Abstract
This study aimed to investigate the effect of different extraction solvents and determine the optimum solvent concentration (in the range of 0–100%) for the recovery of total phenolic content, total flavonoid content and antioxidant capacity of peel, pulp and seed kernel of Mangifera odorata fruit. The total phenolic (TPC) and total flavonoid content (TFC) were determined using Folin–Ciocalteu and aluminum chloride assays, respectively. The highest level of TPC was found in 60% and 80% (v/v) acetone for pulp and seed kernel while peel showed the highest TPC at the concentration of 80% (v/v) acetone. The optimum total flavonoid content was achieved at 100% (v/v) methanol for all fruit parts. Antioxidant activity was measured using ferric reducing antioxidant power assay, and the result showed the highest activity at 60% (v/v) ethanol for all parts of M. odorata fruit. The highest scavenging activity was shown at 60% (v/v) acetone for pulp and seed kernel, respectively. Peel exhibited the highest scavenging activity at 40% acetone. It can be concluded that the recovery of phenolic compounds was dependent on the polarity of the chemical constituents in the different parts of fruit as well as the polarity of the solvent systems used. Seed kernel had the highest TPC, TFC and possessed excellent antioxidant capacity compared to peel and pulp of M. odorata. Therefore, the seed kernel can be incorporated into food products as a functional ingredient for the prevention of oxidative-stress related diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Overproduction of free radicals or reactive oxygen species (ROS) in the human body can lead to the development of oxidative stress. This event causes biomolecules damage and it has been implicated in various pathological and physiological incidents including diabetes, cancer, and numerous other diseases. In Malaysia, 17.5% adult suffered from diabetes mellitus in 2015 (Institute of Public health 2015), while 13.9% and 2.3% of deaths in 2018 were due to ischaemic heart disease and lung cancer, respectively (Mahidin 2018). Antioxidants are capable of scavenging ROS and protecting against oxidative stress-induced diseases. The protection can be enhanced by increasing intake of the dietary antioxidants. Even a lower concentration of antioxidants than oxidants had a significant impact on delaying oxidative damage (Ahmadi et al. 2007). Numerous epidemiological studies have reported that plant sources contain varieties of compounds that exhibit antioxidant properties (Xu et al. 2017). These include micronutrients such as vitamins and minerals as well as phytochemicals. The major group of phytochemicals is phenolic compounds which can be found in all plant parts. They do not only act as defence to the plants but possess biological activity. As an antioxidant compound, they act as reducing agents, hydrogen or electron donors, metal cations chelators, and singlet oxygen quenchers (Rice-Evans et al. 1996).
Extraction procedures are essential for the recovery and isolation of polyphenols from plant materials (Boeing et al. 2014). Phenolic compounds are diverse in structures (Złotek et al. 2016). Therefore, solvent polarity plays a crucial role in influencing phenols solubility (Naczk and Shahidi 2006), which may have a significant impact on the extraction yield and their activity. Solid–liquid extraction is the most commonly used method for extracting and isolating the bioactive constituents from plant sources (Cottica et al. 2011). Typically, acetone is the best solvent for extracting proanthocyanidins and tannins; ethanol efficiently extracts flavonoids and their glycosides, catechol, and tannins; whereas phenolic acids and catechin were better extracted with methanol (Chirinos et al. 2007; Tabart et al. 2007). Antioxidant activity is also dependent on the solvent compositions (Dhanani et al. 2017). An aqueous mixture of acetone, ethanol, and methanol has been widely used for extracting bioactive compounds from botanical materials, especially medicinal plants (Tabart et al. 2007; Wang et al. 2008). However, some studies reported that the optimal solvent selection and condition is typically varied with different food matrices analysed (Rababah et al. 2010; Michiels et al. 2012). Thus, it is vital for investigating the ideal extraction solvent for respective sample type.
Mangifera odorata belongs to the family Anacardiaceae and is classified as an underutilized fruit. It is primarily cultivated in Southeast Asia. The fruit is known as kuini in Malaysia and is commonly consumed by local communities (Lim 2012). The fruit is a hybrid between M. foetida (bacang) and M. indica (mango) (Teo et al. 2002). It has green to yellowish-green colour, and usually sparingly spotted with dark brown lenticel. The flesh is orange–yellow, firm, fibrous, sourish-sweet, juicy with a pungent smell and taste of turpentine (Lim 2012). The fruit is highly nutritious, whereby it contains higher protein and calcium than the other Mangifera species as well as an acceptable amount of carotenoids (Khoo et al. 2008; Mirfat et al. 2015). Besides, our recent findings showed that the M. odorata peel rich in fiber and minerals, while the seed kernel rich in protein and fat (Lasano et al. 2019). Interestingly, M. odorata contains higher isoflavones and polyphenols compared to M. pajang and M. foetida (Khoo and Ahmad 2008; Ikram et al. 2009;). The fruit also had higher antioxidant activity than M. pajang (Mirfat et al. 2015).
Antioxidant properties of several species of Mangifera had been studied in previous research (Khoo et al. 2008; Ikram et al. 2009; Mirfat et al. 2015), however, to the best of our knowledge none of these reports cover the antioxidant activity of the other fruit parts. On the other hand, it is not clear which solvent systems provides the optimal condition for extracting bioactive constituents from M. odorata fruit. Hence, this study aimed to investigate the effect of different extraction solvents on polyphenols and antioxidant activity of peel, pulp, and seed kernel of M. odorata fruit. Besides, the total phenolic content and antioxidant activity of different fruit parts were also examined and compared.
Materials and methods
Chemicals
Methanol, ethanol and acetone were purchased from Sigma-Adrich Company, USA. Folin–Ciocalteu reagent, DPPH (2,2-diphenyl-1-picrylhydrazyl), gallic acid, quercetin, aluminum chloride were from Merk (Darmstadt, Germany). All chemicals and reagents used were of analytical grade.
Plant materials and sample preparation
Mangifera odorata fruit was obtained from the fresh market in Serdang, Selangor, Malaysia. The fruit was authenticated by Mr. Mohd Hafizi Adzmi Hanafi, a botanist at Institute of Bioscience. The voucher number is SK 3179/1. The fresh fruits were then washed with tap water and wiped using tissues. The peel and seed kernel was separated from the pulp and cut into small pieces. The initial weight of the fruits before the peel was separated is 176.97 ± 18.91 g. The sample were dried using freeze dryer and further ground into a fine powder using the electronic grinder at room temperature (24 ± 3 °C). The powder was kept in an airtight opaque container and stored at − 20 °C until further analysis.
Sample extraction
The extraction procedures were conducted according to the method described by Addai et al. (2013). The extraction was carried out by mixing 1.0 g of freeze-dried sample with 10 ml of solvent under magnetic stirring for 1 h in the dark at room temperature (24 ± 3 °C). Then, the solutions were centrifuged for 15 min at 6000 rpm and the supernatant was collected. The procedure was repeated by washing the sediment using 5 ml of the same solvent. The extract was shaken for 15 min and centrifuged again for 15 min at 6000 rpm. The supernatants were pooled and stored in the airtight container and kept at − 20 °C until further analysis. Three solvent–water extraction systems were used (methanol, ethanol and acetone) at five different concentrations (20%, 40%, 60%, 80% and 100%). All analyses were performed in triplicate.
Determination of total phenolic content (TPC)
Total phenolic content was determined by using Folin–Ciocalteu reagent which was adapted from Velioglu et al. (1998). 300 µl extract was mixed with 2.25 ml of Folin Ciocalteu reagent (previously diluted 10 fold (1:9) with distilled water) and allowed to stand at room temperature (24 ± 3 °C). for 5 min. Then, 2.25 ml of sodium carbonate (60 g/L) solution was added to the mixture. After 90 min incubation at room temperature (24 ± 3 °C), absorbance was measured at 725 nm using spectrophotometer. The standard curve was plotted using gallic acid monohydrate at the concentration of 0.05- 0.25 mg/ml. The results were expressed as mg gallic acid equivalent (GAE) per g dry extract using the prepared standard curve. All measurements were conducted in the triplicate analysis.
Determination of total flavonoids content (TFC)
Total flavonoid content was estimated by using aluminum chloride colorimetric method (Bhaigyabati et al. 2014). The assay was conducted by mixing 0.5 ml of each sample extract, 1.5 ml of methanol/ethanol, 0.1 ml of 10% aluminum chloride solution, 0.1 ml of 1 M potassium acetate solution and 2.8 ml distilled water. The mixture was incubated for 30 min and the measurement of absorbance was conducted at 415 nm using a UV–Vis spectrophotometer. Distilled water was used as a sample blank. A calibration curve of quercetin (0–100 µg/ml) was prepared and the TFC was expressed as mg quercetin equivalent (QE) per g dry extract. The samples were assayed in triplicate.
Determination of antioxidant activity by the ferric reducing antioxidant power (FRAP) assay
Antioxidant activity of samples was analysed by using FRAP assay described by Benzie and Strain (1996). The working FRAP reagent was prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2, 4, 6-tripyridyl-s-triazine (TPTZ) solution and 20 mM FeCI3·6H2O in a ratio of 10:1:1. The mixture solution was heated at 37 °C in a water bath prior to use. A total of 3 ml FRAP reagent was added to a cuvette and blank reading was taken at 593 nm using a UV–Vis spectrophotometer. A total of 100 µl of plant extract and 300 µl of distilled water were mixed in a test tube, followed by the addition of freshly prepared FRAP reagent. The second reading was performed after 90 min of incubation at 37 °C in a water bath using a UV–Vis spectrophotometer at the same wavelength. The changed in absorbance after 90 min from the initial blank reading were compared to a standard curve. The standard curve of known Fe(II) concentrations was prepared using several concentrations ranging from 0.1 to 1.5 mM. The result was expressed as the concentration of antioxidant having a ferric reducing ability in 1 g of sample (mM/g).
Determination of radical scavenging activity by the DPPH assay
The scavenging activity of the extract was measured by using 1,1-diphenyl-2-picrylhydrazyl (DPPH) as a free radical model. The method was adapted from Magalhães et al. (2006). An aliquot of 500 µl of samples or control (solvent) at different concentrations were mixed with 500 µl of 500 µm (DPPH) (5.9 mg/100 ml methanol) in 24-well microplates. The mixture was left to stand at room temperature (24 ± 3 °C) for 30 min in the dark. The mixture was measured spectrophotometrically using the microplate reader at 517 nm. The free radical scavenging activity was calculated using the following formula:
The % inhibitions were plotted against the respective concentrations used and IC50 was calculated based on the graph. The IC50 of ascorbic acid and butylated hydroxytoluene (BHT) as positive control were also determined.
Statistical analysis
All experiments were carried out in triplicates, and data were reported as mean ± standard deviation. The differences of mean values among solvent systems were determined using analysis of variance (ANOVA) followed by Tukey’s HSD tests at the significant level of p < 0.05. Correlation analysis was performed between TPC, TFC, and antioxidant activity of sample extracts using Pearson’s correlations coefficient. All statistical analyses were conducted using Minitab version 16.0.
Results
Total phenolic and total flavonoid content
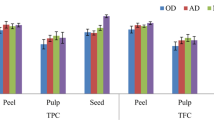
Total phenolic contents of peel, pulp, and seed kernel of M. odorata fruit extracted by using different concentrations of acetone, ethanol and methanol are presented in Fig. 1. The results showed that the recovery of phenolic compounds is significantly varied depending on the type and ratios of the solvents used. The TPC of acetone-based extract ranged from 9.13 mg GAE/g to 959.16 mg GAE/g, from 13.79 mg GAE/g to 693.43 mg GAE/g for the ethanol-based extract and from 21.86 mg GAE/g to 906.50 mg GAE/g for the methanol-based extract, respectively. Besides, all the water extracts of peel, pulp, and seed kernel showed the lowest amounts of TPC. For acetone extract, the highest amounts of TPC were obtained with 60% and 80% acetone for pulp and seed kernel, and 80% acetone for peel, respectively (p < 0.05). In the case of ethanol extract, the highest level of TPC was achieved by using 60% ethanol for all fruit parts. However, there was no significant difference between 40% and 60% ethanol for seed kernel (p > 0.05). For methanol extract of the peel, 60% and 80% solvents gave similar values and showed no significant difference between TPC values of the two extracts (p > 0.05). Nevertheless, all fruit parts extracted using 60% and 80% methanol showed the highest value of TPC compared to other methanol ratios. Our results clearly showed that the order of the extracts with the highest level of TPC in pulp was as follows: 60% and 80% acetone > 60% ethanol > 80% methanol > water extract. In the case of peel, the order was as follows: 80% acetone > 60% ethanol > 80% methanol > water extract. On the other hand, the highest level of TPC in the seed kernel was found in 80% acetone, closely followed by 80% methanol, 60% ethanol and water extract, respectively.
The total flavonoid contents of peel, pulp and seed kernel of M. odorata fruit extracted using different ratios of acetone, ethanol, and methanol were examined and presented in Fig. 2. The results were expressed as mg quercetin equivalent (QE) per gram dry extract. The TFC of acetone-based extract, ethanol-based extract, and methanol-based extract ranged from 2.17 mg QE/g to 43.95 mg QE/g, from 3.67 mg QE/g to 45.21 mg QE/g and from 2.17 mg QE/g to 53.09 mg QE/g, respectively. Among the combination of acetone–water mixtures, peel and pulp extracted using 80% acetone showed the highest amount of TFC. On the hand, the seed kernel extracted using 60% acetone had the highest amount of TFC. For ethanol, the maximum amount of TFC was obtained with 40% ethanol for peel and seed kernel while 80% ethanol for pulp. In the case of methanol, the highest level of TFC was achieved by using 100% methanol for all fruit parts. Among the solvents, the order of high TFC were 100% methanol > 80% ethanol > 80% acetone for pulp; 100% methanol > 40% ethanol > 80% acetone for peel; and 100% methanol > 40% ethanol > 60% acetone for seed kernel. Similar to TPC, the water extracts of peel, pulp, and seed kernel showed the lowest amounts of TFC in comparison with other solvents. In addition, it was found that the TPC and TFC of seed kernel were the highest among the fruit parts irrespective of the solvents used. Whilst, pulp contained the lowest amount of TPC and TFC compared to peel.
Antioxidant activity
In the present study, ferric reducing/antioxidant power (FRAP) and DPPH free radical-scavenging assays were used to determine the antioxidant capacity of different parts of the fruit extracts. FRAP assay is widely used to determine the antioxidant activities of various plant extracts (Złotek et al. 2016). The method is based on the donation of a hydrogen atom to the ferric complex by the antioxidant compounds that act as reducing agents. This leads to the breakage of radical chain reaction (Halliwell 1991). The reducing power of the extracts was compared to ascorbic acid as a positive control. The results were expressed as mM Fe/g dry extract and presented in Table 1. The FRAP value for ascorbic acid was 2350.93 mM Fe/g. The FRAP values for acetone-based extract, ethanol-based extract, and methanol-based extract ranged from 140.7 mM Fe/g to 1076.5 mM Fe/g, from 520.6 mM Fe/g to 1377.8 mM Fe/g and from 425.2 mM eF/g to 1100.8 mM Fe/g, respectively. Acetone extract at concentration of 60% showed the highest values of FRAP for peel and seed kernel while pulp had the highest FRAP value at a concentration of 20% acetone. For ethanol, all fruits parts extracted with 60% ethanol exhibited the highest FRAP value. Among methanol extracts, 100% methanol showed the highest FRAP value for peel and pulp while seed kernel obtained maximum FRAP value at concentration of 60% methanol. Overall, it can be seen that peel, pulp and seed kernel extracted using 60% ethanol demonstrated the highest antioxidant activity compared to other solvent extracts.
DPPH radical scavenging assay measures the ability of antioxidant compounds in the extracts to scavenge the DPPH radicals. The antioxidant activity is usually expressed either as percentage inhibition of DPPH or IC50,which indicates 50% inhibition of DPPH radicals. The lowest IC50 value shows the most potent antioxidant activity. In the present work, the scavenging activity was expressed as IC50 (mg/ml). The values were compared to ascorbic acid (0.0052 mg/ml) and BHA (0.0054 mg/ml) as positive controls. Table 2 shows IC50 values of peel, pulp and seed kernel of M. odorata fruit extracted by using different solvents. It can be seen from the results that the seed kernel showed the lowest IC50 value, demonstrating high antioxidant activity, followed by peel and pulp, respectively. Among seed kernel extracts, the order of high antioxidant activity was 60% acetone > 60% ethanol > 60% methanol. Similarly, the order of pulp extracts with the highest antioxidant activity was as follows: 60% acetone > 60% ethanol > 80% methanol. In the case of peel, among solvents investigated the highest antioxidant activity was seen in 40% acetone, followed by 40% ethanol and 80% methanol, respectively. Regardless of fruit parts, extracts with a concentration of 60% acetone showed significantly higher antioxidant activity as evidenced from the lower IC50 values compared to other solvent mixtures and their respective absolute ones.
Correlation between total phenolic content, total flavonoid content and antioxidant activity
The correlation between the assays was analysed using the Pearson correlation test and r-values were presented in Table 3. It should be noted that the correlations between polyphenols and antioxidant activity assayed by FRAP and DPPH varied with different parts of M. odorata analysed. The results demonstrated that correlations between TPC and DPPH was negatively strong for peel (r = − 0.738) and seed kernel (r = 0.803) (p < 0.05) while negatively moderate correlation was shown for pulp (r = − 0.502) but not significant (p > 0.05). The negative correlations were due to the inverse relationship between IC50 value and antioxidant activity whereby the present study used IC50 values for the correlation analysis. On the other hand, a positively weak correlation was obtained between TPC and FRAP value for pulp (r = 0.243) (p < 0.05). In addition, positive moderate correlations were found between TPC and TFC with antioxidant activity assayed by FRAP for peel and seed kernel (0.427 ≤ r ≥ 0.382) (p < 0.05). The lowest correlation was found between TFC and scavenging activity assayed by DPPH (r = − 0.238) (p < 0.05) and between TFC and FRAP (r = 0.041) (p > 0.05) for pulp. These results indicated that total phenolic and flavonoid contents are the important contributors for antioxidant activity of peel and seed kernel extracts but not in the pulp, suggesting the presence of other significant antioxidant constituents in the pulp of M. odorata.
Discussion
The present work investigates the effect of solvents (acetone, ethanol, and methanol) concentrations (in the range of 0–100%) on the recovery of polyphenols and antioxidant activity of different parts of Mangifera odorata, namely peel, pulp and seed kernel. The selection of an appropriate solvent is very important for extracting bioactive compounds especially in the development of functional food products. Difference solvent has difference polarities that might influence the solubility of the chemical constituents in a sample (Naczk and Shahidi 2006; Dhanani et al. 2017; Mohammedelnour et al. 2017). Generally, solvents with similar polarities to phytochemicals able to penetrate and extract the compounds from the plant cell walls (Nurul and Asmah 2014). Hence, this study would be beneficial for the selection of the most suitable solvents for optimum recovery of antioxidant compounds. Methanol, ethanol, acetone, ethyl acetate, and their water mixture, acidified or not are the most commonly used solvents for extracting phenolic compounds (Pinelo et al. 2004; Lafka et al. 2007). The polarity of pure acetone, ethanol, and methanol is 0.355, 0.654 and 0.762, respectively (Tan et al. 2013). The present results demonstrated that acetone–water mixtures (60% and 80% for pulp, whilst 80% for peel and seed kernel) were the best extracting solvent for TPC from peel, pulp and seed kernel which agrees with other studies of different plant extracts (Turkmen et al. 2006; Al-Farsi and Lee 2008; Meneses et al. 2013; Downey and Hanlin 2016; Ngo et al. 2017). Alasalvar et al. (2006) reported that acetone is more effective for extracting high molecular weight polyphenols such as condensed tannins. This may be attributable to the higher solubility of tannins in acetone as both of them are high molecular weight compounds (Uma et al. 2010). It may also be caused by the breakdown of polyphenol-protein complexes with the combined use of acetone and water. This might be explained by the fact that water increased the bulge of plant material, enlarged the contact and surface area between the solvent and plant matrix (Rostagno et al. 2003), and consequently released the bound polyphenols into the extract. This process may also produce a moderately polar environment which enhances the extraction efficiency (Spigno and De Faveri 2007). It can thus be suggested that the phenolic compounds in aqueous acetone extract of peel, pulp and seed kernel of M. odorata fruit may have more phenol groups, or bound polyphenols or possess higher molecular weights than in the absolute solvents extracts. In a study carried out by Złotek et al. (2016), acetone–water mixtures were also found more efficient in extracting phenolics from fresh and freeze-dried basil leaves compared to methanol-based mixtures. However, Michiels et al. (2012) and Rababah et al. (2010) proposed that the food matrices of the sample analysed should be considered when selecting the best solvent for polyphenols extraction.
A different trend was observed in the amount of TFC as peel, pulp and seed kernel showed the highest amount at 100% methanol. Pure ethanol was shown to extract only hydrophobic polyphenols from fruit, leaving hydrophilic compounds in the plant matrices (Sulaiman et al. 2017). However, in Do et al. (2014) the best solvent for extraction of flavonoids from Limnophilia aromatica was 100% ethanol, closely followed by 75% methanol, 100% methanol and 50% aqueous methanol extract, respectively. Both methanol and ethanol possess almost similar polarities. Chirinos et al. (2007) and Tabart et al. (2007) suggested that methanol is the best solvent for the extraction of phenolic acids and catechin in fruits. The main flavonoids component in Mangifera species was catechin (Dorta et al. 2012) which explained the findings of the present study. This can be the results of higher salvation of antioxidant compounds due to bond interactions between polar positions of the compounds in the fruits and the solvent. By contrast, ethanol provides lower salvation of antioxidant molecules due to the presence of longer ethyl radical compared to the methyl radical present in methanol (Boeing et al. 2014). In brief, the present work concluded that absolute methanol was more efficient for the extraction of flavonoids from M. odorata fruit.
It is well known that phenolic compounds had a significant effect on the radical scavenging activity of the plant extracts (Turkmen et al. 2006). In this study, it was observed that 60% acetone extracts showed the highest radical scavenging activity in pulp and seed kernel, and peel at 40% acetone. This result was correlated with the highest phenolic content in the acetone–water mixture at 80% concentration. Acetone–water mixtures have been proven as the best solvents combination for the extraction of polar antioxidants (Lu and Foo 1999; Sun et al. 2002; Luximon-Ramma et al. 2003; Lien et al. 2015; Mokrani and Madani 2016; Ngo et al. 2017). Therefore, we proposed that polar phenolic molecules contribute to radical scavenging activity of the studied samples except for the pulp of M. odorata. It was observed in the present study that no significant correlation was found between TPC and DPPH for pulp. Our results correspond with the study conducted by Złotek et al. (2016) in which a strong correlation was found between phenolic content and antioxidant activity measured by DPPH assay. Similarly, de Sousa et al. (2018) found high correlation between flavonoid and phenolic and antioxidant capacity of Oenocarpus distichus Mart. fruits. Besides, our study showed that peel, pulp and seed kernel extracted using 60% ethanol had the highest reducing power. The obtained results may be due to the fact that the aqueous ethanol mixtures are able to dissolve in a wide range of phenols group. According to Zhou and Yu (2004) and Dhanani et al. (2017) the alteration in solvent polarity influences the estimation of antioxidant activity as the solvents extract a selected group antioxidant compounds. It can be proposed that the extracts obtained using a more polar solvent had better reducing power than the less polar solvent.
Comparing the TPC, TFC and antioxidant activity of different parts of M. odorata fruit, peel and seed kernel had more phenolic contents and possess excellent antioxidant activity compared to pulp. Phenolic compounds tend to accumulate in the dermal tissues of the plant body due to their role in plant protections and as attractants in fruit dispersal (Toor and Savage 2005). Similar results were reported in previous study whereby the grape seed showed the highest antioxidant capacity in comparison with grape pulps (Guo et al. 2003). In addition, seed kernel rich in tocopherols, carotenoids and minerals such as copper, zinc and selenium which were very effective in scavenging reactive oxygen species (Kothari and Seshadri 2010). Since total phenolic and flavonoid compounds did not show any correlation with antioxidant activity of pulp, it is recommended to conduct future study to determine bioactive compounds in pulp of M. odorata.
The ideal condition for the extraction of polyphenols and antioxidant compounds from Mangifera odorata fruit were depending on the fruit parts analysed and the polarity of solvent system. In general, the mixture of solvents and water is the most efficient for extracting polyphenols and showed potent antioxidant activity compared to their respective pure organic solvents. Acetone at either 60% or 80% was the most efficient in extracting TPC from pulp while peel and seed kernel obtained optimum TPC at 80% acetone. 100% methanol was efficient in extracting TFC from each parts of M. odorata fruit. 60% ethanol and 60% acetone from all fruit parts (except for 40% acetone for the peel) showed the highest reducing power and scavenging activity, respectively. There was a good correlation between total phenolic content and antioxidant activities of the fruit extracts which contradicts to our previous finding on Ficus carica leaves (Ghazi et al. 2012). It should be also noted that seed kernel has the potential to be develop as an ingredient in functional food products and nutraceuticals since it contained more bioactive compounds compared to other fruit parts studied. Hence, it is recommended that future studies should focus on phytochemicals identification of seed kernel and either 60% acetone or 60% ethanol should be used for further utilisation and isolation.
References
Addai ZR, Abdullah A, Mutalib SA (2013) Effect of extraction solvents on the phenolic content and antioxidant properties of two papaya cultivars. J Med Plants Res 7(46):3354–3359
Ahmadi F, Kadivar M, Shahedi M (2007) Antioxidant activity of Kelussia odoratissima Mozaff. in model and food systems. Food Chem 105(1):57–64
Alasalvar C, Karamać M, Amarowicz R, Shahidi F (2006) Antioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy cover. J Agric Food Chem 54(13):4826–4832
Al-Farsi MA, Lee CY (2008) Nutritional and functional properties of dates: a review. Crit Rev Food Sci Nutr 48(10):877–887
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Bhaigyabati T, Devi PG, Bag GC (2014) Total flavonoid content and antioxidant activity of aqueous rhizome extract of three hedychium species of Manipur valley. Res J Pharm Biol Chem Sci 5(5):970–976
Boeing JS, Barizão ÉO, e Silva BC, Montanher PF, de Cinque Almeida V, Visentainer JV (2014) Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem Cent J 8(1):1–9
Chirinos R, Rogez H, Campos D, Pedreschi R, Larondelle Y (2007) Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep Purif Technol 55(2):217–225
Cottica SM, Sawaya AC, Eberlin MN, Franco SL, Zeoula LM, Visentainer JV (2011) Antioxidant activity and composition of propolis obtained by different methods of extraction. J Braz Chem Soc 22(5):929–935
de Sousa SHB, de Andrade Mattietto R, Chisté RC, Carvalho AV (2018) Phenolic compounds are highly correlated to the antioxidant capacity of genotypes of Oenocarpus distichus Mart. fruits. Food Res Int 108:405–412
Dhanani T, Shah S, Gajbhiye NA, Kumar S (2017) Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem 10:S1193–S1199
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22(3):296–302
Dorta E, Lobo MG, Gonzalez M (2012) Reutilization of mango byproducts: study of the effect of extraction solvent and temperature on their antioxidant properties. J Food Sci 77(1):C80–C88
Downey MO, Hanlin RL (2016) Comparison of ethanol and acetone mixtures for extraction of condensed tannin from grape skin. S Afr J Enol Vitic 31(2):154–159
Ghazi F, Rahmat A, Yassin Z, Ramli NS, Buslima NA (2012) Determination of total polyphenols and nutritional composition of two different types of Ficus carica leaves cultivated in Saudi Arabia. Pak J Nutr 11(11):1061–1065
Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y (2003) Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res 23(12):1719–1726
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 91(3):S14–S22
Ikram EHK, Eng KH, Jalil AMM, Ismail A, Idris S, Azlan A, Mokhtar RAM (2009) Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J Food Compost Anal 22(5):388–393
Institute for Public Health (2015) National health and morbidity survey 2015 (NHMS 2015) Volume III: Non-communicable disease. Ministry of Health, Kuala Lumpur
Khoo HE, Ahmad I (2008) Determination of daidzein and genistein contents in mangifera fruit. Malays J Nutr 14(2):189–198
Khoo HE, Ismail A, Mohd Esa N, Idris S (2008) Carotenoid content of underutilized tropical fruits. Plant Foods Hum Nutr 63:170–175
Kothari V, Seshadri S (2010) Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Nutr Food Sci 40(4):403–408
Lafka TI, Sinanoglou V, Lazos ES (2007) Extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem 104(3):1206–1214
Lasano NF, Hamid AH, Karim R, Dek MSP, Shukri R, Shazini Ramli N (2019) Nutritional composition, anti-diabetic properties and identification of active compounds using UHPLC-ESI-orbitrap-MS/MS in Mangifera odorata L. peel and seed kernel. Molecules 24(2):320–340
Lien DTP, Tram PTB, Toan HT (2015) Effects of extraction process on phenolic content and antioxidant activity of soybean. J Food Nutr Sci 3(1–2):33–38
Lim TK (2012) Mangifera odorata. In: Lim TK (ed) Edible medicinal and non-medicinal plants, vol 1. Springer, Amsterdam, pp 127–130
Lu Y, Foo LY (1999) The polyphenol constituents of grape pomace. Food Chem 65(1):1–8
Luximon-Ramma A, Bahorun T, Crozier A (2003) Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J Sci Food Agric 83(5):496–502
Magalhães LM, Segundo MA, Reis S, Lima JL (2006) Automatic method for determination of total antioxidant capacity using 2, 2-diphenyl-1-picrylhydrazyl assay. Anal Chim Acta 558(1):310–318
Mahidin U (2018) Statistics on causes of death Malaysia. Department of Statistics Malaysia https://www.dosm.gov.my/v1. Accessed 13 June 2019
Meneses NG, Martins S, Teixeira JA, Mussatto SI (2013) Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep Purif Technol 108:152–158
Michiels JA, Kevers C, Pincemail J, Defraigne JO, Dommes J (2012) Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem 130:986–993
Mirfat AHS, Razali M, Salma I, Kalsum HZ (2015) Antioxidant and nutritional values of selected under-utilised Mangifera Species in Malaysia. Indian J Plant Genet Resour 28(1):72–79
Mohammedelnour AA, Mirghani MES, Kabbashi NA, Alam MZ, Musa KH, Aminah A (2017) Effect of solvent types on phenolics content and antioxidant activities of Acacia polyacantha gum. Int Food Res J 24(Suppl.):S369–S377
Mokrani A, Madani K (2016) Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep Purif Technol 162:68–76
Naczk M, Shahidi F (2006) Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed 41(5):1523–1542
Ngo TV, Scarlett CJ, Bowyer MC, Ngo PD, Vuong QV (2017) Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J Food Quality 2017:1–8
Nurul SR, Asmah R (2014) Variability in nutritional composition and phytochemical properties of red pitaya (Hylocereus polyrhizus) from Malaysia and Australia. Int Food Res J 21(4):1689–1697
Pinelo M, Rubilar M, Sineiro J, Nunez MJ (2004) Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem 85(2):267–273
Rababah TM, Banat F, Rababah A, Ereifej K, Yang W (2010) Optimization of extraction conditions of total phenolics, antioxi- dant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J Food Sci 75(7):C626–C632
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20(7):933–956
Rostagno MA, Palma M, Barroso CG (2003) Ultrasound-assisted extraction of soy isoflavones. J Chromatogr A 1012(2):119–128
Spigno G, De Faveri DM (2007) Antioxidants from grape stalks and marc: influence of extraction procedure on yield, purity and antioxidant power of the extracts. J Food Eng 78(3):793–801
Sulaiman ISC, Basri M, Masoumi HRF, Chee WJ, Ashari SE, Ismail M (2017) Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem Cent J 11(1):54
Sun J, Chu YF, Wu X, Liu RH (2002) Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem 50(25):7449–7454
Tabart J, Kevers C, Sipel A, Pincemail J, Defraigne JO, Dommes J (2007) Optimisation of extraction of phenolics and antioxidants from black currant leaves and buds and of stability during storage. Food Chem 105(3):1268–1275
Tan MC, Tan CP, Ho CW (2013) Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int Food Res J 20(6):3117–3123
Teo LL, Kiew R, Set O, Lee SK, Gan YY (2002) Hybrid status of kuwini, Mangifera odorata Griff. (Anacardiaceae) verified by amplified fragment length polymorphism. Mol Ecol 11:1465–1469
Toor RK, Savage GP (2005) Antioxidant activity in different fractions of tomatoes. Food Res Int 38(5):487–494
Turkmen N, Sari F, Velioglu YS (2006) Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem 99(4):835–841
Uma DB, Ho CW, Wan Aida WM (2010) Optimization of extraction parameters of total phenolic compounds from Henna (Lawsonia inermis) leaves. Sains Malays 39(1):119–128
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46(10):4113–4117
Wang J, Sun B, Cao Y, Tian Y, Li X (2008) Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem 106(2):804–810
Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Li HB (2017) Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Mol Sci 18(1):96
Zhou K, Yu L (2004) Effects of extraction solvent on wheat bran antioxidant activity estimation. Lebennsmittel-Wissenschaft und-Technologie 3:717–721
Złotek U, Mikulska S, Nagajek M, Świeca M (2016) The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J Biol Sci 23(5):628–633
Acknowledgements
The research has been supported by Universiti Putra Malaysia under Putra Initiative Grant (GP-IPM/2016/9481800).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Nur Fatimah Lasano has no conflict of interest. Nurul Shazini Ramli has no conflict of interest. Azizah Hj Hamid has no conflict of interest. Roselina Karim has no conflict of interest. Mohd Sabri Pak Dek has no conflict of interest. Radhiah Shukri has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lasano, N.F., Ramli, N.S., Hamid, A.H. et al. Effects of different extraction solvents on polyphenols and antioxidant capacity of peel, pulp and seed kernel of kuini (Mangifera odorata). Orient Pharm Exp Med 19, 277–286 (2019). https://doi.org/10.1007/s13596-019-00383-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-019-00383-z