Abstract
This study reports for the first time the total extractive matter yield, total phenolics and total flavonoids content (TPC and TFC, respectively), chemical composition, and ability to scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals of the black mustard seed (BMS) extracts obtained by deep eutectic solvents (DESs). The following DESs: triethanolamine:glycerol (TEOA:G), triethanolamine:propylene glycol (TEOA:PEG), and choline chloride:urea (ChCl:U), as well as their mixtures with water and ethanol at a ratio of 1:4 (v/v), were used. Their extraction efficiencies were compared with the efficiency of ethanol that is most frequently used to extract phenolic compounds from BMS. The TPC and TFC of the obtained extracts were in the ranges of 19.9 ± 0.1 to 32.2 ± 0.2 mg gallic acid equivalents/g and 3.9 ± 0.1 to 7.4 ± 0.3 mg quercetin equivalents/g, respectively. The BMS extracts obtained with the pure ChCl:U and the mixtures of ChCl:U or TEOA:G with ethanol showed the best antioxidant activity (IC50 = 100 mg/mL). The obtained extracts contained syringic acid, ellagic acid, gallic acid, caffeic acid, caftaric acid, rutin, kaempferol, apigenin, taxifolin, hyperoside, and vitexin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few years, the extraction and identification of phenolics from various plant materials have become an interesting research area due to the health-promoting effects of these compounds [1]. Plant phenolics are characterized by a wide range of beneficial properties such as anti-inflammatory, antimicrobial, antioxidative, anticancer, and so on [2, 3]. Gallic acid is a phenolic compound showing multiple pharmacological activities, such as antibacterial, antimicrobial, antifungal [4], and antidiabetic [5] properties. Caftaric and caffeic acids are also types of phenolic acids that are capable of lowering hyperlipidemia and preventing atherosclerosis [6]. He et al. [7] have demonstrated the pharmacological effect of apigenin, a flavonoid, in the treatment of pulmonary hypertension. Another flavonoid, rutin, on the other hand, has been extensively studied as an anticancer agent [8,9,10]. Also, it has a wide range of biological activities like antifungal activity against the strain of Candida gattii [11].
Black mustard (Brassica nigra L.), botanically included in the Brassicaceae family, is an annual, aromatic weedy plant cultivated worldwide. Black mustard seed (BMS) is used as a spice, rich in both nutritional and pharmacologically active compounds. It contains valuable oil, glucosinolates, and phenolic compounds [12, 13]. Several studies have reported the composition of BMS regarding phenolic compounds [14,15,16,17].
One of the essential parameters of the extraction of phenolic compounds from plants is the selection of the appropriate extraction solvent. Traditional organic solvents used for this extraction are methanol, ethanol, acetone, and ethyl acetate. However, this method has several drawbacks, such as long duration, high energy consumption, and high solvent consumption, as well as negative impact on humans and the environment. Therefore, a growing interest of the researchers worldwide is focused on designing new and more environmentally-friendly extracting solvents [18,19,20]. Over the last few years, deep eutectic solvents (DESs), prepared from cheap and easily available components, have been tested as promising alternatives to traditional organic solvents. Besides simple preparation, they show several advantages in terms of liquid state at room or lower temperatures, non-volatility, non-flammability, non-toxicity, thermal stability, tunable physical properties, and high biodegradability [21,22,23]. The high thermal stability of DESs enables their use in various processes that require a temperature above 100 °C [24]. DESs have been proven to be very successful extraction agents for the biocomponents found in different plant materials [20].
Many phenolic compounds show better stability and solubility in DESs than in water or lipids [20]. The choline chloride (ChCl)-based DESs are effective media for the extraction of phenolic compounds from different plant materials [14,15,16, 21, 25,26,27,28,29,30,31]. In addition to its excellent dissolution of quercetin [32], the ChCl:urea (ChCl:U, 2:1 mol ratio) DES is recommended as a solvent for extracting vitexine from the flowers of Trollius chinensis Bunge [33], rutine from Lycium barbarum L. fruits, as well as different flavonoids from Cyclocarya paliurus [34]. The ChCl:propylene glycol (ChCl:PEG, 1:1 mol ratio) DES provided the chickpea sprouts extract with the highest total flavonoids content (TFC) and the best antioxidant activity, compared to the other twenty ChCl-based DESs [35]. The application of the triethanolamine (TEOA)-based DESs in the extraction of phenolic compounds from plant materials has not been studied yet. In addition, to the best authors’ knowledge, the use of DESs for the extraction of phenolic compounds from BMS has not been reported elsewhere. Therefore, the aim of the present study was to study the effect of different DESs like TEOA:glycerol (G), TEOA:PEG, and ChCl:U and their water and ethanolic mixtures on the total extractive matter (TEM) yield, total phenolics content (TPC), TFC, chemical composition, and antioxidant activity of the BMS extracts. The main goal was to compare the extraction abilities of the tested DESs and ethanol towards BMS phenolic compounds.
Materials and methods
Chemicals
G and PEG (both Ph Eur grade) were from MeiLab (Belgrade, Serbia) while ethanol (96%), TEOA (99%), U (99.5%), sodium carbonate anhydrous (99%, Na2CO3), and aluminum chloride anhydrous (99%, AlCl3) were purchased from Zorka-Pharma (Šabac, Serbia). Folin-Ciocalteu reagent (2 M), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ChCl, syringic acid, ellagic acid, gallic acid, caffeic acid, caftaric acid, rutin, kaempferol, apigenin, taxifolin, hyperoside, and vitexin were provided from Sigma Aldrich (Germany). Formic acid was provided by (Carlo Erba, France) while acetonitrile and water were from Fisher Chemical (LC–MS and HPLC grade, respectively).
Preparation of DESs
The DESs were prepared by mixing the selected components (Table 1) in a round-bottomed flask and stored in closed bottles in a desiccator, as reported elsewhere [32].
Plant material
The BMS was produced by crossing the local species by the pedigree breeding method at the Institute of Field and Vegetable Crops (Novi Sad, Serbia). It was stored in paper bags in a dark room. The moisture content of the BMS was 4.2 ± 0.2 wt% (determined by drying the seed at 105 °C to constant weight). The BMS was ground in an electric mill (Iskra, Slovenia) for 1 min (the average particle diameter was 0.31 mm) [36].
Extraction
Ethanol and different DESs like TEOA:G, TEOA:PEG, and ChCl:U, as well as the mixtures of these DESs with water and ethanol, at a ratio of 1:4 (v/v) were used for the extraction of phenolics from ground BMS. The extraction was performed at the seed-to-solvent ratio of 1:10 w/v and 65 °C within 3 h, employing a magnetic stirrer (1000 rpm). The extract obtained with ethanol was filtered under a weak vacuum and dried at 40 °C to constant weight under vacuum using a rotary vacuum evaporator (Hei-VAP, Heidolph, Germany). The obtained extracts were centrifuged (centrifuge Sigma, Typ 2-6E, Germany) at 3900 rpm for 10 min to separate the liquid from the residual solid phase, filtered through a 0.45 µm nylon membrane, and stored in a refrigerator at 4 °C. The TEM yield was expressed as the amount of TEM obtained from 100 g of seed (g/100 g). The extractions were done in triplicate. The TEM recovery was determined based on the highest TEM yield obtained by the TEOA:PEG mixture with water. It was calculated as follows [36]:
where R is the TEM recovery (%), A1 is the TEM yield achieved by the solvent extraction (g/100 g BMS), and A is the best TEM yield obtained by the TEOA:PEG mixture with water (g/100 g BMS).
Total phenolics content
The TPC of the obtained extracts was determined according to the modified Folin-Ciocalteu method [37]. Distilled water (5.4 mL) and the Folin-Ciocalteu reagent (0.5 mL) was added to 0.1 mL of the extract (0.005 mg/mL). After 3 min, 1 mL of 7% Na2CO3 was added to the sample solution. The test tube was kept in dark for 1 h at room temperature. The absorbance was measured at 725 nm against a blank (distilled water) by using a UV–VIS spectrophotometer (JENWAY 6305, UK). The standard curve range was 0.05–0.0005 mg/mL gallic acid (the coefficient of determination R2 = 0.982). The results were expressed as mg of gallic acid equivalents (GAE) per g of seed (mg GAE/g).
Total flavonoids content
The TFC of the obtained extracts was determined spectrophotometrically according to Quettier-Deleu et al. [38]. As recommended, 1.5 mL of 2% AlCl3 was added to 1.5 mL of the extract (0.01 mg/mL) and mixed. The absorbance was measured at 415 nm after 1 h against a blank (distilled water) by a UV–Vis spectrophotometer (JENWAY 6305, UK). The standard curve range was 0.01–0.0005 mg/mL quercetin (R2 = 0.999). The results were expressed as mg of quercetin equivalents (QE) per g of seed (mg QE/g).
DPPH test
The percentage of antioxidant activity of the extracts obtained from BMS was determined by the DPPH free radical assay [39]. The ethanolic solution of DPPH radicals (1 mL, 3 × 10−4 mol/L) was added to the ethanol solutions of the extracts (2.5 mL) of various concentrations (1–0.0075 mg/mL). This mixture was incubated at room temperature for 20 min and the absorbance was measured at 517 nm against a blank (ethanol) using a UV/VIS spectrophotometer (JENWAY 6305, UK). The absorbance was also measured for the control that was prepared with 2.5 mL of ethanol and 1 mL of the DPPH solution. The scavenging activity of the free radicals percentage (AA%) was calculated according to the following equation:
where AU is the absorbance of the ethanol solution of the extract treated with the DPPH radical, AB is the absorbance of the ethanol solution of the extract not treated with the DPPH radical and AC is the absorbance of the “control”.
The concentration of the test material that inhibited 50% of the free radicals (IC50 value) was calculated as mg/mL. All tests were run in triplicate. The antioxidant activity is expressed as IC50, which represents the concentration of the extract necessary to neutralize 50% of the initial DPPH radicals’ concentration. The lower the IC50 value, the better the antioxidant activity.
HPLC analysis
An Agilent Technologies 1100 Series chromatograph equipped with a degasser, a binary pump, a thermostated column (Zorbax Eclipse XDB-C18, 4.6 × 150 mm, 5 μm), and a UV/VIS detector was used for the quantification of the phenolic compounds. A gradient profile using two solvents was applied at 25 °C. The solvents used were as follows: solvent A: 0.1% formic acid in water and solvent B: acetonitrile. A flow rate of 0.5 mL/min was applied. The gradient was as follows: 0 min—20% B; 45 min—90% B (the modified method described elsewhere [20]). The wavelengths of the diode array detector were set at 254, 280, 320, and 350 nm for monitoring of the phenolic compounds. The identification of constituents was performed by comparing their UV spectra and retention times with those of standards. The concentrations of all the compounds in the extracts were quantified using the standard curves and expressed as mg per g dry extract (mg/g).
Statistical analysis
The TEM yield, TPC, TFC, and IC50 values of the BMS extracts obtained with different solvents were evaluated by Duncan’s multiple range test. The multivariate statistical analysis of the data was performed by the hierarchical cluster analysis method using Statistica version 10.0, the analysis of variance among the clusters was carried out using Ward’s method, and Euclidean distance was selected as the hierarchical cluster analysis measurement.
Results and discussion
TEM yields, TPC, and TFC
The values of TEM yield, TPC, and TFC obtained with different solvents are given in Table 2. According to Duncan’s multiple range test, there are no statistically significant differences among the TEM yields obtained by the mixtures of the TEOA:G DES with water or ethanol and the pure TEOA:PEG DES or its combinations with either water or ethanol at the 95% confidence level. These solvents provided the recovered TEM yields in the range of 17.3–18.4% with the highest value of 18.4 ± 0.9% achieved using the mixture of the TEOA:PEG DES and water. Adding water to all three DESs increased the recovered TEM yield from BMS, compared to those obtained using the corresponding pure DESs. The lowest recovered TEM yields were obtained by the ChCl:U DES alone (13.3 ± 0.8%) or its mixture with ethanol (14.3 ± 0.6%).
Among the pure extracting solvents, the TEOA:PEG DES provided the highest TEM yield (17.3 ± 0.8 g/100 g BMS), followed by the TEOA:G DES and ethanol having the same extraction efficiency of TEM from BMS (about 16.0 g/100 g BMS). The differences in the TEM yields are the result of different capabilities of the DESs to dissolve TEM from BMS and might indicate the differences in their selectivity towards certain BMS components like total phenolics and flavonoids.
Duncan’s multiple range test showed statistically significant differences at the 95% confidence level for the TPC of the BMS extracts obtained with different solvents, except for the BMS extracts obtained with ethanol and the pure TEOA:PEG and ChCl:U DESs, which proved a great impact of the type of solvents on the TPC. The highest TPC was reported for the BMS extract obtained with TEOA:G and TEOA:PEG with 25% ethanol (32.2 and 29.3 mg GAE/g BMS, respectively). Adding 25% of ethanol to all three DESs increased the TPC of the BMS extracts, compared to the TPC obtained with pure DESs and ethanol. Also, adding 25% water to the TEOA:G and ChCl:U DESs increased the TPC, compared to the TPC obtained with the pure DESs. According to the literature, 20–25% of water is considered an ideal water content in DESs as it affects positively the extraction efficiency of phenolic compounds [25, 40, 41]. On the other hand, adding 25% water to the pure TEOA:PEG DES negatively affected the TPC of the BMS extract, which could be ascribed to the disintegrating and weakening of the DES’s nanostructure, reducing its capacity to extract phenolic compounds [42].
The differences in TPC can be explained by the difference in the polarity of the applied DESs and their mixtures (both aqueous and ethanolic). Moreover, adding water or ethanol to pure DESs reduces the viscosity of the extraction systems, thus improving the mass transfer between the solvent and the plant particles and consequently the TPC [43]. The TPC values of the BMS extracts obtained in the present study are smaller than those reported for the ethanolic BMS extracted with a help of ultrasound irradiation (80 mg GAE/g BMS) [17] and are close to or higher than those reported for the methanolic BMS extracts (5.16–16.1 mg GAE/g BMS) [44,45,46,47].
These differences may be due to different variety, maturity stage, growing conditions of the plant [48], or extracting conditions. According to Zheng and Wang [49], the range of TPC-value in medicinal and culinary plants is 0.23–2.85 and 0.26–17.51 mg GAE/g BMS, respectively so black mustard can be classified as a plant with a relatively high content of phenolic compounds.
Duncan’s multiple range test showed statistically significant differences at the 95% confidence level for the TFC in most of the BMS extracts, indicating that the choice of a solvent has a major impact on TFC. The BMS extracts obtained by pure DESs had a lower TFC compared to the BMS extract obtained with ethanol (Table 2). The 25% ethanol added to the DESs causes a greater extraction of phenolic compounds, unlike the 25% water addition to the DESs that has the opposite effect, i.e. reduces the extraction of phenolic compounds. This effect is the greatest in the case of the TEOA:PEG and ChCl:U DESs and the least with the TEOA:G DES. The TEOA:PEG DES with 25% ethanol gave the highest TFC value. Also, all BMS extracts obtained with the mixtures of all tested DESs with 25% water had a lower TFC compared to the extracts obtained with the corresponding pure DES or ethanol.
Duncan’s multiple range test showed no statistically significant differences at the 95% confidence level for the TFC of the BMS extract obtained with ethanol and the ethanolic mixtures of the TEOA:G and ChCl:U DESs. According to the TPC, the highest TFC was found for the BMS extract of the ethanolic mixtures of the TEOA:PEG and TEOA:G DESs (7.4 and 7.2 mg QE/g BMS, respectively). The highest TFC reported for a methanolic black mustard leaves extract was 7.75 mg QE/g [13].
Generally, regarding selectivity, the best solvents for total phenolics and flavonoids were the mixtures of all three DESs with ethanol and the ChCl:U DES that provided the highest average values of the TPC/TEM ratio (Table 2). The addition of 25% ethanol to the DESs increased their selectivity with respect to both TPC and TFC. Among these solvents, the highest average TPC/TEM ratio was achieved with the mixture of the TEOA:G DES and ethanol (18.5 ± 0.8%) whereas the mixture of the ChCl:U DES and ethanol provided the highest average TFC/TEM ratio (4.5 ± 0.2%), confirming the earlier observances [32, 33, 50]. Besides its mixtures with the DESs, ethanol, applied alone, was very selective in dissolving total flavonoids. The addition of 25% water to the DESs significantly reduced their selectivity with respect to both TPC and TFC, probably because the water polarity of those solvents causes a limited ability to dissolve some phenolic compounds [20, 51, 52]. These results suggested the use of the mixtures of TEOA:G, TEOA:PEG, or ChCl:U DESs and ethanol as the solvent for extracting total phenolics or flavonoids from BMS.
Based on the results presented in Table 2, no clear relationship between the TPC and TFC with the TEM yield is observed. This is expected since the applied solvents extract not only phenolic and flavonoid compounds but also other components present in BMS, such as proteins, terpenoids, alkaloids, etc. [20]. However, it seems that a direct relationship might exist between the TFC and the TPC as their ratio (Table 2) is around the average value of 24.3 ± 4.0%.
DPPH test
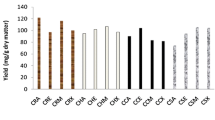
The IC50 values of the BMS extracts obtained with different solvents are shown in Fig. 1. According to Duncan’s multiple range test, there is a statistically significant difference at the 95% confidence level among the IC50 values of the BMS extract obtained with ethanol, the pure DESs, and the DESs mixtures with ethanol and water. The highest antioxidant activity was reported for the extracts obtained with the pure ChCl:U DES and with ChCl:U and TEOA:G DESs ethanolic mixtures (100 µg/mL), which was better by 33.3% than the antioxidant activity of the extract obtained with pure ethanol. The antioxidant activity of the BMS extract obtained with the TEOA:PEG DES was almost the same as the antioxidant activity of the extract obtained with pure ethanol. As expected according to the TPC of the BMS extracts, the addition of ethanol increased the antioxidant activity compared to those of the extracts obtained with the corresponding DESs while adding water to the pure DESs had an opposite effect. To the best authors’ knowledge, there are no literature data on the antioxidant activity of BMS extract, obtained by this method, except for the methanolic black mustard leaves extract (134.6 ± 6.42 µg/mL) [13].
Hierarchical cluster analysis of BMS extracts
Hierarchical cluster analysis is an algorithm that groups similar objects into groups called clusters. The endpoint is a set of clusters, where each cluster is distinct from each other cluster, and the objects within each cluster are broadly like each other. Figure 2 presents a dendrogram of 30 different solvents based on the contents of the seven selected variables, i.e. seven different extracts parameters (TEM yield, TPC, TFC, IC50, TFC/TPC, IC50/TPC, and IC50/TFC values). The clustering results illustrate that all the tested samples can be divided into two main clusters. The first cluster has five subclusters of the extracts obtained by ethanol, TEOA:PEG DES (first subcluster), TEOA:PEG DES with ethanol (second subcluster), pure TEOA:G DES, and TEOA:G DES with water (third subcluster), TEOA:PEG DES with water (fourth subcluster), and ChCl:U DES with water (fifth subcluster). In the second cluster, the extracts obtained by ChCl:U DES, ChCl:U DES with ethanol (first subcluster) and TEOA:G DES with ethanol (second subcluster) are grouped. The BMS extracts of the second cluster have the same or better antioxidant activity than the extracts of the first cluster. In addition, the extracts obtained by TEOA:PEG DES with ethanol and TEOA:G DES with ethanol are clearly distinct in their cluster because of their highest TPC and TFC. Hierarchical cluster analysis shows the same observations that coincide with the results and discussion discussed above.
Hierarchical cluster analysis dendrograms of thirty BMS extracts, obtained by different solvents (ethanol, pure DESs, and DESs mixtures with ethanol or water), based on testing TEM, TPC, TFC, IC50, TFC/TPC, IC50/TFC, and IC50/TFC values. The hierarchical clustering was done by Statistica. Ward’s method was applied, and Euclidean distance was selected as a measure
HPLC analysis
The contents of individual phenolic compounds of the BMS extracts obtained with different solvents are given in Table 3. All the BMS extracts obtained with the pure DESs or their aqueous and ethanolic mixtures contained more identified phenolic compounds than the ethanolic extract. The highest content of the identified phenolic compounds was reported in the BMS extract obtained with the ChCl:U DES with 25% ethanol, which is in accordance with its antioxidant activity.
Among the phenolic acids, gallic acid was the most abundant in the BMS extracts. The highest gallic acid content was determined in the BMS extract obtained with TEOA:G DES with 25% ethanol and with ChCl:U and ChCl:U DESs with 25% ethanol (17.53 ± 0.17 mg/g of dry extract, 11.45 ± 0.11 mg/g of dry extract and 11.45 ± 0.11 mg/g of dry extract, respectively). Rutin was the most abundant flavonoid in all BMS extracts. The highest rutin content was determined in the BMS extract obtained with the pure ChCl:U DES (4.22 ± 0.31 21 mg/g of dry extract), ChCl:U DES with 25% ethanol (4.76 ± 0.21 mg/g of dry extract) and 25% water (4.12 ± 0.24 21 mg/g of dry extract). The presence of rutin and gallic acid in BMS extracts has been previously reported in the literature [17, 46]. The values for rutin, gallic acid, and caffeic acid obtained in the present study are in the range of 1.24–4.76, 1.83–17.53, and 0.07–1.47 mg/g of dry extract, respectively, which is similar to the contents of these compounds in the methanolic black mustard leaves extract (0.36, 4.31, and 0.55 mg/g of dry extract, respectively) reported by [13]. Adding water to the TEOA:PEG DES increased the content of syringic acid, caffeic acid, caftaric acid, and apigenin in the corresponding BMS extracts, compared to their contents in the extracts obtained by pure DESs while adding ethanol reduced their contents. The content of other phenolic compounds after adding water or ethanol to this DES is reduced except at apigenin where it remained almost the same. The addition of water to the ChCl:U DES improved its extraction ability for caffeic acid, caftaric acid, apigenin, taxifolin, and vitexin. Compared to the pure ChCl:U DES, its aqueous mixture extracted twice to ninefold more these compounds. Also, the addition of ethanol to the ChCl:U DES improved its extraction ability for caffeic acid, caftaric acid, rutin, apigenin, taxifolin, and vitexin. The extraction efficiency of other phenolic compounds was almost the same with the addition of either water or ethanol to the ChCl:U DES. ChCl-based DESs have been successfully used for the extraction of catechin [16], rutin, and kaempherol [15].
Conclusions
In this study, the extraction of phenolic compounds from BMS by the pure TEOA:G, TEOA:PEG, and ChCl:U DESs, as well as their mixtures with 25% water or ethanol, was investigated and compared with the extraction efficiency of pure ethanol. To the best of the authors’ knowledge, this is the first study of using DESs in the extraction of phenolic compounds from BMS. Based on the TPC, TFC, and IC50 values of the obtained BMS extracts, the use of the tested DESs in the extraction of phenolic compounds from BMS was proved to be very successful. The most suitable solvents were the DESs with 25% ethanol as their BMS extracts had the highest TPC and TFC values (25.8 ± 0.1–32.22 ± 0.2 mg GAE/g and 6.4 ± 0.3–7.4 ± 0.3 mg QE/g, respectively) and the lowest IC50 values of 100 mg/ml. According to the HPLC analysis, these extracts are rich in phenolic compounds, with gallic acid and rutin being the most abundant. Although only three DESs have been studied, these results indicate the need to test a broader range of DESs for the extraction of phenolic compounds from BMS and to further investigate the impact of the addition of a secondary solvent (such as water and ethanol) to a pure DES.
References
M.N. Zourgui, M. Hfaiedh, D. Brahmi, W. Affi, N. Gharsallah, L. Zourgui, M. Amri, J. Food Meas. Charact. 14, 2721–2733 (2020)
I.C.F.R. Ferreira, N. Martins, L. Barros, Adv. Food Nutr. Res. 82, 1–44 (2017)
A. Septembre-Malaterre, F. Remize, P. Poucheret, Food Res. Int. 104, 86–99 (2018)
R.S. Carvalho, C.A. Carollo, J.C. de Magalhães, J.M.C. Palumbo, A.G. Boaretto, I.C. Nunes e Sá, A.C. Ferraz, W.G. Lima, J.M. de Siqueira, J.M.S. Ferreira, S. Afr. J. Bot. 114, 181–187 (2018)
B.C. Variya, A.K. Bakrania, S.S. Patel, Phytomedicine 73, 152906 (2020)
H. Harnafi, M. Ramchoun, M. Tits, J.-N. Wauters, M. Frederich, L. Angenot, M. Aziz, C. Alem, S. Amrani, Biomed. Prev. Nutr. 3, 393–397 (2013)
Y. He, X. Fang, J. Shi, X. Li, M. Xie, X. Liu, Chem. Biol. Interact. 317, 108942 (2020)
C.Y. Chen, H. Li, Y.N. Yuan, H.Q. Dai, B. Yang, BMC Complement. Altern. Med. 13, 99 (2013)
J.P. Lin, J.S. Yang, J.J. Lin, K.C. Lai, H.F. Lu, C.Y. Ma, R.S.C. Wu, K.C. Wu, F.S. Chueh, W.G. Wood, J.G. Chung, Environ. Toxicol. 27, 480–484 (2012)
G. Scambia, F.O. Ranelletti, P.B. Panici, G. Bonanno, R. De Vincenzo, M. Piantelli, S. Mancuso, Anti-Cancer Drugs 1, 45–48 (1990)
S. Johann, B.G. Mendes, F.C. Missau, M.A. Rezende, M.G. Pizzollati, Braz. J. Microbiol. 42, 1065–1075 (2011)
M. Kumar, S. Sharma, N. Vasudeva, Adv. Pharm. Bull. 3, 359–365 (2013)
R. Rajamurugan, N. Selvaganabathy, S. Kumaravel, C.H. Ramamurthy, V. Sujatha, C. Thirunavukkarasu, Nat. Prod. Res. 26, 2208–2210 (2012)
D.R. Belén-Camacho, I. López, C. Sojo, O. Linares, C. Medina-Martínez, D. García-Pantaleón, M.J. Moreno-Álvarez, Rev. Fac. Agron. 1, 628–635 (2011)
Q. Cui, J.-Z. Liu, L.-T. Wang, Y.-F. Kang, Y. Meng, J. Jiao, Y.-J. Fu, J. Clean. Prod. 184, 826–835 (2018)
N. Fu, R. Lv, Z. Guo, Y. Guo, X. You, B. Tang, D. Han, H. Yan, K. Ho Row, J. Chromatogr. A 1492, 1–11 (2017)
Y.H. Lee, C. Choo, V.Y. Waisundara, Int. J. Food Prop. 18, 2500–2507 (2015)
M.C. Bubalo, S. Vidović, I. Radojčić Redovniković, I. Jokić, Food Bioprod. Process. 109, 52–73 (2018)
C.Y. Hamany Djande, L.A. Piater, P.A. Steenkamp, N.E. Madala, I.A. Dubery, S. Afr. J. Bot. 115, 81–89 (2018)
J. Huang, X. Guo, T. Xu, L. Fan, X. Zhou, S. Wu, J. Chromatogr. A 1598, 1–19 (2019)
Y. Huang, F. Feng, J. Jiang, Y. Qiao, T. Wu, J. Voglmeir, Z.G. Chen, Food Chem. 221, 1400–1405 (2017)
M. Jablonský, A. Škulcová, A. Malvis, J. Šima, J. Biotechnol. 282, 46–66 (2018)
S. Khandelwal, Y.K. Tailor, M. Kumar, J. Mol. Liq. 215, 345–386 (2016)
H.G. Liao, Y.X. Jiang, Z.Y. Zhou, S.P. Chen, S.G. Sun, Angew. Chem. Int. Ed. 47, 9100–9103 (2008)
M.C. Bubalo, N. Ćurko, M. Tomašević, K. Kovačević Ganić, I. Radojčić Redovniković, Food Chem. 159–166 (2016)
J.B. Barbieri, C. Goltz, F.B. Cavalheiro, A.T. Toci, L. Igarashi-Mafra, M.R. Mafra, Ind. Crops Prod. 144, 112049 (2020)
S. Chanioti, C. Tzia, Innov. Food Sci. Emerg. Technol. 48, 228–239 (2018)
M-Z. Gao, Q. Cui, Q.L-T. Wang, Y. Meng, L. Yu, Y-Y. Li, Y-J. Fu, Microchem. J. 154, 104598 (2020)
M. Ivanović, M.E. Alañón, D. Arráez-Román, A. Segura-Carretero, Food Res. Int. 111, 67–76 (2018)
S. Koutsoukos, T. Tsiaka, A. Tzani, P. Zoumpoulakis, A. Detsi, J. Clean. Prod. 241, 118384 (2019)
Z. Meng, J. Zhao, H. Duan, Y. Guan, L. Zhao, J. Pharm. Biomed. Anal. 161, 246–253 (2018)
B.S. Đordević, Z.B. Todorović, D.Z. Troter, L.J.P. Stanojević, V.B. Veljković, Adv. Technol. 7, 5–10 (2018)
L. Duan, W.-H. Zhang, Z.-H. Zhang, E.-H. Liu, L. Guo, Microchem. J. 145, 180–186 (2018)
X. Shang, J.-N. Tan, Y. Du, X. Liu, Z. Zhang, Molecules 23, 2110 (2018)
X. Shang, Y. Dou, Y. Zhang, J.-N. Tan, X. Liu, Z. Zhang, Ind. Crops Prod. 140, 111724 (2019)
B.S. Đorđević, D. Troter, Z.B. Todorović, I.G. Đalović, Lj.P. Stanojević, P.M. Mitrović, V.B. Veljković, J. Food Meas. Charact. 14, 2570–2577(2020)
T. Gutfinger, J. Am. Oil. Chem. Soc. 58, 966–968 (1981)
C. Quettier-Deleu, B. Gressier, J. Vasseur, T. Dine, C. Brunet, M.C. Luyckx, J.C. Cazin, F. Bailleul, F. Trotin, J. Ethnopharmacol. 72, 35–42 (2000)
W.C. Choi, C.S. Kim, S.S. Hwang, K.B. Choi, J.H. Ahn, Y.M. Lee, H.S. Park, K.S. Kim, Y.M. Lee, Plant Sci. 163, 1161–1168 (2002)
V. Vieira, M.A. Prieto, L. Barros, J.A.P. Coutinho, I.C.F.R. Ferreira, O. Ferreira, Ind. Crops Prod. 115, 261–271 (2018)
Z. Wei, X. Qi, T. Li, M. Luo, W. Wang, Z. Yuangang, F. Yujie, Sep. Purif. Technol. 149, 237–244 (2015)
S.K. Saha, S. Dey, R. Chakraborty, J. Mol. Liq. 287, 110956 (2019)
T. Wang, J. Jiao, Q.-Y. Gaia, P. Wanga, N. Guoa, L.-L. Niua, Y.-J. Fu, J. Pharm. Biomed. Anal. 145, 339–345 (2017)
M.D. Borș, S. Socaci, M. Tofană, V. Mureșan, A.V. Pop, M. Nagy, R. Vlaic, Bull. UASVM Food Sci. Technol. 71, 205–206 (2014)
M.D. Borș, C.A. Semeniuc, S. Socaci, R. Vlaic, O.P. Moldovan, A.V. Pop, M. Tofană, Roman. Biotechnol. Lett. 22, 12827–12833l (2017)
G.B. Rasera, M.H. Hilkner, S.M. de Alencar, R.J.S. de Castro, Ind. Crops Prod. 135(2019), 294–300 (2019)
D. Sreeramulu, M. Raghunath, Food Nutr. Sci. 2, 422–427 (2011)
X. Wu, L.W. Gu, J. Holden, D.B. Haytowitz, S.E. Gebhardt, G. Beecher, R.L. Prior, J. Food Compos. Anal. 17, 407–422 (2004)
W. Zheng, S.Y. Wang, J. Agric. Food Chem. 49, 5165–5170 (2001)
M.C. Ali, J. Chen, H. Zhang, Z. Li, L. Zhao, H. Qiu, Talanta 203, 16–22 (2019)
M.C. Bubalo, S. Vidović, I. Radojčić Redovniković, S. Jokić, J. Chem. Technol. Biotechnol. 90, 1631–1639 (2015)
A. Mišan, J. Nađpal, A. Stupar, M. Pojić, A. Mandić, R. Verpoorte, Y.H. Choi, Crit. Rev. Food Sci. Nutr. 1–29 (2019)
Acknowledgements
This work has been funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project assigned to the Faculty of Technology, Leskovac, University of Niš, researchers' group III 45001, No. 451-03-68/2020-14/200133) and the Serbian Academy of Sciences and Arts (the Project F-78).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Đorđević, B.S., Todorović, Z.B., Troter, D.Z. et al. Extraction of phenolic compounds from black mustard (Brassica nigra L.) seed by deep eutectic solvents. Food Measure 15, 1931–1938 (2021). https://doi.org/10.1007/s11694-020-00772-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00772-y