Abstract
Iran is among the main exporters of pistachio (Pistacia vera L.). Thus, evaluation of phytochemical properties of this product is of crucial importance. In this study, effects of three types of solvents namely, polar protic solvents (i.e. ultrapure water, methanol and ethanol), polar aprotic solvents (i.e. acetone and ethyl acetate) and a non-polar solvent (i.e. hexane), on total phenolic compounds, total flavonoids, and total proanthocyanidins extractability as well as antioxidant activity (as evaluated by 2,2-diphenyl-1-picrylhydrazyl and ferric reducing antioxidant power assay) of P. vera var. Sarakhs hull and kernel, were investigated. High extraction yields were observed following utilization of less polar solvents (43.14 and 34.19% for hull and kernel, respectively). There were significant differences among solvents in terms of the amount of antioxidant compounds extracted. Ethanolic hull and kernel extract showed the maximum amounts of total phenols (113.21 and 169.53 mg of gallic acid equivalent per g of dried plant, respectively), total flavonoids (87.03 and 139.47 mg of quercetin equivalent per g of dried plant, respectively) and total proanthocyanidins (110.60 and 150.32 mg of catechin equivalents per g of dried plant, respectively) followed by methanol > ultrapure water > ethyl acetate > hexane. Assessment of antioxidant activity showed that the ethanol extract of hull and kernel had the least IC50 (3.04 and 6.8 µg/ml, respectively) and the greatest value when assessed by FRAP (8.80 and 5.59 mmol/g, respectively). The results suggested that P. vera var. Sarakhs hull and kernel extract can be regarded as a promising alternative for synthetic antioxidants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iran is considered as one of the main pistachio (Pistacia vera L.) producing countries [1]. Genus Pistacia approximately comprises 10 species among which, some are economically valuable (e.g. P. vera L., P. terebinthus L. commonly known as terebinth) and P. lentiscus L. (commonly known as mastic tree) [2]. Pistacia vera var. Sarakhs as a diploid deciduous tree of the order Sapindales from the family Anacardiaceae, is a wild variety of pistachio which is widely distributed in Khwaja forest of Sarakhs region, Razavi Khorasan province, Iran along Tajan river. Wind is responsible for pollination. The ripe fruits have exocarp, mesocarp (hull) and hard endocarp (shell) that surrounds the kernel. The kernel of this variety is smaller than usual commercial pistachios [3].
Turkey is the main customer of P. vera var. Sarakhs and uses it in food industry. Fruits protective effects against diseases are attributed to the presence of phenolic and other natural antioxidant compounds that reduce the burden of oxidative stress [4]. Wild pistachio kernels and hulls are rich in phenolic compounds and exert marked antioxidant activity. In this regard, the methanolic extract of the hull of Bene (Pistacia atlantica subsp. mutica) could be considered a good source of chemicals with anticancer properties [5]. The first step in formulation of phytochemicals in supplements, as well as medicinal and therapeutic products, is bioactive compounds extraction [6].
The type of solvent used for this purpose is of crucial importance as its physicochemical characteristics such as viscosity and other molecular traits e.g. being a hydrogen donor/acceptor, play key roles in the extraction process and different results might be achieved following application of different solvents [7].
Determination of the most efficient solvent for extraction of naturally occurring bioactive compounds, has always been difficult. For extraction of the phenolic compounds from the hull of P. vera var. Fandoghi, methanol and ultrapure water are more efficient as compared to ethyl acetate [8]. In 2015, Rezaie et al. observed a higher extraction yield by using less polar solvents for preparation of Bene (Pistacia atlantica subsp. mutica) hull extract [9].
Since natural phenolic compounds are able to inhibit the LDL oxidation and consequently prevent thrombus development, finding novel antioxidant compounds and/or determination of the antioxidant potential of plants extracts, is beneficial [10]. So far, although the phenolic compounds and antioxidant activities of pistachio kernels and hulls have been widely studied, all these reports focused on limited number of commercial or other types of wild pistachio like Pistacia atlantica subsp. mutica [11].
Noteworthy, to the best of our knowledge, no phytochemical study has been done on Pistacia vera var. Sarakhs. The main purpose of this investigation was to study the effects of different solvents (i.e. ultrapure water, methanol, ethanol, acetone, hexane and ethyl acetate) on the extraction of total phenolic, total flavonoid, and total proanthocyanidin compounds from P. vera var. Sarakhs kernel and hull extracts, and assess their antioxidant activity using DPPH and FRAP assays.
Materials and methods
Plant materials and chemical reagents
The ripe fruits of P. vera var. Sarakhs were collected from Khwaja forest, Sarakhs region, Razavi Khorasan province, Iran with 36°32′N and 61°10′E at 235 meters above sea level, in September 2015. The hulls of fresh fruits were separated from shells. Both kernels and hulls were air-dried at room temperature and then stored at − 18 °C. All chemicals and solvents used in the present study were of analytical grade and purchased from Merck (Darmstadt, Germany).
Preparation of extracts
Dried kernels and hulls were grounded to powder. Thirty grams of each samples were separately extracted using 300 ml of each solvent i.e. polar protic solvents (ultrapure water, 96% methanol, and 96% ethanol), polar aprotic solvents (acetone and ethyl acetate) and non-polar solvents (hexane) for 48 h at room temperature. The extracts were filtered by using Whatman No. 3. Using the same method, the extraction process was repeated for three times until the extracts became colorless. The solvents were removed by using a rotary evaporator (Buchi V-850, Switzerland) and a freezer dryer (Operon, FDB-5503, Korea); then, samples were stored at -80 °C until further analysis.
Yield of extraction
In order to calculate the yield of extraction, the following equation was used:
where We is either the weight of kernel extract or that of the hull extract (g) and Wt is the weight of sample (g).
Determination of total phenolic content (TPC)
Total phenolic content was calorimetrically determined by using the Folin–Ciocalteau regent, as previously described by Derakhshan et al. [12]. Here, equal amounts of dried extracts were diluted with their corresponding solvents (i.e. ultrapure water, methanol, ethanol, ethyl acetate, acetone or hexane); then, 100 µL of the diluted extract was mixed with 0.5 ml Folin–Ciocalteau regent. After 5 min, 400 µl 7.5% sodium bicarbonate solution was added and the mixture was left for 30 min. The absorbance of each sample was read at 765 nm against blank using UV–Visible spectrophotometer (Cecil, UK). In order to determine TPC of each extract, a standard curve was plotted for gallic acid using standard solutions (0.2–1 mg/ml) of this compound and the following formula was achieved: y = 0.0645x + 0.144 (R2 = 0.9913). Results were expressed as mg gallic acid equivalents per g of dried plant (w/w %). All experiments were done in triplicate.
Determination of total flavonoid content (TFC)
TFC was measured using a colorimetric assay reported by Huang et al. with minor changes. Briefly, 5 ml of 2% aluminium trichloride (AlCl3) was mixed with 0.4 mg/ml of the extract [13]. Absorption was read at 367 nm using a UV–Visible spectrophotometer (Cecil, UK). The absorbance of samples was measured against blanks. For each extract, TFC was expressed as mg of quercetin equivalents per g of dried plant (w/w %) using a standard curve plotted for standard solutions (0.5–2 mg/ml) of quercetin from which, the following formula was obtained: y = 0.01x + 0.0045 (R2 = 0.9921). All measurements were carried out in triplicate.
Determination of total proanthocyanidin content (TPrAC)
Equal amounts of dried extracts were diluted with their corresponding solvents (i.e. water, methanol, ethanol, ethyl acetate or acetone), and 0.5 ml of each sample was mixed with 1.5 ml 4% vanillin-methanol solution and 0.75 ml M HCl. After 15-min incubation at room temperature, the absorbance was read at 500 nm against blank by a UV–Visible spectrophotometer (Cecil, UK). Proanthocyanidin content was expressed as mg of catechin equivalents per g of dried plant (w/w %) based on a standard curve plotted for different concentrations (0.125–2 mg/ml) of catechin using the following formula: y = 0.0942x + 0.1064 (R2 = 0.9906). All experiments were performed in triplicate.
Determination of antioxidant activity
Several methods are utilized for evaluation of antioxidant potential [14]. In this experiment, in order to measure the antioxidant activity of each extract, two assays were used; the FRAP assay was carried out according to the method reported by Razli et al. and the radical scavenging ability (DPPH) assay was performed based on the study done by Fernández–Agulló et al. [15, 16]. In the FRAP assay, 300 mM acetate buffer, 10 mM TPTZ in 40 mM HCl and 20 mM FeCl3.6H2O (10:1:1 v:v:v) were used. This is a colorimetric assay based on the reduction of a ferric-tripyridyltriazine (Fe (III)-TPTZ) complex to ferrous (Fe II) subsequently forming a complex with intense blue color. The fresh solution was warmed at 37 °C for 5 min. Each dry extract was separately added to FRAP reagent, and incubated at room temperature for 0 and 4 min until the reaction was started; then, the absorbance of each time point was read against a blank by a UV–Visible spectrophotometer (Cecil, UK). The differences between the absorbance of the two time points were calculated and compared with that of ferrous sulfate as the standard. In DPPH assay, the color of the complex varied from dark violet to colorless. All of the solvents used for extraction were dissolved in methanol, except for hexane dissolved in dichloromethane. Afterwards, each extract was mixed with the DPPH solution. The mixture was incubated at room temperature for 1 h while being shaken. Absorbance was read at 517 nm by a UV–Visible spectrophotometer (Cecil, UK). This test was carried out in triplicate and antioxidant activity was calculated as follows:
where A0 is the absorbance of the blank and A1 is the absorbance of each sample. A curve of percentage of inhibition was plotted against samples concentrations; then, the concentration of the sample required for 50% inhibition (IC50) was determined. The assay was carried out in triplicate. Butylated hydroxytoluene (BHT), was used as a standard antioxidant.
Statistical analysis
Data were analyzed as a complete randomized design (CRD) with three replications and reported as mean ± S.D. Analysis of variance was performed by JMP 8 (SAS Campus Drive, Cary, NC 27513) and Excel software was used as appropriate. Differences among mean values were examined by using LSD and considered significant if p < 0.05.
Results and discussion
Solvents properties
Selection of a suitable solvent for extraction, depends on the type of plant, compounds that are planned to be isolated, physiochemical properties of the solvents, method of extraction and other assay conditions. Therefore, in this study, various solvents with different levels of polarity including non-polar (with low dielectric constants which are not water-miscible), polar aprotic (polar but not a hydrogen bond donor) and polar protic (polar and also a hydrogen bond donor), were used. In this regard, hexane (as a non-polar solvent), acetone and ethyl acetate (as a polar aprotic solvents which have carbonyl groups) and water, ethanol, and methanol (as a polar protic solvents which have hydroxyl groups) were used [17].
The physiochemical properties of selected solvents such as polarity index (P’), proton acceptor parameter (Xe), proton donor parameter (Xd), strong dipole parameter (Xn), viscosity at 20° (η), vapor pressure at 20° mmHg (Pv), are shown in Table 1.
Extraction yield
There were significant differences in extraction yield among different solvents used for extraction of pistachios kernel and hull (hexane > ethyl acetate > acetone > water > methanol > ethanol). As shown in Tables 2 and 3, extraction yields ranged from 14.02 to 34.19% for kernel and from 16.22 to 43.14% for hull. There was no direct correlation between the polarity and extraction yield; though methanol and acetone have the same polarity index (i.e. 5.1), the extraction yield achieved by acetone was higher for both kernel and hull as compared to that of methanol. This finding might be attributed to other physiochemical properties of the solvents such as higher selectivity and lower viscosity. Similarly, ethyl acetate, despite having an almost equal polarity index, had a higher extraction yield compared to ethanol which might be due to its more marked selectivity and less viscosity resulting in low ability to extracted lipid compound. Generally, selection of a solvent for extraction is based on the chemical structures of the existing compounds. For instance, it was reported that aqueous solutions of ethanol, methanol and acetone were the most efficient solvents for extraction of phenolic compounds from Vitis rotundifolia seeds [18]. Also, the effect of solvents’ polarity on extraction yield was demonstrated by Fernández–Agulló [16].
Our results were in agreement with those reported by Rezaie et al., which showed that as the polarity increases from ethanol to water, the extraction yield enhances [9].
Total phenolic content
Phenolic compounds can scavenge free radicals and combat overproduction of reactive oxygen species (ROS), in our body. The restoration of oxidant/antioxidant balance leads to prevention from atherosclerosis, coronary heart diseases and cancers [19].
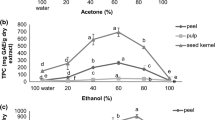
The type of solvent plays a crucial role in extraction of natural phenolic compounds. Results showed that utilization of ethanol as extraction solvent resulted in extraction of higher total phenolic contents (TPC) from kernel and hull (Tables 2, 3). There were significant differences in TPC among different solvents used for hull and kernel extraction. High amounts of phenolic compounds in kernel and hull were found in ethanol extracts (113.21 and 169.53 mg of gallic acid equivalents per g of dried plant, respectively). Methanol, water, acetone, ethyl acetate and hexane extracts, respectively followed the ethanolic extract with respect to the TPC. Among the polar protic solvents, ethanol with a low polarity index (i.e. 4.3) was the most potent solvent in extracting the total phenols. Methanol (polarity index: 5.1) had similar results to that of ethanol in terms of phenols extraction followed by water (polarity index: 10.2). So, in our study, the efficiency of different solvents in extraction, varied based on their polarity. These results were in accordance with those reported by Pinelo et al., concerning preparation of almond hull extract [20]. In another experiment conducted on peanut skin, compared to a combination of water and ethanol, ethanol and methanol alone were more efficient in terms of phenolic compounds extraction with ethanol presenting the highest efficiency [21].
TFC
Flavonoids including chalcones, flavones, flavonols, flavandiols, anthocyanins and condensed tannins (or proanthocyanidins), can inhibit metal-induced lipid oxidation through scavenging free radicals [22]. Results showed that total flavonoid levels significantly vary among different extracts. Based on our results, the highest values for TFC in kernel and hull extracts were obtained following extraction using ethanol (87.03 and 139.47 mg of quercetin equivalents per g of dried plant, respectively). Despite having similar polarities (i.e. 5.1), methanol and acetone yielded different results; this might be attributed to different levels of selectivity, viscosity and vapor pressure. In terms of TFC, our results were similar to those reported by Wang and Helliwell who showed that the efficiency of aqueous ethanol for extraction of the flavonoids compounds from tea, was more pronounced than that of aqueous methanol and acetone [23]. Furthermore, Yu et al. indicated that ethanol is the most efficient solvent for extraction of antioxidant compounds from peanut skin [21].
TPrAC
Condensed tannins (proanthocyanidins) are known as antioxidant compounds which have antiradical activity [24]. Besides, these compounds have antiviral, antimicrobial, anti-HIV, antioxidant, anti-complementary and anti-tumor properties, as well as cardiotonic and anti-arteriosclerotic activities [25]. As shown in Tables 2 and 3, following utilization of different solvents, the TPrAC of kernel and hull extracted by different solvents was in the following order: ethanol > methanol > water > acetone > ethyl acetate > hexane. Statistical analysis showed significant differences in TPrAC among solvents used. The highest levels of TPrAC were obtained following extraction by polar protic solvents indicating a positive relationship between solvent polarity and TPrAC. The ethanol extracts of kernel and hull had the highest TPrAC (110.60 and 150.32 mg of catechin equivalents per g of dried plant, respectively). The selectivity of ethanol for extraction of this group of phenolic compounds may be related to carbon–oxygen and oxygen–hydrogen bonds, as oxygen atoms in alcohols are more available compared to water molecules.
Antioxidant activity
As a synthetic antioxidant, usage of BHT has been restricted due to its probable toxic effects. Therefore, plants materials that exert high levels of antioxidant activity can protect the body from free radicals and many diseases caused by lipid peroxidation, and they could be considered safer substitutes for synthetic ones. Numerous antioxidant assays have been used for evaluation of plants antioxidant capacity [26]. Among various assays used for evaluation of the antioxidant properties, FRAP and DPPH were chosen in this study.
Ferric reducing antioxidant power (FRAP) assay
In the presence of an antioxidant, FRAP solution reduces Fe3+-TPTZ (2, 4, 6-tris (2-pyridyl)-5-triazine) complex to Fe2+–TPTZ at low pH. The absorbance of the mixture was measured spectrophotometrically at 595 nm and BHT was used as the standard. Tables 2 and 3 show that the absorbance was directly associated with the reducing power. Ethanol was the most potent solvent in terms of antioxidant compounds extraction from kernel and hull (5.99 and 8.80 mmol/g, respectively). As mentioned earlier, extraction of secondary metabolites was mainly affected by the polarity, vapor pressure, viscosity, and proton acceptor/donor parameters. Overall, different solvents used for extraction of pistachio kernel and hull, had significantly different electron donating capacities. This probably led to higher efficiencies of polar protic solvents like methanol and ethanol.
DPPH radical scavenging assay
Antioxidant compounds eliminate DPPH radicals through their hydrogen-donating ability or radical scavenging activity [27]. In this assay, compounds with antioxidant properties can reduce the DPPH radical to the yellow-colored diphenyl-picryl hydrazine [28]. Results of DPPH assay were expressed as IC50 and percentage of inhibition. Lower IC50 values indicate higher antioxidant activities. In the present study, the results of DPPH assay were close to those of FRAP assay (Tables 2, 3). In comparison to BHT, the IC50 values of kernel and hull ethanol extracts (6.8 and 3.04 µg/ml, respectively) were less than those of the other extracts; so, ethanol extract had the highest percentage of DPPH inhibition. Due to existence of an ortho-dihydroxy structure in the B ring of phenolic compounds, which make them effective hydrogen donors, there is a positive correlation between TPC and the level of antioxidant activity [29,30,31]. It seems that, this positive correlation between antioxidant activity and phenolic content, is related to the presence of -OH moieties which are potent H donors. Another characteristic of phenolic compounds is the planarity of the molecule, which allows electron conjugation and delocalization [32]. Also, from a toxicological view, polyphenolic compounds are considered safe for human use [33].
Conclusion
Natural antioxidants can protect humans against overproduction of ROS and ROS-induced diseases. In this study, antioxidant properties of Pistacia vera var. Sarakhs as a wild variety of pistachio, were evaluated. Determination of TPC, TFC, TPrAC, FRAP and DPPH values showed that the hull and kernel of this pistachio variety could be considered an antioxidant-rich waste product. Moreover, among the tested solvents, ethanol was the most efficient solvent with respect to extraction of polyphenolic compounds. In conclusion, we found that the extracts of hull and kernel of this pistachio variety are good sources of antioxidant compounds particularly if ethanol is used as the extraction solvent.
Abbreviations
- DPPH:
-
1,1-Diphenyl-2-picryl-hydrazil
- BHT:
-
Butylhydroxytoluene
- FRAP:
-
Ferric reducing antioxidant power assay
- TPC:
-
Total phenolic content
- TFC:
-
Total flavonoid content
- TPrAC:
-
Total proanthocyanidin content
- HCl:
-
Hydrogen chloride
- TPTZ:
-
2, 4, 6-Tris (2-pyridyl)-5-triazine
- P′:
-
Polarity index
- X e :
-
Proton acceptor parameter
- X d :
-
Proton donor parameter
- X n :
-
Strong dipole parameter
- η :
-
Viscosity at 20
- P v :
-
Vapor pressure at 20° mm Hg
References
S.F. Taghizadeh, G. Davarynejad, J. Asili, S.H. Nemati, R. Rezaee, M. Goumenou, A.M. Tsatsakis, G. Karimi, Food Chem. Toxicol. 107, 99–107 (2017)
M. Adams, I. Plitzko, M. Kaiser, R. Brun, M. Hamburger, Phytochem. Lett. 2, 159–162 (2009)
A. Esmail-Pour, Report of the IPGRI Workshop (1998) pp. 16–26
J. Moskaug, H. Carlsen, M.C. Myhrstad, R. Blomhoff, Am. J. Clin. Nutr. 81, 277S–283S (2005)
P.F. Rezaei, S. Fouladdel, S.M. Ghaffari, G. Amin, E. Azizi, DARU J. Pharm. Sci. 20, 101–106 (2012)
J. Dai, R.J. Mumper, Molecules 15, 7313–7352 (2010)
C.H. Gu, H. Li, R.B. Gandhi, K. Raghavan, Int. J. Pharm. 283, 117–125 (2004)
A.H. Goli, M. Barzegar, M.A. Sahari, Food Chem. 92, 521–525 (2005)
M. Rezaie, R. Farhoosh, M. Iranshahi, A. Sharif, S. Golmohamadzadeh, Food Chem. 173, 577–583 (2015)
S.F. Taghizadeh, G. Davarynejad, J. Asili, S.H. Nemati, G. Karimi, Avicenna J. Phytomed. 8, 33–42 (2017)
G. Ballistreri, E. Arena, B. Fallico, Molecules 14, 4358–4369 (2009)
Z. Derakhshan, M. Ferrante, M. Tadi, F. Ansari, A. Heydari, M.S. Hosseini, G.O. Conti, E.K. Sadrabad, Food Chem. Toxicol. 114, 108–111 (2018)
D.J. Huang, L. Chun-Der, C. Hsien-Jung, L. Yaw-Huei, Bot. Bull. Acad. Sin. 45, 179–186 (2004)
A. Shakeri, J. Akhtari, V. Soheili, S.F. Taghizadeh, A. Sahebkar, R. Shaddel, J. Asili, Microb. Pathog. 109, 39–44 (2017)
N. Razali, S. Mat-Junit, A.F. Abdul-Muthalib, S. Subramaniam, A. Abdul-Aziz, Food Chem. 131, 441–448 (2012)
A. Fernández-Agulló, E. Pereira, M. Freire, P. Valentao, P. Andrade, J. González-Álvarez, J. Pereira, Ind. Crops Prod. 42, 126–132 (2013)
C. Reichardt, T. Welton, Solvents and solvent effects in organic chemistry, 4th edn. (Wiley-VCH, Weinheim, 2010)
Y. Yilmaz, R.T. Toledo, J. Food Compos. Anal. 19, 41–48 (2006)
Y. Yilmaz, R.T. Toledo, Trends Food Sci. Technol. 15, 422–433 (2004)
M. Pinelo, M. Rubilar, J. Sineiro, M. Nunez, Food Chem. 85, 267–273 (2004)
J. Yu, M. Ahmedna, I. Goktepe, Food Chem. 90, 199–206 (2005)
N.V. Yanishlieva-Maslarova, Inhibiting oxidation in Antioxidants in Food. Practical Applications ed. By J. Pokorny, N. Yanishlieva, M. Gordon (Woodhead Publishing Ltd., Cambridge, 2001), pp. 22–70
H. Wang, K. Helliwell, Food Res. Int. 34, 223–227 (2001)
R. Amarowicz, M. Piskuła, J. Honke, B. Rudnicka, A. Troszynska, H. Kozłowska, Pol. J. Food Nutr. Sci. 4, 53–62 (1995)
W. Huemmer, P. Schreier, Mol. Nutr. Food Res. 52, 1381–1398 (2008)
P. Michel, A. Owczarek, M. Kosno, D. Gontarek, M. Matczak, M.A. Olszewska, Phytochem. Lett. 20, 356–364 (2017)
F. Oke, B. Aslim, S. Ozturk, S. Altundag, Food Chem. 112, 874–879 (2009)
A. Benmerache, A.A. Magid, D. Berrehal, A. Kabouche, L. Voutquenne-Nazabadioko, S. Messaili, A. Abedini, D. Harakat, Z. Kabouche, Phytochem. Lett. 18, 23–28 (2016)
F. Alén-Ruiz, M. García-Falcón, M. Pérez-Lamela, E. Martínez-Carballo, J. Simal-Gándara, Food Chem. 113, 53–60 (2009)
D. Di Majo, M. La Guardia, S. Giammanco, L. La Neve, M. Giammanco, Food Chem. 111, 45–49 (2008)
E.F. Gris, F. Mattivi, E.A. Ferreira, U. Vrhovsek, D.W. Filho, R.C. Pedrosa, M.T. Bordignon-Luiz, J. Agric. Food Chem. 59, 7954–7961 (2011)
A.N. Messi, J.N. Mbing, J.T. Ndongo, M.A. Nyegue, A.T. Tchinda, F.L. Yemeda, M. Feredrich, D.E. Pegnyemb, Phytochem. Lett. 17, 119–125 (2016)
J. Shi, H. Nawaz, J. Pohorly, G. Mittal, Y. Kakuda, Y. Jiang, Food Rev. Int. 21, 139–166 (2005)
Acknowledgements
The authors are grateful to Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Seyedeh Faezeh Taghizadeh and Ramin Rezaee have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Taghizadeh, S.F., Rezaee, R., Davarynejad, G. et al. Phenolic profile and antioxidant activity of Pistacia vera var. Sarakhs hull and kernel extracts: the influence of different solvents. Food Measure 12, 2138–2144 (2018). https://doi.org/10.1007/s11694-018-9829-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9829-x