogotsi, Y.: Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotubes in electrical double layer capacitors. Carbon 45, 2511–2518 (2007)
Sharifuddin, S.A.B., Ismail, S.B., Abdullah, I., Mohamad, I., Mohammed, J.S.: Antibacterial evaluation of activated carbon cloth with Ag+ impregnated with ZnO nanoparticles. Res. J. Textile Apparel 23, 232–243 (2019)
Juurlink, D.N.: Activated charcoal for acute overdose: a reappraisal. British J. Clinic. Pharm. 81, 482–487 (2016)
Miriyala, N., Ouyang, D., Perrie, Y., Lowry, D., Kirby, D.J.: Activated carbon as a carrier for amorphous drug delivery: Effect of drug characteristics and carrier wettability. Eur. J. Pharm. Biopharm. 115, 197–205 (2017)
Nanda, S., Gong, M., Hunter, H.N., Dalai, A.K., Gökalp, I., Kozinski, J.A.: An assessment of pinecone gasification in subcritical, near-critical and supercritical water. Fuel Process. Technol. 168, 84–96 (2017)
Azargohar, R., Nanda, S., Rao, B.V.S.K., Dalai, A.K.: Slow pyrolysis of deoiled Canola meal: Product yields and characterization. Energy Fuels 27, 5268–5279 (2013)
Nanda, S., Dalai, A.K., Pant, K.K., Gökalp, I., Kozinski, J.A.: An appraisal on biochar functionality and utility in agronomy. In: Konur, O. (ed.) Bioenergy and Biofuels, pp. 389–409. CRC Press, Florida (2018)
Fathy, N.A.: Carbon nanotubes synthesis using carbonization of pretreated rice straw through chemical vapor deposition of camphor. RSC Adv. 7, 28535–28541 (2017)
Guo, T., Nikolaev, P., Thess, A., Colbert, D.T., Smalley, R.E.: Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 243, 49–54 (1995)
Journet, C., Maser, W.K., Bernier, P., Loiseau, A., Lamy de la Chapelle, M., Lefrant, S., Deniard, P., Lee, R., Fischer, J.E.: Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 388, 756–758 (1997)
Wang, Z., Ogata, H., Morimoto, S., Ortiz-Medina, J., Fujishige, M., Takeuchi, K., Muramatsu, H., Hayashi, T., Terrones, M., Hashimoto, Y., Endo, M.: Nanocarbons from rice husk by microwave plasma irradiation: From graphene and carbon nanotubes to graphenated carbon nanotube hybrids. Carbon 94, 479–484 (2015)
Goodell, B., Xie, X., Qian, Y., Daniel, G., Peterson, M., Jellison, J.: Carbon nanotubes produced from natural cellulosic materials. J. Nanosci. Nanotechnol. 8, 2472–2474 (2008)
Zhu, J., Jia, J., Kwong, F.L., Ng, D.H.L., Tjong, S.C.: Synthesis of multiwalled carbon nanotubes from bamboo charcoal and the roles of minerals on their growth. Biomass Bioenergy 36, 12–19 (2012)
Bernd, M.G.S., Bragança, S.R., Heck, N., da Filho, L.C.P.: Silva Filho: Synthesis of carbon nanostructures by the pyrolysis of wood sawdust in a tubular reactor. J. Mater. Res. Technol. 6, 171–177 (2017)
Bachtold, A., Hadley, P., Nakanishi, T., Dekker, C.: Logic circuits with carbon nanotube transistors. Science 294, 1317–1320 (2001)
Baughman, R.H., Zakhidov, A.A., de Heer, W.A.: Carbon nanotubes–the route toward applications. Science 297, 787–792 (2002)
Karfa, P., De, S., Majhi, K.C., Madhuri, R., Sharma, P.K.: Functionalization of carbon nanostructures. Compr. Nanosci. Nanotechnol. 26, 123–144 (2018)
Karousis, N., Suarez-Martinez, I., Ewels, C.P., Tagmatarchis, N.: Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 116, 4850–4883 (2016)
Pagona, G., Mountrichas, G., Rotas, G., Karousis, N., Pispas, S., Tagmatarchis, N.: Properties, applications and functionalisation of carbon nanohorns. Int. J. Nanotechnol. 6, 176–195 (2008)
Zhu, S., Xu, G.: Single-walled carbon nanohorns and their applications. Nanoscale 2, 2538–2549 (2010)
Morgan, J.L.W., Strumillo, J., Zimmer, J.: Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493, 181–186 (2013)
Ullah, H., Santos, H.A., Khan, T.: Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 23, 2291–2314 (2016)
Lin, S.P., Loira Calvar, I., Catchmark, J.M., Liu, J.R., Demirci, A., Cheng, K.C.: Biosynthesis, production and applications of bacterial cellulose. Cellulose 20, 2191–2219 (2013)
Klemm, D., Schumann, D., Udhardt, U., Marsch, S.: Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog. Polym. Sci. 26, 1561–1603 (2001)
Hasan, N., Biak, D.R.A., Kamarudin, S.: Application of bacterial cellulose (BC) in natural facial scrub. Int. J. Adv. Sci. Eng. Inf. Technol. 2(272), 1–4 (2012)
Amnuaikit, T., Chusuit, T., Raknam, P., Boonme, P.: Effects of a cellulose mask synthesized by a bacterium on facial skin characteristics and user satisfaction. Med. Devices (Auckl) 4, 77–81 (2011)
Almeida, I.F., Pereira, T., Silva, N.H.C.S., Gomes, F.P., Silvestre, A.J.D., Freire, C.S.R., Sousa Lobo, J.M., Costa, P.C.: Bacterial cellulose membranes as drug delivery systems: an in vivo skin compatibility study. Eur. J. Pharm. Biopharm. 86, 332–336 (2014)
Lin, Y.C., Wey, Y.C., Lee, M.L., Lin P.C.: Cosmetic composition containing fragments of bacterial cellulose film and method for manufacturing thereof. United States Patent US20150216784A1 (2015)
Songsurang, K., Pakdeebumrung, J., Praphairaksit, N., Muangsin, N.: Sustained release of amoxicillin from ethyl cellulose-coated amoxicillin/chitosan–cyclodextrin-based tablets. AAPS PharmSciTech 12, 35–45 (2011)
Trovatti, E., Freire, C.S.R., Pinto, P.C., Almeida, I.F., Costa, P., Silvestre, A.J.D., Neto, C.P., Rosado, C.: Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 435, 83–87 (2012)
Sulaeva, I., Henniges, U., Rosenau, T., Potthast, A.: Bacterial cellulose as a material for wound treatment: properties and modifications: a review. Biotechnol. Adv. 33, 1547–1571 (2015)
Sun, B., Zhang, M., Shen, J., He, Z., Fatehi, P., Ni, Y.: Applications of cellulose-based materials in sustained drug delivery systems. Curr. Med. Chem. 26, 2485–2501 (2019)
Weyell, P., Beekmann, U., Küpper, C., Dederichs, M., Thamm, J., Fischer, D., Kralisch, D.: Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 207, 1–10 (2019)
Tamahkar, E., Bakhshpour, M., Denizli, A.: Molecularly imprinted composite bacterial cellulose nanofibers for antibiotic release. J. Biomater. Sci. Polym. Ed. 30, 450–461 (2019)
Acknowledgements
The authors would like to thank the Natural Sciences and Engineering Research Council of Canada (NSERC) and Canada Research Chairs (CRC) program for funding this bioenergy research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okolie, J.A., Nanda, S., Dalai, A.K. et al. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass Valor 12, 2145–2169 (2021). https://doi.org/10.1007/s12649-020-01123-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01123-0