Abstract
Mango seed kernel, a by-product of the processing industry, can be valorized as a potential source of bioactive compounds. Binary mixtures of ethanol and water, used in solid–liquid extraction (SLE), have drawn interest as an effective means of recovering phytochemicals from plant materials because these solvents can be used in food applications and their synergistic effect makes them a superior solvent over their pure counterparts. Total phenolic content (TPC) and HPLC chromatograms of each ethanolic extract revealed that ethanol concentration had a significant effect on phenolic compound recovery, wherein, TPC of mango kernel varied from 18.19 to 101.68 mg gallic acid equivalence (GAE) per gram of sample. Subsequently, the antioxidant activities (AOAc) of the extracts, measured by scavenging activities with the DPPH+ (1,1-diphenyl-2-picrylhydrazyl) radical and ferric reducing antioxidant power (FRAP) assay, ranged from 8.19 to 85.45 mmol/L and 3.82–55.61 mmol/L Trolox equivalence, respectively. The solvent containing 50% (w/w) ethanol–water had the highest TPC and exhibited the most potent reducing and radical scavenging activities. With the use of an HPLC–UV/Vis, gallic acid, caffeic acid, rutin and penta-O-galloyl-β-d-glucose were identified to be present in the mango seed kernel. Results show that the mango seed kernel is a viable source of bioactive compounds which can be recovered with water–ethanol binary solvent systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mango (Mangifera indica L.), is one of the most significant tropical fruits produced and accepted by consumers around the globe. They are either eaten fresh or processed to a variety of products in order to increase its shelf life. In the Philippines, mangoes are processed to produce dried fruit slices, purees, concentrates, juices, jams, candies, jellies either for local consumption or for export. An increasing demand for mangoes has been observed owing to the notable recognition and awareness of consumers of its nutritional and therapeutic value (Da Silva et al. 2013; Ribeiro et al. 2008). Consequently, refuse coming from mango fruit production, consumption and processing is constantly on the rise. Waste generated are comprised mainly of seeds and peels, which make up 35% to 60% of the fruit weight depending on the cultivar (Larrauri et al. 1996; Schieber et al. 2001), that are readily discarded as waste and pose a serious threat to the environment (Da Silva et al. 2013). Generally, these by-products have not received much attention, but studies have shown that they contain valuable bioactive compounds that are natural and can be used as raw materials for specific applications or as alternative to synthetically made counterparts. Utilizing these feedstocks will have minimal costs and consequently reduce waste for disposal (Soong and Barlow 2004). As a result, there is a strong emphasis on investigating the utilization of agricultural waste as a low-cost source for value added applications (Da Silva et al. 2013).
The seed represents 10% to 25% of the entire fruit weight, depending on the mango variety. It is composed of a thick, hard and woody outer shell, called the endocarp, which encloses the seed kernel, making up about 45% to 75% of the whole seed (Abdalla et al. 2007; Maisuthisakul and Gordon 2009). A number of studies have shown that mango seed kernel can be a rich source of different phenolic and antioxidant compounds (Abdalla et al. 2007; Dorta et al. 2013; Maisuthisakul and Gordon 2009). Phenolic compounds have gained substantial interest because of the benefits that are associated with these phytochemicals that are very attractive in the formulation of functional foods and nutraceutical compounds (Abdalla et al. 2007; Da Silva et al. 2013).
Solvent extraction is the most common means of recovering phenolic compounds from plant materials due to simplicity of the technique, efficiency and a wide range of possible applications. Although it is a simple process, chemical extraction is driven by conditions such as time allotted for extraction, extraction temperature and sample-to-solvent ratio, which affect yield and extent of recovery. Under the same conditions, the nature of the compounds to be recovered is an important factor to be considered as it helps in selecting the most suitable solvent for extraction (Robbins 2003; Dai and Mumper 2010).
No single solvent can extract all of the bioactive compounds in a plant material and it is with this premise that binary solvent systems, water and organic solvent, are favored as opposed to their native counterparts (Sun and Ho 2005; Xu and Chang 2007; Wang et al. 2008; Thoo et al. 2010). With the concept of “like dissolves like,” water dissolves polar compounds and the organic solvent recovers less polar constituents (Durling et al. 2007; Dorta et al. 2012). Although a number of organic solvents have been used to extract bioactive compounds from mango seed kernel, ethanol is preferred for food applications (Alothman et al. 2009; Maisuthisakul and Gordon 2009).
So far, no study has been conducted on the systematic solvent extraction using binary systems of ethanol and water for phenolic-compound recovery from mango seed kernel, especially that of the Philippine variety. Thus, the aim of this study is to investigate the effect of ethanol concentration on the recovery of phenolic compounds from mango seed kernel and consequently link this to the antioxidant activities of these extracts. Identification of some of the most common bioactive compounds present in the mango seed kernel is also explored in this work.
Materials and methods
Mango seed kernel powder
Samples of mango seed kernel powder (MSKP) was generously provided by a local mango waste processing facility (Lapu-lapu City, Philippines) and were used as provided.
Chemicals
A 200 proof ethanol was used during extraction (Decon Labs, USA) and deionized water was purchased from Millipore-sigma, USA. Chemicals used as mobile phase in HPLC analysis were, acetonitrile, formic acid (Fisher, USA) and de-ionized water (Millipore, USA). Reagents and chemicals such as Follin-Ciocalteau, (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), citric acid, sodium carbonate, potassium per sulfate, ferric chloride, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Analytical grade standards for gallic acid, caffeic acid, rutin hydrate and penta-O-galloyl-β-d-glucose were purchased from Sigma-aldrich (St. Louis, MO, USA).
Extraction of bioactive compounds
Aqueous solutions containing varied amounts of ethanol, prepared in weight per weight proportions (0%, 10%, 25%, 50%, 75%, 100%), were used as solvent for extraction. 150 mL for each of the aqueous solutions was prepared and poured into separate Erlenmeyer flasks containing 15 g each of the MSKP. Then the flasks containing the samples were placed on a table top shaker (Model MS2, New Brunswick Co., Inc., NJ, USA) and was constantly stirred (200 RPM) for an hour at room temperature. After extraction, the content of each flask was centrifuged (Allegra 25R Centrifuge, Beckman Coultier Inc., CA, USA) at 5000×g for 20 min at 20 °C. Then the liquid phase was decanted and stored in vials and were kept in a freezer prior to sample preparation for subsequent analyses.
Analysis of phenolic compounds in the ethanolic extracts
HPLC–UV/Vis analysis
MSKP extracts were thawed by letting them stand at room temperature and were diluted with an ethanolic solution (1:1 wt/wt water:ethanol) accordingly and filtered with a 0.2 μm syringe filter prior to HPLC analysis. Blank samples, pure ethanol, and water, were also prepared in the same manner.
A Beckman System Gold High Performance Liquid Chromatograph with a 126 programmable solvent delivery module and UV/Vis detector was used for HPLC–UV/Vis analysis. Chromatographic separation of ethanolic extracts (50 μL) was carried out using a C18 reverse-phase (5μ), HAISIL PS Phalanx column (250 × 4.6 mm I.D.; Higgins, CA, USA) with a C18 Phalanx HAICart SS guard cartridge (20 × 3.2 mm I.D.; Higgins, CA, USA) at room temperature. The mobile phase consisted of 1% formic acid in deionized-water (A) and 1% formic acid in acetonitrile (B). Both mobile phases were degassed under house vacuum prior to usage. The gradient profile was 0 min, 85% A; 15 min, 70% A; 35 min, 0% A; 37 min 15% A. The column was equilibrated and cleaned for 15 min prior to injection of the next sample. The mobile phase flow rate was set at 1 mL/min and detection wavelength was set at 280 nm.
Total phenolic content (TPC)
A modified method of Liu et al. (2008) was used in carrying out TPC analysis. About 1 mL of filtered extract was diluted with water to about 7 mL and was added with 0.5 mL of Folin–Ciocalteau phenol reagent. After 5 min, about 1.0 mL of saturated sodium carbonate solution was added to the mixture. The mixture was diluted up to 10 mL with water. The mixture was incubated for 2 h at 30 °C and the absorbance was determined at 765 nm using a Spectroquant Pharo 100 spectrophotometer (Merck, United States). A standard curve was prepared using gallic acid and concentration values were reported as mg gallic acid equivalents per gram of dry mango seed kernel powder (mg GAE/g MSKP).
Antioxidant activity
-
(1)
DPPH (1,1-diphenyl-2-picrylhydrazyl) assay
Ethanol-DPPH solution (0.1 mM DPPH in ethanol) was prepared fresh. The DPPH solution (2.9 mL) was placed in a glass cuvette and the absorbance at 515 nm in time t = 0 min (t0) was measured. 0.1 mL of sample extract was added and the mixture was shaken vigorously and kept in the dark at room temperature for 30 min (t30). The absorbance at 515 nm was then measured using a Spectroquant Pharo 100 spectrophotometer (Merck, United States). A standard curve using Trolox was prepared and the percentage reduction of DPPH was calculated as
-
(2)
Ferric reducing antioxidant power (FRAP) assay
0.1 mL of sample extract and 0.1 mL FeCl3 (3 mM in 5 mM citric acid) were mixed well in a 1.5 mL Eppendorf tube and incubated for 30 min in a waterbath at 37 °C. The mixture was then added to 0.9 mL of 1 mM TPTZ in 50 mM HCl and vortexed. After 1 min, the absorbance is read at 620 nm using a Spectroquant Pharo 100 spectrophotometer (Merck, United States). A standard curve of Trolox is prepared following such procedures and results are expressed as µmol Trolox equivalent antioxidant capacity/100 g dry matter.
Statistical analyses
All analyses conducted in this study were performed at least in triplicate and the values reported are presented as average values along with their standard deviations. Data obtained were analyzed with MS Excel 2016. Statistical comparison for two means were done with T test, whereas, one-way analysis of variance was conducted for comparison of more than two means, and p values < 0.05 were considered as significant. Pearson’s correlation was carried out to determine correlation coefficients (r) between TPC with DPPH and FRAP assays, respectively.
Results and discussion
Extraction of bioactive compounds from mango seed kernel powder
Solvent extraction of phenolic compounds in the mango seed kernel were carried out using water–ethanol mixtures containing different mass ratios of ethanol: 0%, 10%, 25%, 50%, 75%, 90% and 100%. Each ethanolic extract was directly injected into the HPLC for characterization after sample preparation and six peaks from the elution profile were selected in this study for comparative study as shown in Fig. 1. Four of the six peaks were identified with the use of standards and are illustrated in Online Resources 1, 2, 3 and 4: (1) gallic acid, (2) caffeic acid, (3) rutin, (4) Penta-O–galloyl-β-d-glucose. Peaks (5) and (6) were supposedly galloylglucose species with galloyl moieties greater than five (Hexa, Hepta, Octa, etc.) as reported in literature (Berardini et al. 2004; Engels et al. 2012). These compounds vary in polarity as they were separated in a reversed-phase column during HPLC characterization, allowing polar compounds to elute earlier from the column due to a lesser affinity with the stationary phase.
Bioactive compounds in the ethanolic extracts were detected at 280 nm wavelength with a UV–Visible light (UV–Vis) detector. As shown in Fig. 2, changes in absorbance intensity were observed as the concentration of ethanol in the solvent was varied. A clearer presentation of the extraction profiles for the identified peaks in Fig. 1 are shown in Fig. 3, wherein, the relationship between the amount recovered for the identified compound, as expressed in absorbance intensity, and ethanol concentration is illustrated. It is noted that the presence of ethanol in the extraction solvent plays a key role in the recovery of bioactive compounds from MSKP. Previous studies showed that a wide array of phytochemicals, which vary in polarity, can be obtained from plant materials and it was for this reason that no particular solvent can recover all of these compounds efficiently (Sun and Ho 2005; Xu and Chang 2007). Although there are a number of parameters to be considered during solvent extraction, under the same extraction time and temperature conditions, the nature and properties of the phenolic compounds determine the solvent or mixture of solvents to be utilized for recovery. Among the common solvents used for extraction, water and ethanol are highly recommended as they are recognized to be non-toxic and fit for food applications (Alothman et al. 2009; Maisuthisakul and Gordon 2009; Soong and Barlow 2004). In general, the complementary relationship of both solvents, when used in binary systems, can be described in such a way that polar compounds are best recovered with water while less polar constituents were dissolved in ethanol (Durling et al. 2007; Dorta et al. 2012).
Bioactive compounds in seeds are either free or bound in the solid matrix and it follows that free polar phenolic compounds, that are easily dissolved in water and are situated on the surface of the bulk material, will have a good interaction with the extraction solvent and will have an extraction profile similar to that of peak 1 (gallic acid), as seen in Fig. 3. This was consistent with what can be inferred from Figs. 1 and 2 which showed that this compound eluted early (in less than 5 min) during chromatography. Moreover, the extraction profile of gallic acid was also consistent with its solubility data since the compound had been reported to be more soluble in water than in ethanol (Galanakis et al. 2013). Though no significant difference in extraction can be observed from 0% to 75% ethanol concentration (peak 1), it was possible that enough water was available to recover gallic acid, and as the amount of water was diminished with increasing ethanol content in the solvent, less water was available to totally dissolve the compound resulting to a decrease in recovery.
Caffeic acid (peak 2) and Rutin (peak 3) had been reported to be more recoverable with ethanol than water (Zi et al. 2007; Galanakis et al. 2013). However, this was in contrary to the extraction profiles for both compounds as pure ethanol exhibited the least absorbance intensities, as seen in Fig. 3. On the other hand, pentagalloylglucose was sparingly soluble in water and in the study of Dorta et al. (2012), tannins from mango peels were found to be best recovered with a 1:1 ethanol to water solvent, followed by ethanol, then water. This was consistent with the extraction profiles that were obtained (peaks 4–6) wherein, these compounds were recovered in greater amounts with ethanol and water mixtures, and least for both pure water and pure ethanol solvents.
In the extraction of bioactive compounds from plant sources, it was noted that not all of these compounds were freely extracted. Most were in the bound form, such as: conjugates with sugars, fatty acids or in polyphenol-protein complexes, making recovery tougher (Maisuthisakul and Gordon 2009; Dorta et al. 2012; Parmar et al. 2017). This was the case when binary solvents of water and ethanol had an advantage over their pure constituents since a synergistic effect had been recorded for both solvents when used together for phytochemical extraction (Thoo et al. 2010). Robards (2003) had reported that the alcoholic solvent would cause dissolution of the cell membrane by dissolving less polar phenolics in the cell wall, and subsequently exposing endocellular phenolic compounds to the solvent, hence, extracting them. In the study of Dorta et al. (2013), a similar observation was noted where water, with high percentage of organic solvent, was favored for extracting phenolic compounds from protein matrices, such as mango seed, since organic compounds seemed to degrade polyphenol-protein complexes. While it had been established that water dissolves polar compounds, interestingly, it could also cause plant material to swell and resulted in an easier penetration of the solvent in the solid matrix and increase extraction yield (Mukhopadhyay et al. 2006; Galili and Hovav 2014). These accounts were apparent for peaks 2–6, wherein, phenolic extractions were at its peak with solvents having either 25%, 50% or 75% ethanol weight ratio. Peak 2 (caffeic acid), peak 3 (rutin), peak 4 (penta-O–galloyl-β-d-glucose), and peaks 5 and 6 (gallotannins) were examples of bound or complexed phenolics as pure ethanol and pure water extracted the least amount of these phytochemicals. It was also interesting to note that differences in polarity among these compounds can be observed as their extraction profiles shift from a negatively-skewed (peak 2, polar) profile to a positively-skewed (peak 6, less polar) profile.
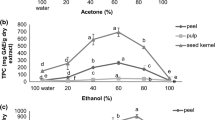
Effect of varying ethanol concentration on the total phenolic content
Total phenolic content (TPC) was measured by the reduction of the Folin-Ciocalteu reagent by phenolic compounds with the formation of a blue complex (Imeh and Khokhar 2002). In this study, TPC values for ethanolic extracts of the mango seed kernel powder were obtained in terms of the concentration of gallic acid solution (μg/mL) expressed as mg gallic acid equivalents per gram of dried mango seed kernel powder (mg GAE/g MSKP).
Results showed that TPC values of the ethanolic extracts ranged from 18.19 to 101.68 mg GAE/g MSKP. As illustrated in Fig. 4, 50% ethanol exhibited the highest value for TPC and the lowest was that of 100% ethanol. It was observed that as ethanol content in the extraction solvent was increased, TPC also increased up to a certain extent, where maximum extraction was attained with 50% ethanol. Beyond this point, an increase in ethanol content was not beneficial for extraction as TPC values decreased with 75% ethanol (93.41 mg GAE/g MSKP), however it should be noted that it had a TPC value greater than that of 25% ethanol (76.52 mg GAE/g MSKP). Further addition of ethanol would significantly lower the TPC value to 38.05 mg GAE/g MSKP (90% ethanol) and eventually lead to the lowest amount of TPC with pure ethanol (18.19 mg GAE/g MSKP). The TPC value at 0% ethanol was not significantly higher compared to that of 100% ethanol (p > 0.05).
The TPC results obtained were consistent with the works of Wang et al. (2008) and Thoo et al. (2010), where, binary solvent systems of ethanol and water were superior compared to pure water and pure ethanol in phenolic compound extraction with respect to relative polarity. This was likewise in congruent to what can be observed in Fig. 2, wherein, absorbance intensities for most peaks were at its highest in the chromatograph for 50% ethanolic extract.
So far, this was the first study to report systematically the total phenolic content of mango seed kernel and its relation to the binary solvent systems used during extraction, noting that the material from which bioactive compounds were recovered was of the Philippine variety. A number of studies made use of other mango cultivars like Chonsa and Langra which were reported to contain 63.89 ± 0.72 mg GAE per g sample and 69.24 ± 0.54 mg GAE per g sample, respectively (Sultana et al. 2012). Soong and Barlow (2004) utilized mangoes in Singapore and found its seed kernel to contain 117 ± 13.5 mg GAE per g sample. While seed kernel for the Ubá variety was reported to contain 82.54 mg GAE per g of total phenolics (Ribeiro et al. 2008). An interesting study done by Sogi et al. (2013) took into account the effect of drying method on total phenolic content of seed kernels from the Tommy Atkins variety and their results were as follows: freeze dried (200.35 mg GAE g−1), cabinet dried (125.35 mg GAE g−1), vacuum dried (150.22 mg GAE g−1) and infrared dried (112.28 mg GAE g−1). Although, it was evident that cultivar was a significant factor on the disparity of TPC values, variation in reported phenol content may also be associated with differences in climate, ripeness, extraction method and other agronomic factors (Moure et al. 2001; Imeh and Khokhar 2002; Barreto et al. 2008; Dorta et al. 2012).
Effect of varying ethanol concentration on the antioxidant activity
As seen in Fig. 5, all of the extracts exhibited antioxidant activities for both ferric reducing antioxidant power (FRAP) and DPPH (1,1-diphenyl-2-picrylhydrazyl) assays. Scavenging activity increased with increasing ethanol content from 0 to 50%, however, further increase in ethanol concentration resulted to a decline in scavenging activity. Extracts using 10%, 25% and 75% ethanol differed significantly in DPPH+ radical scavenging activities and reduction capabilities (p < 0.05), however, it was noteworthy that at 25% and 75% ethanol, there was no significant difference (p > 0.05) in activities for both FRAP and DPPH. In addition, at 0% ethanol (pure water), the extract had a more potent reducing capacity and radical scavenging activity than with 100% ethanol (pure ethanol). In general, it was observed that for all extracts at different ethanol concentrations, the antioxidant activities of the mango seed kernel extracts were greater with the DPPH assay than with the FRAP assay. This result was similar with the work of Maisuthisakul and Gordon (2009), wherein, the extract containing 50% ethanol (w/w) had the highest antioxidant capacities. The disparity in antioxidant activities of the mango seed kernel extracts, at different ethanol concentration, was an indicator that the type of bioactive compounds and amounts recovered from mango seed kernel were affected by ethanol concentration. With solvent polarity varying from being very polar (0% ethanol) to a less polar (100% ethanol), the capacity of the solvent to dissolve selected groups of antioxidants would also vary and this would have an implication on the antioxidant activity, as well (Abbasi et al. 2015; Turkmen et al. 2006; Ribeiro et al. 2008; Spigno et al. 2007). This observation was consistent with the results in this study as depicted in Fig. 5, wherein, the antioxidant capacities of the extracts were sensitive to solvent polarity which can be linked to the phenolic content of each extract, as well.
Correlations
Pearson’s correlation test had been done to check on the relationship between the two responses evaluated which were TPC and antioxidant activities (AOAc), as measured with FRAP and DPPH assays, and the value for r could range from + 1 to − 1. A value of zero would indicate that there was no association between TPC and AOAc. A value greater than 0 showed a positive correlation, which meant that AOAc had a direct relationship with TPC. On the other hand, a negative correlation would mean an inverse relationship between AOAc and TPC, which could be signified by an r value less than 0 (Bluman 2013). Results suggested that there was a strong positive correlation between TPC to both antioxidant activity measurements, exhibiting grater correlation with FRAP (r = 0.9373) than with DPPH (r = 0.8524). This implies that the AOAc of the extracts was greatly influenced by its phenolic content, which could also be observed from Figs. 4 and 5 where the TPC profile of the extracts from mango seed kernel was similar to that of the AOAC profiles. This observation was in agreement with other reports in literature (Abbasi et al. 2015; Alothman et al. 2009; Maisuthisakul and Gordon 2009; Singh et al. 2016; Turkmen et al. 2006).
Conclusion
This study illustrates that binary mixtures of ethanol and water were superior solvents compared to their pure counterparts in recovering bioactive compounds from mango seed kernel. The combination of both solvents in recovering phenolic compounds was mainly due to the principle of “like dissolves like” where water dissolves polar compounds while ethanol dissolves the less polar constituents. Specifically, at 50% (w/w) ethanol concentration, the highest total phenolic content and correspondingly exhibited the highest antioxidant activity with FRAP and DPPH assays, were noted.
References
Abbasi AM, Guo X, Fu X, Zhou L, Chen Y, Zhu Y, Yan H, Liu RH (2015) Comparative assessment of phenolic sontent and in vitro antioxidant capacity in the pulp and peel of mango cultivars. Int J Mol Sci 16(6):13507–13527. https://doi.org/10.1016/j.fluid.2007.07.030
Abdalla AEM, Darwish SM, Ayad EHE, El-Hamahmy RM (2007) Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chem 103(4):1134–1140. https://doi.org/10.1016/j.foodchem.2006.10.017
Alothman M, Bhat R, Karim AA (2009) Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem 115(3):785–788. https://doi.org/10.1016/j.foodchem.2008.12.005
Barreto JC, Trevisan MTS, Hull WE, Erben G, de Brito ES, Pfundstein B, Würtele G, Spiegelhalder B, Owen RW (2008) Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mango indica L.). J Agric Food Chem 56(14):5599–5610. https://doi.org/10.1021/jf800738r
Berardini N, Carle R, Schieber A (2004) Characterization of gallotannins and benzophenone derivatives from mango (Mangifera indica L. cv. ‘Tommy Atkins’) peels, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 18(19):2208–2216. https://doi.org/10.1002/rcm.1611
Bluman AG (2013) Elementary statistics: a step by step approach. In: Correlation and regression, 6th edn. McGraw-Hill, New York, NY, USA, p 533
Da Silva LMR, De Figueiredo EAT, Ricardo NMPS, Vieira IGP, De Figueiredo RW, Brasil IM, Gomes CL (2013) Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem 143:398–404. https://doi.org/10.1016/j.foodchem.2013.08.001
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15(10):7313–7352. https://doi.org/10.3390/molecules15107313
Dorta E, Lobo MG, Gonzalez M (2012) Reutilization of mango byproducts: study of the effect of extraction solvent and temperature on their antioxidant properties. J Food Sci 71(1):80–88. https://doi.org/10.1111/j.1750-3841.2011.02477.x
Dorta E, Lobo MG, Gonzalez M (2013) Optimization of factors affecting extraction of antioxidants from mango seed. Food Bioprocess Technol 6(4):1067–1081. https://doi.org/10.1007/s11947-011-0750-0
Durling NE, Catchpole OJ, Grey JB, Webby RF, Mitchell KA, Yeap Foo L, Perry NB (2007) Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem 101(4):1417–1424. https://doi.org/10.1016/j.foodchem.2006.03.050
Engels C, Gänzle MG, Schieber A (2012) Fast LC-MS analysis of gallotannins from mango (Mangifera indica L.) kernels and effects of methanolysis on their antibacterial activity and iron binding capacity. Food Res Int 45(1):422–426. https://doi.org/10.1016/j.foodres.2011.11.008
Galanakis CM, Goulas V, Tsanoka S, Manganaris GA, Gekas V (2013) A knowledge base for the recovery of natural phenols with different solvents. Int J Food Prop 16(2):328–396. https://doi.org/10.1080/10942912.2010.522750
Galili S, Hovav R (2014) Chapter 16—determination of polyphenols, flavonoids and antioxidant capacity in dry seeds. Polyphen Plants. https://doi.org/10.1016/b978-0-12-397934-6.00016-4
Imeh U, Khokhar S (2002) Distribution of conjugated and free phenols in fruits: antioxidant activity and cultivar variations. J Agric Food Chem 50(22):6301–6306. https://doi.org/10.1021/jf020342j
Larrauri JA, Rupérez B, Borroto B, Saura-Calixto F (1996) Mango peels as a new tropical fibre: preparation and characterization. Lebensm-Wiss u-Technol 29(8):729–733. https://doi.org/10.1006/fstl.1996.0113
Liu X, Cui C, Zhao M, Wang J, Luo W, Yang B, Jiang Y (2008) Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem 109(4):909–915. https://doi.org/10.1016/j.foodchem.2008.01.071
Maisuthisakul P, Gordon MH (2009) Antioxidant and tyrosinase inhibitory activity of mango seed kernel by product. Food Chem 117(2):332–341. https://doi.org/10.1016/j.foodchem.2009.04.010
Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, Núñez MJ, Parajó JC (2001) Natural antioxidants from residual sources. Food Chem 72(2):145–171. https://doi.org/10.1016/S0308-8146(00)00223-5
Mukhopadhyay S, Luthria DL, Robbins RJ (2006) Optimization of extraction process for phenolic acids from black cohosh (Cimicifuga racemose) by pressurized liquid extraction. J Sci Food Agric 86(1):156–162. https://doi.org/10.1002/jsfa.2326
Parmar N, Singh N, Kaur A, Thakur S (2017) Comparison of color, anti-nutritional factors, minerals, phenolic profile and protein digestibility between hard-to-cook and easy-to-cook grains from different kidney bean (Phaseolus vulgaris) accessions. J Food Sci Technol 54(4):1023–1034. https://doi.org/10.1007/s13197-017-2538-3
Ribeiro SMR, Barbosa LCA, Queiroz JH, Knödler M, Schieber A (2008) Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem 110(3):620–626. https://doi.org/10.1016/j.foodchem.2008.02.067
Robards K (2003) Strategies for the determination of bioactive phenols in plants and vegetables. J Chromatogr A 1000:657–691. https://doi.org/10.1016/S0021-9673(03)00058-X
Robbins R (2003) Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem 51:2866–2887. https://doi.org/10.1021/jf026182t
Schieber A, Stintzing FC, Carle R (2001) By-products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci Technol 12:401–413. https://doi.org/10.1016/S0924-2244(02)00012-2
Singh B, Sing N, Thakur S, Kaur A (2016) Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J Food Sci Technol 54(4):921–932. https://doi.org/10.1007/s13197-016-2356-z
Sogi DS, Siddiq M, Greiby I, Dolan KD (2013) Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem 141(3):2649–2655. https://doi.org/10.1016/j.foodchem.2013.05.053
Soong YY, Barlow PJ (2004) Antioxidant activity and phenolic content of selected fruit seeds. Food Chem 88(3):411–417. https://doi.org/10.1016/j.foodchem.2004.02.003
Spigno G, Tramelli L, De Faveri DM (2007) Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng 81(1):200–208. https://doi.org/10.1016/j.jfoodeng.2006.10.021
Sultana B, Hussain Z, Asif M, Munir A (2012) Investigation on the antioxidant activity of leaves, peel, stems bark, and kernel of mango (Mangifera indica L.). J Food Sci 77(8):849–852. https://doi.org/10.1111/j.1750-3841.2012.02807.x
Sun T, Ho CT (2005) Antioxidant activities of buckwheat extracts. Food Chem 90(4):743–749. https://doi.org/10.1016/j.foodchem.2004.04.035
Thoo YY, Ho SK, Liang JY, Ho CW, Tan CP (2010) Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem 120(1):290–295. https://doi.org/10.1016/j.foodchem.2009.09.064
Turkmen N, Sari F, Velioglu YS (2006) Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem 99(4):835–841. https://doi.org/10.1016/j.foodchem.2005.08.034
Wang J, Sun B, Cao Y, Tian Y, Li X (2008) Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem 106(2):804–810. https://doi.org/10.1016/j.foodchem.2007.06.062
Xu BJ, Chang SKC (2007) A Comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci 72(2):159–166. https://doi.org/10.1111/j.1750-3841.2006.00261.x
Zi J, Peng B, Yan W (2007) Solubilities of rutin in eight solvents at T = 283.15, 298.15, 313.15, 323.15, and 333.15 K. Fluid Phase Equilib 261:111–114. https://doi.org/10.1016/j.fluid.2007.07.030
Acknowledgements
K. J. A. Lim would like to thank USAID-STRIDE for awarding the Ph.D. dissertation scholarship grant that supported the completion of this work. Likewise, the Philippine Engineering Research and Development for Technology (ERDT) Program is acknowledged for the doctoral scholarship grant of K. J. A. Lim. Special gratitude is extended to Dr. James Faulk for the assistance in HPLC–UV/Vis Analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, K.J.A., Cabajar, A.A., Lobarbio, C.F.Y. et al. Extraction of bioactive compounds from mango (Mangifera indica L. var. Carabao) seed kernel with ethanol–water binary solvent systems. J Food Sci Technol 56, 2536–2544 (2019). https://doi.org/10.1007/s13197-019-03732-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03732-7