Abstract
Improved productivities of microalgal biomass tend to play a significant role in biorefineries pertaining to multifaceted applications and the inadequate biomass yield in any particular medium is a bottleneck that must be overcome to achieve such sustainability goals. In our present study, we employed new approach to enhance the cell growth of a potential strain Chlorella saccharophila (UTEX 247), i.e., media engineering perspective. For better biomass yields, the fundamental constituents are the macronutrients within the growth medium consisting of nitrogen (as NaNO3, sodium nitrate), phosphorus (as K2HPO4, dipotassium phosphate) with an additional source of carbon supplementation in the form of NaHCO3, sodium bicarbonate. Our preliminary studies by One Factor at a Time demonstrated no effect on growth with additional carbon supplementation but showed that nitrogen and phosphorus ratios play a significant role in the biomass production. Furthermore, we optimized the biomass yields employing the central composite design associated with the response surface methodology tool to illustrate the combinatorial effects of nitrogen (N) and phosphorous (P). Our results have showed an increase up to 131% dcw in biomass production, i.e., 0.84 g L−1 DCW with 26.4 mM and 0.11 mM of NaNO3 and K2HPO4 concentrations, respectively, than the control condition (NaNO3: 17.6 mM; K2HPO4: 0.23 mM) yielding a biomass content of 0.64 g L−1 DCW with a coefficient of variance of 5.12%. In conclusion, the new perspective of media engineering predicts and also evaluates the best condition for the specific strain of interest so that the optimized medium essentially produces higher cell biomass along with other biocommodities of industrial significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
On a global scale, fossil fuel use has significantly increased demand for renewable fuels due to environmental threats [1,2,3], such as global warming and melting glaciers [4,5,6], whereas the development of the first and second generations has been impeded by competition with food production and rising production costs [7,8,9]. Alternatively, microalgae have been extensively investigated as a potential feedstock for third-generation biofuels due to their faster growth rates, use of non-arable land resources, carbon sequestration of flue gases, and treatment of wastewater effluents [10]. In recent times, algal biomass has got significant appreciation in the applied research especially for their advancements in the areas of pharmaceuticals, nutraceuticals, and biofuels, and also in the wastewater treatments [11, 12]. Even though the cost of microalgal cultivation seems to be still high, their economic viability and competitiveness in the market can be taken into consideration only with few specialized products [13, 14]. Henceforth, new perspectives are required to enhance the biomass yields in a cost-effective manner using different strategies which will make the overall process sustainable. In the present study, a potential strain of microalgae with industrial relevance namely Chlorella saccharophila UTEX 247 having better growth rates with remarkable biocommodities such as lipids, proteins, and other high-value added bioproducts [15,16,17,18,19,20,21,22,23] has been further optimized for higher biomass yields. A major hurdle in algal biorefineries is production of cell biomass in an optimized medium that can be efficiently scaled up in the industrial photobioreactors.
In such context, our new approach in this study was employing media engineering perspective via response surface methodology (RSM), which is an effective, efficient, statistical tool involved in modeling, analyzing, and predicting experimental datasets wherein the response of interest will be assessed by several factors involved in maximizing their productivities [24]. Henceforth in this study, we have employed a statistical-based experimental design to unveil the interactions between multiple factors simultaneously, thus providing information on their cumulative effects. Furthermore, the number of experiments used in such approaches seems to be reasonable without jeopardizing the accuracy following systematic studies. Thus, the RSM tool seems to be an effective methodology for optimizing numerous variable combinations at the same time for maximizing the output [25,26,27,28,29]. Previously, studies have shown the relevance of using multivariate dose method [30] to optimize the algal growth rates [31,32,33,34]. For example, Cheng et al. [35], Mubarak et al. [36], and many others demonstrated the use of the RSM tool for optimization of medium components such as sodium nitrate, phosphate, ethylenediaminetetraacetic acid, CaCl2, and KNO3 in Chlorella sp. for improving biomass yields.
In the present study, the optimization tool such as response surface methodology (RSM) has been employed for enhancing the cell growth of the C. saccharophila UTEX 247. The role of essential macronutrients (such as “N” for nitrate, NaNO3; “P” for phosphate, K2HPO4; and “C” as bicarbonate, NaHCO3) was studied by varying their concentrations in the medium in context with improving the biomass productivities. Further, the evaluation of growth under optimized conditions has been illustrated via the chlorophyll fluorescence measurements, which demonstrates the enhanced photosynthetic performance in these microalgal cell factories. Overall, our study focused on the deployment of significant macronutrients, either alone or in combination, to obtain optimized biomass yields with other biomolecules such as lipids and carotenoids.
2 Materials and methods
2.1 Strain, culture conditions, and biomass estimation
A freshwater C. saccharophila UTEX 247 was procured from The University of Texas at Austin (UTEX) Culture Collection of Algae, Austin, TX, USA, and the strain was cultured in the minimal BG-11 medium in 250-mL Erlenmeyer flasks [37, 38]. The culture was maintained under a light regime of 16:8 h and an illumination of 150 μmol photons m−2 s−1 photosynthetically active radiation at 24 °C ± 2 °C. The mid-logarithmic phase cells were centrifuged and resuspended in fresh medium with initial concentration of 50 ± 5 mg L−1 biomass, and its growth was measured at an interval of every 3 days up to 21 days.
Growth and biomass were evaluated by cell counting method using a hemocytometer [39] and correlated with the optical density (O.D.) measured by a SpectraMax M Series Multimode Microplate Reader (Molecular Devices, LLC., San Jose, CA, USA) at 750 nm [40] using the regression equation:
where y corresponds to the cell number and x corresponds to the O.D.
Then the growth rate was determined using the equation below:
where N1 and N2 are the cell count/O.D. at initial (t1) and final time (t2), respectively, and similarly doubling time can be estimated using the Eq. 3.
2.2 Identification of factors influencing algal biomass
Primarily, the following essential macronutrients namely nitrate (NaNO3) and phosphate (K2HPO4) along with addition carbon source as bicarbonate (NaHCO3) were independently evaluated to predict their effect on the biomass yields. Initially, these three abovementioned variables with different concentrations were carried out employing the strategy of the One Factor at a Time (OFAT) with following experimental setup: NaNO3 ranges between 4.4 (0.25 N), 8.8 (0.5 N), 26.4 (1.5 N), and 35.4 (2 N) mM, respectively, where 17.6 mM (as control [C]); K2HPO4 ranges between 0.05 (0.25P), 0.11 (0.5P), 0.34 (1.5P), and 0.46 (2P) mM, respectively, where 0.23 mM (as control [C]); whereas additional carbon was included as follows in the form of NaHCO3: 3.5 (0.5B), 7.0 (B), and 10.5 (1.5B) mM, respectively, where 0 mM (no bicarbonate as control [C]) in BG-11 medium. Summarized tabulation of the OFAT experimental setup with all three variables is clearly illustrated in Table 1. All the OFAT experiments were carried out independently in biological triplicates with all three variables for 21 days to evaluate their effect on the biomass yields in C. saccharophila.
2.3 Response surface methodology
An efficient, user-defined decision-making statistical tool known as RSM was employed in this study for optimizing biomass yields following three steps [41]. In the earlier step, essential medium components namely two macronutrients (nitrogen [NaNO3], phosphorous [K2HPO4]) and bicarbonate (NaHCO3) supplementation (additional carbon source) were evaluated with three important variables to investigate their influence on the cell’s biomass [42]. In the RSM, the specific concentrations of variables which have shown positive effect on enhancement of algal biomass were selected to find the optima using central composite design (CCD) with their corresponding equation. Additionally, a three-dimensional surface plot was reconstructed to assess the interactions of different variables especially nitrate (NaNO3) and phosphate (K2HPO4) with reference to their impact on growth. The final step was employed to validate the deduced model using the equation with varying concentrations, thus further confirming the responses between the predicted (YPred.) and experimental (YExp.) conditions.
2.3.1 Step 1: development of model equation using central composite design
The CCD, a second-order experimental setup, was performed for the optimization process [42,43,44], where the two-level factorials, both the axial and central points, were included in the design to unveil the occurrence and to estimate terms involved in the second-order fitted model equation. The minimum and maximum concentrations were considered at a distance of − 1 and + 1 units, respectively, and the central point of the minimum and maximum concentrations was automatically denoted by the model, which are summarized in Table 2.
A major advantage of setting the experiments with CCD was inclusion of the outliers for each factor at a distance α, thus avoiding any possible error with five replicates at the center point. In this study, the Design-Expert® software, version 13, Stat-Ease, Inc., Minneapolis, MN, USA (www.statease.com), was used to predict the model employing the one-way analysis of variance (ANOVA) analysis. In addition, the model generated the response surface 3D plot with the contour lines, depicting the correlation between the factors and response [45]. The experimental datasets achieve the equation as follows:
where y is the response; β0 is the intercept; βi, βii, and βij are the regression coefficients of different variables in linear and quadratic equations; and Ai and Aj are the coded independent variables.
2.3.2 Step 2: validation of the model
The predicted model has been validated with three different concentrations of both factors designated with validation points as VP1, VP2, and VP3 to determine the biomass yields. Overall, the YPred. and YExp. datasets were compared to analyze the model’s accuracy as stated by Eq. 4.
2.4 Quantification of different biocommodities and Chl a fluorescence measurement
2.4.1 Estimation of total pigments using spectrometry
To quantify other biocommodities such as total pigments and lipids obtained in the RSM optimized biomass, the following modified protocols were employed [46]. For the estimation of total pigments, 1 mL of cells was centrifuged, and the pellet was resuspended in 1 mL of methanol. After vortexing, it was incubated at 55 °C for an hour. Later, the absorbance of the supernatant was measured at specific wavelengths of 665, 652, and 470 nm in the SpectraMax M Series Multimode Microplate Reader (Molecular Devices, LLC., San Jose, CA, USA), and the contents of chlorophyll a, chlorophyll b, and total carotenoids [35] were determined using the following equations:
2.4.2 Quantification of total lipids by sulpho-phospho-vanillin assay
The total lipids were estimated by the sulpho-phospho-vanillin method, wherein 2 mL of cells was pelleted, followed by addition of 2 mL of concentrated H2SO4 (98%) and incubated at 100 °C for 10 min. After cooling the reaction, 5 mL of freshly prepared phospho-vanillin reagent has been added and incubated at 37 °C for 15 min with continuous shaking at 200 rpm. The absorbance was measured at 530 nm in the SpectraMax M Series Multimode Microplate Reader (Molecular Devices, LLC., San Jose, CA, USA) and the quantification was done using canola oil (MilliporeSigma, USA) as the standard [47].
2.4.3 Chl a fluorescence measurement as photosynthetic efficiency
Non-invasive fluorescence measurements were acquired by using dual-pulse amplitude modulation (PAM) 100 chlorophyll fluorometer (Heinz Walz Gmbh, Effeltrich, Germany) to measure the photosynthetic efficiency of photosystem II (PSII) [48]. Each sample corresponding to at least 20 µg of chlorophyll was incubated in dark for 15 min at 25 °C. For the optimal measurements, the sample was transferred into a quartz glass cuvette (10 × 10 × 40 mm) with a magnetic bead, followed by placing the cuvette into the PAM fluorometer to obtain the induction curve. For minimum fluorescence (Fo) measurement, a measuring light was applied (< 0.1 μmol photons m−2 s−1) and for maximum fluorescence measurement (Fm), a saturation pulse light was applied (6000 μmol photons m−2 s−1) for 0.8 s in every 10 s). The maximum photosynthesis efficiency of PSII (Fv/Fm) was calculated based on the equation Fv/Fm = (Fm–Fo)/Fm, where Fv is the variable fluorescence that elucidates the difference between Fm and Fo [48,49,50].
2.5 Software and statistical analysis
The mean and standard deviations for all three independent biological triplicates (n = 3) were calculated by the ANOVA along with the statistical analysis (p < 0.05). The CCD design was performed using the Design-Expert® software, version 13, Stat-Ease, Inc., Minneapolis, MN, USA (www.statease.com). The goodness of fit of these designs was assessed statistically by applying ANOVA to identify the statistically significant terms. The significance of regression coefficients was determined with a confidence level of 95%. Further, the probability plots were drawn between the studentized residual and percent probability of response to confirm data homogeneity.
3 Results
3.1 Optimization of biomass yields using OFAT experiments
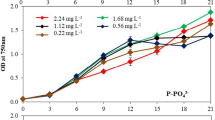
The growth profile of C. saccharophila UTEX 247 in the minimal BG-11 medium with following macronutrients (17.6 mM NaNO3, 0.23 mM K2HPO4, 0 mM NaHCO3—defined as the control [C] in these experiments) demonstrates biomass yields of 640.0 ± 25.0 mg L−1 with a specific growth rate (µ) of 0.57 ± 0.02 day−1 and doubling time 29.0 ± 2.0 h. The time-course experiments were done at regular intervals as follows: 0, 3, 6, and 9 days. As described earlier in Section 2, Table 1 summarizes the key essential factors with varying concentrations the components nitrate (NaNO3), phosphate (K2HPO4), and bicarbonate (NaHCO3), which were evaluated for their effects on biomass yields individually using the OFAT experimental setup in microalgae C. saccharophila (Fig. 1).
Our preliminary data analysis demonstrated that the macronutrients, i.e., nitrogen (as NaNO3, sodium nitrate) and phosphorus (as K2HPO4, dipotassium phosphate), showed significant effect on their biomass yields (Fig. 1a, b). The results on the 9th day showed a significant increase in biomass content, i.e., 690 mg L−1 with a specific growth rate (µ) of 0.6 ± 0.02 day−1 in slightly higher concentration (26.4 mM [1.5 N]) of NaNO3 than the control. In the case of phosphorus (P), the biomass yields ranged between 600 and 680 mg L−1 using different K2HPO4 concentrations of 0.05–0.46 mM, respectively (Fig. 1a). Also, we have observed a significant enhancement in biomass content, i.e., 680 mg L−1 at lower K2HPO4 (0.11 mM [0.5 P]) concentration, and the lowest biomass, i.e., 600 mg L−1 under the highest K2HPO4 (0.46 mM [2.0 P]) concentration on the 9th day (Fig. 1b). Our study demonstrates that higher K2HPO4 concentration showed negative impact on biomass yields, whereas the additional carbon supplementation (NaHCO3; 3.5, 7.0, and 10.5 mM) illustrated no impact on their biomass yields in the C. saccharophila (Fig. 1c). Henceforth, the NaHCO3 was not included as the essential factor in the further experimentation. In summary, our preliminary study demonstrates that NaNO3 and K2HPO4 showed significant effect in enhancing the biomass yields at the concentration of 1.5 N and NaNO3 (26.4 mM) and 0.5 P and K2HPO4 (0.11 mM), respectively.
3.2 Response surface methodology
3.2.1 Model designing with two variables for biomass enhancement
Based on the results obtained in the initial step, two variables with different concentrations were further selected in this study as illustrated in Table 2 (Section 2). The reconstruction of the model using response surface methodology was done by employing the CCD module. Also, we performed experiments for demonstrating the interactions between the two essential macronutrients and their impact on the biomass yields with the best-suited model within the selected range of factors. All the combinations used in the CCD model are shown in Table 3, including the 5 replicates around the center point to avoid any possible errors which may occur due to certain artifacts.
Our experimental data analysis of biomass using the parameters predicted by the model ranges between 630.0 and 840.0 mg L−1 (Table 3). The range predicted indicates that these two nutrients significantly impacted their growth profiles in C. saccharophila. Moreover, the second-order fitted model derived a quadratic equation perfectly suited for the experimentation. In addition, the model also demonstrates non-significant lack of fit (R2 of 0.87 and adjusted R2 0.78), which describes the fitness of the data predicted by the model along with the analysis of variance (ANOVA) to evaluate the model’s significance [51]. Statistical significance for the response surface quadratic model is given in Table 4. F-value (9.09) of the model implies that the model is significant; only 0.57% chance is there that an F-value this large occurs due to noise. p values of less than 0.05 indicates that the model terms are significant. In this case, B, AB, and A2 are significant model terms that affect biomass production. On other hand, values greater than 0.10 indicate that the model terms have no direct significance. Adeq Precision measures the signal-to-noise ratio and a ratio greater than 4 is desirable. Obtained ratio of 10.597 shown in Table 5 indicates an adequate signal so as the model can be used to navigate the design space. All the findings in this study are illustrated in Tables 4 and 5, where the p value < 0.05 indicates the significance of model. For example, K2HPO4 (B; p = 0.004) and NaNO3 × K2HPO4 (AB; p = 0.03) are more significant in terms of the biomass determinants. The quadratic equation has a positive magnitude of A and a negative magnitude of B, indicating their correlation for biomass with the increasing concentration of NaNO3 and the decreasing concentration of the K2HPO4. Furthermore, other quadratic terms such as AB and B2 have a negative magnitude, whereas A2 has a positive magnitude, which clearly states that the higher concentrations of NaNO3 enhance the growth, i.e., increasing the overall biomass yields, whereas the negative magnitude of term B2 indicates that it has a negative impact on biomass when it is in very high concentration and the p value indicates that this model term has no direct significance but when it comes to AB and B with p values 0.03 and 0.004, respectively, it is much more significant.
The correlation between the predicted and actual values is illustrated in Fig. 2a with an excellent coefficient of variation (CV) of 5.12. In addition, the 3D surface plot shown in Fig. 2b demonstrates the contour lines with appropriate optimization values and their effect on the biomass yields. It clearly depicted that the higher NaNO3 or lower K2HPO4 contributes to better biomass content and any change in their concentration will impact the overall biomass in C. saccharophila. With reference to these results, we showed that the highest biomass content obtained was 840 mg L−1 with two factors, i.e., NaNO3 (26.4 mM) and K2HPO4 (0.11 mM). Perhaps, this model accepts some outliers except K2HPO4 (0.25 mM) where the experimental (YExp.) and predicted (YPred.) response showed significant difference with their standard deviation.
a Parity graphs demonstrating the distribution of actual and predicted values of biomass production in C. saccharophila. b Response surface and contour lines indicating the impact of NaNO3 and K2HPO4 on the biomass yields in C. saccharophila with reference to response surface polynomials. Also, the actual data points are shown as red circles
In the present study, we have also estimated the contents of other biocommodities such as total chlorophylls/carotenoids and total lipid content in the optimized biomass to know the changes occurring within the cells subjected to varying macronutrients (Figs. 3a-c). Our results showed biocommodities such as total lipids (120 mg L−1) along with the total chlorophyll and carotenoids, i.e., 10.5 and 6.53 mg L−1, respectively (Figs. 3a-b). In summary, this work also demonstrates the best optimized concentration of two factors, i.e., NaNO3 and K2HPO4, for higher biomass production (131%) without compromising any fitness cost on the yield of other biocommodities (122% TC [total chlorophyll], 127% CT (total carotenoids), and 125% total lipids shown in Fig. 3c).
Biochemical components in C. saccharophila at day 9, in two different concentrations of nitrogen (NaNO3) and phosphorus (K2HPO4) to compare the total pigments (total chlorophyll [TC] and total carotenoids [Ct.]), total lipid yield (represented as a–c respectively]. d Changes in maximum quantum yield of PSII photochemistry (Fv/Fm), i.e., maximum efficiency at which PSII-absorbed light is utilized to reduce QA. All the samples are represented as the average of three biological replicates ± S.D.
Fig. 3d indicates the Fv/Fm ratios, which are extensively used as a representation of the maximal photochemical efficiency of the PSII reaction centers, and it generally correlates with the photosynthetic performance of the cell. In this study, Fv/Fm (PSII quantum yield) was observed to compare the photosynthetic performance of culture in control and in optimized conditions. In the control, cells attained the highest activity on the 3rd day, i.e., 0.67, whereas in the optimized medium the photosynthetic activity observed was 0.75 on day 6, which was maintained at 0.69 until the 9th day (Fig. 3d). In conclusion, our results demonstrate that the optimized medium showed better photosynthetic efficiency than the control, which indicates the better activity of the photosynthetic machinery thus leading to enhanced cell biomass.
3.2.2 Validation of the model for the accuracy and reliability
Table 6 illustrates the validation of the model for demonstrating their accuracy and reliability in terms of the response on the biomass yield with two optimized variables. The validation points (VP1, VP2, VP3) correspond to biomass yields of 640.25, 600.38, and 760.15 mg L−1, respectively, as shown in Table 6. The difference between the responses as YPred. and YExp. ranges within the standard deviation thus demonstrating the accuracy of the model. Moreover, the results also affirmed that such model designing approach for the optimization process is quite accurate and reliable.
4 Discussion
During the past few decades, substantial research has been done with different strains of Chlorella sp. for enhancing their biomass yields [20, 52,53,54,55,56]. These reports clearly indicate that the factors influencing the production of biomass include medium composition especially the macronutrients [57,58,59]. Some of the major nutrients are nitrogen (N), phosphorus (P), and carbon (C); among these, N and P are usually present in the medium and C will be supplemented as additional source. In the present study, we demonstrated the effect of two essential macronutrients including NaNO3 and K2HPO4 along with NaHCO3 (additional carbon) independently via the OFAT experiments to illustrate their effect on biomass yields in C. saccharophila UTEX 247. Among these, NaNO3 (26.4 mM; 1.5 N) showed a positive impact on its biomass content, thus demonstrating that the nitrogen is the significant growth-enhancing factor in microalgae [60]. Thus, the use of nitrate as N source is the most suitable option for biomass production [61] as it is an essential constituent of the structure of amino acids, proteins, and enzymes.
Moreover, K2HPO4 is also important for growth at the lower concentrations. Solovchenko et al. [62] and Lavrinovics et al. [63] demonstrated that there was no effect on the growth when subjected to phosphorus (P) starvation. Moreover, Singh et al. (2021) [64] also reported that less P is giving the better biomass productivity. Other than that, Suthar et al. [65] also reported amount of P for growth is strain specific. In addition, several research groups worked on phosphorous uptake and revealed that microalgae can only absorb additional phosphorus if they develop in a deprived state first [66, 67]. As a result, the subjective reason behind this strategy can be the uptake of inorganic phosphorous and stores it as the poly-P granules. Such poly-P molecules are rich energy source that are able to support the growth of organisms for different metabolic functions within the cells [68]. Furthermore, in the case of higher P, growth was delayed [69]. Henceforth, the P assimilation and/or tolerance is strain-specific. On the other hand, the possibility of P assimilation at higher concentration may lead to irregular N:P ratios, which will significantly impact the biomass yields.
The N:P ratio is known to affect cell proliferation of some micro algae. A research group Zhang et al. (2011) [70] evaluated the effect of N/P ratios on the proliferation and succession of phytoplankton using different nitrogen sources NH4Cl (N1) and urea (N2), and a single source of phosphorous, NaH2PO4(P). The optimal N/P ratio that differed among the five species was affected by the source of nitrogen, being as follows (N1/P, N2/P in order): Thalassiosira sp. (30/1, 20/1), Heterosigma akashiwo (30/1, 30/1), Chroomonas salina (20/1, 30/1), Chaetoceros gracilis (40/1, 60/1), and Alexandrium sp. (10/1, 30/1). Thus, the source of nitrogen must be considered when analyzing the N/P ratio. Other than that, Molina et al. 2011 [71] observed the maximum growth rate for N:P between 2.5 and 80. Furthermore, Armitage et al. (2005) [72] reported N:P > 96:1 for Thalassia testudinum at its natural habitat. But the main feature of BG11 medium is that the N:P ratio is deliberately high (~ 80:1) for simple and convenient cultivation of unicellular photosynthetic organisms [73, 74]. However, species-specific medium optimization is necessary for different aspects such as biomass and lipid [73, 75]. In this study, we optimized the medium for better biomass therefore needed more N. As a result, the best biomass obtained in the N:P ration 240:1 which shown as the 5th run in the Table 3. Furthermore, nitrogen is the medium’s restraining nutrient and the phosphorus concentration might be even higher after N depletion. Thus, leading to a saturation point where the phosphorus cellular absorption might be restricted [63].
But in the case of NaHCO3, there was no significant improvement in biomass content of C. saccharophila. Similar results have been reported earlier [76], where bicarbonate inhibits growth by raising the pH of the medium. Nayak et al. [77] and Richmond et al. [78] investigated the effect of bicarbonate on growth and suggested that the increased pH can be maintained by introducing gaseous CO2. Henceforth, bicarbonate in some instances cannot be considered a growth-enhancing factor. Chi et al. [79] observed that a few strains can tolerate a higher concentration of different salts including NaHCO3 and/or NaCl. Furthermore, White et al. [76] also worked on bicarbonate and examined the bicarbonate supplementation on two strains and found that it either had no effect on growth or delayed it. However, our present study shows that there is no enhancement in the growth patterns of C. saccharophila when subjected with additional carbon supplementation.
Subsequently, two parameters (NaNO3 and K2HPO4) were considered to optimize conditions for the better biomass production in C. saccharophila employing the CCD module. The CCD is essential for determining the effect of each variable alone or in combination with the total response. Nitrogen (N) is a primary factor which is essential for the synthesis of biomolecules besides growth. Also, it is well known that the nitrogen depletion will lead to decrease in overall protein content which ultimately affects the cell’s machinery. In the present study, we demonstrated that the optimal concentration of NaNO3 is required for higher biomass which relates to the work done by Zarrinmehr et al. [80] which stated that increased nitrogen concentration enhances biomass yields. Moreover, our results with the CCD model and the quadratic equation showed that biomass yields in C. saccharophila enhance significantly with the specific concentration of higher NaNO3 and lower K2HPO4.
Taziki et al. [81] demonstrated use of nitrate as a nitrogen source which has been efficiently utilized by Chlorella sp. to produce higher biomass. Furthermore, Ana−Maria and coworkers [82] enhanced both biomass and lipid yield through the CCD model, and found that nitrate concentration was the growth−enhancing factor. Kim et al. [83] showed that the N and P supplementation in Chlorella sp. further enhanced their growth rate to 0.48 day−1. Recently, Rodrigues−Sousa et al. [84] demonstrated that the nitrogen supplementation enhanced biomass content in Chlorella sp. and stated that a single macronutrient with the specific concentration, including the N:P ratio, will act as an excellent growth−enhancing factor. Similarly, in this study, even NaNO3 and K2HPO4 independently influenced the growth but simultaneously in combination they tend to promote better as the growth−enhancing factors. Such technique demonstrates the importance of the CCD model in the medium optimization process, where a precise concentration and/or their combination of both components generates a promising optimized response for higher yields.
In such context, this is a worthy study of its kind which utilizes RSM tool with CCD model for further enhancing the biomass yields along with other biocommodities as we used 1.5 N and 0.5 P in optimized medium, and achieved 131% biomass, 122% TC (total chlorophyll), 127% CT (total carotenoids), and 125% total lipids as shown in Fig. 3. Kirrolia et al. [30] used similar strategy as a decision−making tool for the medium optimization in Chlorella sp. for enhancing biodiesel production. Increasing the biomass yields of C. saccharophila is an important step in making algal biofuels more economical and sustainable [85, 86]. Therefore, the use of optimized medium for producing better biomass productivities was investigated along with other biocommodities simultaneously. Another salient feature in this study is that the optimized medium showed better photosynthetic performance, which states that the activity of cell’s photosystem is functioning at their maximal.
5 Conclusions
In this present study, the microalgae C. saccharophila UTEX 247 was subjected to media engineering approach employing response surface methodology for enhancing their biomass productivities. Essential macronutrients in the BG-11 medium, i.e., NaNO3 and K2HPO4, influence the biomass yields independently but a better enhancement in the biomass content (840 mg L−1) was achieved by the specific combination of these two factors at the optimized concentrations (NaNO3 (26.4 mM) and K2HPO4 (0.11 mM)) as defined by CCD module. The statistical tool used in the current study demonstrated an increase of 131.25% dcw in biomass yields along with other biocommodities such as lipids (125%) and pigments (122% TC [total chlorophyll], 127% CT (total carotenoids) respectively). In conclusion, the optimization of specific growth conditions is essential for each specific strain of industrial relevance for enhancing their growth rates along with other biocommodities, which will lead to sustainable and cost-effective biorefineries.
Data availability
Not applicable.
Abbreviations
- RSM:
-
Response surface methodology
- CCD:
-
Central composite design
- N:
-
Nitrogen
- P:
-
Phosphorus
- NaNO3:
-
Sodium nitrate
- KH2PO4:
-
Phosphate
- NaHCO3:
-
Bicarbonate
- CV:
-
Coefficient of variance
- EDTA:
-
Fe-Ethylenediaminetetraacetic acid
- O.D. :
-
Optical density
- SPV:
-
Sulpho-phospho-vanillin
- PAM:
-
Pulse amplitude modulation
- ANOVA:
-
Analysis of variance
- VP:
-
Validation point
References
Liu J, Song Y, Qiu W (2017) Oleaginous microalgae Nannochloropsis as a new model for biofuel production: review & analysis. Renew Sust Energ Rev 72:154–162. https://doi.org/10.1016/j.rser.2016.12.120
Kabir G, Hameed B (2017) Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew Sust Energ Rev 70:945–967. https://doi.org/10.1016/j.rser.2016.12.001
Katiyar R, Gurjar B, Biswas S, Pruthi V, Kumar N, Kumar P (2017) Microalgae: an emerging source of energy based bio-products and a solution for environmental issues. Renew Sus Energ Rev 72:1083–1093. https://doi.org/10.1016/j.rser.2016.10.028
Kumar S, Shrestha P, Salam PA (2013) A review of biofuel policies in the major biofuel producing countries of ASEAN: production, targets, policy drivers and impacts. Renew Sust Energ Rev 26:822–836. https://doi.org/10.1016/j.rser.2013.06.007
Su Y, Zhang P, Su Y (2015) An overview of biofuels policies and industrialization in the major biofuel producing countries. Renew Sust Energ Rev 50:991–1003. https://doi.org/10.1016/j.rser.2015.04.032
Manirafasha E, Ndikubwimana T, Zeng X, Lu Y, Jing K (2015) Phycobiliprotein: potential microalgae derived pharmaceutical and biological reagent. Biochem Eng J 109:282–296. https://doi.org/10.1016/j.bej.2016.01.025
Song C, Liu Q, Ji N, Deng S, Zhao J, Li S, Kitamura Y (2015) Evaluation of hydrolysis-esterification biodiesel production from wet microalgae. Bioresour Technol 214:747–754. https://doi.org/10.1016/j.biortech.2016.05.024
Noraini M, Ong HC, Badrul MJ, Chong W (2015) A review on potential enzymatic reaction for biofuel production from algae. Renew Sust Energ Rev 39:24–34. https://doi.org/10.1016/j.rser.2014.07.089
Bhuiya M, Rasul M, Khan M, Ashwath N, Azad A (2015) Prospects of 2nd generation biodiesel as a sustainable fuel-Part: 1 selection of feedstocks, oil extraction techniques and conversion technologies. Renew Sust Energ Rev 55:1109–1128. https://doi.org/10.1016/j.rser.2015.04.163
Moncada J, Tamayo JA, Cardona CA (2015) Integrating first, second, and third generation biorefineries: incorporating microalgae into the sugarcane biorefinery. Chem Eng Sci 118:126–140. https://doi.org/10.1016/j.ces.2014.07.035
Caporgno MP, Mathys A (2018) Trends in microalgae incorporation into innovative food products with potential health benefits. Front Nutr 5:1–15. https://doi.org/10.3389/fnut.2018.00058
Matos ÂP (2017) The impact of microalgae in food science and technology. J Am Oil Chem Soc 94:1333–1350. https://doi.org/10.1007/s11746-017-3050-7
Enzing C, Ploeg M, Barbosa M, Sijtsma L, authors Vigani M, Parisi C, Rodriguez Cerezo E, editors. Microalgae-based products for the food and feed sector: an outlook for Europe. EUR 26255. Luxembourg (Luxembourg): Publications Office of the European Union; 2014. JRC85709. https://doi.org/10.2791/3339.
Wijffels RH, Barbosa MJ, Eppink MH (2015) Microalgae for the production of bulk chemicals and biofuels. Biofuel Bioprod Biorefin 4:287–295. https://doi.org/10.1002/bbb.215
Mondal M, Ghosh A, Tiwari O, Gayen K, Das P, Mandal M, Halder G (2017) Influence of carbon sources and light intensity on biomass and lipid production of Chlorella sorokiniana BTA 9031 isolated from coalfield under various nutritional modes. Energy Convers Manag 145:247–254. https://doi.org/10.1016/j.enconman.2017.05.001
Barlow J, Sims RC, Quinn JC (2015) Techno-economic and life-cycle assessment of an attached growth algal biorefinery. Bioresour Technol 220:360–368. https://doi.org/10.1016/j.biortech.2016.08.091
Mondal M, Ghosh A, Sharma AS, Tiwari O, Gayen K, Mandal M, Halder G (2015) Mixotrophic cultivation of Chlorella sp. BTA 9031 and Chlamydomonas sp. BTA 9032 isolated from coal field using various carbon sources for biodiesel production. Energy Convers Manag 124:297–304. https://doi.org/10.1016/j.enconman.2016.07.033
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2015) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278. https://doi.org/10.1016/j.rser.2014.04.007
Pratt R, Daniels T, Eiler JJ, Gunnison J, Kumler W, Oneto JF, Strait LA, Spoehr H, Hardin G, Milner H (1944) Chlorellin, an antibacterial substance from Chlorella. Science 99:351–352. https://doi.org/10.1126/science.99.2574.351
El-Sheekh M, Abu-Faddan M, Abo-Shady A, Nassar MZA, Labib W (2020) Molecular identification, biomass, and biochemical composition of the marine chlorophyte Chlorella sp. MF1 isolated from Suez Bay. J Genet Eng Biotechnol 8:27. https://doi.org/10.1186/s43141-020-00044-8
Gouveia L, Choubert G, Pereira N, Santinha J, Empis J, Gomes E (2002) Pigmentation of gilthead seabream, Sparus aurata (L. 1875), using Chlorella vulgaris (Chlorophyta, Volvocales) microalga. Aquac Res 33:987–993. https://doi.org/10.1046/j.1365-2109.2002.00751.x
Choix FJ, De-Bashan LE, Bashan Y (2012) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II Heterotrophic conditions. Enzyme Microb Technol 51:300–309. https://doi.org/10.1016/j.enzmictec.2012.07.013
Yeh KL, Chang JS (2011) Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: implications for biofuels. Biotechnol J 6:1358–1366. https://doi.org/10.1002/biot.201000433
Ahmad S, Pathak VV, Kothari R, Kumar A, Krishna SBN (2018) Optimization of nutrient stress using C. pyrenoidosa for lipid and biodiesel production in integration with remediation in dairy industry wastewater using response surface methodology. 3 Biotech 8:326. https://doi.org/10.1007/s13205-018-1342-8
Yang J, Astatkie T, He QS (2015) A comparative study on the effect of unsaturation degree of camelina and canola oils on the optimization of bio-diesel production. Energy Rep 2:211–217. https://doi.org/10.1016/j.egyr.2016.08.003
Ahmad S, Kothari R, Pathania D, Tyagi V (2020) Optimization of nutrients from wastewater using RSM for augmentation of Chlorella pyrenoidosa with enhanced lipid productivity, FAME content, and its quality assessment using fuel quality index. Biomass Convers Bioref 10:495–512. https://doi.org/10.1007/s13399-019-00443-z
Saleem A, Hussain A, Chaudhary A, Q-u-A A, Iqtedar M, Javid A, Akram AM (2022) Acid hydrolysis optimization of pomegranate peels waste using response surface methodology for ethanol production. Biomass Convers Bioref 12:1513–1524. https://doi.org/10.1007/s13399-020-01117-x
Dahmen-Ben Moussa I, Masmoudi MA, Choura S, Chamkha M, Sayadi S (2021) Extraction optimization using response surface methodology and evaluation of the antioxidant and antimicrobial potential of polyphenols in Scenedesmus sp. and Chlorella sp. Biomass Convers Bioref. https://doi.org/10.1007/s13399-021-01850-x
Campos L, Moura HO, Cruz AJ, Assumpcao S, de Carvalho LS, Pontes LA (2020) Response surface methodology (RSM) for assessing the effects of pretreatment, feedstock, and enzyme complex association on cellulose hydrolysis. Biomass Convers Bioref. https://doi.org/10.1007/s13399-020-00756-4
Kirrolia A, Bishnoi NR, Singh R (2015) Response surface methodology as a decision-making tool for optimization of culture conditions of green microalgae Chlorella spp. for biodiesel production. Ann Microbiol 64:1133–1147. https://doi.org/10.1007/s13213-013-0752-4
Ghosh S, Roy S, Das D (2015) Improvement of biomass production by Chlorella sp. MJ 11/11 for use as a feedstock for biodiesel. Appl Biochem Biotechnol 175:3322–3335. https://doi.org/10.1007/s12010-015-1503-8
Ho S-H, Chen C-Y, Chang J-S (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Zheng Y, Chen Z, Lu H, Zhang W (2011) Optimization of carbon dioxide fixation and starch accumulation by Tetraselmis subcordiformis in a rectangular airlift photobioreactor. Afr J Biotechnol 10:1888–1901. https://doi.org/10.5897/AJB10.1620
Deamici KM, Santos LO, Costa JAV (2021) Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst Eng 44:1453–1460. https://doi.org/10.1007/s00449-021-02526-6
Cheng K-C, Ren M, Ogden KL (2013) Statistical optimization of culture media for growth and lipid production of Chlorella protothecoides UTEX 250. Bioresour Technol 128:44–48. https://doi.org/10.1016/j.biortech.2012.09.085
Mubarak M, Shaija A, Suchithra T (2019) Cost effective approach for production of Chlorella pyrenoidosa: a RSM based study. Waste Biomass Valori 10:3307–3319. https://doi.org/10.1007/s12649-018-0330-x
Singh R, Paliwal C, Nesamma AA, Narula A, Jutur PP (2020) Nutrient deprivation mobilizes the production of unique tocopherols as a stress-promoting response in a new indigenous isolate Monoraphidium sp. Front Mar Sci 7:575817. https://doi.org/10.3389/fmars.2020.575817
Paliwal C, Jutur PP (2021) Dynamic allocation of carbon flux triggered by task-specific chemicals is an effective non-gene disruptive strategy for sustainable and cost-effective algal biorefineries. Chem Eng J 418:129413. https://doi.org/10.1016/j.cej.2021.129413
Guillard RR, Sieracki MS (2005) Counting cells in cultures with the light microscope. In: Andersen RA (ed) Algal culturing techniques. Elsevier Academic Press, London, pp 239–252
Santos-Ballardo DU, Rossi S, Hernández V, Gómez RV, del Carmen R-U, Caro-Corrales J, Valdez-Ortiz A (2015) A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquac Res 448:87–92. https://doi.org/10.1016/j.aquaculture.2015.05.044
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Yaakob MA, Mohamed RMSR, Al-Gheethi A, Ravishankar GA, Ambati RR (2021) Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: an overview. Cells 10:393. https://doi.org/10.3390/cells10020393
Box GE, Wilson KB. (1992) On the experimental attainment of optimum conditions. Breakthroughs in statistics, Springer Series in Statistics. Springer, New York, NY, pp. 270–310. https://doi.org/10.1007/978-1-4612-4380-9_23.
Slathia PS, Raina N, Kiran A, Kour R, Bhagat D, Sharma P (2020) Dilute acid pretreatment of pine needles of Pinus roxburghii by response surface methodology for bioethanol production by separate hydrolysis and fermentation. Biomass Convers Bioref 10:95–106. https://doi.org/10.1007/s13399-019-00433-1
Baş D, Boyacı IH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 78:836–845. https://doi.org/10.1016/j.jfoodeng.2005.11.024
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, Park MS, Yang J-W (2015) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155:330–333. https://doi.org/10.1016/j.biortech.2013.12.077
Zhou Y, Schideman L, Park D, Stirbet A, Rupassara S, Krehbiel J, Seufferheld M (2015) Characterization of a Chlamydomonas reinhardtii mutant strain with improved biomass production under low light and mixotrophic conditions. Algal Res 11:134–147. https://doi.org/10.1016/j.algal.2015.06.001
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurevplant59.032607.092759
Agarwal A, Patil S, Gharat K, Pandit RA, Lali AM (2019) Modulation in light utilization by a microalga Asteracys sp. under mixotrophic growth regimes. Photosynth res 139:553–567. https://doi.org/10.1007/s11120-018-0526-8
Khuri AI, Mukhopadhyay S (2015) Response surface methodology. Wiley Interdiscip Rev Comput Stat 2:128–149. https://doi.org/10.1002/wics.73
McGaughy K, Abu Hajer A, Drabold E, Bayless D, Reza MT (2019) Algal remediation of wastewater produced from hydrothermally treated septage. Sustainability 11:3454. https://doi.org/10.3390/su11123454
Chinnasamy S, Ramakrishnan B, Bhatnagar A, Das KC (2009) Biomass production potential of a wastewater alga Chlorella vulgaris ARC 1 under elevated levels of CO2 and temperature. Int J Mol Sci 10:518–532. https://doi.org/10.3390/ijms10020518
Zhang X, Yuan H, Guan L, Wang X, Wang Y, Jiang Z, Cao L, Zhang X (2019) Influence of photoperiods on microalgae biofilm: photosynthetic performance, biomass yield, and cellular composition. Energies 12:3724. https://doi.org/10.3390/en12193724
Romanowska-Duda Z, Szufa S, Grzesik M, Piotrowski K, Janas R (2021) The promotive effect of cyanobacteria and Chlorella sp. foliar biofertilization on growth and metabolic activities of willow (Salix viminalis l.) plants as feedstock production, solid biofuel and biochar as C carrier for fertilizers via torrefaction process. Energies 14:5262. https://doi.org/10.3390/en14175262
Doušková I, Kaštánek F, Maléterová Y, Kaštánek P, Doucha J, Zachleder V (2015) Utilization of distillery stillage for energy generation and concurrent production of valuable microalgal biomass in the sequence: biogas-cogeneration-microalgae-products. Energy Convers Manag 51:606–611. https://doi.org/10.1016/j.enconman.2009.11.008
Chia MA, Lombardi AT, Melao MDGG (2013) Growth and biochemical composition of Chlorella vulgaris in different growth media. An Acad Bras Cienc 85:1427–1438. https://doi.org/10.1590/0001-3765201393312
Blair MF, Kokabian B, Gude VG (2015) Light and growth medium effect on Chlorella vulgaris biomass production. J Environ Chem Eng 2:665–674. https://doi.org/10.1016/j.jece.2013.11.005
Couto C, Hernández CP, Alves Sobrinho RCM, Mendes CRB, Roselet F, Abreu PC (2021) Optimization of a low-cost fertilizer-based medium for large-scale cultivation of the coastal diatom Conticribra weissflogii using response surface methodology and its effects on biomass composition. J Appl Phycol 33:2767–2781. https://doi.org/10.1007/s10811-021-02519-8
Costa SS, Miranda AL, Andrade BB, de Jesus AD, Souza CO, de Morais MG, Costa JAV, Druzian JI (2018) Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int J Biol Macromol 116:552–562. https://doi.org/10.1016/j.ijbiomac.2018.05.064
Arumugam M, Agarwal A, Arya MC, Ahmed Z (2013) Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour Technol 131:246–249. https://doi.org/10.1016/j.biortech.2012.12.159
Solovchenko A, Khozin-Goldberg I, Selyakh I, Semenova L, Ismagulova T, Lukyanov A, Mamedov I, Vinogradova E, Karpova O, Konyukhov I (2019) Phosphorus starvation and luxury uptake in green microalgae revisited. Algal Res 43:101651. https://doi.org/10.1016/j.algal.2019.101651
Lavrinovičs A, Murby F, Zīverte E, Mežule L, Juhna T (2021) Increasing phosphorus uptake efficiency by phosphorus-starved microalgae for municipal wastewater post-treatment. Microorganisms 9:1598. https://doi.org/10.3390/microorganisms9081598
Singh P, Kumar D (2021) Biomass and lipid productivities of cyanobacteria-Leptolyngbya foveolarum HNBGU001. BioEnergy Res 14:278–291. https://doi.org/10.1007/s12155-020-10170-3
Suthar S, Verma R (2018) Production of Chlorella vulgaris under varying nutrient and abiotic conditions: a potential microalga for bioenergy feedstock. Process Saf Environ Prot 113:141–148. https://doi.org/10.1016/j.psep.2017.09.018
Hernandez J-P, de-Bashan LE, Bashan Y (2006) Starvation enhances phosphorus removal from wastewater by the microalga Chlorella spp. co-immobilized with Azospirillum brasilense. Enzyme Microb Technol 38:190–198. https://doi.org/10.1016/j.enzmictec.2005.06.005
Powell N, Shilton AN, Pratt S, Chisti Y (2008) Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ Sci Technol 42:5958–5962. https://doi.org/10.1021/es703118s
Achbergerová L, Nahálka J (2011) Polyphosphate-an ancient energy source and active metabolic regulator. Microb cell factories 10:63. https://doi.org/10.1186/1475-2859-10-63
Ernst A, Deicher M, Herman PM, Wollenzien UI (2005) Nitrate and phosphate affect cultivability of cyanobacteria from environments with low nutrient levels. Appl Environ Microbiol 71:3379–3383. https://doi.org/10.1128/aem.71.6.3379-3383.2005
Zhang Q, Hu G (2011) Effect of nitrogen to phosphorus ratios on cell proliferation in marine micro algae. Chin J Oceanol Limnol 29:739–745. https://doi.org/10.1007/s00343-011-0503-y
Molina E, Martínez E, Sańchez S, García F, Contreras A (1991) The influence of temperature and the initial N: P ratio on the growth of microalgae Tetraselmis sp. Process Biochem 26:183–187. https://doi.org/10.1016/0032-9592(91)80016-I
Armitage AR, Frankovich TA, Heck KL, Fourqurean JW (2005) Experimental nutrient enrichment causes complex changes in seagrass, microalgae, and macroalgae community structure in Florida Bay. Estuaries 28:422–434. https://doi.org/10.1007/BF02693924
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Thomas DL, Mantes JG (1978) Spectrophotometrically assayed inhibitory effects of mercuric compounds on anabaena flos-aquae and anacystis nidulans (cyanophyceae). J Phycol 14:494–499. https://doi.org/10.1111/j.1529-8817.1978.tb02475.x
Cordeiro RS, Vaz IC, Magalhaes S, Barbosa FA (2017) Effects of nutritional conditions on lipid production by cyanobacteria. An Acad Bras Cienc 89:2021–2031. https://doi.org/10.1590/0001-3765201720150707
White D, Pagarette A, Rooks P, Ali S (2013) The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J Appl Phycol 25:153–165. https://doi.org/10.1007/s10811-012-9849-6
Nayak M, Suh WI, Lee B, Chang YK (2018) Enhanced carbon utilization efficiency and FAME production of Chlorella sp. HS2 through combined supplementation of bicarbonate and carbon dioxide. Energy Convers Manag 156:45–52. https://doi.org/10.1016/j.enconman.2017.11.002
Richmond A, Karg S, Boussiba SJP (1982) Effects of bicarbonate and carbon dioxide on the competition between Chlorella vulgaris and Spirulina platensis. Plant Cell Physiol 23:1411–1417. https://doi.org/10.1093/oxfordjournals.pcp.a076489
Chi Z, Elloy F, Xie Y, Hu Y, Chen S (2015) Selection of microalgae and cyanobacteria strains for bicarbonate-based integrated carbon capture and algae production system. Appl Biochem Biotechnol 172:447–457. https://doi.org/10.1007/s12010-013-0515-5
Zarrinmehr MJ, Farhadian O, Heyrati FP, Keramat J, Koutra E, Kornaros M, Daneshvar E (2020) Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt J Aquat Res 46:153–158. https://doi.org/10.1016/j.ejar.2019.11.003
Taziki M, Ahmadzadeh H, Murry MA (2015) Growth of Chlorella vulgaris in high concentrations of nitrate and nitrite for wastewater treatment. Curr Biotechnol 4:441–447. https://doi.org/10.2174/2211550104666150930204835
Aguirre AM, Bassi A (2013) Investigation of biomass concentration, lipid production, and cellulose content in Chlorella vulgaris cultures using response surface methodology. Biotechnol Bioeng 110:2114–2122. https://doi.org/10.1002/bit.24871
Kim S, Lee Y, Hwang S-J (2013) Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. Int Biodeterior Biodegr 85:511–516. https://doi.org/10.1016/j.ibiod.2013.05.025
Rodrigues-Sousa AE, Nunes IV, Muniz-Junior AB, Carvalho JCM, Mejia-da-Silva LC, Matsudo MC (2021) Nitrogen supplementation for the production of Chlorella vulgaris biomass in secondary effluent from dairy industry. Biochem Eng J 165:107818. https://doi.org/10.1016/j.bej.2020.107818
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531. https://doi.org/10.1016/j.apenergy.2011.04.018
Hildebrand M, Abbriano RM, Polle JE, Traller JC, Trentacoste EM, Smith SR, Davis AK (2013) Metabolic and cellular organization in evolutionarily diverse microalgae as related to biofuels production. Curr Opin Chem Biol 17:506–514. https://doi.org/10.1016/j.cbpa.2013.02.027
Acknowledgements
Senior Research Fellowships for A.M. supported by the Council for Scientific Research (CSIR) (Award No. 09/512(0232)/2017-EMR-I), New Delhi, India, and funding by the Department of Biotechnology (DBT) (Ref ID: DBT/2017/ICGEB/930), New Delhi, India, for S.U.Z. are duly acknowledged.
Funding
The work was supported by the grants provided from the Department of Biotechnology (DBT) and Biotechnology Industry Research Assistance Council (BIRAC), India (Grant No. BT/PB/Center/03/2011-Phase II, BT/SB0078/02/19).
Author information
Authors and Affiliations
Contributions
Conceptualization, analysis, writing—original draft, A.M., S.U.Z; conceptualization, funding acquisition, supervision, project administration, writing—review and editing, P.P.J. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehra, A., Zafar, S.U. & Jutur, P.P. Optimization of biomass production by Chlorella saccharophila UTEX 247 employing response surface methodology. Biomass Conv. Bioref. 14, 8549–8561 (2024). https://doi.org/10.1007/s13399-022-02966-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02966-4