Abstract
The present study was aimed to evaluate the differences among anti-nutritional factors in relation to mineral absorption and protein digestibility of Easy-to-cook (ETC) and Hard-to-cook (HTC) grains from different kidney bean (KB) accessions.HTC grains showed lower a* (redness to yellowness) and b* (greenness to blueness) values and L* value than ETC grains. HTC grains had significantly higher Ca and Zn and lower Cu, Mn and Fe than ETC grains. ETC and HTC grains showed significant variation in mineral, total phenolic content (TPC), tannin and phytic acid content. TPC and tannin content were significantly higher for HTC grains, on the contrary phytic acid content was lower than counterpart ETC grains. Protein and in vitro protein digestibility (IVPD) also varied significantly between HTC and ETC grains and was found to be lower for HTC grains. Majority of phenolic compounds (PCs) were present in bound state in both ETC and HTC grains. Moreover, HTC grains showed higher amount of chlorogenic acid and catechin content than ETC grains in bound form. ETC and HTC grains from dark color accessions showed higher catechin content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulse grains are mainly composed of carbohydrates, proteins, lipids, mineral matter and water. In addition to these, the grains contain small quantities of vitamins, enzymes and phytochemicals, which are important for human health (Tiwari and Singh 2012). Pulses are the main source of protein in a primarily vegetarian Indian diet. Pulses utilization in human diets is considered benificial as the pulse intake is believed to play central role in reducing the risk of certain diseases, like, prostate and colon cancers (Mathers 2002). The presence of potentially bioactive constituents like phenolic acids, flavanols, isoflavones, condensed tannins and anthocyanins in pulses has been reported to exhibit antioxidant and anticarcinogenic characteristics (Tiwari and Singh 2012; Dinelli et al. 2006; Champ 2002).
Hard-to-cook (HTC) defect is the major factor which limits the consumption of pulses and is illustrated by prolonged cooking time succeeding high humidity and high temperature storage conditions along with incorrect handling (Garcia et al. 1998; Reyes-Moreno et al. 1993). The inappropriate storage conditions caused adverse changes in nutritional, functional and physicochemical properties of chickpea seeds (Reyes-Moreno et al. 2001). HTC grains do not absorb sufficient water and require longer time to soften under normal cooking conditions (Tiwari and Singh, 2012) and characterized to have limited cell separation as well as starch gelatinization during cooking (Liu and Bourne 1995). Various factors contributing to HTC defect include seed size, ripening degree, genetic factor and environmental factors, while the most important is the storage of the grains in adverse conditions post-harvest (Jackson and Varriano-Marston 1981). Both enzymatic as well as non-enzymatic reactions contribute towards this defect, simultaneously, leading to the toughness in grains. Reports have also been found which includes the protein denaturation and pectin insolubilization leading to the inability of grains to soften during cooking. Limited inter as well as intra-cellular water availability is attributive of this defect (Hincks and Stanley 1987). β-eliminative degradation of pectin is also an important factor which resulted in the HTC defect. The reduction in antioxidant activity and free and acid hydrolyzed phenolics due to HTC phenomenon was reported by Machado et al. (2008) that affected the nutritional properties of beans. The different genotypes were reported to vary in susceptibility to the development of HTC and this change was dependent upon the environmental factors. The genetic variability amongst common beans in tannins and phytate content is well reported (Wassimi et al. 1988; Wang and Daun 2004).
The limited information on the differences in chemical constituents and protein digestibility of easy-to-cook (ETC) and hard-to-cook (HTC) grains in beans was available. Therefore, the present study was designed to compare the color, anti-nutritional constituents, mineral, phenolics and protein digestibility of HTC and ETC grains from different kidney bean (KB) accessions.
Materials and methods
Materials
Eleven accessions of KB namely EC500655, EC500661, EC500347, EC500522, EC500256, EC530916, EC500634, EC500669, PLB14-1, EC-400396, EC398520, EC398525 and EC398513were procured from National Bureau of Plant Genetic Research (NBPGR), Shimla, Himachal Pradesh, for the present study. KB grains were soaked overnight at room temperature. Swollen grains were separated from the rest and considered as ETC while the other grains were termed as HTC. Both were freeze dried and analyzed for different properties. HTC phenomenon is also related to the poor quality of legume seeds.
Preparation of pulse flour
Both ETC and HTC grains (50 g) from different KB accessions after freeze drying were ground to pass through 72 mesh sieve to obtain the flour of uniform particle size, which was then packed in airtight containers for further analysis.
Color characteristics
Color characteristics (L*, a* and b*) of cleaned grains from different KB accessions were determined by using a Ultra Scan VIS Hunter Lab (Hunter Associates Laboratory Inc., Reston, VA, U.S.A.). The L* value indicates the lightness, 0–100 representing dark to light. The a∗ value determines the degree of red-green color, with a higher positive a∗ value indicating more red. The b* value indicates the degree of yellow–blue color, with a higher positive b* value indicating more yellow.
Total phenolic content
Total phenolic content (TPC) content of legume flour was determined using the method of Yu et al. (2002) with certain modifications. The sample (500 mg) was weighed and PCs were extracted in methanol (80%) for 2 h, followed by centrifugation (3000 rpm, 10 min). The supernatant (100 μl) was mixed with 900 μl distilled water and 0.5 mL Folin–Ciocalteu reagent. The mixture was allowed to equilibrate (5 min) followed by addition of 1.5 mL of sodium carbonate (20%) and incubated for 2 h at room temperature in dark. The volume was made up to 10 mL and absorbance was measured at 765 nm. Gallic acid was used as a standard and the total phenolics were expressed as mg gallic acid equivalents (GAE)/g. Standard curve was plotted between absorbance and gallic acid equivalents. The equation for TPC estimation was y = 0.0012x–0.0079 with r2 value of 0.998.
Tannin content
Tannin content was measured according to the method described by Schanderl (1970). Ground sample was weighed (0.5 g) and transferred to a 250 mL conical flask which was heated for 30 min after adding 75 mL of distilled water. The solution was centrifuged at 2000 rpm for 20 min and the supernatant was collected and the volume was made to 100 mL. Sample extract (1 mL) was transferred to 100 mL volumetric flask containing 75 mL water to which 5 mL of Folin-Denis reagent, 10 mL of sodium carbonate solution was added and the volume was made to 100 mL with distilled water. Absorbance was measured at 700 nm after 30 min interval.
Phytic acid content
Phytic acid content was quantified using spectrophotometric method (Haug and Lantzsch 1983). The decrease in iron content (determined calorimetrically with 2, 2′-bipyridine) in the supernatant was measured. Ferric (III) chloride solution (1 mL) was added to 0.5 mL extract. The solution was heated for 30 min in a boiling water bath. After being cooled to room temperature, the solution was centrifuged for 30 min at 4500 rpm. Then, 1 mL of the supernatant was transferred to another test tube and mixed with 1.5 mL of 2, 2′-bipyridine (0.1 g 2, 2′-bipyridine dissolved in 1 mL of thioglycolic acid and the volume was made to 100 mL with distilled water. The absorbance of the reaction mixture was measured at 519 nm against distilled water. The method was calibrated with standard phytic acid solutions for each set of analysis and expressed as mg of phytic acid equivalent/g of sample. The amount of ferric ions precipitated the quantity of phytic acid P was obtained by the standard graph. Phytic acid concentration was quantified by multiplying phytic acid P with the factor 3.55.
Mineral composition
Mineral composition was determined by using Atomic Absorption Spectrophotometer (Agilent Technologies) as described earlier (Parmar et al. 2014).
Protein content and in vitro protein digestibility
Protein content (%) was determined in triplicate by following AACC International Approved Method 46-13.01. In-vitro protein digestibility (IVPD) was determined by using the method of Akeson and Stahmanna (1964). Sample (250 g) in 0.1 mol/L HCl-pepsin solution was incubated at 37 °C for 3 h followed by neutralization and treatment with pancreatin in 0.2 mol/L phosphate buffer (pH 8.0) for 24 h. After incubation, the sample was treated with 10 g/100 g TCA and centrifuged at 10,000×g for 20 min. Protein digestibility (g/100 g) was calculated by the ratio of protein in supernatant to protein in sample as equation:
Extraction of soluble phenolic compounds
Extraction of PCs from ground samples was done using the method of Luthria and Pastor-Corrales (2006) with slight modifications. The ground samples were treated with methanol containing 10% acetic acid. The mixture was sonicated for 30 min and the volume was adjusted to 10 mL with distilled water. Aliquot (1 mL) of extract was filtered and analyzed for free phenolics. The remaining 9 mL of the extract was used for sequential hydrolysis with base followed by an acid. 10 mL of distilled water and 5 mL of 10 M NaOH with 2% ascorbic acid were added to the extract. The mixture was stirred overnight and then pH was adjusted to 2 by using 6 N HCl. The liberated phenolic acids were extracted with 15 mL of diethyl ether-ethyl acetate (DE/EA). The DE/EA organic layer containing the phenolic acids liberated from base hydrolysis was collected and evaporated to dryness under rotary vacuum. The residue was re-dissolved in methanol (80%). The samples were then filtered and analyzed for base hydrolysed PCs.
All extracts were quantified using high performance liquid chromatogram (HPLC) (1260 infinity, Agilent technollogies, USA) equipped with diode array detector (DAD), quadratic pump & auto-sampler at wavelength of 280 nm. The separation was carried out by using zorbax 300SB C18 reverse phase column (4.6 mm × 150 mm, 5 µm particle size) at 35 °C. A 5 µl of pre-filtered sample volume was injected into HPLC system. The mobile phase was consisted of two solvents: HPLC grade water acidified with 0.1% acetic acid (A) and 100% HPLC grade acetonitrile (B). The solvent gradient in volume ratios was as follows: 98% (A), 2% (B) for 0–20 min, it was increased to 40% B at 20 min, then it was increased to 100% (B) at 25 min and decreased to 5% (B) at 30 min. Identification of the PCs was achieved by comparing retention times of the unknowns with the standards.
Statistical analysis
The statistical analysis of data was carried out using ‘MINITAB’ (Statistical software; State College, PA). Pearson correlation was used to determine the relationship between different constituents. Analysis of variance (ANOVA) was also used to determine the statistical significance of data.
Results and discussion
Color characteristics
Color characteristics of HTC grains were significantly different from that of ETC grains (Table 1). Among ETC grains, L*, a* and b*ranged from 32.82 to 48.78, 0.28 to 11.23 and 1.48 to 10.83, respectively, while for HTC grains these ranged from 29.57 to 44.39, 0.01 to 8.17 and −0.79 to 5.75 (Table 1). Average L*, a* and b*values were higher for ETC grains than their corresponding HTC grains. This implies that dark color accessions had more HTC grains. The highest L* value was shown by EC500916 among ETC grains, on the contrary, EC398520 showed the highest L* value among HTC grains. The highest values of a* and b* were shown by EC398513 and EC500256, respectively, for both ETC and HTC grains. During the development of HTC defect, low molecular weight PCs polymerized to dark colored high molecular weight tannins (Nozzolillo and De Bezada 1984). This may have contributed to the dark color of HTC grains.
Total phenolic content
TPC varied significantly among ETC and HTC grains. HTC grains showed higher average phenolic content (1.58 mgGAE/g) than that of ETC grains (1.43 mgGAE/g) (Table 2).TPC content varied from 1.00 to 2.29 mgGAE/g and 1.18 to 2.87 mgGAE/g, respectively for ETC and HTC grains. ETC grains from different accessions with high a* and b* showed high TPC (Table 7). Whereas HTC grains from different accessions with higher a* showed high TPC (Table 8). This implies that the accessions with more redness tend to have more TPC, which was consistent with earlier results (Oomah et al. 2008). TPC varied significantly among ETC and HTC grains from different accessions (Table 10). Phenolic content of seed coats and cotyledons was reported to be changed during development for HTC defect (Jones and Boulter 1983). HTC grains cell wall reported to have smaller intercellular spaces (Gracia et al. 1993) which attributed to the changes occurred in cell wall components during hardening (Hincks and Stanley 1987; Liu and Bourne 1995). This led to decrease in leaching of phenolic content during soaking among hard grains as compared to ETC grains (Xu and Chang 2008). Srisuma et al. (1989) revealed the fact that during the bean hardening, phenolic derivatives released from their bound form and resulted in the increase in free phenolic content in HTC grains.
Tannin content
ETC grains had higher tannin content than HTC grains (Table 2). ETC grains from different accessions had tannin content ranged from 0.03 to 1.26 mg/g and from 0.03 to 1.18 mg/g for HTC grains (Table 2). EC398520 had the lowest while EC530916 had the highest tannin content amongst ETC grains. EC398520 had the lowest while EC500634 had the highest tannin content amongst HTC grains. ETC and HTC grains from different accessions showed positive correlation of tannin content with a* and TPC (Table 4a, b). However, tannin content had negative correlation with L* (Tables 7, 8). Tannin content varied with the color of seed coat or testa (Reddy et al. 1985). Lower amount of tannins was found in white than in black, red and bronze colored varieties of common beans (Bressani and Elias 1980). Reddy et al. (1985) reported that tannin content varied with color of grains and species of Phaseolus v. A negative correlation between the development of HTC defect and tannins was found in previous studies, accessions having low susceptibility to develop HTC defect were reported to have lower tannin content in common beans (Reyes-Moreno et al. 2000). Tannin crosslinked with the proteins and caused a reduction in in vitro protein digestion of beans (Gupta and Haslam 1980; Bressani and Elias1980). Despite having deleterious effect on digestibility, tannins also resulted in the inhibition of digestive enzymes, increased excretion of endogenous protein and effect on digestive tract.
Phytic acid content
Phytate is known to be the anti-nutritional factor as it acts as a potent metal chelator of mineral cations. HTC grains from different accessions had lower phytic acid content as compared to ETC grains (Table 2). ETC and HTC grains from different KB accessions showed an average phytic acid content of 2.09 and 1.89 mg/g, respectively. Phytic acid content varied significantly among ETC and HTC grains (Table 10). Phytic acid content of ETC grains was highly positively correlated to tannin content, b* and L* (Table 7), while for HTC grains, phytic acid content was positively correlated to L* (Table 8). This can be inferred that phytic acid content decreased with the increase in HTC defect. During the development of HTC defect, phytase acted upon phytin which resulted into the hydrolysis of the substrate and release of inorganic phosphorus content (Jones and Boulter 1983). HTC defect has been correlated with the decrease in phytic acid content (Sievwright and Shipe 1986; Reyes-Moreno et al. 2000). Liu and Bourne (1995) reported that storage of grains in adverse conditions activated the enzyme phytase, which further produced an insoluble pectate complex during cooking by attaching to the free carboxyl groups of pectin in middle lamella, hence increased the cooking time of the grains.
Mineral composition
Mineral content varied significantly amongst ETC and HTC grains (Table 9). Mean values of Cu, Mn, Fe, Zn and Ca were found to be 0.081, 0.14, 0.74, 0.91 and 42.09 ppm, respectively, for ETC grains against 0.07, 0.11, 0.53, 1.04 and 79.27 ppm, respectively, for HTC grains (Table 3). HTC grains had significantly higher Ca and Zn while Cu, Mn and Fe were lower than ETC grains. Majority of minerals were correlated with the color of the grains.HTC grains from accessions having light color had lower Zn content (Table 8). White varieties of common beans had low accumulation of Zn and Fe and higher of Ca and Mg (Akond et al. 2011). Accessions having higher amount of tannin content was found to have lower Zn content among ETC grains and low Cu content among HTC grains. Same results were reported in common beans by Beebe et al. (2000). ETC grains from different accessions showed negative correlation of phytic acid with Ca and Fe while negative correlation of phytic acid with Fe was observed among HTC grains. Akond et al. (2011) reported that higher level of phytic acid led to the low accumulation of Fe, Ca and Mg in common beans. Among HTC grains, TPC was negatively correlated to Zn. Earlier, it has been reported that Fe and Zn bio-availability was negatively affected by the presence of PCs in Phaseolus v. (Donangelo et al. 2003). Bean grains were considered to be the best inexpensive source of minerals but their bioavailability was reported to constrain by the presence of anti-nutritional constituents like phytic acid and PCs (Nyombaire et al. 2007). Phytic acid was reported to chelate several elements such as Zn, Fe, Ca and Mg in its dephosphorylated form (Feil 2001) by interfering with their absorption (Ologhobo 1980). The divalent minerals were reported to move to middle lamella of cell wall after getting released from phytate-mineral complex (Phillips et al. 2003), and form insoluble pectate complex by replacing monovalent minerals (Mattson 1948). This may prevent cell separation during cooking, hence contributing towards the HTC defect.
Protein content and In-vitro protein digestibility
HTC grains from different accessions had lower protein content as compared to their counterpart ETC grains (Table 4). Protein content of HTC grains from different accessions ranged from 19.06 to 28.89% and for ETC grains, it ranged from 21.01 to 29.24%. HTC grains showed similar average protein content (25.64%) as reported by Tuan and Phillip (1991). It was observed that protein content of HTC grains was lower than that from ETC grains which was in consistence with the earlier research (Ruiz–Ruiz et al. 2012). Hardened grains may be used as a good source of protein because of their less suitability for the consumption and low cost (Morales-De Leon et al. 2007). Segura-Campos et al. (2014) reported that protein isolates from hardened grains can be used in different functional foods.
HTC grains from different accessions had lower in vitro protein digestibility (IVPD) than ETC grains (Table 4). IVPD of ETC and HTC grains from different KB accessions ranged from 37.50 to 55.17% and 20.49 to 57.02%, respectively. The lowest value was observed for HTC grains from EC398525 and the highest for ETC grains from EC398513. The changes in the complexes of protein with other biomolecules during storage and in protein structure influenced in vitro digestibility in grains (Segura-Campos et al. 2014). Tuan and Phillip (1991) and Reyes-Moreno et al. (2000) reported the same results when the storage conditions of grains favored the development of HTC defect. Mbofung et al. (1991) demonstrated that digestibility of kokis was decreased with the increase in the percentage of HTC grains. High level of tannin and PCs present in HTC grains interacted with proteins, hence, lowered its digestibility (Segura-Campos et al. 2014). The protein digestibility seemed to be governed majorly by the genetic makeup of the grains (Table 10). Higher accumulation of 27 kDa polypeptide in protein isolated from HTC grains might also contribute towards the lower digestibility of HTC grains as compared to ETC grains (Parmar et al. 2017).
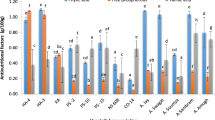
Phenolic profile
HTC and ETC grains from different pulse accessions showed the presence of both free and bound PCs (Fig. 1). PCs in ETC and HTC grains from different KB accessions in free and bound form are given in Tables 5, 6. Phenolics were observed to bind with cell wall structural proteins and carbohydrates. Bound PCs were released by dismantling the cell wall during the extraction by changing the pH (Shibuya 1984).
Base hydrolysis of grains from different KB accessions showed the presence of catechin, chlorogenic acid, protocatechuic acid and p-caumaric acid in both ETC and HTC grains. Gallic acid was observed only in EC500522, EC530196 and EC398525 KB accessions where ETC grains showed a higher amount than HTC grains. Chlorogenic acid was observed in higher amount in HTC grains than that from ETC grains. Non-significant difference was observed for p-caumaric acid, resveratrol and ferulic acid between ETC and HTC grains of KB accessions.
ETC grains from EC530916, EC400396 and EC398525; and HTC grains from EC530916, EC500256, EC500669, EC400396 and EC398525 being darker in color, showed high catechin content. It has been reported earlier that colored beans were a major source of PCs when compared with the white beans (Madhujith et al. 2004). High accumulation of catechin in HTC grains than their corresponding ETC grains could be a reason for darker color of colored beans. Khanbabaee and Van Ree (2001) also reported that catechin was colorless belonging to the class of condensed tannins, contributing towards the color of beans. The presence of these phenolics was also governed by a group of genes which also contributed to dark color to beans (Beninger and Hosfield 2003). Singh et al. (2016b) also concluded that polyphenols were highly accumulated in hull portion than the cotyledon part. Singh et al. (2016a) reported that seed coat color resulted from variation in flavonol glycosides, tannins and anthocyanins. Changes in the phenolic composition were also reported to occur due to the activation of phenolic metabolism (Khandelwal et al. 2010). These changes were dependent on pulse type and germination duration (Lopez-Amoros et al. 2006).
Conclusion
HTC grains had low L* value and phytic acid content, while, had high tannin content and TPC than ETC grains. This reflected that dark colored accessions had greater tendency to develop HTC defect. High TPC and tannin content in HTC grains resulted in the decrease in IVPD may be due to the binding to the protein and digestive enzymes.HTC grains had high chlorogenic and catechin content than ETC grains. Colored accessions showed high accumulation of catechin.
References
AACC International. Approved methods of analysis, 11th (ed.) Method46-13.01.Crude protein-Micro-Kjeldahl method. Approved October 8, 1976; reapproved November 3, 1999. AACC International: St. Paul,MN. http://dx.doi.org/10.1094/AACCIntMethod-46-13.01
Akeson WR, Stahmanna AA (1964) Pepsin pancreatin digest index of protein. J Nutri 83:257–261
Akond ASMGM, Crawford H, Berthold J, Talukder ZI, Hossain K (2011) Minerals (Zn, Fe, Ca and Mg) and antinutrient (phytic acid) constituents in common beans. Am J Food Technol 6:235–243
Beebe S, Gonzalez AV, Rengifo J (2000) Research on trace minerals in the common bean. Food Nutri Bull 21:387–391
Beninger CW, Hosfield GL (2003) Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J Agri Food Chem 51:7879–7883
Bressani R, Elias LG (1980). Polyphenols in cereals and legumes. Hulse JH (ed.) IDRC-145e, Ottawa
Champ MMJ (2002) Non-nutrient bioactive substances of pulses. Br J Nutr 88:307–319
Dinelli G, Bonetti A, Minelli M, Marotti I, Catizone P, Mazzanti A (2006) Content of flavonols in Italian bean (Phaseolus Vulgaris L.) ecotypes. Food Chem 99:105–114
Donangelo CM, Woodhouse LR, King SM, Toffolo G, Shames DM, Viteri FE, Cheng Z, Welch RM, King JC (2003) Iron and zinc absorption from two bean (Phaseolus vulgaris L.) genotypes in young women. J Agri Food Chem 51:5137–5143
Feil B (2001) Phytic acid. J New Seeds 3:1–35
Garcia E, Filisetti T, Udaeta J, Lajolo F (1998) Hard-to-cook beans (Phaseolus Vulgaris): involvement of phenolic compounds and pectates. J Agric Food Chem 46:2110–2116
Gracia E, Lajolo FM, Swanson BG (1993) A comparative study of normal and hard-to-cook brazilian common bean (Phaseolus vulgaris L): ultrastructural and histochemical aspects. J Food Struct 12:147–154
Gupta RK, Haslam E (1980) Vegetable tannins-structure and biosynthesis. In: Polyphenols in cereals and legumes symposium, St. Louis, Missouri, IDRC
Haug W, Lantzsch HJ (1983) Sensitive method for the rapid determination of phytic acid in cereals and cereals products. J Sci and Food Agric 34:1423–1426
Hincks MJ, Stanley DW (1987) Hard-to-cook defect in black beans: protein and starch consideration. J Agric Food Chem 35:517–576
Jackson GM, Varriano-Marston E (1981) Hard-to-cook phenomenon in beans: effects of accelerated storage on water absorption and cooking time. J Food Sci 46:799–803
Jones PMB, Boulter D (1983) The cause of reduced cooking rate in Phaseolus vulgaris following adverse storage conditions. J Food Sci 48:623
Khanbabaee K, Ree T (2001) Tannins: classification and definition. Nat Prod Rep 18:641–649
Khandelwal S, Udipi SA, Ghugre P (2010) Polyphenols and tannins in Indian pulses: effect of soaking, germination and pressure cooking. Food Res Int 43:526–530
Liu K, Bourne MC (1995) Cellular, biological, and physicochemical basis for the hard-to-cook defect in legume seeds. Crit Rev Food Sci Nutri 35:263–298
Lopez-Amoros ML, Hernandez T, Estrella I (2006) Effect of germination on legume phenolic compounds and their antioxidant activity. J Food Comp Anal 19:277–283
Luthria DL, Pastor-Corrales MA (2006) Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J Food Comp Anal 19:205–211
Machado CM, Ferruzzi MG, Nielsen SS (2008) Impact of the hard-to-cook phenomenon on phenolic antioxidants in dry beans (Phaseolus vulgaris). J Agric Food Chem 56:3102–3110
Madhujith T, Naczk M, Shahidi F (2004) Antioxidant activity of common beans (Phaseolus vulgaris L.). J Food Lipids 11:220–233
Mather J (2002) Pulses and carcinogenesis: potential for the prevention of colon, breast and other cancers. Br J Nutr 88:S273–S279
Mattson S (1948) The cookability of yellow peas. Soil Sci 66:77
Mbofung CMF, Rigby N, Waldron K (1991) Use of two varieties of hard-to-cook beans (Phaseolus vulgaris) and cowpeas (Vigna unguiculata) in the processing of koki (a steamed legume product). Plant Foods Hum Nut 54:131–150
Nozzolillo C, De Bezada M (1984) Browning of lentil seeds, concomitant loss of viability, and the possible role of soluble tannins in both phenomena. Can J Plant Sci 64:815–824
Nyombaire G, Siddiq M, Dolan K (2007) Effect of soaking and cooking on the oligosaccharides and lectins of red kidney beans (Phaseolus vulgaris L.). Bean Improv Coop Ann Rep 50:31–32
Ologhobo AD (1980) Biochemical and nutritional studies of cowpea and limebean with particular reference to some inherent antinutritional components. Ph.D. Thesis, University of Ibadan, Ibadan, Nigeria
Oomah BD, Blanchard C, Balasubramanian P (2008) Phytic acid, phytase, minerals and antioxidant activity in canadian dry bean (Phaseolus vulgaris L.) cultivars. J Agric Food Chem 56:11312–11319
Parmar N, Virdi AS, Singh N, Kaur A, Bajaj R, Rana JC, Agrawal L, Nautiyal CS (2014) Evaluation of physicochemical, textural, mineral and protein characteristics of kidney bean grown at Himalayan region. Food Res Int 66:45–57
Parmar N, Singh N, Kaur A, Virdi AS, Shevkani K (2017) Protein and microstructure evaluation of Harder-to-cook and Easy-to-cook grains from different Kidney bean accessions. LWT- Food Sci Tech (In Press)
Phillips RD, McWatters KH, Chinnan MS, Hung YC, Beuchat LR, Sefa-Dedeh S, Sakyi-Dawson S, Ngoddy P, Nnanyelugo D, Enwere J, Komey SM, Liu K, Mensa-Wilmot Y, Nnanna IA, Okeke C, Prinyawiwatkul W, Saalia FK (2003) Utilization of cowpeas for human food. Field Crops Res 82:193–213
Reddy NR, Pierson MD, Sathe SK, Salunkhe DK (1985) Dry bean tannins: a review of nutritional implications. J Am Oil Chem Soc 62:541–549
Reyes-Moreno C, Paredes-Lopez O, Gonzalez E (1993) Hard-to-cook phenomenon in common beans—a review. Crit Rev Food Sci Nutr 33:227–286
Reyes-Moreno C, Okamura-Esparza J, Armienta-Rodelo E, Gomez-Garza RM, Milan-Carrillo J (2000) Hard-to-cook phenomenon in chickpeas (Cicer arietinum L): effect of accelerated storage on quality. Plant Food Hum Nut 55:229–241
Reyes-Moreno C, Rouzaud-Sandez O, Milan-Carrillo J, Garzon-Tiznado JA, Camacho-Hernandez L (2001) Hard-to-cook tendency of chickpea (Cicer arietinum L) varieties. J Sci Food Agric 81:1008–1012
Ruiz-Ruiz J, Davila-Ortiz G, Chel-Guerrero L, Betancur-Ancona D (2012) Wet fractionation of hard-to-cook bean (Phaseolus vulgaris L.) seeds and characterization of protein, starch and fibre fractions. Food Bioprocess Technol 5:1531–1540
Schanderl SH (1970) Methods in food analysis. Academic Press, New York, p 709
Segura-Campos MR, Garcia-Rodriguez K, Ruiz-Ruiz JC, Chel-Guerrero L, Betancur-Ancona D (2014) In vitro bioactivity, nutritional and sensory properties of semolina pasta added with hard-to-cook bean (Phaseolus vulgaris L.) protein hydrolysate. J Func Foods 8:1–8
Shibuya N (1984) Phenolic acids and their carbohydrate esters in rice endosperm cell walls. Phytochemistry 23:2233–2237
Sievwright CA, Shipe WF (1986). Effect of storage conditions and chemical treatments on firmness, in vitro protein digestibility, condensed tannins, phytic acid and divalent cations of cooked black beans (Phaseolus vulgaris). J Food Sci 51(4):982–987
Singh B, Singh JP, Shevkani K, Singh N, Kaur A (2016a) Bioactive constituents in pulses and their health benefits. J Food Sci Technol. doi:10.1007/s13197-016-2391-9
Singh B, Singh N, Thakur S, Kaur A (2016b) Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J Food Sci Technol. doi:10.1007/s13197-016-2356-z
Srisuma N, Hammerschmidt R, Uebersax MA, Ruengsakulrach S, Bennink MR, Hosfield GL (1989) Storage induced changes of phenolic acids and the development of hard-to-cook in dry beans (Phaseolus vulgaris var. Seafarer). J Food Sci 54:311
Tiwari BK, Singh N (2012) Pulse chemistry and technology. Royal Society of Chemistry, Cambridge
Tuan Y, Phillips RD (1991) Effect of the hard-to-cook defect and processing on protein and starch digestibility of cowpeas. Cereal Chem 68:413–418
Wang N, Daun JK (2004) Effect of variety and crude protein content on nutrients and certain antinutrients in field peas (Pisum sativum). J Sci Food Agric 84:1021–1029
Wassimi NN, Hosfield GL, Uebersax MA (1988) Combining ability of tannin content and protein characteristics of raw and cooked dry beans. Crop Sci 28:452–458
Xu B, Chang SKC (2008) Effect of soaking, boiling, and steaming on total phenolic content and antioxidant activities of cool season food legumes. Food Chem 110:1–13
Yu L, Halley S, Perret J, Harris M, Wilson J, Qian M (2002) Free radical scavenging properties of wheat extracts. Food Chem 50:1619–1624
Acknowledgements
Financial assistance from department of biotechnology (DBT) is acknowledged. The authors acknowledge Dr. Jai Chand Rana for providing the samples for research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parmar, N., Singh, N., Kaur, A. et al. Comparison of color, anti-nutritional factors, minerals, phenolic profile and protein digestibility between hard-to-cook and easy-to-cook grains from different kidney bean (Phaseolus vulgaris) accessions. J Food Sci Technol 54, 1023–1034 (2017). https://doi.org/10.1007/s13197-017-2538-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2538-3