Abstract
This study aimed to evaluate the influence of a magnetic field (MF) intensity of 25 mT on Chlorella fusca cultivation in outdoor and indoor conditions, and evaluate the changes in the macromolecules, pigment content and protein profile. C. fusca was cultivated for 15 d in raceway photobioreactor. MF was applied for 24 h d−1 and 1 h d−1. In outdoor cultivation, MF applied for 24 h d−1 increased 23% in the biomass concentration, while indoor assays resulted in an increase in both modes, with biomass production increasing between 70 and 85%. Biomass composition was altered when MF was applied for 1 h d−1 in indoor assays; the highest protein content was achieved (32.7%). Nitrate consumption was higher in outdoor assays, while MF application did not alter the protein profile. The results showed that combining the outdoor conditions with MF is advantageous, as higher biomass concentration can be achieved with lower energy expenditure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorella sp. is a unicellular eukaryotic green alga that has been extensively studied. Chlorella fusca (C. fusca), which belongs to the chlorophyceae phylum, can be found in freshwater and marine environments and can exist as autotrophic, heterotrophic, mixotrophic or photoheterotrophic [1]. The biomass obtained is typically rich in high-quality proteins, vitamins, liposoluble compounds, glycolipids and compounds used as food additives. However, is necessary to evaluate the viability of outdoor cultivation systems for the industrial production of microalgae products. The use of sunlight and uncontrolled environmental conditions can reduce the overall costs of the biomass production process [2]. Raceway ponds or photobioreactors are open systems that are commonly used for large-scale outdoor cultivation due to their low construction and operational costs.
Although several studies have been carried out on the cultivation of microalgae in open systems, studies with C. fusca are scarce. To cultivate in this way, it is important to understand the variations in growth and metabolism between the different modes of cultivation (outdoor and indoor) to determine the viability of outdoor cultivation of this microalga.
New techniques to increase biomass production have recently been studied and the use of magnetic fields (MF) has been shown as an alternative to increasing the productivity of microalgae biomass and the production of biomolecules. Microalgae studies have shown that MF effect can alter protein content [3], lipid production [4], oxygen production [5], growth rate [6], and CO2 biofixation [7]. Besides, the increased growth of Chlorella fusca was previously discovered due to MF action in a closed system [7] that aroused the interest to describe how the MF can act in the cell in uncontrolled environmental conditions. The use of MF in bioprocess can usually be stimulated under particular conditions adopted in each assay [8] even if the optimal conditions for the microalgae growth are used, such as luminosity, temperature and photoperiod. In this context, the aim of this study was to evaluate the influence of 25 mT magnetic field intensity applied at different exposure times on the cultivation of Chlorella fusca LEB 111 in raceway ponds, in outdoor and indoor conditions, as well as to evaluate the expressed changes in macromolecules, pigment content and protein profile.
Material and methods
Microalga
The evaluated microalga was Chlorella fusca LEB 111 (C. fusca) which was isolated from lakes near the President Médici Thermoelectricity Plant, located in Candiota, RS, Brazil. The strain was obtained from a Collection of Cultures in the Laboratory of Biochemical Engineering of the Federal University of Rio Grande (FURG). The strain studied below the genetic collection at the Aidar & Kutner Microorganism Bank (BMA&K) of the Oceanographic Institute of the University of São Paulo (IOUSP), under registration number BMAK-D14.
Cultivation conditions
Assays were performed on raceway photobioreactor (0.7 m long, 0.18 m wide, 0.075 m depth and 4.5 L working volume) and mixed using paddle wheels turning at 24 revs min−1. The initial biomass concentration was 0.3 g L−1 [6], and the amount of inoculum necessary for that concentration was calculated based on the standard curve for this microalga. C. fusca was maintained and cultivated in BG 11 medium [9]. The assays were performed in batch mode for 15 d.
Outdoor cultivation
In outdoor cultivation mode (uncontrolled environmental conditions), the assays were carried out between March and April in a greenhouse covered with transparent film with daylight sunlight (~ 300 μmolphotons m−2 s−1) and room temperature (~ 34 °C).
Indoor cultivation
In the indoor cultivation mode, the assays were performed in a thermostat chamber with the following working conditions: a constant temperature of 30 ºC, a 12 h light/dark photoperiod and illumination provided by four fluorescent tubes of 32 W, with 81.3 µmolfotons m−2 s−1. Evaporation of water was controlled by adding distilled water daily, and the cultures were performed in duplicate.
Use of magnetic fields in the assays
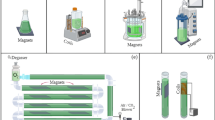
MFs were applied using six ferrite magnets (150 × 50 × 10 mm) coupled outside each raceway. A gauss meter (TLMP–HALL-05 k-T0, Brazil) placed inside the photobioreactor at different points was used to measure the intensity of the magnets. The mean intensity was 25 mT. MF application was studied for two different exposure times, 24 h and 1 h d−1, respectively. In the outdoor mode, the assays were performed at different periods, so that the environmental conditions in each culture varied. Thus, it was necessary to perform a control culture for each condition studied (control/MF for 24 h d−1 and control/MF for 1 h d−1). In the indoor assays, a control culture (without MF application) was performed under similar conditions of temperature, luminosity, photoperiod and initial biomass concentration as the outdoor assays.
Biomass concentration and pH
Biomass concentration and pH were measured daily. The biomass concentration (X, g L−1) was monitored by determining the absorbance (670 nm) in a UV–Vis spectrophotometer (QUIMIS Q998U, Brazil) in relation to the optical density using the standard curve for C. fusca LEB 111. The pH values were obtained directly using a digital pH meter (QUIMIS Q400MT, Brazil).
Consumption of nitrate
Nitrogen in the form of NO3 was quantified in the culture medium every 72 h, according to the methodology proposed by Cataldo et al. [10]. In a 0.25-mL culture medium, 0.8 mL of 5% salicylic acid solution in sulphuric acid was added, the reaction was allowed for 2o min after which 19-mL NAOH (2 M) was added. The samples were kept at room and spectrophotometer measurements were performed at 410 nm in relation to a standard N-NO3 curve.
Recovery of the biomass from the liquid medium
At the end of the assays, the biomass was recovered from the liquid medium by centrifugation (HITACHI, Himac CR-GIII, Japan). The centrifuged biomass was frozen for 48 h at − 80 °C and then lyophilized for 48 h at − 4 °C under vacuum (LABCONCO, USA). The lyophilized samples were kept in freezer at -20 °C for further characterization of the protein, lipid and carbohydrate contents.
Macromolecules in the biomass
The biomass of the assays was analyzed for protein, carbohydrate and lipid content. Unclarified biomass extracts were prepared for protein and carbohydrate determination. These extracts were obtained using 5 mg of lyophilized biomass and 10 mL of distilled water, sonicated in an ultrasonic probe (COLE PARMER, CPX 130, USA) for 10 min in a 59 s cycle (on/off) at 25% amplitude. This procedure was carried out to release the intracellular material into the liquid medium. Lipid concentration was obtained directly from the lyophilized biomass. Protein content in the biomass was determined using the colorimetric method described by Lowry et al. [11], with a standard curve of bovine serum albumin. Carbohydrate content was determined using the phenol–sulfuric method described by Dubois et al. [12], with a standard glucose curve. Lipids in the lyophilized biomass were extracted according to the method proposed by Folch et al. [13], using chloroform and methanol solvents.
Chlorophyll content
Extraction of chlorophyll (Chl) from the C. fusca biomass was done every 3-d using methanol 99.8% (v v−1), according to the method proposed by Litchtenthaler [14]. The chlorophyll (a + b) content was obtained according to the following equation:
Extraction and protein profile
Proteins were extracted every 72 h by adding buffer with the following composition: 80 mM Tris–HCl (pH 6.8), 0.1 M 2-mercaptoethanol, 2% (w v−1) sodium dodecyl sulfate-SDS, 15% (v v−1) glycerol and 0.006% (w v−1) m-purple cresol, the lysates were then heated for 5 min at 100 °C. After centrifugation at 10,000 g for 1 min, the samples were subjected to discontinuous SDS polyacrylamide gel electrophoresis (SDS-PAGE) according to the method proposed by Laemmli [15], using a 5% acrylamide stacking gel and 12.5% acrylamide resolving gel.
Growth parameters
The growth parameters were determined using biomass concentration (X, g L−1). The biomass productivity (Pmax, g L−1 d−1) was calculated by the equation P = (Xt – X0)/(t – t0), where Xt is the biomass concentration at time t (d), and X0 is the biomass concentration at time t0 (d). The maximum specific growth rate (µmax, d−1) was obtained by exponential regression in the logarithmic phase of cell multiplication of each assay on the ln X (g L−1) versus t (d) plot. The doubling time (Dt, d) was determined at the exponential phase by the equation: Dt = ln (2)/µmax.
The evaluation of the MF effect compared to the control was calculated using the following equation: ɳ = (CMF − Cc)/CMF·100, where ɳ corresponds to the difference in the percentage of responses evaluated with MF (CMF) in relation to the responses obtained in the control cultures (Cc). The values are expressed in % throughout the text.
Statistical analysis
The responses were evaluated by analysis of variance (ANOVA), followed by the Tukey test at 95% confidence level. Statistical analyses were carried out by Statistica 10.0 software.
Results
The effects of different application times of MF (25 mT) were investigated during the cultivation of Chlorella fusca LEB 111. The growth curve of this microalga under indoor and outdoor conditions is showed in Fig. 1a, b, where it is related to the biomass concentration (g L−1) and cultivation time (d). C. fusca grew in all conditions evaluated and presented cell adaption phase (lag phase) that lasted approximately 2 d, followed by the exponential phase. When the MF was applied throughout the assay (24 h d−1), the microalga growth was similar to that of the respective control assay, but the biomass concentration was higher (p ≤ 0.05). When MF was applied for a shorter duration (1 h d−1), there was no positive impact on cell development, as microalgae growth was close to the control assay, and biomass concentrations showed no significant difference (p ≥ 0.05) at all time of culture.
Microalgae grown in outdoor conditions produced 64% more biomass than when cultured under controlled conditions (indoor) at the same time (15 d). As observed, the Xmax in outdoor assays was 2.30 g L−1 (MF 24 h d−1), while the maximum biomass produced in indoor assays was approximately 1.40 g L−1 under the influence of MF (Table 1). However, MF application had positive effect on microalgae growth cultured in indoor conditions, with 75% more biomass than in culture without MF action.
Growth parameters in the C. fusca assays were influenced by MF and time. Xmax increased by 32% when MF was applied for 24 h d−1 in outdoor assay, while in indoor cultivation, it increased by 70% when MF was applied for 1 h d−1. This value increased by more than 85% when MF was applied for 24 h d−1. Productivity in all conditions did not differ statistically (p ≥ 0.05), whereas µmax and Dt were statistically different (p ≤ 0.05) for each condition evaluated. The highest µmax and the lowest Dt were obtained in the outdoor assays when MF was applied for 1 h (0.23 d−1 and 2.97 d, respectively).
Regarding the macromolecules in the biomass, MF when applied for 1 h d−1 induced lipid production (Table 2). Protein content was statistically higher (p ≤ 0.05) in indoor assays when MF was applied for 1 h d−1, 26.6% higher than the control assay. In both cultivation methods (outdoor and indoor), C. fusca LEB 111 presented approximately 30% (m m−1) of lipids in its biomass, presenting potential for the production of biofuels. Applying MF for 1 h d−1 achieved 36% of lipids in the biomass of indoor assays.
During the assays, the pH value varied and reached values higher than those obtained on the last day. The pH in outdoor assays ranged from 10.50 to 12.30, while in indoor assays, the value was between 10.53 and 10.76. Although this factor (pH) was not controlled in these experiments, the pH varied slightly during the 15 days of cultivation.
Table 3 presents the total chlorophyll concentration (µg mL−1) obtained every 72 h.
In outdoor assays, on the 6th day, all conditions presented the highest chlorophyll content (~ 10 µg mL−1) which decreased (~ 4 µg mL−1) until the end of the assay.
The highest values observed over the whole time were at 4 d (~ 20 µg mL−1) and 6 d (~ 11 µg mL−1) in MF culture for 1 h d−1. MF when applied in indoor assays, induced the total chlorophyll production (10.36 µg mL−1 in the 12th day), which was higher than all other conditions evaluated.
The N–NO3 concentration (Fig. 2) decreased gradually in each culture. Until the end of the assays (15 days), the microalgae did not consume the entire nitrogen source under any of the evaluated conditions. However, the consumption rate (CR) was calculated by relating the contents from the 3rd to the 15th day, and the assays under uncontrolled conditions (outdoor) presented higher nitrate CR than indoor assays. In outdoor assays, the MF applied for 24 h d−1 did not increase the nitrate CR, but when applied only for 1 h d−1, the nitrate CR was 32% higher than the control, reaching 38% of the consumption. Regarding the indoor assays, the highest nitrate CR was obtained when MF was applied for 1 h d−1.
Protein profile of the outdoor assays is presented in Fig. 3. The blank circles mark the bands in which they remained or appeared after MF application. The SDS-PAGE technique provides estimated values of protein molecular weight. From the protein profile shown by SDS-PAGE, the protein levels remained unchanged and MF applied for 24 h d−1 did not induce stress proteins.
SDS-PAGE showed intense protein bands of approximately 100 kDa molecular weight in the control culture (Fig. 3-A) when MF was applied for 24 h d−1, and the bands did not change in the experimental culture when MF was applied for 24 h d−1(Fig. 3-b).
Figure 3-d shows higher degradation of the protein bands in the culture with MF application for 1 h d−1. Proteins with molecular weight between 50 and 70 kDa did not appear in this condition. In addition, the low-molecular weight proteins (~ 30 kDa) were slightly degraded, whereas the high-molecular weight proteins (100–150 kDa) remained when MF was applied for 1 h. The enzymes D1 (38.9 kDa) and D2 (39.5 kDa) constitute the reaction center of photosystem II, which is the key complex in the electron transport chain [16]. In the biomass of C. fusca, these enzymes remained when MF was applied to the assays (Fig. 3b–d).
Discussion
The biomass concentration with MF application for 24 h d−1 (1.48 g L−1) was 85% higher than that of the control in indoor assays (Table 1). This effect became noticeable from 4 d and remained unchanged until 15 days. Also, C. fusca showed a tendency to achieve the stationary phase with MF applied for 1 h after the 10th day. The results of a study conducted by Deamici et al. [17] with Arthrospira platensis SAG 21.99 showed similar effect with the present study, since the MF effect was only noticeable on day 4 for both conditions with MF application (1 h d−1 and 24 h d−1). The possible answer to MF presenting effect on microalgae growth only after some days (usually after 4 days), is that the microalgae needs to adapt to this new condition, and after this adaptation phase, the microalgae is able to change the metabolism and increase or decrease the growth.
The highest overall specific growth rate (μmax) in the outdoor assay is due to the increased luminosity in the environment that stimulates photosynthesis. In outdoor assays, the irradiance typically varies greatly throughout the day and the media luminosity to which the microalgae were exposed was 300 μmolphotons m−2 s−1. According to Masojídek et al. [18], the availability of light is fundamental for the cultivation of photosynthetic microorganisms, because the energy used in photosynthesis is provided by light. However, Torzillo and Vonshak [19] mentioned that cultivation at high light intensities can induce photoinhibition, which is the reduction in photosynthetic activity of microalgae. According to Acién et al. [20], in most species, this phenomenon occurs in irradiances above 1000 μE m−2 s−1, although it can occur in irradiances up to 300 μE m−2 s−1.
Some studies have shown that increased protein content in Chlorella sp. and other microalgae species is also related to the high level of irradiance and long photoperiod [21, 22]. In the studies of Duarte and Costa [23], the same microalgae under the highest intensity (100 µmol m−2 s−1) when evaluated presented 23% of lipids in the biomass, a value lower than the lipid content obtained in the present study, while Deamici et al. [7] showed that C. fusca cultivated with CO2 supply achieved just approximately 18% of lipids content in the biomass. Depending on the purpose of microalgal biomass, the accumulation of storage compounds (such as lipids) is of interest to use biomass as biodiesel source. Besides, under the conditions used in the present study, 30% of lipids were obtained, higher than the amount obtained in other studies that were between 20 and 22% (g g−1) in biomass of Chlorella kessleri [24] and Chlorella pyrenoidosa [25] under MF treatment. However, the conditions under which MF were evaluated did not induce lipid production, but it is possible to study other intensities and application times to induce the production of this molecule, which has a potential application to biofuels. As is well known, pH influences the transfer of CO2 to microalgae cultivation, and sub-optimal pH values may limit the microalgae growth [26]. In addition, the increased concentration of dissolved CO2 causes a reduction in the pH of the assays and it may decrease the activity of the rubisco enzyme, which is responsible for catalyzing the CO2 fixation in the Calvin cycle [27, 28].
Chlorophyll content in outdoor assays was not linear (Table 3), as chlorophyll content varies in response to physical factors, such as light intensity, agitation and temperature and chemical factors, such as nutrient availability [29]. In outdoor assays these parameters are not controlled which induces the variation in chlorophyll content.
In microalgal cultivation, nitrogen is an important macro-nutrient that can influence the growth and production of lipids. Nitrogen is also used in the synthesis of proteins and other essential cellular components [30] and a hypothesis about the MF influences on microalgae growth is that the MF may change the membrane permeability and, consequently, increase the associated nutrient uptake [31]. The consumption of nitrogen in form of nitrate was evaluated to evaluated whether the influence of MF could alter the consumption of this nutrient. In the present study, nitrate consumption showed similar behavior with MF application in the studies of Deamici et al. [29] with Spirulina sp., since the MF application for 24 h d−1 positively influenced the growth, but it did not influence nitrate consumption and when MF was applied for 1 h d−1 in outdoor assays, the nitrate consumption rate was higher (~ 84%, p ≤ 0.05).
MF altered many parameters evaluated during microalgae cultivation and Chlorella responded to these changes, mainly in indoor cultivations as shown in the present study. In another study, Chlorella pyrenoidosa was cultivated outdoor in municipal wastewater with MF, and the results demonstrated the feasibility of using MF to enhance lipid production with higher waste–water treatment efficiency [32]. Depending on the strain and microalgae genre, different effects may occur from MF application, as reported in other studies [16, 24, 31, 33]. Besides, this study demonstrated that is advantageous to combine the assays in outdoor conditions with MF, since the highest biomass concentration may be obtained with less energy expenditure, at the same cultivation time.
Conclusion
Biomass concentration was increased by MF effect in all conditions evaluated. In outdoor assays, Xmax was increased by 32% when MF was applied for 24 h d−1. In indoor assays, Xmax was increased by 70% when MF was applied for 1 h d−1 and it exceeded 85% when MF was applied for 24 h d−1. Protein content was positively affected when MF was applied for 1 h d−1 (35.7%) in indoor assays, which was higher than the control (p ≤ 0.05). Nitrate consumption was higher in outdoor assays, but was not affected by MF, while the protein profile was altered when MF was applied for 1 h d−1.
References
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Feng PZ, Deng ZY, Fan L, Hu ZY (2012) Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. J Biosci Bioeng 114:405–410
Yang G, Wang J, Mei Y, Luan Z (2011) Effect of magnetic field on protein and oxygen-production of Chorella vulgaris. Math Phys Fish Sci 9:116–126
Small DP, Hüner NPA, Wan W (2012) Effect of static magnetic fields on the growth photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 33:298–308
Tu R, Jin W, Xi T, Yang Q, Han S-F, Abomohra AE (2015) Effect of static magnetic field on the oxygen production of Scenedesmus obliquuscultivated in municipal wastewater. Water Res 86:132–8
Deamici KM, Costa JAV, Santos LO (2016) Magnetic fields as triggers of microalga growth: evaluation of its effect on Spirulina sp. Bioresour Technol 220:62–67
Deamici KM, Santos LO, Costa JAV (2019) Use of static magnetic fields to increase CO2 biofixation by the microalga Chlorella fusca. Bioresour Technol 276(2019):103–109
Hunt RW, Zavalin A, Bhatnagar A, Chinnasamy S, Das KC (2009) Electromagnetic biostimulation of living cultures for biotechnology, biofuel and bioenergy applications. Review Int J Mol Sci 10:4515–4558
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. J Gen Microbiol 111:1–61
Cataldo DA, Haroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plan 6:71–80
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Folch J, Lees M, Stanley GHS (1957) A Simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Lichtenthaler HK (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol 122:127–136
Deamici KM, Cuellar-Bermudez SP, Muylaert K, Santos LO, Costa JAV (2019) Quantum yield alterations due to the static magnetic fields action on Arthrospira platensis SAG 21.99: evaluation of photosystem activity. Bioresour Technol 292:121945
Masojídek J, Torzillo G, Koblízek M (2013) Photosynthesis in microalgae. In: Richmond A, Hu C (eds) Handbook of microalgal culture: applied phycology and biotechnology, 2nd edn. Wiley Blackwell, Chicester
Torzillo G, Vonshak A (2013) Environmental stress physiology with reference to mass cultures. In: Richmond A, Hu C (eds) Handbook of microalgal culture: applied phycology and biotechnology, 2nd edn. Wiley Blackwell, Chicester, West Sussex
Acién FG, Sevilla JMF, Grima EM (2013) Photobioreactors for the production of microalgae. Rev Environ Sci Bio 12:131–151
Ogbonda K, Aminigo RE, Abu G (2007) Influence of aeration and lighting on biomass production and protein biosynthesis in a Spirulina sp. isolated from an oil-polluted brackish water marsh in the Niger Delta Nigeria. Afr J Biotechnol 6:2596–2600
Seyfabadi J, Ramezanpour Z, Amini Khoeyi Z (2011) Protein, fatty acid, and pigment content of Chlorella vulgarisunder different light regimes. J Appl Phycol 23:721–726
Duarte JH, Costa JAV (2018) Blue light emitting diodes (LEDs) as an energy source in Chlorella fusca and Synechococcus nidulans cultures. Bioresour Technol 247:1242–1245
Bauer LM, Costa JAV, Rosa APC, Santos LO (2017) Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour Technol 244:1425–2143
Han S, Jin W, Chen Y (2016) Enhancement of lipid production of Chlorella Pyrenoidosa cultivated in municipal wastewater by magnetic treatment. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-016-2151-3
Suh IS, Lee SB (2003) A light distribution model for an internally radiating photobioreactor. Biotechnol Bioeng 82:180–189
S. Tebbani, F. Filipa Lopes, R. Filali, D. Dumur, D. Pareau (2014) CO2 biofixation by microalgae: modeling, estimation and control, 1 edn. New Jersey: Wiley.
Voet D, Voet JG, Pratt CW (2014) Fundamentos de Bioquímica: a vida em nível molecular, 4th edn. Porto Alegre, Artmed
Deamici KM, Santos LO, Costa JAV (2018) Magnetic field action on outdoor and indoor cultures of Spirulina: evaluations of growth, medium consumptions and protein profile. Bioresour Technol 249:168–174
Sirisansaneeyakul S, Singhasuwan S, Choorit W, Phoopat N, Garcia JL, Chisty Y (2011) Photoautotrophic production of lipids by some Chlorella strains. Mar Biotechnol 13:928–941
Zhi-Yong L, Si-Yuan G, Lin L, Miao-Yan C (2007) Effects of electromagnetic field on the batch cultivation and nutritional composition of Spirulina platensis in an air-lift photobioreactor. Bioresour Technol 98:700–705
Han S, Jin W, Chen Y, Tu R, Abomohra AE (2016) Enhancement of lipid production of Chlorella pyrenoidosa cultivated in municipal wastewater by magnetic treatment. Appl Biochem Biotechnol 180:1043–1055
Shao W, Ebaid R, Abomohra AEF, Shahen M (2018) Enhancement of Spirulina biomass production and cadmium biosorption using combined static magnetic field. Bioresour Technol 265:163–169
Author information
Authors and Affiliations
Contributions
KMD conception and design, analysis and interpretation of the data, methodology and writing. LOS and JAVC final approval of the article, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deamici, K.M., Santos, L.O. & Costa, J.A.V. Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst Eng 44, 1453–1460 (2021). https://doi.org/10.1007/s00449-021-02526-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02526-6