Abstract
We have evaluated process optimization and the interactive effects of a number of variables using a Box–Behnken design of response surface methodology (RSM). The process variables nitrate, phosphate, glucose and pH were optimized to enhance the cell growth rate, lipid accumulation and other biochemical parameters of Chlorella spp. The most significant increase in lipid production (dry cell weight basis) occurred at limited concentrations of nitrate and phosphate, 1 % glucose and pH 7.5. The addition of nitrates during the mid-lag and mid-exponential phases produced the maximum inhibitory effect on lipid accumulation and the presence of yeast extract led to a further enhancement of lipid accumulation. Of all the media tested, BG-11 was the best suited medium for algal biomass production and chlorophyll content. A significant increase in algal biomass was observed in BG-11 supplemented with bicarbonate and glucose (1 %). The maximum specific growth rate observed was on 9th day of culturing. Results of optimization of process variables through response surface methodology and optimization of various other conditions reflect cutting edge research directed towards increasing algal biomass and lipid content for biodiesel production using an efficient economical technological approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Demand for liquid transportation fuels continues to rise amidst concerns of climate change, air pollution, ecosystem destruction and national security challenges that are related to petroleum production and combustion (Levine et al. 2011). The rate of depletion of fossil fuels and the effect of greenhouse gas emissions on global climate change have created an interest in biodiesel as an alternate energy-efficient and environmentally safe fuel (Lang et al. 2000; Lee et al. 2010). Feedstocks for biodiesel production currently range from soya bean and rapeseed oil to oils from plants and fruits, but given the inherent limitations of sufficient agricultural land and feedstocks, there is little chance that these oil seed crops will be able to replace a significant fraction of fossil fuels (Singh et al. 2011). Several obstacles, such as space considerations, low efficiency of lipid production and long cultivation periods, together with other major non-technical barriers have to be solved for the industrialization and commercialization of biodiesel from traditional agriculture and forestry sectors. The major non-technical barriers are restrictions or prior claims on land use (food, energy, amenity use, housing, commerce, industry, leisure or designations as areas of natural beauty, special scientific interest, etc.) and the environmental and ecological effects of large areas of monoculture (Demirbas and Demirbas 2011).
Microalgae are receiving increasing worldwide attention for their possible exploitation as biofuels. In fact, several species of this group are able to accumulate large amounts of lipids and can act as a suitable feedstock for biodiesel production. In the case of algae, productivities are estimated to be around 10-fold higher than that of conventional crops, making these organisms the more promising source for biomass production on a long-term perspective (Chisti 2008). Microalgae have a great potential to serve as a source for one of the renewable biofuels which depends mainly on lipid yields and composition of the algal cells (Hu et al. 2008; Pienkos and Darzins 2009). Due to their high lipid content, photosynthesis efficiency and CO2 reduction efficiency, microalgae are considered to be an attractive source for biodiesel production (Zhao et al. 2012). They have the potential to produce more oil per acre than any other feedstock currently used to make biodiesel and they can be grown in fresh or marine waters, thereby avoiding landuse (Anthony et al. 2012; Halim et al. 2012). Naturally occurring fatty acid methyl esters (FAMEs) or fatty acid ethyl esters (FAEEs) obtained from the transesterification of triacylglycerides (TAGs) have been reported in Chlamydomonas reinhardtii, a green microalgae, and these FAMEs and FAEEs are very essential for biodiesel production (Herrera-Valencia et al. 2012).
The production of lipids in microalgal cells is affected by many environmental conditions, such as light and ultraviolet-B irradiation (Xue et al. 2005; Hader et al. 2007), temperature and salinity (Xu and Beardall 1997; Azachi et al. 2002; Converti et al. 2009), nutrients and CO2 concentration (Chu et al. 2009; Chen et al. 2011; Praveenkumar et al. 2012). Further research is required to understand why and how, under certain environmental conditions, some species of algae upregulate neutral lipids, which can be readily converted to biodiesel and other biofuels (Hu et al. 2008; Scott et al. 2010). In contrast to non-oleaginous algal species, oleaginous species use excess carbon and energy to synthesize storage lipids under nitrogen stress (Rodolfi et al. 2009). The most significant distinguishing characteristic of algal oil is its production and, in turn, its conversion efficiency to biodiesel. The yield (per acre) of oil from algae is over 200-fold higher than that from the best-performing plants (vegetable oils) (Sheehan et al. 1998). It is well documented that certain species of microalgae, particularly Chlorella (Lv et al. 2010), Scenedesmus (Li et al. 2011) and Nannochloropsis (Rodolfi et al. 2008), have relatively faster growth rates, easier cultivation characteristics and great oil producing capabilities (Song et al. 2013). Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors designed for biodiesel application have shown that the highest total lipid content was achieved with Neochloris oleoabundans [25–37 % dry cell weight (dcw)], while the highest TAG content was found in Chlorella vulgaris (11–14 % dcw) (Pruvost et al. 2011). Nutrient stress conditions, such as low nitrogen content of medium, were found to significantly increase the lipid content by 40 % in Chlorella vulgaris (Illman et al. 2000), and manipulated culture conditions of 1 mM KNO3, 1.0 % CO2, 60 μmol photon m−2 s−1 and 25 °C incubation temperature were shown to increase the lipid production of Chlorella vulgaris by 2.5-fold (Lv et al. 2010). Moreover, Widjaja et al. (2009) reported for Chlorella vulgaris that changing from a normal nutrient medium to a nitrogen depletion medium gradually changed the lipid composition from free FA-rich lipids to lipid in the form of TAG. An increased lipid content in Chlorella has also been achieved through silicon deficiency (Griffiths and Harrison 2009) and iron supplementation (Liu et al. 2008). In their study on the effect of iron on growth and lipid accumulation in Chlorella vulgaris, Liu et al. (2008) found that when the culture was supplemented with Fe3+ at different concentrations in the late-exponential growth phase, there was increase in lipid content up to 56.6 % biomass by dry weight. Chlorella has oil levels between 20-50 %, but higher productivities can be reached by optimizing the environmental conditions and growth phases. Taking all these factors into consideration, Chlorella would appear to be a good option for biodiesel production. Although other species are as efficient and productive as Chlorella in terms of oil production, as a potential biodiesel candidate, other factors need to be taken into account, such as the ability to develop using available nutrients or under specific environmental conditions (Sharma et al. 2012).
We selected Chlorella spp. for study because of their interesting FA composition (Spolaore et al. 2006). Bellou and Aggelis (2012) reported that Chlorella spp. cultivated in a lab-scale reactor that simulated an open pond grew well and produced 350–500 mg L−1 of biomass containing approximately 40 % lipid content; in continuous culture, the highest biomass and lipid productivity were 0.7 and 0.06 mg L−1 h−1, respectively, at D = 0.0096 h−1. It is important that all of the parameters and optimized conditions should be considered simultaneously in the selection of the most adequate species or strains for biodiesel production.
The aim of our study was to examine how the lipid yields of Chlorella spp. vary under different environmental conditions and during different growth phases. Process optimization using response surface methodology (RSM) was performed and interactions between the operating variables were elucidated to identify the optimum conditions for algal growth. The effects of cultivation time, yeast extract, shaking speed, temperature, salinity, nitrate and different carbon sources on various growth parameters of Chlorella spp. were also determined.
Materials and methods
Isolation and maintenance of algae culture
Chlorella spp. used in the present study were isolated from the freshwater pond of Ladwi village, Haryana (India). The pure culture of the isolate was obtained by repeated streaking on solidified (1.5 % agar) BG-11 medium {in g L−1: NaNO3, 1.5; K2HPO4, 0.04; MgSO4⋅7H2O, 0.075; CaCl2⋅2H2O, 0.036; citric acid, 0.006; ferric ammonium citrate, 0.006; EDTA (disodium salt), 0.001; Na2CO3, 0.02; 1 mL trace elements solution [in g L−1: H3BO3, 2.86; MnCl2⋅4H2O, 1.81; ZnSO4⋅7H2O, 0.222; NaMoO4⋅2H2,O 0.39; CuSO4⋅5H2O, 0.079; Co (NO3)2⋅6H2O, 0.0494]; pH 7.0 ± 1} using standard isolation and culture techniques. The purity of the culture was ensured by repeated plating and by regular observation under the compound microscope. Media and flasks were sterilized by autoclaving for 20 min at 15 psi and 121 °C before use. All other essential equipment was also sterilized in the autoclave. All glassware was cleaned thoroughly with water, dried and sterilized in a hot air oven at 180 °C for 6–7 h prior to use.
Inoculation of the culture was carried out in the vertical laminar flow cabinet under sterilized conditions to avoid bacterial and fungal contamination. Cultures were maintained at a light intensity of 3,000 lx using cool-fluorescent tubes at 25 ± 1 °C in a culture room. Growth experiments were carried in BG-11 medium in 250-mL Erlenmeyer flasks (100 mL medium per flask) at 25 °C in an orbital shaker–incubator at 120 rpm. The medium and flasks were sterilized prior to inoculation in an autoclave for 20 min at 121 °C. To maintain the broth cultures, we transferred the old culture into new flasks containing the same growth media at regular intervals to avoid deterioration of the culture due to the depletion of nutrients and accumulation of waste products.
Measurement of growth rate of Chlorella spp. by various analytical methods
All growth experiments were carried out in BG-11 medium in 250-mL Erlenmeyer flasks (100 mL medium per flask) at 25 ± 1 °C in a culture room. Prior to inoculation, the medium and flasks were sterilized in an autoclave for 20 min at 121 °C. Fresh inoculum (1 mL of a 10-day-old culture) was used for all experiments; care was taken to ensure the cultures do not get too old and reach the late-stationary phase as depletion of nutrients and accumulation of waste products would causes the cultures to deteriorate and be damaged. The growth of the algal isolate was measured in terms of various physiological and biochemical parameters, such as biomass, chlorophyll, protein, carbohydrate and lipid contents.
Cell growth rate measurement
Dry cell biomass was measured as the cell density (dcw, g L−1) at OD625 of an 11-day-old culture at dilutions ranging from 0.2 to 1.0. The dry biomass was calculated using the regression equation as the relationship given by Yount (2006):
where, Y (g L−1) is the cell density and x is the optical density at OD625.
The specific growth rate (μ; day−1) was calculated from the relationship given by Guillard (1973). The growth of the culture was measured as
where, N 1 and N 2 are the biomass (g L−1) on day t 1 and t 2, respectively.
Total lipid estimation
The total algal lipid content in the samples was extracted using methanol–chloroform (2:1.5, v/v) following the modified Bligh and Dyer (1959) method. Algal biomass was collected by centrifuging 50 mL of the algal culture at 5,000 rpm for 10 min. The supernatant was discarded, and the algal biomass was incubated for 24 h at 25 °C in a mixture of 2 mL methanol and 1.5 mL chloroform. The mixture was then vortexed for 2 min, followed by the addition of 1.5 mL of chloroform and agitation again for 1 min. The mixture was amended with 1.8 mL distilled water followed by 2 min of vigorous agitation. It was then centrifuged for 10 min at 2,000 rpm, and a lower lipid layer was separated carefully using Eppendroff micropipettes in a clean previously dried (104 °C) and pre-weighed 15-mL glass centrifuge tube. The chloroform phase was evaporated near to dryness in a water bath at 70 °C, and the residue was dried further at 104 °C for 30 min. Lipid content was described as percentage dcw.
Total chlorophyll estimation
Total chlorophyll content of the algae was estimated spectrophotometrically at 650 and 665 nm by the hot extraction method (Tandeau de Marsac and Houmard 1988). Total chlorophyll content (μg mL−1) was calculated using the following formula:
.
Total protein estimation
Protein content was estimated by the standard method of Lowry et al. (1951). Algal filtrate was treated with alkaline reagent and Folin–Ciocalteu reagent, and absorbance was read after 30 min at 660 nm. Protein concentration was calculated from the standard curve prepared with bovine serum albumin (BSA). A known volume (10 mL) of sample was collected and centrifuged at 7,500 rpm for 10 min at 4 °C and the supernatant and pellets separated. Calibration was performed using a 1 mg mL−1 BSA standard diluted with double distilled water to a concentration range of 0–1.0 mg mL−1.
Process variable optimization using RSM
In conventional one-factor time experiments, a single factor is varied, while other factors are held as constant, and the effect of interaction among the variables is ignored. The RSM is a systematic statistical design approach aimed at exploring the relationships between design variables and responses in order to provide a better overall understanding with a minimal number of experiments. The general form for the second-order model is expressed as:
where, Y is the response, βn is the coefficient associated with factor n, and the letters, A, B, C, … represent the variables in the model.
Lipid content, carbohydrate, chlorophyll, protein and biomass produced were studied under varied pH (6–9), nitrate (NaNO3) (0.0–20.0 mM), phosphate (K2HPO4) (1.0–15.0 mM) and glucose (1–5 %; organic carbon source) conditions with the aim to establish the actual potential of the organism under heterotrophic conditions. The Box–Behnken design (BBD) (Design-Expert 6.0.10 trial version; Stat-Ease, Minneapolis, MN) of four variables and three levels, each with three concentric point combinations, was used to identify the optimum variable level for the indigenous isolate of Chlorella spp. The minimum, central and maximum levels for each design variable used in the Box–Behnken for various responses are listed in Table 1. The experimental design was applied after selecting a range of each variable (maximum and minimum), as shown in Table 2. The required pH of the medium was adjusted using 1 N NaOH/H2SO4 prior to autoclaving. The appropriate nitrate and phosphate concentrations were obtained using 1,000 mM stock solutions of NaNO3 and K2HPO4. Optimization of lipid content was established on the basis of Design Expert software using the BBD model, which is the standard RSM.
Optimization studies for enhancement of biomass and lipid content in Chlorella spp.
All experiments were performed in triplicate. Unless stated otherwise, the Chlorella spp. isolate (1 mL fresh inoculum) was cultured in sterile BG-11 medium having pH 7.in 250-mL Erlenmeyer flasks (100 mL medium per flask) under continuous white fluorescent light (intensity 3,000 lx) at 25 ± 1 °C.
To determine temporal changes under different growth parameters, the Erlenmeyer flasks inoculated with 1 mL of Chlorella spp. culture were placed in a BOD incubator-cum-shaker and cultured up to 21 days with shaking (120 rpm). Samples were harvested after regular intervals on culture days 3, 6, 9, 12, 17 and 21 for analysis of the various parameters. On each day of analysis algae were harvested from three flasks.
In order to determine whether yeast extract supported the growth of Chlorella spp., the isolate was grown in BG-11 medium amended with varying doses of yeast extract (0.5–3.0 g L−1) under the above-mentioned culture conditions for 11 days. A control culture was run parallel in unamended BG-11 medium under similar conditions. The pH of the medium was adjusted to 7.5 prior to autoclaving using 1 N NaOH/H2SO4
The effect of shaking was evaluated at 25 °C and 120 rpm in BG-11 medium, and the control culture was also run parallel under the static condition. Culture conditions for both arms were similar as described above.
The optimal growth temperature varies from species to species. Therefore, to determine the optimum temperature for Chlorella spp. experiments were performed at five temperatures (range 25–35 °C) under the above-mentioned culture conditions for 11 days.
Effect of salinity was studied by varying NaCl concentration from 1.0 to 10 mM in BG-11 medium. Appropriate dosing was made using 1,000 mM NaCl stock solution. The culture conditions followed were similar as described above.
The effect of nitrate dosing on the culture was studied by adding 1 mM sodium nitrate (NaNO3) to BG-11 medium at various cultivation times (0, 2, 5, 9 and 11 days, respectively). Separate sets of the 250-mL Erlenmeyer flasks received different nitrate dosing. A control was also run in parallel without nitrate. The culture conditions were similar as described above.
Different carbon sources (glucose, fructose, lactose, methanol, ethanol, bicarbonate, carbonate, sucrose, mannitol) were added to the culture to determine the effect of carbon sources. A control culture was also run in parallel. The culture conditions were similar as described above.
To determine the best culture medium suitable for growth of Chlorella spp., chemical composition of different test media [BG-11, Bold’s Basal Medium (BBM), Chu#10 medium, Half-strength (HS) Chu#10 medium and Allen media] having different pH were used. All media were prepared and autoclaved separately in reagent bottles. All chemicals used for preparation of different media compositions were of analytical grade. pH of the different culture media was adjusted accordingly using 1 N NaOH/H2SO4 prior to autoclaving. The experiments were performed in sterilized plastic culture tubs of 5,000 mL capacity containing 1,000 mL of each of the different sterilized media to which 10 mL of a 10-day-old algal culture had been added. These experiments were performed in duplicate. All tubs were completely covered with muslin cloth to avoid any contamination. Experiments were performed under continuous illumination of 3,000 lx and incubated for 7 days at 25 ± 2 °C in culture racks.
Results and discussion
RSM for optimization of process variables
The BBD of the RSM was aimed at identifying the best levels of the selected variables, such as nitrate (0.0–20.0 mM), phosphate (1.0–15.0 mM), glucose (1.0–5.0 %) and pH (6–9). The BBD and its results are presented in Table 1. The second-order polynomial equation was used to determine the relationship between variables and responses. The regression equation coefficients were calculated and data were fitted to a second-order polynomial equation. A flat surface on the three-dimensional response graphs indicates an optimum condition for responses such as lipid accumulation, biomass production, carbohydrate consumption and chlorophyll and protein contents. Analysis of variance was used in order to ensure a good model as explained in Table 3. A Prob > F value of <0.05 and >0.05 indicated model terms which were significant and non-significant, respectively.
In the BBD, glucose (C) in the linear and quadratic term and pH (D) in the quadratic term were the significant model terms for lipid content (Table 3), with the remaining ten non-significant model terms with values of >0.05 contributing relatively less towards responses. For chlorophyll contents, all of the linear (A, B, C and D), quadratic (A2, B2, C2) and interactive terms (AB, AC, AD, BC, CD) were significant model terms, whereas for carbohydrate consumption and glucose concentration, the linear (C) and quadratic (C2) terms were significant model terms. For protein contents, two linear (A and B), three quadratic (A2, C2 and D2) and two interactive terms (AD and BD) acted as significant model terms. All of the significant terms were found to contribute more towards the responses than the non-significant terms.
The diagnostic details provided by Design-Expert from the analysis of all the data points of the normal probability and the studentized residual were approximately linear and found to lie around the zero line, indicating the suitability of the model and showing that the variance was associated with prediction changes over the design space. A flat bowl-shaped bottom surface of the standard error is desirable for an RSM design with no signs of data problems. On the basis of all the statistical output for design evaluations and three-dimensional plots, we determined that the maximum prediction variance at a design point was 0.583, whereas the average prediction variance was 0.517. The minimum value of the standard error design (0.422) around the centroid and the maximum prediction variance (0.447) at the design central points indicated that present model can be used to navigate the design space and provide suitable conditions for the study (Fig. 1).
The residuals were used to check the homogeneous variance assumption by plotting the studentized residuals against the predicted probability values. Homogeneously spread data around either side of the zero line indicated the suitability of the model for our study (Fig. 2). The adequate precision ratio of 7.855 was an adequate signal and could, therefore, be used to navigate the design space. The very small value of the coefficient of variation (28.38) and the low standard deviation value (5.17) clearly indicated the very high degree of precision and good reliability of the experimental values (Table 3). The model F values of 5.15, 42.13, 356.09, 36.96 and 77.17 for lipid accumulation, chlorophyll, carbohydrate consumption, protein and biomass genesis, respectively, implied that the model was suitable for our study. The predicted R 2 and adjusted R 2 values closer to 1.0 were in a reasonable agreement, indicating the better fitness of the model to the experimental data (Table 3). The final responses for lipid accumulation, chlorophyll content, carbohydrate consumption, protein and biomass genesis in terms of coded factors are presented in the equations below.
Effect of optimized process variables on various growth parameters
Statistical analysis of the positive linear coefficient indicated that incubation time was the most important factor affecting all responses (Eqs. 4–8). Therefore, the relationship of lipid accumulation with variables such as nitrate concentration, phosphate concentration, glucose and pH in Chlorella spp. can be inferred from equation presented in coded factors (Eq. 4). Three-dimensional graphs were included to determine the sensitivity of all of the responses of two interacting variables by holding the other variable at the central values. The higher pH value in the linear coefficient terms illustrates the significant, positive effect of the variable on all of the responses. The positive values for nitrate and pH indicated that of the selected variables, these two parameters had the most effect. The higher value of 1.23 for the linear coefficient of nitrate concentration (Eq. 4) illustrates the significant, positive effect of this variable on lipid accumulation. The negative interactive effect coefficient of the independent variables (AC and BD) on lipid accumulation was observed as a function of these variables by keeping all other variables at a fixed level, indicating a higher level for both these factors gave a smaller response. The non-significant value (>0.05) for all the interactive variables indicated that there was no interaction among the variables i.e. a change in one variable did not affect another variable.
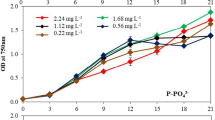
The lipid accumulation properties of Chlorella spp. under different initial nitrate and phosphate levels is shown in a three-dimensional graph (Fig. 3a) which indicates higher lipid accumulation under a limited condition of both variables. The higher levels of nitrate and phosphate individually had an inhibitory effect on lipid accumulation. However, the increased and positive value (4.63) of the interactive effect coefficient of nitrate and phosphate enhanced the accumulation of lipids with increasing levels of both variables (Eq. 4). These results indicate that the nitrate/phosphate ratio is an important factor affecting lipid accumulation in Chlorella spp., and that the appropriate ratio of both variables was also able to enhance lipid accumulation to a significant level. The significant Prob > F values for the linear, quadratic and interactive effects for nitrate and phosphate by Chlorella spp. indicate an increase in chlorophyll contents with an increase in the value of the variables (Table 3). Most of the non-significant values [linear 0.1485 (A) and 0.2852 (B); quadratic 0.1219 (A2) and 0.0879 (B2) and interactive 0.7131 (AB)] of Prob > F for carbohydrates indicate that nitrate and phosphate are not very significant factors determining its consumption by the isolate. The addition of an external carbon source, such as glucose alone, reduced lipid accumulation. However, the addition of glucose and nitrate and phosphate together enhanced lipid accumulation as inferred from the interactive effect of glucose with nitrate, phosphate and pH (Figs. 3 and 4). The addition of an external carbon source (glucose) alone reduced chlorophyll contents. The biomass was mainly controlled by the carbon and yeast extract sources that were essential for the buildup of biomass. Significant values for nitrate (A), phosphate (B) and glucose (C) for the linear terms indicated their very influential effect on biomass production and increased algal growth with increased levels of the variables.
Interactive effect of all the variables
Interactive effects of all variables were simultaneously studied by using the perturbation graphs to study the lipid production ability of Chlorella spp. (Fig. 5). The perturbation plot illustrates the percentage lipid content (dcw) as each variable moves from the preferred reference, with all other factors held constant at the coded zero level of the design space. The perturbation plots show the deviation of the factorial level from the adjusted reference point of all variables. The parabolic path of the pH curve indicates that the lipid production was higher at the reference zero point, indicating deviation from either side of the zero point and resulting in a decrease in lipid content. Curve C depicts the decreasing effect of lipid production with the addition of glucose. Flat curves for nitrate (A) and phosphate (B) indicate that these variables have little effect relative to other variables.
Confirmation experiments
Our model will have no significance until its desirability is checked. To this end, we conducted confirmatory experiments at different levels of the variables, as suggested by the numerical modeling to support the optimized data under optimized conditions. These experiments were conducted to maximize lipid content while keeping other responses within the reference range. The model for growth media with low nitrate (0.1 mM), phosphate (1.0 mM), glucose concentration (1.0 %) and pH (7.6) closely agreed with the data, and 30.5–33.8 % dcw lipid content was observed against the highest predicted level of 36.76 % dcw lipid. However, at a high nitrogen concentration the biomass content increased but with a decrease in total lipid content.
Nitrogen (Dean et al. 2010; Yeh and Chang 2011) and phosphorus deficiency (Xin et al. 2010) as well as extreme environmental conditions, such as high salinity (Dean et al. 2010; Yeh and Chang 2011), high light intensity (Zhukova and Titlyanov 2006) and extreme temperatures (Converti et al. 2009), have been reported to induce the accumulation of lipids in several microalgae. Generally, microalgae accumulate lipid under nutrient limitation when the energy source (light) and carbon source (CO2) are available due to the cellular mechanisms of active photosynthesis (Courchesne et al. 2009). Our findings are in agreement with those of these earlier studies as we found a higher lipid accumulation under limited conditions of nitrogen and phosphorus. Other researchers have also observed higher lipid contents in green algae Chlorella vulgaris and Neochloris oleoabundans at lower NaNO3 and KNO3 (Tornabene et al. 1983; Lv et al. 2010). Gardner et al. (2011) observed that nitrate and pH limitation resulted in the accumulation of TAGs by two members of the chlorophyta. In our study the sole addition of an external carbon source such as glucose reduced lipid accumulation whereas the addition of glucose concomitantly with nitrate and phosphate enhanced lipid accumulation. We also found that the addition of an external carbon source (glucose) reduced the chlorophyll content. Similar findings in relation to a decline in chlorophyll content were observed by Tanoi et al. (2010) who explained this decline is due to an inhibition of chloroplast development by the presence of glucose or by a relative increase in the level of another substance other than chlorophyll. Our results also show that algal growth and in turn biomass production increase with increasing levels of variables, such as nitrate, phosphate and glucose. Similar observations of high lipid accumulation and algal biomass were observed at lower initial concentration of nitrogen or phosphorus in Scenedesmus sp. LX1 (Xin et al. 2010).
Effect of cultivation time
Variation in the biochemical composition of the medium as a function of culture time was initially investigated to determine appropriate time to harvest the cells i.e. the time of maximum lipid production. The Chlorella spp. showed a short lag phase (up to 3 days), steep log phase (up to 12 days) and a stationary phase (>12 days) in BG-11 medium. Accordingly, cell growth phase was found to have a critical effect on cellular lipid yield and composition (Mansour et al. 2003). The maximum rate of biomass production (3.765 g L−1), as determined from the rate of increase in culture density, was obtained on culture day 12, and the maximum specific growth rate of −1.0473 day−1 was obtained on culture day 9. A reduction in growth rate was observed from culture day 9 onwards, reaching −3.8076 day−1 on culture day 18.
Effect of yeast extract
An increase in chlorophyll content was observed with increasing levels of yeast extract, whereas lipid content increased initially with increase in yeast extract up to 1.5 g L−1 followed by a decrease (Fig. 6). Protein content increased continuously from 0.082 mg mL−1 (control without yeast extract) to 0.23 mg mL−1 (at 3.0 g L−1 yeast extract). A higher growth rate of Chlorella spp. was observed with increased dosing of yeast extract, as inferred from the biomass contents. These results indicate that at 2.5 g L−1 yeast extract, a maximum biomass of 1.44 g L−1 was produced. However, Chlorella spp. growth fell along with a reduction in biomass following further increases in yeast extract above 2.5 g L−1. Leesing and Kookkhunthod (2011) in their study on Chlorella sp. KKU-S2 found that maximum values for the volumetric lipid production rate, specific yield of lipid, lipid concentration and specific rate of lipid production 0.220 g L−1day−1, 0.203 g lipid/g cells, 0.881 g L−1 and 0.093 g L−1day−1, respectively were obtained. Recently, Haque et al. (2012) observed in Chlorella spp. TISTR 8990 that for high biomass production (2.2 g L−1), the most effective and significant factors were yeast extract, KH2PO4, FeSO4 and ZnSO4 at concentrations of 0.3, 0.3, 3 and 0.3 mg L−1, respectively. In contrast, for high lipid accumulation (19.59 % dcw), KH2PO4, pH and yeast extract at a level of 1.7, 6.0 and 0.1 g L−1, respectively, were the most effective. Cheirsilp et al. (2012) showed that co-culture of an oleaginous yeast Rhodotorula glutinis TISTR 5159 and a microalga Chlorella vulgaris var. vulgaris TISTR 8261 enhanced biomass and lipid production from glycerol and the lipid so obtained was composed mainly of palmitic acid (C16:0; 40.52 %) and oleic acid (C18:1; 21.30 %), both of which have a high potential to be used as a biodiesel feedstock.
Effect of static/shaking conditions
Our comparison of shaking and static culture showed that the later was characterized by higher values of lipid accumulation (17.40 % dcw) when compared with shaking conditions (12.99 % dcw). However, chlorophyll (11.75 μg mL−1), protein (0.065 mg mL−1), carbohydrate (0.066 mg mL−1) and biomass (2.3 g L−1) contents were higher under shaking conditions. Similar findings of the highest lipid accumulation in the static phase were also reported in Chlorella pyrenoidosa (Nigam et al. 2011). The results of higher lipid accumulation and lower carbohydrate, chlorophyll and protein yield in static culture have also been recently demonstrated in Chlorococcum spp. (Kirrolia et al. 2012).
Effect of incubation temperature
The effects of varying incubation temperature on different growth parameters of Chlorella spp. are depicted in Fig. 7. The lipid content accumulated was 18.02, 20.14, 18.32, 12.53 and 11.93 % dcw at 23, 25, 27, 30 and 35 °C, respectively (Fig. 7a). A higher algal biomass of 2.54 and 2.44 g L−1was observed at 27 and 30 °C, respectively (Fig. 7a). However, higher chlorophyll (22.99 μg mL−1), protein (0.074 mg mL−1) and carbohydrate (0.088 mg mL−1) contents were observed at 30 °C (Fig. 7a, b). These results indicate that lipid accumulation and chlorophyll content were favoured at 25 and 30 °C, respectively. Temperature has been found to have a major effect on the fatty acid (FA) composition of microalgae (Guschina and Harwood 2006; Morgan-Kiss et al. 2006). A general trend towards increasing FA unsaturation with decreasing temperature and increasing saturated FA with increasing temperature has been observed in many microalgae and cyanobacteria (Murata et al. 1975; Sato and Murata 1980; Lynch and Thompson 1982; Renaud et al. 2002). The effects of temperature on lipid content in our study are in accordance with the findings of Sushchik et al. (2003), who reported that the relative content of unsaturated FA first increased with increasing temperature and then decreased with further increases in temperature in Chlorella vulgaris and Botryococcus braunii. Similar findings of increases in the lipid content of Chlorella vulgaris from 5.9 to 14.7 % with decreases in growth temperature from 30 to 25 °C were also observed by Converti et al. (2009). Increases in growth rate and total lipid production were obtained in Nannochloropsis salina with an increase in temperature (Boussiba et al. 1987). In a culture of Isochrysis galbana tk1 grown at 30 °C, total lipids accumulated at a higher rate with a slight decrease in the proportion of nonpolar lipids (Zhu et al. 1997). Recently, Crowe et al. (2012) compared the growth performance of Nannochloropsis salina in two outdoor pond designs (conventional raceways vs. the Arid pond with superior temperature management) and found that mitigation of temperature extremes and increased daily average temperatures led to increased biomass production in the Arid culture. The average temperature in the Arid pond culture was 19.5 °C throughout its batch growth phase as compared to 10.5 °C in the conventional raceways over the same period and 13.2 °C over the entire experiment.
Effect of salinity stress
The effect of salinity stress i.e. NaCl dosing from 1.0 to 10 mM, on various growth parameters of Chlorella spp. are described in Table 4. Our results indicate that an increase of the initial NaCl concentration from 1 to 8 mM decreased lipid accumulation from 7.46 to 5.15 % dcw. There was little difference in the lipid content between cells grown in cultures containing 10 mM NaCl (7.56 % dcw) and the control culture (7.46 % dcw). The amount of biomass harvested from the culture and protein content increased from 1.08 to 1.18 g L−1 and from 0.039 to 0.11 mg mL−1, respectively, with increases in the NaCl concentration from 1.0 to 10 mM, but chlorophyll content decreased from 16.62 to 14.56 μg mL−1 with subsequent increase in NaCl concentration. However, the chlorophyll content was found to be higher than that in the control cultures at 1.0 mM NaCl.
An increase in lipid content at higher NaCl concentrations has been reported in other studies. Takagi and Yoshida (2006) proposed that this increase may be due to adaptation under stress conditions which facilitates the accumulation of lipid content. In a study by Azachi et al. (2002), cells of Dunaliella salina were transferred from 0.5 to 3.5 M (29 to 205 g L−1) NaCl, following which there was a significantly higher ratio of C:18 (mostly unsaturated) to C:16 (mostly saturated) FAs in the cells grown in 3.5 M (205 g L−1) NaCl compared with those grown at 0.5 M (29 g L−1) NaCl. In an another study in Dunaliella spp., an even stronger increase in salinity from 0.4 M to 4 M (23 to 234 g L−1) increased the proportion of total saturated fatty and monounsaturated FAs, whereas the proportion of polyunsaturated FAs (PUFAs) decreased (Xu and Beardall 1997). Zhu et al. (2007) analyzed the growth, lipid content and FA composition of heterotrophic microalga Schizochytrium limacinum OUC88 at different temperatures (16, 23, 30 and 37 °C) and salinities (0, 9 27 and 36 g L−1). The highest lipid content was obtained at salinities of 9-36 g L−1 at a temperature range of 16–30 °C, and the content of saturated FAs C15:0 and C17:0 increased greatly (Zhu et al. 2007). Shah et al. (2013) recently studied the effects of photoperiod, salinity and pH on cell growth and lipid content of Pavlova lutheri, a microalga for biodiesel production in small-scale and large-scale open-pond tanks. They observed that in a 250-mL flask, the cultures grew well under 24-h constant illumination, with a maximum specific growth rate, μmax of 0.12 days−1 and a lipid content of 35 % as compared to 0.1 day−1 and 15 % lipid content in cultures grown in the dark. The salinity was optimum for the cell growth at 30–35 ppt, but the lipid content of 34–36 % was higher at 35–40 ppt. Algal growth and lipid accumulation were optimum at pH 8–9. Large-scale cultivation in 5-L and 30-L tanks achieved a μmax of 0.13–0.14 day−1 as compared to 0.12 day−1 in small-scale and 300-L cultures (Shah et al. 2013).
Effect of nitrate dosing at different cultivation times
The effect of 1.0 mM nitrate dosing at different cultivation times is given in Table 5. Maximum lipid content (20.8 % dcw) was observed in the control cultures (without nitrate) followed by the culture amended with nitrate on culture day 11 (14.51 % dcw). The maximum inhibitory effect of nitrate dosing was observed on culture days 5 and 9, and the lipid content accumulation observed was 12.9 and 13.0 % dcw, respectively. Nitrate dosing at various stages of the lag phase also inhibited lipid accumulation. The inhibitory effect was greater when nitrate dosing occurred at inoculation time (day 0) than on culture day 2 (13.99 vs. 10.59 % dcw, respectively). Maximum chlorophyll content of 5.14 and 6.30 μg mL−1 was observed in cultures amended on culture days 2 and 5, respectively. The relatively greater inhibitory effect on lipid accumulation in the culture amended on culture day 9 (maximum growth rate) indicated that growing cells were more affected than other phases of the cell cycle. Nigam et al. (2011) observed a similar finding of the highest lipid accumulation at lower nitrate dosing in Chlorella pyrenoidosa with decreasing levels of nitrate in the medium, biomass production also decreased but lipid content increased. Moreover, at the same concentration of the nitrate source, there was a tendency for more lipid to accumulate in the stationary phase than in the exponential phase. The highest lipid accumulation (26 %) was recorded in the culture with 0.05 g L−1 KNO3, which is one-fourth of the basal nitrogen source concentration (Nigam et al. 2011). A net increase of lipids was observed by Sforza et al. (2012) and Rodolfi et al. (2009) in Nannochloris salina growing under nitrogen deficiency. The optimum results from the RSM also indicated that higher levels of NO3- inhibited lipid accumulation but enhanced the chlorophyll, protein and carbohydrate contents. Mujtaba et al. (2012) reported that a small amount of nitrogen and phosphate, a moderate degree of aeration, light intensity and carbon supply were helpful in increasing the lipid productivity of Chlorella vulgaris in the second stage of a two-stage lipid production process consisting of rapid cell growth under nutrient repletion and incubation under nitrogen-depleted conditions.
Effect of different carbon sources
All of the carbon sources tested supported the growth of Chlorella spp. to some extent, with glucose and bicarbonate providing the highest biomass yield. Lipid content was found to be maximum in Chlorella spp. cultured in medium with glucose as the carbon source, followed more or less by medium containing mannitol, lactose and ethanol. Chlorophyll content was highest in Chlorella spp. grown in medium containing ethanol as the carbon source, followed by medium containing methanol, glucose and fructose. There was no apparent effect of carbon source on the carbohydrate content of Chlorella spp., with the carbohydrate content being more or less equal for all of the carbon sources tested (range 0.04–0.05 mg mL−1). The protein content was maximum when sucrose was the carbon source, followed by glucose, fructose and lactose. These effects of different carbon sources on lipid and biomass content are in accordance with those reported by Lee (1997) who found that compared with other heterotrophic cultures, commercial heterotrophic cultivation of Chlorella spp. in conventional stirred tank fomenters was common and that glucose and acetate were the most utilized carbons in this fermentation process. Glucose and acetate are known as basic nutrients of Chlorella spp. in an autotrophic medium (Kaplan et al. 1986) and are used for heterotrophic cultivation of microalgae for other objectives (Chen and Johns 1996). Yeh et al. (2010) showed that the biomass production by Chlorella vulgaris ESP-31 increased with increasing sodium bicarbonate concentration in the culture medium. There have been some reports that Chlorella minutissima can use organic carbon, such as glucose, acetate and methanol as a heterotroph (Vazhappilly and Chen 1998; Kotzabasis et al. 1999). In addition Liu et al. (2009) observed that organic carbon sources such as glycerol, acetate and glucose were able to significantly increase specific growth rate of a marine diatom Phaeodactylum tricornutum.
Influence of different media compositions
In our study, algal biomass yield was highest (0.98 g L−1) in BG-11 medium, and chlorophyll content was also highest (11.8 μg mL−1) in BG-11 medium followed by BBM (3.01 μg mL−1). The highest total chlorophyll content of cells in BG-11 medium reflected the enhanced capacity of the medium to maintain heterotrophic cultures. In the green algae Chlorella spp. the lipid content was maximum in Allen medium (15.7 % dcw) followed by Chu medium (10.2 % dcw). The higher values of lipid content might be due to the lower level of nitrates in the media. Carbohydrate content was maximum in BG-11 medium (0.05 mg mL−1) followed by Chu#10 medium. The BG-11 medium also supported the best protein content (0.034 mg mL−1) followed more or less equally by BBM. In the BG-11 medium, with an increase in algal biomass there was also an increase in the chlorophyll content, similar to the findings reported for Botryococcus braunii (Eroglu and Melis 2010). Similar results for higher algal biomass yield in the BG-11 medium for green alga Botryococcus braunii have also been observed (Dayananda et al. 2007; Seshadri and Thomas 2010).
Conclusions
In conclusion, the results of our study reveal that the addition of a small amount of carbon source (glucose), a pH of 7.5 and limited nitrate and phosphate sources played crucial roles in affecting the growth and lipid accumulation of Chlorella spp. The best performance was obtained when the Chlorella spp. was grown in medium containing yeast extract at 2.5 g L−1 and 10.0 mM NaCl. All of the carbon sources used in our study supported the growth of Chlorella spp. to some extent, with glucose and bicarbonate providing the highest biomass yield and glucose providing the maximum lipid content. The algal biomass yield and chlorophyll content were highest in BG-11 medium and the lipid content was maximum in Allen medium followed by Chu medium. The good agreements between the predicted and experimental results are demonstrated with a squared correlation coefficient (R 2) for various responses. We conclude that the use of inexpensive optimization tools along with proper strategies lead to a great potential for commercial microalgae cultivation and lipid production.
References
Anthony WD, Larkum, Ross IL, Kruse O, Hankamer B (2012) Selection, breeding and engineering of microalgae for bioenergy and biofuel product ion. Trends Biotechnol 30:4198–4205
Azachi M, Sadka A, Fisher M, Goldshlag P, Gokhman I, Zamir A (2002) Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina. Plant Physiol 129:1320–1329
Bellou S, Aggelis G (2012) Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J Biotechnol 164:318–329
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Boussiba S, Vonshak A, Cohen Z, Avissar Y, Richmond A (1987) Lipid and biomass production by the halotolerant microalga Nannochloropsis salina. Biomass 12:37–47
Cheirsilp B, Kitcha S, Torpee S (2012) Co-culture of an oleaginous yeast Rhodotorula glutinis and a microalga Chlorella vulgaris for biomass and lipid production using pure and crude glycerol as a sole carbon source. Ann Microbiol 62:987–993
Chen F, Johns MR (1996) Heterotrophic growth of Chlamydomonas reinhardtii on acetate in chemostat culture. Process Biochem 31:601–604
Chen M, Tang H, Ma H, Holland TC, Ng KYS, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Chu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Converti A, Casazza AA, Ortiz EY, Perego P, Del BM (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41
Crowe B, Attalah S, Agrawal S, Waller P, Ryan R, Wagenen JV, Chavis A, Kyndt J, Kacira M, Ogden KL, Huesemann M (2012) A Comparison of Nannochloropsis salina growth performance in two outdoor pond designs: conventional raceways versus the ARID pond with superior temperature management. Int J Chem Eng 9:1–9
Dayananda C, Sarada R, Rani MU, Shamal TR, Ravishankar GA (2007) Autotrophic cultivation of Botryococcus braunii for the production of hydrocarbons in exosaccharides in various media. Biomass Bioenerg 31:87–93
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507
Demirbas A, Demirbas MF (2011) Importance of algae oil as a source of biodiesel. Energy Convers Manage 52:163–170
Dubois M, Gilles AK, Hamilton JK, Rebes PA, Smith F (1956) Colorimetric method for detemination of sugars and related substances. Anal Chem 28:350–356
Eroglu E, Melis A (2010) Extra cellular terpenoid hydrocarbon extraction and quantitation from the green microalgae Botryococcus braunii Showa. Bioresour Technol 101:235–236
Gardner R, Peters P, Peyton B, Cooksey KE (2011) Medium ph and nitrate concentration effects on accumulation of triacylglycerol in two members of the chlorophyta. J Appl Phycol 23:1005–1016
Griffiths M, Harrison S (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guillard RRL (1973) Methods for microflagellates and nanoplankton. In: Stein J (ed) Handbook of phycological methods. Cambridge University Press, Cambridge, pp 80–81
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hader DP, Kumar HD, Smith RC, Worrest RC (2007) Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem Photobiol Sci 6:67–285
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30:709–732
Haque MA, Bangrak P, Sirisansaneeyakul S, Choorit W (2012) Factors affecting the biomass and lipid production from Chlorella sp. TISTR 8990 under mixotrophic culture. Walailak J Sci Technol 9:347–359
Herrera-Valencia VA, Andres Us-Vazquez R, Larque-Saavedra FA, Barahona-Perez LF (2012) Naturally occurring fatty acid methyl esters and ethyl esters in the green microalga Chlamydomonas reinhardtii. Ann Microbiol 62:865–870
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–663
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym Microb Technol 27:631–635
Kaplan D, Richmond AE, Dubinsky Z, Aaronson S (1986) Algal nutrition. In: Richmond A (ed) Handbook of microalgal mass culture, Florida. CRC Press, Boca Raton, pp 147–198
Kirrolia A, Bishnoi NR, Singh R (2012) Effect of shaking, incubation temperature, salinity and media composition on growth traits of green microalgae Chlorococcum sp. J Algal Biom Utlzn 3:46–53
Kotzabasis K, Hatziathanasiou A, Ruigomez MVB, Kentouri M, Divanach P (1999) Methanol as alternative carbon source for quicker efficient production of the microalgae Chlorella minutissima: role of the concentration and frequence of administration. J Biotechnol 70:357–362
Lang X, Dalai AK, Bakhshi NN, Reaney MJ, Hertz PB (2000) Preparation and characterization of bio-diesels from various bio-oils. Bioresour Technol 80:53–62
Lee YK (1997) Commercial production of microalgae in the Asia-Pacific rim. J Appl Phycol 9:403–411
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 10:75–77
Leesing R, Kookkhunthod S (2011) Heterotrophic growth of Chlorella sp. KKU-S2 for lipid production using molasses as a carbon substrate. Int Conf Food Eng Biotechnol 9:87–91
Levine RB, Robinson MSC, Spatafor GA (2011) Neochloris oleoabundans grown on anaerobically digested dairy manure for concomitant nutrient removal and biodiesel feedstock production. Biom Bioen 35:40–49
Li X, Hu HY, Zhang YP (2011) Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour Technol 102:3098–3102
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Liu X, Duan S, Li A, Xu N, Cai Z, Hu Z (2009) Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J Appl Phycol 21:239–246
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin–Phenol reagent. J Biol Chem 193:265–275
Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 106:6797–6804
Lynch DV, Thompson TGJ (1982) Low temperature-induced alterations in the chloroplast and microsomal membranes of Dunaliella salina. Plant Physiol 69:1369–1375
Mansour MP, Volkman JK, Blackburn SI (2003) The effect of growth phase on the lipid class, fatty acid and sterol composition in the marine dinoflagellate. Gymnodinium sp. in batch culture. Phytochem 63:145–153
Morgan-Kiss RM, Priscu JC, Pocock T, Gudynaite-Savitch L, Huner NPA (2006) Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev 70:222–252
Mujtaba G, Choi W, Lee CG, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283
Murata N, Troughton JH, Fork DC (1975) Relationships between the transition of the physical phase of membrane lipids and photosynthetic parameters in Anacystis nidulans and lettuce and spinach chloroplasts. Plant Physiol 56:508–517
Nigam S, Rai MP, Sharma R (2011) Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. Am J Biochem Biotechnol 7:124–129
Pienkos PT, Darzins A (2009) The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod Bioref 3:431–440
Praveenkumar R, Shameera K, Mahalakshmi G, Akbarsha MA, Thajuddin N (2012) Influence of nutrient deprivations on lipid accumulation in a dominant indigenous microalga Chlorella sp. bum11008: Evaluation for biodiesel production. Biomass Bioenerg 37:60–66
Pruvost J, Van Vooren G, Le Gouic B, Couzinet-Mossion A, Legrand J (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102:150–158
Renaud SM, Thinh LV, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2008) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Sato N, Murata N (1980) Temperature shift-induced responses in lipids in the blue-green alga, Anabaena variabilis: the central role of diacyl mono galactosyl glycerol in thermo-adaptation. Biochim Biophys Acta Lipids Lipid Metabol 619:353–366
Scott S, Davey M, Dennis J, Horst I, Howe C, Smith DL (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:1–10
Seshadri CV, Thomas S (2010) National solar energy conservation. Bhavnagar, India, pp 41–46
Sforza E, Bertucco A, Morosinotto T, Giacometti GM (2012) Photobioreactors for microalgal growth and oil production with Nannochloropsis salina: from lab scale experiments to large-scale design. Chem Eng Res Des 90:1151–1158
Shah SMU, Radziah CC, Ibrahim S, Latiff F, Othman MF, Abdullah MA (2013) Effects of photoperiod, salinity and pH on cell growth and lipid content of Pavlova lutheri. Ann Microbiol 4:645–656
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the US Department of Energy’s Aquatic Species Program-biodiesel from algae. National Renewable Energy Laboratory (NREL) report NREL/TP-580-24190. National Renewable Energy Laboratory, Golden
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102:10–16
Song M, Pei H, Hu W, Ma G (2013) Evaluation of the potential of 10 microalgal strains for biodiesel production. Bioresour Technol 141:245–251
Spolaore P, Cassan CJ, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Sushchik NN, Kalacheva GS, Zhila NO, Gladyshev MI, Volova TG (2003) A temperature dependence of the intra- and extracellular fatty-acid composition of green algae and cyanobacterium. Russ J Plant Physiol 50:374–380
Takagi M, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and Triacylglyceride in marine Microalgae Dunaliella cells. J Biosc Bioeng 101:223–226
Tandeau de Marsac N, Houmard J (1988) Complementary chromatic adaptation: physiological conditions and action spectra. Methods Enzymol 167:318–328
Tanoi T, Kawachi M, Watanabe MM (2010) Effects of carbon source on growth and morphology of Botryococcus braunii. J Appl Phycol 10:9528–9540
Tornabene TG, Holzer G, Lien S, Burris N (1983) Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enz Microbiol Technol 5:435–440
Vazhappilly R, Chen F (1998) Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. J Am Oil Chem Soc 75:393–397
Widjaja A, Chien CC, Ju YH (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40:13–20
Xin L, Hong-Ying H, Ke G, Ying-Xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus spp. Bioresour Technol 101:5494–5500
Xu X, Beardall J (1997) Effect of salinity on fatty acid composition of a green microalga from an Antarctic hypersaline lake. Phytochem 45:655–658
Xue L, Zhang Y, Zhang T, An L, Wang X (2005) Effects of enhanced ultraviolet-B radiation on algae and cyanobacteria. Crit Rev Microbiol 31:79–89
Yeh KL, Chang JS (2011) Nitrogen starvation strategies and photobioreactor design for enhancing lipid production of a newly isolated microalga Chlorella vulgaris esp-31: implications for biofuels. Biotechnol J 6:1358–1366
Yeh KL, Chang JS, Chen WM (2010) Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng Life Sci 10:201–208
Yount R (2006) Advanced statistical procedures, research design and statistical analysis in Christian Ministry. Southwestern Baptist Theological Seminary,Fort Worth
Zhao G, Yu J, Jiang F, Zhang X (2012) The effect of different trophic modes on lipid accumulation of Scenedesmus quadricauda. Bioresour Technol 114:466–471
Zhu C, Lee Y, Chao T (1997) Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana tk1. J Appl Phycol 9:451–457
Zhu LY, Zhang XC, Ji L, Song XJ, Kuang CH (2007) Changes of lipid content and fatty acid composition of Schizochytrium limacinum in response to different temperatures and salinities. Process Biochem 42:210–214
Zhukova N, Titlyanov E (2006) Effect of light intensity on the fatty acid composition of dinoflagellates symbiotic with hermatypic corals. Bot Mar 49:339–346
Acknowledgments
The authors are highly thankful to Haryana State Council of Science & Technology (HSCST), Panchkula, Haryana (India) for Sir C.V. Raman Research Fellowship and to the Council of Scientific and Industrial Research, New Delhi (India) for awarding Senior Research Fellowship to one of the authors (Ms. Anita Kirrolia). They are also thankful to Professor B.B. Chaugule, Department of Botany, University of Pune, Pune, India for providing the necessary help in the identification of algal species.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the Electronic Supplementary Material.
ESM 1
(JPEG 494 kb)
Rights and permissions
About this article
Cite this article
Kirrolia, A., Bishnoi, N.R. & Singh, R. Response surface methodology as a decision-making tool for optimization of culture conditions of green microalgae Chlorella spp. for biodiesel production. Ann Microbiol 64, 1133–1147 (2014). https://doi.org/10.1007/s13213-013-0752-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-013-0752-4