Abstract
In this study, extraction of polyphenols using different solvents (acetone, ethanol, methanol and water) with ultrasound and conventional method from whole mung bean (WMB), hull and cotyledon was conducted. Total phenolic content (TPC), total flavonoids content (TFC), total antioxidant activities (TAA), ferric reducing power (FRP) and DPPH radical scavenging activity were determined. Ultrasound treated extracts exhibited higher TPC, TFC, TAA, FRP and DPPH in different mung bean fractions than CSE. Among the solvents, acetone showed better TPC, TFC, TAA, FRP and DPPH. Hull had significantly higher TPC, TFC, TAA, FRP and DPPH than WMB and cotyledon. Sinapic acid (SA) was the major polyphenol in different fractions. Acetone extract of hull showed high polyphenol content. SA, ferulic acid, catechin, p-coumaric acid, resveratrol, quercetin and luteolin were the major contributors to antioxidant activity of acetone extract. Mung bean hull contained the maximum polyphenols and acetone was observed to be the best extraction medium for polyphenols in combination with ultrasound.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mung bean is a fast-growing, warm-season Indian originated legume belonging to the family Fabaceae which is also known as vigna radiate, green gram, haricot mungo, oregon pea, mungo and chicksaw pea across the globe. The world’s 90 % of mung bean is produced by southern and eastern Asian countries followed by Australia, Canada, Southern Europe and Southern United States (Nair et al. 2013; Kim et al. 2015). Mung bean is not only low price legume, rich source of carbohydrates, protein, essential amino acids, minerals and vitamins but it also contains polyphenols like phenolic acids and flavonoids which are beneficial for curing and preventing major chronical ailments viz. cancer, diabetes, cardiovascular diseases due to their antioxidant properties (Luo et al. 2016; Singh et al. 2016). The major polyphenols, such as, caffeic acid, syringic acid, chlorogenic acid, ferulic acid and p-coumaric acid are present in mung bean. These compounds are generally linked to cellulose, lignin and protein through ester bonds (Yao et al. 2013).
The dried seeds of mung bean are consumed either whole or after splitting popularly known as dal or dhal. The splitting of mung bean produces two major milling fractions i.e. cotyledon (75 %) and hull mix (25 % contains seed coat, germ, aleurone layer and plumule). The hull mix is either used as an animal feed or discarded (Girish et al. 2012). But, hull has a high concentration of polyphenols as compared to cotyledon. So, such bio-waste could be used to extract these polyphenols which can be further used as nutraceuticals to cure various ailments in human, in general or food preservation as a natural antioxidant.
Polyphenols can be degraded during conventional solvent extraction (CSE) method due to high temperature, oxidation conditions and/or longer time, therefore, the proper extraction of these compounds is important. There are number of other techniques like accelerated solvent extraction, microwave-assisted extraction and supercritical fluid extraction, used for extraction of polyphenols from different biomaterials but the best alternative is ultrasound assisted extraction (UAE) due to simplicity, inexpensiveness and higher efficiency (Sahin et al. 2013). During the ultrasound extraction, the collapsing of acoustic cavitation bubbles releases high energy that accelerates the microstructure breakdown of conjugating bond in phenolic esters, glycosides and bound complexes (Wanga and Zuo 2011). Although UAE showed better extraction of polyphenols from different biomaterials (Dent et al. 2015; Sahin et al. 2013), however, the optimization of other variables, such as, type and concentration of solvent, extraction duration, extraction temperature and solvent to solid ratio are also required. Thus, the study of one variable at a time can lead towards wrong conclusion, which can be overcome by studying the multi-variable effects on the response using central composite face centered design (CCFCD) of response surface methodology (RSM). CCFCD not only helps to know the individual or combined effects of the variables but it also helps to get the efficient results with minimum experimental run (Madhujith and Shahidi 2006; Sahin et al. 2013; Ilaiyaraja et al. 2015; Karami et al. 2015).
Therefore, the aim of this study was to determine the best extraction solvent for polyphenols and to compare UAE method with CSE method for polyphenols distribution in both whole mung bean (WMB) and fractions.
Materials and methods
Materials and reagents
Mung bean (var. SML 668) grains of 2014 harvests were procured. All chemicals were either purchased from Hi-Media, Bombay or Merck USA. Ascorbic acid (AA), ferulic acid (FA), p-coumaric acid (p-CoA), caffeic acid (CA), sinapic acid (SA), chlorogenic acid (CHA), quercetin (QE), luteolin (LU), procatechuic acid (PCA), vanillic acid (VA), resveratrol (RS), trans-stilbene (T-SB), gallic acid (GA) and catechin (CAT) standards were purchased from Sigma Aldrich, USA. All chemicals used in the study were either AR grade or extra pure.
Optimization of ultrasound-assisted extraction
Sample preparation
WMB was grounded and passed through a 60 mesh sieve to obtain uniform particle size flour. The grounded flour was stored in an airtight container under refrigerated condition for further analysis.
Ultrasound-assisted extraction
Polyphenols from WMB flour were extracted using ultrasonic bath along with pulse sweep energy (USC-100, Titus) at 40 kHz. Extraction of WMB flour (1 g) in 40 ml of different solvents for required time–temperature combination as suggested by CCFCD of RSM was done (Table S1–S5). The mixture was centrifuged at 4000g for 5 min and supernatant was concentrated at 40 °C using a rotary evaporator and the residue was re-suspended with 5 ml of methanol (80 % v/v). All extracts were kept in amber coloured vials under refrigerated conditions until further analysis of total antioxidant activity as a response variable.
Total antioxidant activity (TAA)
TAA was evaluated by phosphor-molybdenum method (Prieto et al. 1999). Ascorbic acid was used as standard curve and results were expressed as μmol ascorbic acid equivalents per gram of sample (μmol AAE/g).
Preliminary experiment
The four extraction solvents (acetone, ethanol, methanol and water) were selected to find out the optimized conditions for extraction of polyphenols. The experimental range of these solvent concentrations was determined using one factor design. WMB flour (1 g) was subjected to the ultrasound extraction of polyphenols by using 40 ml of different organic solvents (acetone, ethanol and methanol) in range of 5–100 % (% v/v; solvent/water) at 50 °C for 65 min. Subsequently, the solvent concentration of 30–90 %, 10–40 % and 5–35 % v/v for acetone, ethanol and methanol, respectively was selected on the basis of maximum TAA detected. Distilled water (100 %) was also evaluated for extraction of polyphenols. Extraction temperature (ET) ranged from 40 to 60 °C was selected since this range was proven to be the best for obtaining maximum TAA by using various solvents (Liyana-Pathirana and Shahidi 2005; Dent et al. 2015). In last step, a series of extraction were also performed for the duration of 10–300 min at 50 °C to find the best extraction duration (ED). The ED ranged from 40 to 120 min, 30 to 90 min, 30 to 90 min and 120 to 240 min, respectively, for acetone, ethanol, methanol and water was selected.
Experimental design
After evaluating the preliminary experimental range of extraction variables, CCFCD of RSM was used for optimizing three independent variables i.e. solvent concentration (% v/v), ET (°C), and ED (min) with the specific ranges (Table S1) to evaluate the TAA as a response variable. The coded and corresponding actual values were used to determine the actual levels of variables with 20 experiments for acetone, ethanol and methanol models and 13 experiments for water model as given in Table S2–S5.
Optimization and validation of the model
‘Design expert software (version 8.0.7.1, Stat-Ease, Inc., Minneapolis, MN) was used for regression and graphical analysis of the data obtained. The optimum values of the selected independent variables were obtained by using ANOVA, regression equation and response surface contour plots. The experiments were performed in triplicate at suggested optimized conditions to validate the models. The obtained experimental values were compared with theoretically predicted values to verify the prediction power of models.
Comparison of UAE with CSE method
The cotyledon and hull mix were obtained after de-hulling of WMB followed by grinding into flour (60 mesh). The UAE of WMB, hull and cotyledon flour was done by using optimum conditions obtained in previous section for all models. In CSE method, the optimum conditions were used without ultrasound to extract polyphenols from WMB, hull and cotyledon flour.
Analysis of polyphenols
TPC of different extracts was determined by using folin-ciocalteu assay (Velioglu et al. 1998). Gallic acid was used as calibration standard and results were calculated as mg of gallic acid equivalents per gram of sample (mg GAE/g).
TFC of different extracts was determined using colorimetric method as described by Chang et al. (2002). Quercetin was used as a standard compound to construct a standard curve and results were expressed as Quercetin equivalent (mg QE/g sample).
HPLC analysis
Individual polyphenols of different extracts obtained from WMB, hull and cotyledon flour were quantified by using High Performance Liquid chromatography (Model: 1260 infinity, Agilent Technologies, USA) equipped with diode array detector (DAD), quadratic pump and auto-sampler. The separation was carried out by using zorbax 300SB C18 reverse phase column (4.6 mm × 150 mm, 5 µm particle size) at 35 °C. The extracts were pre-filtered using a 0.22 µm membrane and 5 µl of sample volume was injected into the HPLC system. The mobile phase consisted of two solvents: HPLC grade water acidified with 0.1 % acetic acid (A) and 100 % HPLC grade acetonitrile (B). The solvent gradient in volume ratios was as follows: 2 % (B) at 0 min,; increased 2 % B to 40 % B in 0–20 min, then it was increased to 100 % (B) in 20–25 min and decreased to 5 % (B) in 25–30 min at 0.8 ml/min flow rate. The photodiode array detector was operated at multiple wavelengths ranging from 190 to 800 nm for acquiring the spectra. The individual polyphenols were identified by comparing retention times (tR) and UV spectra of the unknowns with the standards and quantified from peak area at 280 nm. The standards used for identification were GA, FA, CAT, p-CoA, CA, SA, CHA, QE, LU, PCA, VA, RS and T-SB.
Analysis of antioxidant activity
TAA of different extracts was evaluated as reported earlier.
The FRP of different extracts was determined using colorimetric method as described by Oyaizu (1986). Ascorbic acid was used as standard and the reducing power was expressed as μmol ascorbic acid equivalents per gram (μmol AAE/g).
DPPH radical scavenging activity of different extracts was estimated by using colorimetric method as described by Brand-Williams et al. (1995). The results were expressed in percentage reduction (%).
Statistical analysis
All values were expressed as mean ± standard deviation. Statistical analysis was performed by using Minitab Statistical Software (MINITAB® v 14.12.0, State College, PA) and Tukey Honest Significant Differences test was used to determine the significant differences between group means at p < 0.05. The correlation between the variables was determined by Pearson correlation test.
Results and discussion
Optimization of ultrasound-assisted extraction
Modelling and fitting the model
Experimental and predicted TAA of WMB is reported in Table S6. Experimental and predicted values indicated that models developed for different solvents were appropriate. ANOVA showed that TAA data best fitted with quadratic polynomial models for all the solvents (Table S7). The regression coefficients of the linear, quadratic and interaction terms of all solvent models are reported in Table S8. All the models directly correlated with the measured data and were statistically significant. High R2 values (>97 %), insignificant lack-of-fit (p > 0.05) and adjusted R2 close to R2 indicated that the models were appropriate and can be used for efficient extraction of polyphenols.
Acetone, ethanol and methanol concentration was observed to be the major factor influencing TAA, both in linear and quadratic terms (Table S8). TAA increased with increase in the acetone, ethanol and methanol concentration up to 50.47, 26.53 and 22.98 % v/v, respectively, but, further increase in concentration caused the negative effect (Fig. 1a–f). This was consistent with earlier findings (Dahmoune et al. 2015; Liyana-Pathirana and Shahidi 2005). Water resulted into increase in polarity of the solvents and surface area of cell material due to the swelling that may have resulted into more extraction of polyphenols (Singh et al. 2012; Tan et al. 2013). The results reflected that the aqueous solvents were better for extraction of antioxidant compounds than pure solvents and greatly depended upon polarity of solvent.
Response surface plots of whole mung bean showing the effect of solvent, temperature and extraction duration on TAA (a acetone concentration and temperature at 80 min; b ethanol concentration and temperature at 60 min; c methanol concentration and temperature at 60 min; d acetone concentration and duration at 50 °C; e ethanol concentration and duration at 50 °C; f methanol concentration duration at 50 °C)
ET showed a highly significant effect on TAA in linear terms, for acetone, methanol and water models followed by ethanol model (Table S8). TAA increased with increasing the ET up to 56 °C for acetone model (Fig. 1a) and up to 54 °C for ethanol and methanol models (Fig. 1b, c). This was followed by a decrease in TAA. Solubility and diffusion rate of polyphenols may be increased with increase in temperature due to decrease in viscosity of extraction solvent followed by possible loss due to decomposition. (Singh et al. 2012; Bi et al. 2013; Carciochi et al. 2014). TAA for water model was maximum (39.48 μmol AAE/g) at 60 °C which may be due to more extraction of thermally stable polyphenols (Singh et al. 2012). ET showed highly significant effect in quadratic term on TAA for methanol model followed by acetone model. Ethanol and water models showed less pronounced quadratic effect of ET on TAA (Table S8).
ED showed the highly significant linear and quadratic effect, for water model and linear effect for acetone model on TAA (Table S8). ED showed only linear effect on TAA for methanol model (Table S8). TAA increase with increase in ED up to 71 min and 196 min, respectively in ethanol and water models, followed by a decline (Fig. 2b, d). Lower yield during extraction at higher temperature for prolonged duration may be associated with polymerization or degradation of thermal sensitive polyphenols (Liyana-Pathirana and Shahidi 2005; Dent et al. 2015; Parmar et al. 2016). Acetone and methanol models exhibited the positive relationship between TAA and ED with the highest TAA at maximum ED (Fig. 2a, c). Among various models studied, solvent concentration and ET interaction effect on TAA for methanol was significant. While ET and ED interaction effect on TAA was significantly higher for acetone model followed by ethanol and water models.
Response surface plots of whole mung bean showing the effect of temperature and extraction duration on TAA (a temperature and duration at 60 % v/v acetone concentration; b temperature and duration at 25 % v/v ethanol concentration; c temperature and duration at 20 % v/v methanol concentration; d temperature and duration at 100 % water)
Optimization and validation of the models
The optimum UAE conditions of TAA for different models are given in Table 1. Experimental values were very close to the predicted values which indicated that models developed were appropriate. The highest TAA was found for acetone (51.93 ± 0.60 μmol AAE/g) followed by ethanol (47.45 ± 0.85 μmol AAE/g), methanol (44.67 ± 0.69 μmol AAE/g) and water (37.55 ± 0.74 μmol AAE/g) (Table 1).
Comparison of UAE with CSE method
The efficiency of UAE for extraction of polyphenols from WMB, hull and cotyledons was compared with CSE on the basis of TPC, TFC, TAA, FRP, and DPPH radical scavenging activity (Table 2). TPC and TFC of WMB and fractions of different solvents were higher for UAE as compared to CSE (Table 2). The higher extraction of polyphenols with ultrasound treatment may be associated with breakdown of mung bean cell walls into small fragments due to the cavitation power of ultrasound (Kanatt et al. 2011).
TPC
TPC of WMB, hull and cotyledon differed significantly (Table 2). ANOVA showed significant differences between fractionations, solvents and treatment on TPC (Table S9). Ultrasound treated WMB and fractions showed higher TPC as compared to conventional extraction. Ultrasonic extraction showed an increase in TPC to the extent between 11.58 and 99 % for hull, 35.43 and 71.18 % for cotyledon and 5.44 and 59.13 % for WMB in comparison to similar extract of CSE. Different solvent extracts showed that TPC ranged from 16.05 ± 0.47 to 79.65 ± 0.83 mg GAE/g for hull, 7.62 ± 0.30 to 15.33 ± 0.71 mg GAE/g for cotyledon and 14.06 ± 0.55 to 31.31 ± 0.68 mg GAE/g for WMB. Hull showed significantly higher TPC as compared to cotyledon and WMB, consistent with earlier results reported by Luo et al. (2016). CSE of extracts from WMB and cotyledon using different solvents did not show significant variation in TPC. Acetone extract with UAE showed higher TPC as compared to other which is shown in boxplot (Fig. S1). TPC of both WMB and fractions extracts obtained using water was significantly lower than that of organic solvent extracts.
TFC
Mung bean fractions had highly significant effect on TFC followed by treatment and solvents (Table S9). TFC for hull extracted with different solvents was higher than cotyledon (Luo et al. 2016). Solvents did not show significant effect on TFC for cotyledon. TFC of WMB and fractions extracted with ultrasound treatment was higher as compared to conventional extraction. UAE increased TFC of hull, WMB and cotyledons in range from 7.95 to 378.43 %, 25.86 to 137.51 % and 15.36 to 63.56 %, respectively. Hull extracted with acetone using ultrasound showed the highest TFC of 1.40 ± 0.05 mg CE/g (Table 2). Like TPC, different extracts showed the lower TFC when extracted using water. Boxplot clearly shows that water was poor solvent for flavonoids extraction (Fig. S1). The results reflected that hull contained higher polyphenols and acetone was the best solvent for their extraction.
Phenolic compounds
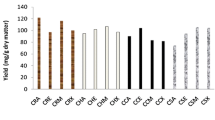
Polyphenolic profile of WMB and fractions extracted using different solvents under conventional and ultrasonic treatments is reported in Table 3 and Fig. 3.
HPLC chromatograms of hull, cotyledons and whole mung bean at 280 nm wavelength. The peaks correspond to: 1 gallic acid; 2 protocatechuic acid; 3 catechin; 4 chlorogenic acid; 5 caffeic acid; 6 vanillic acid; 7 p-coumaric acid; 8 ferulic acid; 9 sinapic acid; 10 resveratrol; 11 quercetin; 12 luteolin; 13 trans-stilbene; UAE ultrasound assisted extraction; CSE conventional solvent extraction
Hydroxybenzoic acid derivatives
The hydroxybenzoic acid viz. GA, PCA, CHA and VA showed tR of 2.20 ± 0.02 min, 2.52 ± 0.04 min, 7.16 ± 0.1 min and 9.28 ± 0.04 min, respectively. Both CSE and UAE treated extracts showed GA, PCA, CHA and VA content in range from 29.68 ± 0.68 to 84.29 ± 0.76 μg/g, 411.22 ± 1.64 to 3414.40 ± 2.80 μg/g, 57.39 ± 1.06 to 142.43 ± 1.02 μg/g, and 0.84 ± 0.01 to 40.30 ± 0.25 μg/g, respectively (Table 3). ANOVA results revealed highly significant variation of GA, PCA, CHA and VA between fractions, solvents and treatment. GA varied highly with treatment followed by fraction and solvents. VA and CHA showed the highest difference among fractions followed by treatment and solvents (Table S10).
Ultrasound treated mung bean fractions showed higher GA content than CSE. GA content was increased between 5.51 and 62.79 % in ultrasound treated extracts. Ultrasonic treated hull showed that the highest extraction of GA in acetone (61.68 ± 0.98 μg/g) followed by methanol (57.11 ± 0.85 μg/g), ethanol (53.69 ± 0.71 μg/g) and water (47.78 ± 0.61 μg/g). Cotyledon and WMB methanol extract using ultrasound showed the higher GA (52.52 ± 0.53 μg/g and 84.29 ± 0.76 μg/g, respectively) than other solvents (water, ethanol and acetone). Hull extract of acetone showed an increase in PCA content with ultrasound treatment, whereas, reduction was recorded for hull extracts of ethanol and methanol as compared to similar extract from CSE. The highest PCA content (3414.40 ± 2.80 μg/g) was observed in ultrasonic treated WMB extract of acetone followed by conventionally extracted cotyledon acetone extract (2287.71 ± 1.91 μg/g). CHA content was differentially modulated by different solvents and treatments in different fractions. Hull acetone extraction using ultrasound reported the highest CHA content (142.67 ± 0.80 μg/g). Ultrasound increased CHA content of hull in acetone and water extracts, whereas, a decrease in methanol extract as compared to similar extract of CSE. WMB acetone extract exhibited the highest amount of CHA (136.48 ± 0.84 μg/g), whereas, the lowest value (91.62 ± 1.00 μg/g) was recorded for ultrasound treated water extract. Cotyledon showed the lowest CHA content in comparison to hull and WMB. VA was only recorded in hull and WMB extracts in range from 0.84 ± 0.01 to 31.94 ± 0.40 μg/g and 3.41 ± 0.32 to 40.30 ± 0.25 μg/g, respectively. Ultrasound treated hull acetone extract demonstrated a significant increase (534.25 %) in VA as compared to similar extract of CSE. Other solvents indicated a reduction in VA content with ultrasound treatment.
Hydroxycinnamic acid derivatives
CA, p-CoA, FA and SA standards of hydroxylcinnamic acid showed tR at 7.81 ± 0.06 min, 10.05 ± 0.05 min, 11.3 ± 0.1 min and 11.6 ± 0.1 min, respectively. CA, p-CoA, FA and SA content were present in range from 20.44 ± 0.01 to 66.24 ± 0.08 μg/g, 291.74 ± 0.20 to 326.91 ± 0.25 μg/g, 37.22 ± 0.32 to 1634.54 ± 1.81 μg/g and 1034.33 ± 0.21 to 3258.62 ± 27.66 μg/g), respectively in different extracts (Table 3).
Different fractions showed lower CA content than other hydroxycinnamic acid derivatives (Table 3). ANOVA results showed that CA varied significantly amongst solvents, fractions and treatment (Table S10). A significant increase in CA was observed with ultrasound treatment in hull extracts of acetone, methanol and water. The ultrasound treatment increased the CA content of cotyledon extract of acetone and ethanol, whereas, a decrease was recorded in cotyledon extract of methanol and water. p-CoA content was highest for ultrasound treated hull extract of acetone and water. Ultrasound treated hull extract of ethanol showed a decrease in p-CoA as compared to similar extract of CSE which may be due to either degradation of p-CoA or decrease in solubility of p-CoA in ethanol. Cotyledon ethanol, methanol and water extracts also showed decrease in p-CoA as compared to similar extract of CSE. The higher amount of p-CoA in mung bean was also reported by Yao et al. (2013). WMB acetone and ethanol extracts had the highest p-CoA content followed by methanol and water extracts. FA and SA varied significantly amongst solvents, fractions and treatment. Fraction showed the highest variations in both phenolic acids (Table S10). The highest FA and SA content was found in hull followed by WMB and cotyledon. Ultrasound treated hull acetone, methanol and water extracts showed higher FA and SA content as compared to similar extract of CSE. UAE treated hull acetone extract contained the highest FA (634.54 ± 1.81 μg/g), SA (3258.62 ± 27.66 μg/g), CA (66.24 ± 0.08 μg/g) and p-CoA (326.91 ± 0.25 μg/g) content. Hence, results clearly indicated that hull contained the maximum amount of hydroxylcinnamic phenolic acids and acetone was found to be the best solvent for extraction of hydroxylcinnamic phenolic acids in combination with ultrasound.
Flavonoids and stilbene
The flavonoids viz. CAT, QE, and LU showed tR of 5.8 ± 0.2 min, 16.27 ± 0.06 min, and 16.37 ± 0.05 min, respectively and stilbene viz. RS and T-SB at 14.56 ± 0.02 min and 26.25 ± 0.03 min respectively. QE, LU, CAT and RS varied significantly between fraction and treatment whereas, solvent showed less pronounced effect. RS varied significantly with fraction followed by solvents and treatment. QE and CAT varied significantly with fraction and treatment followed by solvents, whereas, LU did not show variation among solvents (Table S10). Different fractions ultrasonically extracted with different solvent contained higher QE, CAT, LU and RS content as compared to CSE, whereas, T-SB was increased only in ultrasound treated acetone extract of hull and cotyledon but reduction was recorded in all other extracts. QE and LU ranged from 1.10 ± 0.10 to 37.02 ± 0.72 μg/g and 1275.65 ± 0.38 to 1290.77 ± 0.21 μg/g, respectively, in various extracts of different fractions, whereas, CAT (2.69 ± 0.24–49.94 ± 0.76 μg/g) was detected only in hull and WMB (Table 3). Ultrasound treated hull acetone extract showed the highest QE content, whereas, LU content was the highest in ultrasound treated hull water extract. RS and T-SB content ranged from 6.81 ± 0.18 to 30.58 ± 0.61 μg/g and 57.20 ± 0.15–212.31 ± 0.65 μg/g, respectively, in various extracts of different fractions were present (Table 3). The highest RS and T-SB was found in hull followed by WMB and cotyledon. The highest RS content was present in ultrasound treated hull extract of acetone. The higher T-SB content was recorded in conventionally extracted hull water extract as compared to UAE which indicated that ultrasound caused the negative effect on T-SB.
Antioxidant activity
Antioxidant activity of different mung bean fractions determined using TAA, FRP and DPPH radical scavenging activity varied significantly (Table 2). ANOVA results showed significant difference of antioxidant activity (TAA, FRP and DPPH) between fractions, solvents and treatments. Solvents showed more pronouced effect on FRP followed by TAA and DPPH, whereas, treatment had the highest significant effect on DPPH and the lowest on FRP (Table S9). Ultrasound treatment increased the antioxidant activity which may be attributable to a higher amount of polyphenols extraction. TAA, FRP and DPPH radical scavenging activity were highly correlated with TPC and TFC. These results clearly indicated that the antioxidant activity is directly associated with TPC and TFC. This implied that an extract with higher TPC and TFC showed higher antioxidant activity and vice versa.
Hull exhibited the highest antioxidant activity followed by WMB and cotyledons. Kanatt et al. (2011) and Madhujith and Shahidi 2006 also demonstrated that hull of mung bean had higher antioxidant activity as compared to that from cotyledon. Hull extracted with acetone using ultrasonic treatment showed higher TAA (98.85 ± 0.73 μmol AAE/g), FRP (57.11 ± 0.74 μmol AAE/g) and DPPH radical scavenging activity (86.31 ± 0.57 %) as compared to other solvents. The high phenolic content and DPPH scavenging activity of mung bean hull as compared to cotyledon was also reported by Kanatt et al. (2011). TAA and DPPH radical scavenging activity of hull extracted using ethanol and methanol varied insignificantly for both UAE and CSE methods, whereas, FRP had significantly different electron donor properties to neutralize free radicals by forming stable products in both solvents. Acetone, ethanol and methanol extraction of cotyledons showed the highest value of TAA (43.01 ± 0.79 μmol AAE/g), FRP (11.30 ± 0.42 μmol AAE/g) and DPPH radical scavenging activity (36.49 ± 0.48 %), respectively. Yao et al. (2013) and Ramesh et al. (2011) also reported the DPPH scavenging activity of mung bean. Acetone extract of WMB showed the highest TAA (51.93 ± 0.60 μmol AAE/g) and DPPH radical scavenging activity (65.27 ± 0.35 %), whereas, ethnaol had the highest FRP (15.07 ± 0.49 μmol AAE/g) value when compared with methanol and water extracts. This might be due to qualitative and quantitative variation of polyphenols with different solvents, thus, they have different free radical scavenging capacity. Different acetone extracts showed that TAA and FRP were highly correlated with different polyphenols except PCA and T-SB, whereas, DPPH did not show significant correlation with PCA, T-SB and CHA. Different ethanol extracts showed that TAA and FRP were highly correlated with different polyphenols except GA, VA and T-SB, whereas, GA, VA, CHA and T-SB did not show any correlation with DPPH. Methanol and water extracts showed that GA, PCA, CA, VA and TSB contribute minimum to TAA, FRP and DPPH.
Conclusion
UAE was more efficient than CSE to extract polyphenols from different fraction of mung bean. Hull contained more polyphenols than cotyledon which contributed more antioixdant activity. Acetone was the best solvent to extract polyphenols. GA, PCA, CHA, VA, CA, p-CoA, FA, SA, CAT, QE, LU, and T-SB were present in WMB and fractions. Hydroxycinnamic phenolic acid was present in the highest amount followed by hydroxybenzoic phenolic acid, whereas, flavonoids and stilbene were observed to be the lowest.
References
Bi W, Tian M, Row KH (2013) Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J Chromatogr A 12:22–30
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28(1):25–30
Carciochi RA, Manrique GD, Dimitrov K (2014) Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J Food Sci Technol 52(7):4396–4404
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Dahmoune F, Nayak B, Moussi K, Remini H, Madani K (2015) Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem 166:585–595
Dent M, Dragović-Uzelac V, Elez Garofulić I, Bosiljkov T, Ježek D, Brnčić M (2015) Comparison of conventional and ultrasound-assisted extraction techniques on mass fraction of phenolic compounds from Sage (Salvia officinalis L.). Chem Biochem Eng Q 29(3):475–484
Girish TK, Pratape VM, Rao UJSP (2012) Nutrient distribution, phenolic acid composition, antioxidant and alpha-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products. Food Res Int 46:370–377
Ilaiyaraja N, Likhith KR, Babu GRS, Khanum F (2015) Optimisation of extraction of bioactive compounds from Feronia limonia (wood apple) fruit using response surface methodology (RSM). Food Chem 1173:348–354
Kanatt SR, Arjun K, Sharma A (2011) Antioxidant and antimicrobial activity of legume hulls. Food Res Int 44(10):3182–3187
Karami Z, Emam-Djomeh Z, Mirzaee HA, Khomeiri M, Mahoonak AS, Aydani E (2015) Optimization of microwave assisted extraction (MAE) and soxhlet extraction of phenolic compound from licorice root. J Food Sci Technol 52(6):3242–3253
Kim SK, Nair RM, Lee J, Lee S-H (2015) Genomic resources in mungbean for future breeding programs. Front Plant Sci 6:626
Liyana-Pathirana C, Shahidi F (2005) Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem 93:47–56
Luo J, Cai W, Wua T, Xu B (2016) Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiata L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem 201:350–360
Madhujith T, Shahidi F (2006) Optimization of the extraction of antioxidative constituents of six barley cultivars and their antioxidant properties. J Agric Food Chem 54(21):8048–8057
Nair RM, Yang R-Y, Easdown WJ, Thavarajah D, Thavarajah P, Hughes Jd’A, Keatinge J (2013) Biofortification of mungbean (Vigna radiata) as a whole food to enhance human health. J Sci Food Agric 93(8):1805–1813
Oyaizu M (1986) Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Parmar N, Singh N, Kaur A, Virdi AS, Thakur S (2016) Effect of canning on color, protein and phenolic profile of grains from kidney bean, field pea and chickpea. Food Res Int. doi:10.1016/j.foodres.2016.07.022
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant Capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Ramesh CK, Rehman A, Prabhakar BT, Avin BRV, Rao SJA (2011) Antioxidant potentials in sprouts vs. seeds of Vigna radiata and Macrotyloma uniflorum. J App Pharm Sci 1(7):99–103
Sahin S, Aybastıer O, Işık E (2013) Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem 141(2):1361–1368
Singh B, Sharma HK, Sarkar BC (2012) Optimization of extraction of antioxidants from wheat bran (Triticum spp.) using response surface methodology. J Food Sci Technol 49(3):294–308
Singh JP, Kaur A, Singh N, Nim L, Shevkani K, Kaur H, Arora DS (2016) In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT Food Sci Technol 65:1025–1030
Tan MC, Tan CP, Ho CW (2013) Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int Food Res J 20(6):3117–3123
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46:4113–4117
Wanga C, Zuo Y (2011) Ultrasound-assisted hydrolysis and gas chromatography–mass spectrometric determination of phenolic compounds in cranberry products. Food Chem 128(2):562–568
Yao Y, Yang X, Tian J, Liu C, Cheng X, Ren G (2013) Antioxidant and antidiabetic activities of black mung bean (Vigna radiata L.). J Agric Food Chem 61(34):8104–8109
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, B., Singh, N., Thakur, S. et al. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J Food Sci Technol 54, 921–932 (2017). https://doi.org/10.1007/s13197-016-2356-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2356-z