Abstract
Agro-industrial wastes can be processed into valuable products. Successively, current investigation is an effort to optimize the acid hydrolysis of pomegranate peels waste (PPW) using central composite design (CCD) of response surface methodology (RSM) for ethanol production. Concentration of sulfuric acid, temperature, and time of hydrolysis were used as dependent variables, whereas reducing sugars, total carbohydrates, extractives, weight loss, hemicellulose, cellulose, and lignin contents were recorded as responses for PPW decomposition. The highest glucose level of 0.56 ± 0.04 mg mL−1 (with 5% acid concentration at 100 °C for 30 min) and carbohydrate contents of 1.53 ± 0.07 mg mL−1 (with 3% acid concentration at 75 °C for 45 min) were obtained. Subsequently, detoxification of hydrolysate was conducted employing 2.5% activated charcoal that reduced 62% of phenolic compounds. Detoxified hydrolysate was subjected to fermentation by ethanologenic yeasts: Metschnikowia sp. Y31, Metschnikowia cibodasensis Y34, and Saccharomyces cerevisiae K7 for 10 days experiment. Significant ethanol yield of 0.42 ± 0.08 g g−1 was noticed by Metschnikowia sp. Y31 on day 5 and 0.41 ± 0.07 g g−1 for Metschnikowia cibodasensis Y34 on day 2. The results demonstrated the hopeful prospect for bioethanologenesis using cellulosic wastes at marketable level.

Statement of novelty
The focus of the current study was to develop low-cost ethanologenesis by using pomegranate peels waste. Initially, the biomass hydrolysis, being extremely a critical step, was optimized through central composite design using response surface methodology by Design Expert Software. The pretreated and detoxified biomass hydrolysate was then subjected to ethanol production via fermentative yeast. The low-cost ethanol production from wastes of pomegranate can be highly valuable not only for sustainable energy production but also for effective waste management. Significant yield of ethanol was achieved while using treated pomegranate peels waste as substrate. Our findings of the present study will be helpful in developing efficient and economical strategies tending to valorize cellulosic wastes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Waste management measures comprise of all the proceedings requisite to handle waste from its collection to disposal. The indecent disposal of wastes consequences the unhygienic environment that leads to pollution. Incineration and land filling are the general practices executed for waste management in Pakistan [1,2,3]. To reduce the piles of agro-industrial wastes, 3 R (reduce, reuse, recycle) strategy was also proposed to recuperate energy and management of municipal waste. Although these strategies can lessen the amount of waste to a huge amount, they also cause significant ecological contamination [4,5,6]. Alternately, an excellent way of waste management is its conversion into such valuable products that can be further used for mankind. In this regard, production of biofuels such as bioethanol from waste biomass, in the current scenario of depleting fossil fuels’ reservoirs, can play a pivotal role in boosting the country’s economy [7,8,9].
The main part of agricultural wastes comprises biodegradable substances that are composed of lignocellulosic biomass (LCB) [10]. Bioethanol produced through fermentation of the abovementioned wastes while utilizing microorganisms can be considered the chief liquid biofuel and an alternative additive to gasoline [11, 12]. Ethanol is a highly demanded fuel worldwide. Mixing of bioethanol and gasoline lessens the emission of greenhouse gasses to about 40–50% [13]. Being an agricultural country, abundant fruit wastes are produced in Pakistan throughout the year. The fruit wastes can be used as a potential feedstock for the production of bioethanol. Alternately, if the generated fruit wastes are not disposed of properly, they can lead to severe environmental issues [14].
Pomegranate is a tropical fruit and cultivated in Iran, Afghanistan, Northern India, Pakistan, Russia, Azerbaijan, California, and Mediterranean region. According to Food Agricultural Organization (FAO), approximately 1.5 million tons of waste is produced annually during industrial processing of pomegranates and has immense nutritional values. Pakistan is cultivating pomegranate in 13,000 ha, and the annual pomegranate fruit yield in Pakistan appeared about 0.5 million tons in 2010–2011 [15,16,17].
Basically, pomegranate fruit consists of peel, seeds, and aril. The exocarp/peel of a mature pomegranate fruit weighs up to 500 g kg−1. The weight of edible part and arils reaches 100 and 400 g kg−1, respectively [18,19,20]. The chief components of arils are water (80–85%), sugars (predominately C6 sugars: 10–14% glucose and fructose), few organic acids (ascorbic acid, malic acid, and citric acid), and other bioactive compounds (anthocyanins, antioxidants, pectin, phenolics, etc.) [21].
All parts of the pomegranate plant (roots, stem, leaves, and fruit) are utilized to treat various diseases. The plant consists of different dyes, alkaloids, antioxidants, flavonoids, and tannins [22, 23]. Pomegranate peels waste (PPW) is a good source of phenolic (tannins and flavonoids) compounds that contribute to antioxidant activity [24]. Due to having antioxidant potential, PP extract is used in food recipes, animal feedstocks, cosmetics, tinctures, and certain therapeutic formulae [25]. The compositional analysis of PP revealed the percent presence of proteins (5.1), fats (2.4), total sugars (30.5), crude fibers (12.61), insoluble fibers (30.003), lignin (29.4), phenolic compounds (40.53), tannins [26], and minerals [26,27,28,29]. PPW is used for biorefineries to get value-added products. Waste is modified physically, chemically, and microbially to get industrially important products such as dietary fibers, color pigments, dyes, medicinal components, and bioactive compounds. The modified PPW is also used for solvent extraction and as heavy metal absorbent. Dried PPW serves as substrate for the production of certain enzymes, biogas, and single-cell proteins [29].
Different statistical approaches have been employed in the last few years to optimize various steps involved in the pretreatment of LCB and production of different enzymes and biofuels [30,31,32,33,34]. Statistical approach of optimization proves to be a quicker and economical approach and provides real optima for the desired results [35].
A critical step for the production of bioethanol from LCB is the scarcity of efficient hydrolysis for releasing fermentable sugars [36]. The methodology of dilute acid hydrolysis for LCB proved to be an efficient and economical way to degrade biomass [37,38,39]. However, the drawback of this technique is the production of inhibitors such as hydroxyl methyl furfural (HMF), phenolic compounds, and acetic acid that reduces the growth of organisms used to ferment ethanol. Various methods have been used to enhance the yields of sugars and decrease the amount of toxins using diverse types of detoxification procedures [40]. Activated charcoal adsorption can prove to be very useful to palliate the inhibitors of hydrolysates, thus improving the microbial growth [41]. The focus of the current study was to develop low-cost ethanologenesis by using PPW. Initially, the biomass hydrolysis, being extremely a critical step, was optimized through central composite design (CCD) of response surface methodology (RSM) by Design Expert Software. The pretreated and detoxified biomass hydrolysate was then subjected to ethanol production via fermentative yeast keeping in view the incredible worth of some previously reported fermentative yeast species Metschnikowia sp. and Metschnikowia cibodasensis [42]. The low-cost ethanol production from wastes of pomegranate can be highly valuable not only for sustainable energy production but also for effective waste management.
2 Materials and methods
2.1 Proximate compositional analysis of PPW
PPW was collected from various locations in Lahore, Punjab, Pakistan, and rinsed with water. The waste was then placed in a hot-air oven at 60 °C for 2 days. The substrate after drying was ground and sieved to obtain fine powder (particle size ~ 1 mm). Different compositional contents such as carbohydrates, lipids, proteins, and reducing sugars were then estimated following phenol sulfuric acid method [43], method of Zollner and Kirsch [44], method of Folin Ciocalteu [45], and DNS method [46], respectively. The ash and moisture contents were estimated following protocols of AOAC [47]. Lignin, cellulose, and hemicellulose contents were measured by following the method proposed by Lin et al. [48].
2.2 Dilute sulfuric acid hydrolysis optimization by CCD
Sulfuric acid was used in dilute form to hydrolyze PPW. Peels and acid ratio for hydrolysis was 1:10. The reaction was carried out in conical flasks of 100-mL capacity covering with aluminum foil. Three parameters, i.e., acid concentration, hydrolysis temperature, and time, were executed by CCD into 20 runs. Experiments were performed in triplicates. The mixture in the flasks was agitated in shaking incubator at 100 rpm for specified temperature and time. After completion of reaction, the mixture was filtered and neutralized by NaOH pellets to keep the volume same. The neutralized mixture was filtered again and proceeded for detoxification step.
The PPW hydrolysis was executed using CCD by design expert software (version 6.0.8) for obtaining the highest reducing sugar contents that were vital for bioethanol production through fermentation [49].
Experimental plan for three dependent factors, viz., acid concentration (X1, %) along with hydrolysis temperature (X2, °C) and time (X3, min), was described in Table 1, while 20 runs of the experimental design were shown in Table 2. The model was designed based on low and high levels for parameters with central points as 3% (X1), 75 °C (X2), and 45 min (X3). The dependent parameters were selected on the basis of previous research emphasizing the influential impact of some key factors affecting biomass hydrolysis [50,51,52,53]. The subsequent optimization of the parameters for acid saccharification of PPW by RSM was performed in this investigation.
The following general quadratic equation “Y” [1] illustrated the relationship of input variables and different responses [reducing sugars (Y1) and total carbohydrates (Y2)] with the help of RSM as:
where
Y = predicted response
ß0 = constant coefficient
ß1, ß2, and ß3 = linear coefficients
ß11, ß22, and ß33 = quadratic coefficients
ß12, ß13, and ß23 = cross products coefficients
X1, X2, and X3 = input variables
e = residual error between the observed Y and the prediction (Ŷ)
2.3 Saccharification of PPW hydrolysate
After chemical hydrolysis, the PPW hydrolysate obtained was processed for the estimation of reducing and non-reducing sugars. The saccharification of PPW was estimated by the following formula [54]:
2.4 Detoxification of PPW hydrolysate
Some toxic phenolic compounds are considered an obstacle for microbial strains to ferment sugars. Thus, hydrolysate detoxification was well thought out a compulsory step prior to fermentation. For the purpose, PPW hydrolysate was detoxified with 2.5% activated charcoal [55]. The charcoal was removed with filter paper after agitation at 200 rpm for 1 h at 30 °C. Then it was centrifuged at 2000 rpm for 20 min. The supernatant was neutralized with pellets of NaOH. Total phenolic estimation of the hydrolysate was carried out by Folin-Ciocalteu method as described by Gonzalez et al. [56].
2.5 Production of ethanol from PPW hydrolysate
Bioethanol production was carried out by S. cerevisiae K7, Metschnikowia sp. Y31, and M. cibodasensis Y34 strains for optimum saccharification. Saccharomyces cerevisiae K7 granted by the Brewing Society in Japan (Tokyo, Japan) was considered standard yeast strain. Metschnikowia sp. Y31 and M. cibodasensis Y34 (isolated from flowers) have been evaluated previously for ethanol production [42].
The synthetic medium was prepared by following the protocol used by Bonciu et al. [57]. For the culturing of ethanologenic yeasts, Malt Yeast Glucose (MYG) medium was prepared for different inocula. All the yeast strains were revived separately in MYG medium (10 mL) by incubating overnight at 30 ± 0.2 °C.
The fermentation medium was composed of 5 mL of yeast as inoculum in 45 mL of the synthetic medium and 50 mL of the detoxified hydrolysate with the condition of having maximum reducing sugar contents as evaluated previously. The inoculated fermentation medium was placed in incubator without agitation for 10 days at 30 ± 0.2 °C and taken out after every 24-h interval on regular basis and evaluated for variations in reducing sugar contents and ethanol production. All the experiments were performed in triplicates.
Reducing sugars were measured by DNS method, and acid dichromate test was performed for ethanol estimation [58]. The growth of fermentative microorganisms was measured spectrophotometrically (CE-2041UK) by examining optical densities of fermentation media at 600 nm [59].
2.6 Statistical analysis
All runs in CCD for optimization experiment were performed in triplicates. For CCD data analysis, Design Expert Software (ver. 6.0.8 Software, Stat-Ease, Minneapolis, MN 55413) was used by following ANOVA and regression for the response surface quadratic model. The experimental data obtained from fermentation experiments were analyzed by one-way ANOVA following Duncan multiple range test (SPSS Version. 16.0. Software, Chicago, IL, USA).
3 Results
3.1 Proximate compositional analysis of PPW
The compositional analysis of PPW showed that it has good potential for the growth of microorganisms (Table 3). PPW has been appeared as good a substrate for ethanologens due to the presence of fermentable sugars in considerable amount [60, 61]. Cellulose and hemicellulose contents present in PPW were also previously subjected to chemical and enzymatic hydrolysis for better ethanol production by different microbes [62]. This study depicted 0.767 ± 0.08 mg mL−1 of moisture contents, 0.235 ± 0.01 mg mL−1 reducing sugars, 0.786 ± 0.016 mg mL−1 total sugars, 0.033 ± 0.002 mg mL−1 total lipids, and 0.166 ± 0.005 mg mL−1 total proteins in PPW in addition to hemicellulose (2.93 ± 0.126 mg mL−1), lignin (1.43 ± 0.125 mg mL−1), and cellulose (3.53 ± 0.030 mg mL−1) contents.
3.2 Dilute sulfuric acid hydrolysis optimization by CCD
For optimization of acid hydrolysis of biomass, different parameters were investigated and tabulated with statistical interpretation of the model (Table 4). The quadratic regression (Eq. (2)) for the release of reducing sugars (Y1) was:
The plus signs represented the synergistic association, whereas minus signs indicated antagonistic relations among variables. In determining the relationship between the response Y and the factors Y = f (X1, X2) in the statistical model, X1, X2 showed linear interaction, while X12, X22 exhibited quadratic relationship. In the current study, the equation is useful for identifying the relative impact of hydrolysis parameters, i.e., acid concentration (X1), hydrolysis temperature (X2), and hydrolysis time (X3), on reducing sugars (Y1) by comparing the factor coefficient. Individual factor, for instance, acid concentration (− 0.040 X1 + 0.053 X2), increased the level of reducing sugars by hydrolyzing PPW. It did not have any interaction effects with other factors studied in the experiment, thus showing synergistic relationship. Similarly, same rule applies for negative signs.
The linear interactive effect between X1 and X2 on Y1 and Y2 corresponds to the B3 slope. If B3 is positive (the interactive effect is positive), then it means that X2 is more positive, and the effect of X1 on response becomes more positive. This is interpreted as synergistic association. Alternatively, the more negative X2 is, the more negative effect of X1 on Y becomes, i.e., antagonistic effect. These effects are also dependent on high/low levels of X1 or high/low levels of X2. In this study, linear relationship of two factors (+X1X2, X2X3, X1X3) mutually had positive effect on reducing sugars in saccharification process as explained above.
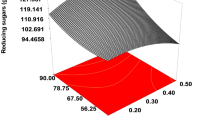
The optimum experimental and predicted value (g L−1) was 52.3 ± 0.01 and 47, respectively, at 3% of H2SO4 concentration at 100 °C temperature for 30 min of hydrolysis. The highest reducing sugar contents observed were 56.3 ± 0.04 g L−1 at 5% H2SO4 concentration at 100 °C for 30 min. The reliability of model was explored by ANOVA (Table 5) by RSM. The F value of model was 4.42, and the p value was 0.0187 showing significance of the model. The values of coefficient of R2 and Adj R2 appeared as 0.8154 and 0.6308, respectively (Table 6). For “Adeq Precision,” a value of 8.782 was calculated. Basically, R-squared (R2) is a statistical measure that represents the proportion of the variance for a dependent variable that is explained by an independent variable or variables in a regression model. R-squared values range from 0 to 1 and are commonly stated as percentages from 0 to 100%. The R-squared values such as 0.3 < r < 0.5, 0.5 < r < 0.7, and r > 0.7 are generally considered weak/low, moderate, and strong effect size. The values for R2 were calculated by regression using Design Expert Software (ver. 6.0.8 Software, Stat-Ease, Minneapolis, MN 55413). Usually, the larger the R2, the better the regression model fits the observations. In the current study, the R2 value was 0.8154 for response Y1.
Adequate precision measures the signal-to-noise ratio. It compares the range of the predicted values at the design points to the average prediction error. A ratio greater than 4 is desirable. The present investigation showed ratios of 8.782 for Y1, which indicated an adequate signal to confirm that the model can be used to navigate the design space. A ratio greater than four is desirable. Adequate precision was calculated by regression analysis using design expert software. In the current model, value 8.782 for Y1 was calculated.
Response surface graph exhibited the outcome of optimum value and variables by response using sulfuric acid hydrolysates. Figure 1a showed an increase in reducing sugar contents because of a decrease in the acid concentration as well as an increase in temperature of hydrolysis. Figure 1b depicted a decrease in reducing sugars with increase in both acid concentration plus time. The results elucidate that increase in temperature exhibited sharp elevation in contents of reducing sugars, whereas increased time showed slight elevation (Fig. 1c).
Total carbohydrates (Eq. (3)) were also observed in PPW hydrolysate and demonstrated by an equation that explained the effect of different variables.
The more positive symbols are the indications for a consistent equation. The experimental value recorded for total carbohydrates was 1.12 ± 0.01 mg mL−1, while the predicted value was 1.21 mg mL−1 at optimum conditions. The maximum total carbohydrates were 1.53 ± 0.07 mg mL−1 with 3% sulfuric acid concentration for 45 min at 75 °C. The model was significant with F value (3.27) and p value (0.0463), while R2 (0.7656) and Adj R2 (0.5312) were also calculated. In the current study, the R2 value was 0.7656 for response Y2, i.e., total carbohydrates after saccharification. The model accounts for 76.56% of the variance/variations that indicated reliability in predicting increase in carbohydrates after saccharification. The more variance of model indicated that the data points will fall closer to the fitted regression line. The model explained a lot of variation within the data and is significant. The three parameters in the model supported for the response.
Statistical methods are required to ensure that data are interpreted correctly and that apparent relationships are meaningful (significant) and not simply chance occurrences. The significance of model helps to interpret hypothesis that cellulosic and hemicellulosic biomass of PPW was hydrolyzed into monomers (reducing sugars). The reducing sugars were subjected to fermentation for ethanologenesis. The values of F, p, R-squared, and adequate precision will help to interpret the significance of model. The desired values will be > 4 for F and adequate precision, near to 1 for R2, and < 0.05 for probability. The model’s values for F (4.42), p (0.0187), coefficient of R2 (0.8154), and adequate precision (8.782) indicated the significance of model and predicted the optimum values for hydrolysis.
It can been seen from the Fig. 2a that increase in sugar contents was achieved by increasing the temperature. In case of studying the effect of acid concentration, maximum total sugars were recorded at 3% H2SO4. The sugar yield decreased above/below this value. Figure 2b showed that slight increase in carbohydrates contents was obtained by increasing hydrolysis time, whereas sharp increase was obtained at 4% acid concentrations. Figure 2c exhibited that maximum carbohydrates contents were recruited at initial values of hydrolysis temperature and time (i.e., 50 °C, 30 min) and increase in both parameters resulted in decreased carbohydrate contents.
3.3 Percent saccharification and detoxification of PPW hydrolysate
Percent saccharification yield of reducing sugars estimated after hydrolysis was 2.26%. The 62% reduction in phenol contents was estimated after detoxification by using 2.5% activated charcoal. The amount of phenolic compounds (mg mL−1) in PPW hydrolysate before detoxification was 1.50 ± 0.01, and the amount lessened to 0.93 ± 0.06 after detoxification.
3.4 Production of ethanol from PPW hydrolysate
Maximum ethanol yield (0.42 ± 0.08 g g−1) was noticed with Metschnikowia sp. Y31 at day 5 and 0.41 ± 0.07 g g−1 with M. cibodasensis Y34 at days 2, 7, and 10. The S. cerevisiae K7 manifested 0.44 ± 0.09 g g−1 ethanol yield at day 6 (Fig. 3). In terms of percentage, 12.18, 11.89, and 12.76% contents of ethanol were obtained from three yeast isolates, i.e., Metschnikowia sp. Y31, M. cibodasensis Y34, and S. cerevisiae K7, respectively. The reduction in reducing sugar contents was noticed day wise because of ethanol bioproduction (Fig. 4). Stability in growth of yeast (Metschnikowia sp. Y31 and M. cibodasensis Y34) subsequent to day 5 and 7 envisaged that these organisms could be promising candidates as they have the capacity to tolerate ethanol (Fig. 5).
4 Discussion
The waste management performance through recycling has been increased and being used extremely in various countries nowadays [68]. The chief renewable fuel to meet the measurement of waste management is ethanol [69]. In the current study, management of LCB after pretreatment with dilute acid then fermentation with yeast isolates for the production of ethanol was carried out.
Dilute acid pretreatment was performed with sulfuric acid. The same pretreatment was also reported by Jennings and Schell [70] to break down the cellulose and hemicellulose into simple sugars. The CCD was applied by Design Expert Software for the sake of optimization of conditions for hydrolysis. The same design was reported for the optimization to produce bioethanol from glycerol [71] and wastes of citrus fruits [72].
The highest level of reducing sugars was 52.3 ± 0.10 g L−1 measured at 3% sulfuric acid concentration at 100 °C for 30 min, while the value predicted was 47.95 g L−1. The F value (4.42) and p value (0.0187) showed that the model was significant. The reducing sugars were increased because acidic pretreatment of PPW resulted in conversion of cellulose and hemicellulose into soluble sugars [73]. Previously, similar results with maximum release of reducing sugar contents (56.07 g L−1) with 2.0% HCl concentration for 45 min were reported for the hydrolysis of durian peels [74]. In durian peels, glucose was the main sugar released after pretreatment. According to Aguilar et al. [75], acid hydrolysis helped to release glucose along with other sugars in the liquor. Dilute acid pretreatment not only converted hemicellulose into monomers but also caused structural changes to form hollow porous zones in lignocellulosic biomass to be made accessible for better enzymatic activity for cellulose [76,77,78].
The optimum carbohydrate contents (1.12 ± 0.01 mg mL−1) were observed at the same optimum condition. The predicted value (1.21) in case of carbohydrate contents was close to the experimental value. The results were significant having the F value and p value of 3.27 and 0.0463, respectively. Unhasirikul et al. [74] reported the increase in reducing sugars, total sugars, and acid hydrolysis efficiency by performing HCl hydrolysis with durian peels. In the present investigation, the increase in acid concentration contributed to release more sugars (g L−1), i.e., 52.3 ± 0.01 (3%) and 56 ± 0.04 (5%) at 100 °C for 30 min of sulfuric acid hydrolysis. The increase in acid leads to release more sugar and produce more inhibitors by degradation of sugars into hydroxymethylfurfural (HMF) and furfural and levulinic acid [79,80,81,82]. The production of toxic compounds such as furans and phenols that inhibit microbial fermentation of sugars could be removed by using suitable detoxification methods like activated charcoal treatment [55]. By performing acid hydrolysis, lignin is partially hydrolyzed into phenolics and became the part of fermentation medium by retaining in hydrolysate. Consequently, acid hydrolysis of lignocellulose resulted in formation of inhibitors that have negative impact on microbial metabolism in fermentation. The concentration of inhibitors not only affects the end product of fermentative metabolism but sometimes blocks the process completely [83, 84]. The production of inhibitors at low temperature is lower than the higher temperature for same time of hydrolysis. At higher temperature, the sugar degradation rate into inhibitors is high in hydrolysis. But at temperature higher than 100 °C, HMF, furfural, and phenolics are destructed along with sugar degradation. This effect is likely to increase by increasing hydrolysis time [85].
The growth rate of yeasts in hydrolysate showed the same log phase up to day 8 then stationary phase on days 9 and 10. Significant ethanol yield was recorded in log phase from day 5 to day 7. A study reported that the high substrate inhibited the growth rate of yeast because of high medium osmolality [86]. The significant amount of ethanol (0.42 ± 0.08 g g−1) was observed on day 5 by Metschnikowia sp. Y31, while it was 0.44 ± 0.09 g g−1 on day 6 by S. cerevisiae K7. In terms of percentage, 12.18, 11.89, and 12.76% contents of ethanol were obtained from three yeast isolates, i.e., Metschnikowia sp. Y31, M. cibodasensis Y34, and S. cerevisiae K7, respectively. These results are comparable with the previous findings (Table 6). In the present study, Metschnikowia sp. Y31 showed 12.18% of ethanol employing PPW that is significantly improved as compared to some previously performed fermentative studies [62, 68]. A study using pomegranate peel under statistically optimal condition was also recorded by using a different strain Candida tropicalis [48].
5 Conclusions
The present study arrived at the conclusion that maximum reducing sugars’ release was 0.56 ± 0.04 mg mL−1 recorded by dilute sulfuric acid hydrolysis at 5% acid concentration with 100 °C of hydrolysis temperature for 30 min with ethanol production of 0.42 ± 0.08 g g−1 using Metschnikowia sp. Y31 as fermentative yeast after an incubation period of 5 days. Significant yield of ethanol was achieved while using treated pomegranate peels waste as substrate. In prospects to the control of agro-industrial waste and its bioconversion into value-added products such as ethanol, the optimal way of hydrolyzing the PPW as necessitated in this study will be highly valuable scientifically as well as economically. The main obstacle in utilizing cellulosic biomass as fuels’ substrate is the selection of optimally efficient pretreatment and hydrolysis methodology. Our findings of the present investigation deals with the efficient employment of locally abundant LCB; i.e., PPW, involving low-cost acid hydrolysis, will be extremely helpful in developing efficient and economical way to valorize cellulosic wastes into bioethanol.
References
Bernstad A, Jansen IC (2011) A lifecycle approach to the management of household food waste - a Swedish full-scale case study. Waste Manag 31(8):1879–1896
Cia C, Li B, Lu H, Wu W (2012) Research advances in control of N2O emission from municipal solid waste landfill sites. J Appl Ecol 23(5):1415–1422
Khan K, Shaheen S, Iqbal H, RohinaArif GMR, Khalil M, Munawaa A, Batool M, Farooq M, Khan H, Sattar AA, Ilyas K (2020) Assessment of waste management practices in hospitals of Islamabad and Abbottabad-Pakistan. Pure Appl Biol 9(1):282–289
Forastiere F, Badaloni C, Hoogh KD, Kraus MK, Martuzzi M, Mitis F, Palkovicov L, Porta D, Preiss P, Ranzi A, Perucci CA, Briggs D (2011) Health impact assessment of waste management facilities in three European countries. J Environ Health 10(53):10–53
Mahmoudkhani R, Valizadeh B, Khastoo H (2014) Greenhouse gases lifecycle assessment (GHGLCA) as a decision support tool for municipal solid waste management in Iran. J Environ Health Sci Eng 12:17
Paul S, Choudhury M, Deb U, Pegu R, Das S, Bhattacharya SS (2019) Assessing the ecological impacts of ageing on hazard potential of solid waste landfills: a green approach through vermitechnology. J Clean Prod 236:117643
Valerio F (2010) Environmental impacts of post-consumer materials managements: recycling, biological treatments, incineration. Waste Manag 30(11):2354–2361
Buratti C, Barbanera M, Testarmata F, Fantozzi F (2015) Life cycle assessment of organic waste management strategies: an Italian case study. J Clean Prod 89:125–136
Correa DF, Beyer HL, Fargione JE, Hill JD, Possingham HP, Thomas-Hall SR, Schenk PM (2019) Towards the implementation of sustainable biofuel production systems. Renew Sust Energ Rev 107:250–263
Colazo A, Sanchez A, Font X, Colon J (2015) Environmental impact of rejected materials generated in organic fraction of municipal solid waste anaerobic digestion plants: comparison of wet and dry process layout. Waste Manag 43:84–97
Bowen K, Kennedy SC, Miranda K (2010) Ethanol from sugar beets: a process and economic analysis. A Project Report of Worcester Polytechnic Institute
Kim JH, Kim HJ, Yoo SH (2020) External benefits of increasing bioethanol consumption: a choice experiment study. Appl Econ Lett 27(6):447–450
Sarkar N, Kumar GS, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27
Tahany GM, Amal A (2015) Effect of agriculture waste: pomegranate fruits peel on some important phytopathogenic fungi and control of tomato damping off. J Appl Life Sci 3(3):103–113
FAO (2012) Statistical database. Food and Agriculture Organization of the United Nations. Codex Alimentarius Commission, Tunis http://www.fao.org. (Accessed May 23, 2012)
Farag RS, Abdel-Latif MS, Emam SS, Tawfeek LS (2014) Phytochemical screening and polyphenol constituents of pomegranate peels and leave juices. Agric Soil Sci 1:86–93
Malviya S, Arvind JA, Hettiarachchy N (2014) Antioxidant and antibacterial potential of pomegranate peel extracts. J Food Sci Technol 51(12):4132–4137
Aviram M, Dornfeild L, Rosenblat M, Volkova N, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman B (2000) Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr 71:1062–1076
Orzuaa MC, Mussattob SI, Contreras-Esquivela JC, Rodrigueza R, Garzaa H, Teixeirab JA, Aguilara CN (2009) Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind Crop Prod 30(1):24–27
Christaki EV, Bonos EM, Florou-Paneri PC (2011) Dietary benefits of pomegranates in humans and animals. J Food Agric Environ 9:142–144
Viuda-Martos M, Fernández-López J, Pérez-Álvarez J (2010) Pomegranate and its many functional components as related to human health: a review. Com Reviews Food Sci Food Safe 9:635–654
Mirdehghan SH, Rahemi M (2007) Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci Hortic 111:120–127
Miguel MG, Neves MA, Antunes MD (2010) Pomegranate (Punica granatum L.) a medicinal plant with myriad biological properties—a short review. J Med Plants Res 4(25):2836–2847
Ismail T, Sestili P, Akhtar S (2012) Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol 143:397–405
Yasoubi P, Barzegar M, Sahari MA, Azizi MH (2007) Total phenolic contents and antioxidant activity of pomegranate (Punica granatum L.) peel extracts. J Agric Sci Technol 9:35–42
Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S (2006) Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem 96:254–260
Suryawanshi PC, Kirtane RD, Chaudhari AB, Kothari RM (2009) Conservation and recycling of pomegranate seeds and shells for value addition. J Renew Sustain Energy 1:013107
Spilmont M, Leotoing L, Davicco MJ, Lebecque P, Mercier S, Miot-Noirault E, Pilet P, Rios L, Wittrant Y, Coxam V (2014) Pomegranate and its derivatives can improve bone health through decreased inflammation and oxidative stress in an animal model of postmenopausal osteoporosis. Eur J Nutr 53:1155–1164
Pathak PD, Mandavgane SA, Kulkarni BD (2017) Valorization of pomegranate peels: a biorefinery approach. Waste Biomass Valor 8:1127–1137
Irfan M, Asghar U, Nadeem M, Nelofer R, Syed Q, Shakir HA, Qazi JI (2016) Statistical optimization of saccharification of alkali pretreated wheat straw for bioethanol production. Waste Biomass Valor 7(6):1389–1396
Imran M, Anwar Z, Irshad M, Javid A, Hussain A, Ali S (2017) Optimization of cellulase production from a novel strain of Aspergillus tubingensis IMMIS2 through response surface methodology. Biocatal Agric Biotechnol 12:191–198
Gebremedhin M, Mishra S, Mohanty K (2018) Augmentation of native microalgae based biofuel production through statistical optimization of campus sewage wastewater as low-cost growth media. J Environ Chem Eng 6(5):6623–6632
Imran M, Hussain A, Anwar Z, Irshad M, Jabeen F (2019) Beta-glucosidase production optimization from newly isolated Aspergillus tubingensis IMMIS2 using Taguchi statistical design. Iran J Sci Technol Trans Sci 43:701–707. https://doi.org/10.1007/s40995-017-0462-z
Pandey A, Shah R, Yadav P, Verma R, Srivastava S (2020) Harvesting of freshwater microalgae Scenedesmus sp. by electro–coagulation–flocculation for biofuel production: effects on spent medium recycling and lipid extraction. Environ Sci Pollut Res 27(3):3497–3507
Dyaee N, Luti KJK (2019) Classical and statistical optimization by response surface methodology for enhancing biomass and bacteriocin production by Lactobacillus plantarum. Iraqi J Sci 60(3):494–508
Taherzadeh MJ, Karimi K (2007) Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources 2(3):472–499
Ribeiro JAB (2010) Hydrolysis of lignocellulosic residues using cellulolytic enzyme extract produced by Trichoderm reesei ATCC 2768. Dissertation(Federal University of Rio Grande do Norte), Natal/RNBrazil (iIn Portuguese)
Avci A, Saha BC, Dien BS, Kennedy GJ, Cotta MA (2013) Response surface optimization of corn stover pretreatment using dilute phosphoric acid for enzymatic hydrolysis and ethanol production. Bioresour Technol 130:603–612
Jeong SY, Lee JW (2016) Optimization of pretreatment condition for ethanol production from oxalic acid pretreated biomass by response surface methodology. Ind Crop Prod 79:1–6
Kim D (2018) Physico-chemical conversion of lignocellulose: inhibitor effects and detoxification strategies: a mini review. Molecules 23(2):309
Silva-Fernandes T, Santos JC, Hasmann F, Rodrigues RCLB, Izario Filho HJ, Felipe MGA (2017) Biodegradable alternative for removing toxic compounds from sugarcane bagasse hemicellulosic hydrolysates for valorization in biorefineries. Bioresour Technol 243:384–392
Chaudhary A, Karita S (2017) Screening of yeast isolates from flowers for effective ethanol production. Turk J Biol 41(6):890–900
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Zollner N, Kirsch K (1962) Uber die quantitative Bestimmung von Lipoiden (Mikromethode) mittels der vielen naturlichen Lipoiden (allen bekannten Plasmalipoiden) gemeinsamen Sulfophosphovanillin-Reaktion. Zeitschrift Für Die Gesamte Experimentelle Medizin 135:545–561
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
AOAC (2016) Official methods of analysis (Latimer GW Jr. Ed.), 20th ed, Association of Official Analytical Chemists, Washington
Lin L, Yan R, Liu Y, Jiang W (2010) In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: cellulose, hemicellulose and lignin. Bioresour Technol 101(21):8217–8223
Myers RH, Montgomery DC, Vining GG, Borror CM, Kowalski SM (2004) Response surface methodology: a retrospective and literature survey. J Qual Technol 36(1):53–77
Amjad M (2016) Optimization of acid saccharification by RSM and fermentation of mango peels for ethanol production. Dissertation, University of Education, Lahore, Pakistan
Ijaz A (2016) Use of melon peels for ethanol production as remedy for waste management. Dissertation, University of Education, Lahore, Pakistan
Pervaiz S (2016) Potential of yeast isolates for ethanol production from mango and banana peels. Dissertation, University of Education, Lahore, Pakistan
Siddique N (2016) Watermelon peels: a promising raw material for ethanol production. Dissertation, University of Education, Lahore, Pakistan
Begum MF, Alimon AR (2011) Bioconversion and saccharification of some lignocellulosic wastes by Aspergillus oryzae ITCC-4857.01 for fermentable sugar production. Electron J Biotechnol 14(5):1–9
Mussatto SI, Roberto IC (2005) Evaluation of nutrient supplementation to charcoal-treated and untreated rice straw hydrolysate for xylitol production by Candida guilliermondii. Braz Arch Biol Technol 48(3):497–502
Gonzalez M, Guzman B, Rudyk R, Romano E, Molina MA (2003) Spectrophotometric determination of phenolic compounds in propolis. Acta Farm Bonaer 22(3):243–248
Bonciu C, Tabacaru C, Bahrim G (2010) Yeasts isolation and selection for bioethanol production from insulin hydrolysates. Innov Rom Food Biotech 6:29
Bennete C (1971) Spectrophotometric acid dichromate method for the determination of ethyl alcohol. Am J Med Tech 37(6):217–220
Yang E, Fan L, Yan J, Jiang Y, Doucette C, Fillmore S, Walker B (2018) Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Expr 8:10. https://doi.org/10.1186/s13568-018-0536-0
Hasnaoui N, Wathelet B, Jiménez-Araujo A (2014) Valorization of pomegranate peel from 12 cultivars: dietary fiber composition, antioxidant capacity and functional properties. Food Chem 160:196–203
Zhu CP, Zhai XC, Li LQ, Wu XX, Li B (2015) Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem 177:139–146
Demiray E, Karatay SE, Dönmez G (2018) Evaluation of pomegranate peel in ethanol production by Saccharomyces cerevisiae and Pichia stipitis. Energy 159:988–994
Demiray E, Ertuğrul Karatay S, Dönmez G (2019) Efficient bioethanol production from pomegranate peels by newly isolated Kluyveromyces marxianus. Energy Sources Part A Recover Util Environ Eff 42:709–718. https://doi.org/10.1080/15567036.2019.1600621
Maina MB, Oluwole FA, Ngala GM, Abdulrahman SA (2017) Comparison of the properties and yield of bioethanol from mango and orange waste. Arid Zone J Eng Technol Environ 13(6):779−789
Magyar M, da Costa SL, Jin M, Sarks C, Balan V (2016) Conversion of apple pomace waste to ethanol at industrial relevant conditions. Appl Microbiol Biotechnol 100(16):7349–7358
Boulal A, Kihal M, Khelifi C, Benali B (2016) Bioethanol production from date palm fruit waste fermentation using solar energy. Afr J Biotechnol 15(30):1621–1627
Arumugam R, Manikandan M (2011) Fermentation of pretreated hydrolyzates of banana and mango fruit wastes for ethanol production. Asian J Exp Biol Sci 2:246–256
Baud ISA, Post J, Furedy C (2006) Solid waste management and recycling: actors, partnerships and policies in Hyderabad, India and Nairobi, Kenya (Vol. 76). Springer Science and Business Media
Licht FO, Agra CEAS (2007) World biodiesel markets: the outlook to 2010. Agra Informa Limited, Kent, p 200
Jennings EW, Schell DJ (2011) Conditioning of dilute-acid pretreated corn Stover hydrolysate liquors by treatment with lime or ammonium hydroxide to improve conversion of sugars to ethanol. Bioresour Technol 102(2):1240–1245
Adnan NAA, Suhaimi SN, Abd-Aziz S, Hassan MA, Phang LY (2014) Optimization of bioethanol production from glycerol by Escherichia coli SS1. Renew Energy 66:625–633
Talebnia F, Bafrani MP, Lundin M, Taherzadeh M (2008) Optimization study of citrus wastes saccharification by dilute acid hydrolysis. BioResources 3(1):108–122
Asli AE, Qatibi A-I (2009) Ethanol production from olive cake biomass substrate. Biotechnol Bioprocess Eng 14:118–122
Unhasirikul M, Naranong N, Narkrugsa W (2012) Reducing sugar production from durian peel by hydrochloric acid hydrolysis. World Acad Sci Eng Technol 6(9):394–399
Aguilar R, Ramirez JA, Garrote G, Vazquez M (2002) Kinetic study of the acid hydrolysis of sugar cane bagasse. J Food Eng 55:309–318
Gil N, Ferreira S, Amaral ME, Domingues FC, Duarte AP (2010) The influence of dilute acid pretreatment conditions on the enzymatic saccharification of Erica spp. for bioethanol production. Ind Crop Prod 32:29–35
Toquero C, Bolado S (2014) Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour Technol 157:68–76
Loow Y, Wu TY, Md. Jahim J, Mohammad AW, Teoh WH (2016) Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 23:1491–1520
Chaudhary G, Singh LK, Ghosh S (2012) Alkaline pretreatment methods followed by acid hydrolysis of Saccharum spontaneum for bioethanol production. Bioresour Technol 124:111–118
Kupiainen L, Ahola J, Tanskanen J (2014) Kinetics of formic acid-catalyzed cellulose hydrolysis. BioResources 9:2645–2658
Baadhe RR, Potumarthi R, Mekala NK (2014) Influence of dilute acid and alkali pretreatment on reducing sugar production from corncobs by crude enzymatic method: a comparative study. Bioresour Technol 162:213–217
Castro E, Nieves IU, Mullinnix MT (2014) Optimization of dilute-phosphoric-acid steam pretreatment of Eucalyptus benthamii for biofuel production. Appl Energy 125:76–83
Taherzadeh MJ, Karimi K (2011) Fermentation inhibitors in ethanol processes and different strategies to reduce their effects. In: Larroche C, Ricke SC, Dussap C-G, Gnansounou E (eds) Pandey a. Academic Press, Biofuels, pp 287–311
Kim SK, Park DH, Song SH, Wee YJ, Jeong GT (2013) Effect of fermentation inhibitors in the presence and absence of activated charcoal on the growth of Saccharomyces cerevisiae. Bioprocess Biosyst Eng 36:659–666
Łukajtis R, Kucharska K, Hołowacz I, Rybarczyk P, Wychodnik K, Słupek E, Nowak P, Kamiński M (2018) Comparison and optimization of saccharification conditions of alkaline pre-treated triticale straw for acid and enzymatic hydrolysis followed by ethanol fermentation. Energies 11:639
Moawad EY (2012) Optimizing bioethanol production by regulating yeast growth energy. Syst Synth Biol 6(3–4):61–68
Acknowledgments
The authors are thankful to the University of Education, Township Campus, Lahore, for providing research facilities to accomplish the investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleem, A., Hussain, A., Chaudhary, A. et al. Acid hydrolysis optimization of pomegranate peels waste using response surface methodology for ethanol production. Biomass Conv. Bioref. 12, 1513–1524 (2022). https://doi.org/10.1007/s13399-020-01117-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01117-x