Abstract
A decline in northern pike (Esox lucius) abundance in the upper St. Lawrence River is thought to be related to hybrid cattail invasion and disruption of natural water-level periodicity leading to spawning and nursery habitat degradation. Channel-connectivity and spawning-pool excavations were implemented in a tributary to the upper St. Lawrence River to enhance spawning and nursery site complexity. We assessed the effects of excavations on pike by comparing percent survival (from advanced larvae to fall emigration), water quality factors, and larval diets at excavated and non-excavated sites. Lab-reared pike, 16 days post-hatch and 14 mm in length, were released at three site types: non-excavated, spawning pool, and channel. Percent survival in channels and spawning pools after a minimum of 26 days was nearly identical (2.4%), and greater than non-excavated sites (0.13%). Spawning pools displayed higher water temperatures and dissolved-oxygen concentrations than either channel or non-excavated sites. Larvae primarily consumed zooplankton and diets of larvae from spawning pools and non-excavated sites were similar, whereas larvae in channels consumed different taxa. Differences in diet were not reflected in survival, suggesting larvae were not prey-limited. Observed differences in percent survival among sites demonstrates created spawning pool and channel-connectivity sites enhanced conditions for pike.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal wetlands of the Laurentian Great Lakes serve as crucial habitats for many fishes during their spawning and nursery periods (Jude and Pappas 1992). Wetlands are typically warmer and more productive than their associated main water bodies and provide young-of-year (YOY) fishes ample forage, protection from predation, and ideal, warm conditions for rapid growth (Gutreuter et al. 1999; Nunn et al. 2007). Dynamics occurring during the larval and juvenile stages may influence the year-class strength of fish populations (Cushing 1990). Larval survival is influenced by several factors, including water quality factors (e.g. temperature, water level, and dissolved oxygen; Casselman and Lewis 1996), abundance and suitability of prey items (Mayer and Wahl 1997; Burrow et al. 2011), predation (Houde 1987; Skov et al. 2003), and growth (Houde 1987; Oele et al. 2019).
Northern pike (Esox lucius) are apex predators that use seasonally flooded wetlands for spawning and nursery habitats (Bry 1996; Casselman and Lewis 1996). In the St. Lawrence River, the spawning period is protracted, and begins in seasonally flooded meadows in early spring, transitions to nearshore areas of bays, and is completed in offshore sites in late spring (Farrell 2001; Farrell et al. 2006). The protracted spawning behavior is thought to occur in response to water-level management (Cooper et al. 2008), which has disrupted the natural hydroperiod, lowering spring water levels and altering wetland-plant communities (Wilcox and Xie 2007). Typha x. glauca is a robust cattail hybrid of T. angustifolia and T. latifolia that has expanded in the upper St. Lawrence River following water-level regulation (Rippke et al. 2010; Farrell et al. 2010b). Spawning northern pike prefer sedge and grass vegetation (Bry 1996), and tend to avoid cattail (Franklin and Smith 1963; Farrell 2001). The encroachment of T. x. glauca into sedge meadows and changes in the hydroperiod are thought to have cumulatively altered pike spawning distributions (Farrell 2001; Farrell et al. 2006).
Northern pike egg hatch is temperature dependent, occurring as early as 7 days post-spawn (Farrell et al. 2006; Cooper et al. 2008). Newly hatched larvae attach to vegetation via adhesive papillae and feed off their yolk sac for approximately 10 days, until exogenous feeding begins, but development is temperature dependent and therefore varies (Raat 1988). Larvae are gape-limited (Nilsson and Brönmark 2000) and consume primarily zooplankton until they are large enough to consume insects and other fishes. Larval diets consist of primarily copepods and cladocerans (Massa and Farrell 2019), and the coincidence of exogenous feeding and abundant zooplankton prey is crucial (Cushing 1974, 1990; Burrow et al. 2011). Larvae remain in nurseries for several weeks before emigrating to deeper areas (Casselman and Lewis 1996). Emigration may occur in response to declining water levels, which forces juvenile pike to exist closer in space to one another, increasing opportunities to be cannibalized (Skov et al. 2003; Nilsson et al. 2014), and lowering dissolved oxygen (Casselman 1978).
Declining catch rates of YOY pike during emigration have been documented in critical spawning and nursery habitats in the upper St. Lawrence River (Smith et al. 2007; Farrell et al. 2015). Declines in adults have also been observed and are believed to be related to poor juvenile recruitment (McCullough and Gordon 2015). Efforts to restore the population have focused on improving wetland connectivity and enhancing the amount of available spawning and nursery habitat (Neveldine et al. 2019). Excavation of dense stands of cattail is a technique that has been implemented in the Great Lakes and designs aim to create an interspersion of open water and vegetated habitats (Mathers and Hartley 1995; Vincent 1995). Channel-connectivity excavations reconnect main channels of rivers with their seasonally flooded sedge meadows and allow fauna access to these productive habitats (McKenna 2003b; Neveldine et al. 2019).
Environmental factors influence growth and survival of YOY pike (Mingelbier et al. 2008; Cucherousset et al. 2009) and ultimately affect juvenile recruitment (Oele et al. 2019). Evaluation of the environmental conditions created through habitat enhancement and the effects on YOY pike are critical to determining project success, and help develop and advance effective strategies for sustaining fish populations. The purpose of this study was to test whether conditions created following channel-connectivity and spawning-pool excavations improved larval pike survival. We evaluated this research question by (1) measuring water quality factors, larval diets, prey selection, and prey availability in excavated (channel-connectivity and spawning pool) versus non-excavated sites, (2) comparing relative abundance, size, and timing of emigration among wild and lab-reared pike, and (3) evaluating survival of stocked larvae to their fall juvenile stage.
Methods
Study Area
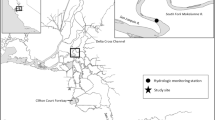
French Creek is a meandering, drowned river mouth tributary of the St. Lawrence River, approximately 8 km long and located near Clayton, NY. The watershed is sparsely developed, and French Creek possesses a broad floodplain including over 280 ha of extensive wetland habitat (Fig. 1). A 930-ha section of the watershed is protected and managed as a New York State Department of Environmental Conservation (NYSDEC) Wildlife Management Area (WMA). Large areas of submerged and emergent aquatic vegetation exist for fish spawning and nursery habitats, including seasonally flooded sedge meadows. Juvenile pike emigration has been monitored in French Creek since 2003 and declines in catch per unit effort (CPUE) have been observed (Farrell et al. 2015).
Excavations
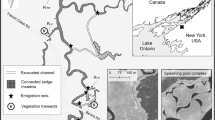
Beginning in 2008, two types of wetland excavations were implemented in the French Creek WMA with the goal of increasing YOY pike production. The work was planned in fulfillment of the Fish Habitat Conservation Strategy and in partnership with the U.S. Fish and Wildlife Service. Channel-connectivity excavations (abbreviated channels) were completed in 2010 and 2011 using an aquatic excavator with a cutting head that removed dense cattail mats blocking former channels, side-casting extracted material and reconnecting the main channel with remnant sedge meadows further upslope. Spawning-pool excavations (abbreviated pools) were completed by Ducks Unlimited in the winters of 2012 and 2013 using a long-arm excavator. The excavator removed sediment and vegetation, and created complexes of connected channels and pools within monotypic cattail stands, constructing new habitat. The complexes also were designed to create connectivity to remnant sedge meadows and increase diversity of emergent and submerged aquatic vegetation. Further detail on these habitat excavations are given in a companion study by Neveldine et al. (2019).
Sixteen spawning/nursery sites within French Creek were selected for this study, including eight excavated and eight non-excavated (Fig. 2). Non-excavated sites were defined as existing side channels within cattail stands that did not receive excavation. Non-excavated and excavated sites were in close proximity to each other throughout the entire drainage. The set of eight excavated sites comprised five pools and three channels. Excavations were geographically separate from one another where channels were present in the upper watershed and pools were present in the middle and lower watershed (Fig. 2).
Larvae Propagation and Marking
Adult pike spawners were caught in fyke nets at the mouth of French Creek between April 14 and April 17, 2016. Eggs were stripped from females and fertilized with milt from males using the dry method (Sorenson et al. 1966; Klingbiel 1986) at the Thousand Islands Biological Station. Fertilized eggs were incubated in hatching jars, which received a continuous flow of well water for 2 weeks. Following hatch, yolk-sac larvae were transferred to raceways and fed Artemia spp. for an additional 2 weeks after the transition to exogenous feeding. Ten days after hatching, larvae were marked with oxytetracycline (OTC) using the methods described by Fielder (2002). The OTC leaves a fluorescent ring visible on the otolith when removed, sectioned, and viewed under a transmitted ultraviolet light microscope under 100x-400x magnification (Farrell and Werner 1999). Twenty control larvae were euthanized by immersion in buffered Tricaine Methanesulfonate (MS-222) following OTC marking, and otoliths were viewed to validate mark success.
Larvae Stocking
Lab-reared larvae were stocked 16 days post-hatch at a mean length of 13.8 mm (sd = 0.65). All 16 sites were stocked on May 17 and 18, 2016, allowing a minimum of 26 days between release and capture of larvae in the field. Wetted marsh areas were estimated using Google Earth Pro, and stocking occurred at a rate of 3000 larvae per hectare of marsh (Table 1). Larvae were acclimated by the slow addition of water from the site to transport coolers, while monitoring temperature and dissolved-oxygen with a YSI ProDSS multiprobe. Larvae were distributed evenly throughout the site once temperatures in the coolers and the site equilibrated. Onset HOBO U26 dissolved-oxygen data loggers were placed in the middle of the water column at each site on the stocking day, and logged temperature (°C) and dissolved oxygen (mg/L) at 15 min intervals, 24 h a day.
Emigration Trapping
Peak emigration of juvenile pike occurs from June 1 to July 1 in upper St. Lawrence River tributaries (Farrell unpublished data) and trapping for this study occurred from June 13 to July 1, 2016. We used two types of traps: modified minnow traps and mini hoop nets. The minnow traps had a 2.54 cm throat and were wrapped with 0.8 mm nylon mesh. Traps were centered and sewn to the top of 0.8 mm nylon mesh wings, 200 cm in length on each side, and 122 cm in height. These traps are fished similar to hoop nets, and are buoyed with floats on the top line and weighted with lead core line on the bottom, to span the entire water column. The trap is staked with a reinforcing rod attached on the downstream end. Mini hoop nets were made of four connected hoops, 60 cm in diameter, with 2.54 cm throats, wrapped with 1.6 mm mesh, and outfitted with the same wing design as the modified minnow traps. Larger mesh size encouraged water flow through the trap to maintain high oxygen levels and protect fish. All distinct outlet channels for both non-excavated and channel sites were blocked. The large number of outlets (n = 21) from pools prevented complete coverage, so blocked outlets were selected using a random number generator. The opportunity for juvenile pike to leave via a different route (i.e., through the cattail) was possible but unlikely because the shallow water depth and possibility of stranding. Moreover, these trapping techniques are effective for sampling pike during the emigration period (Nilsson et al. 2014; Oele et al. 2019). We did not have enough traps of a single type to cover all sites and the decision to use two sampling gears was made to increase our sampling effort. Each trap was deployed at random to minimize any potential gear bias. Both types blocked outlets entirely and were assumed to catch pike at similar rates. Traps were checked daily, and all fish were identified to species and counted. Total length of all northern pike was measured (mm). One hundred and forty-eight juvenile pike were caught and 121 (81.8%) were retained in 95% ethanol for otolith examination. Differences in total length and emigration date of stocked and wild fish were compared using t-tests (α = 0.05). Catches of pike were compared among site types using a standardized metric of CPUE (pike/net night). Daily CPUE was calculated for each site type, averaged for the entire sampling period, and compared using one-way ANOVA (α = 0.05).
Otolith Mark Determination
To determine pike origin (wild or stocked), otoliths were dissected from retained pike (n = 121) and examined for OTC marks indicative of stocked larvae. Otoliths were removed by splitting the head laterally at the caudal portion of the braincase with scissors, to access the semi-circular canals. Otoliths were extracted with a fine-tipped probe and secured to a glass microscope slide with a thermo-polymer. Once dried, otoliths were hand sanded on both sides with 600-grit sandpaper followed by 1000-grit, until daily rings were visible, and viewed with an epi-fluorescent microscope. An Amscope B600 compound microscope with 4x-100x objectives (40x-1000x total magnification), outfitted with an EPI Fluorescence microscopy kit (510-nm dichroic mirror, 350–580-nm exciting filter, and 530-nm barrier filter), and a 100-watt mercury UV light source was used for viewing. The sagittal otolith was used for mark inspection and all otoliths were viewed by two observers. Marked otoliths displayed a gold ring that followed a daily growth ring near the center of the otolith. Wild northern pike were assumed to be those observed without an OTC mark. Each observer decided whether the otolith was marked or not and revealed the decision after viewing was completed. If observers disagreed, a second otolith was prepared and viewed using this same protocol.
Percent Survival Estimates
Survival was estimated using methods similar to those described by Farrell and Werner (1999) and Farrell (2001). First, we estimated the total number of surviving stocked fish (survivors) at each site (Estimate 1). Using OTC mark results, the proportion of survivors was calculated by dividing the number of survivors by the total number of retained fish. The proportion of survivors was multiplied by the number of released fish, to estimate the number of survivors that were released. The number of survivors was added to the number of released survivors:
-
(1)
Estimate 1 = (proportion survivors · # released) + # survivors
Juvenile northern pike were assumed to emigrate equally from all outlets. To estimate total number of survivors in sites where only some of the outlets were blocked, we divided Estimate 1 by the number of blocked outlets. Then we multiplied that estimate by the total number of outlets from the site (Estimate 2).
-
(2)
Estimate 2 = (Estimate 1 / blocked outlets) · total outlets
Long-term data from emigration surveys was examined prior to this study to identify the temporal distribution and peak timing of emigration over the entire season (May–August), which guided the sampling period selected for this study (Farrell, unpublished data). The proportion of emigration occurring during the sampling period was calculated to be 0.38. Estimate 2 was divided by 0.38 and multiplied by 1–0.38 (0.62) to estimate the survivors not sampled (outside the sampling period) and added to Estimate 2 to equal the total number of survivors for the entire season:
-
(3)
Estimate 3 = [(Estimate 2 / 0.38) · (0.62)] + Estimate 2
The estimated number of survivors (Estimate 3) was divided by the number of larvae stocked at each site and multiplied by 100 resulting in an estimated percent survival (Farrell and Werner 1999; Farrell 2001). Percent survival was combined for site type and a two-sample t-test was done to test for differences between percent survival occurring at pools and channels (α = 0.05). Statistical testing revealed no differences in mean percent survival between channels and pools (t(4) = 0.02, p = 0.98), so percent survival for these sites were combined to represent “excavated” sites. A two-sample t-test was done to test for differences between excavated and non-excavated sites (α = 0.05).
Water Quality Factors
Water temperatures (°C) and dissolved-oxygen concentrations (mg/L) were recorded using Onset HOBO U26 data loggers that were deployed during stocking and logged at 15-min intervals. Mean water temperature and mean dissolved oxygen were calculated for each site from the stocking day (May 17) to the end of emigration sampling (July 1), and combined for site type. Differences among site type were tested for using one-way ANOVA (α = 0.05). A logger placed in one of the channel sites (CH-1) malfunctioned during sampling, only collecting temperature data, and was omitted from the dissolved-oxygen analysis.
Larval Diet Analysis
Duplicate grab samples were taken using a three liter pitcher at each site concomitantly with stocking to examine the prey assemblage available to larvae. Effervescent tablets were used to carbonate water and anesthetize zooplankton. Zooplankton were then filtered through a 53-μm sieve and preserved in 95% ethanol. During processing, zooplankton were filtered out using a 53-μm sieve, rinsed with well water, and viewed using a Leica MZ 10x dissecting microscope. Zooplankton densities from duplicate samples were averaged and differences among site type were tested for using a one-way ANOVA (α = 0.05).
Stocking sites were revisited six days after stocking to recapture larvae using dipnets for diet analysis. Netters worked from canoes and focused their efforts on shallow, vegetated areas of the site. Total length (mm) of captured larvae were measured, and specimens were preserved in 95% ethanol. During processing, stomachs were removed using a fine-tipped probe and a Leica MZ 10x dissecting microscope. Stomach contents were rinsed and all prey items were counted and identified. All cladocerans were identified to lowest possible taxonomic group and copepods were identified to order. Ward and Whipple (1959) and Thorp and Covich (2001) were used as references for identification of all zooplankton.
Prey Availability and Selection
A hierarchical agglomerative cluster analysis was performed using the BOOTCLUS program (McKenna 2003a) to assess similarities in larval diets and available zooplankton assemblages. Raw zooplankton abundance data were analyzed using the Bray-Curtis similarity index and Unweighted Pair Group Method with Arithmetic Mean (UPGMA) with 1000 bootstrap samples to test for significance difference between clusters (α = 0.05). The cluster analysis results in a dendrogram which identifies distinct assemblages (clusters) and describes their spatial relationships.
Prey selection was evaluated using the Chesson index (Manly 1974; Chesson 1978, 1983). Selectivity coefficients (α) were calculated for prey items found in the diet:
where r = the proportion of prey i in the diet, p = the proportion of prey i in the environment, and m = the number of prey types (Manly 1974; Chesson 1978, 1983). Selectivity coefficients range from 0 to 1 where α ˂ 1/m indicates avoidance and α ˃ 1/m indicates selection. When α = 1/m, prey are consumed in proportion to their abundance in the environment (neutral selection). The Chesson index allows for comparisons in selectivity across spatial scales when the available prey items vary. Selectivity coefficients were calculated for individual larvae and averaged for site type. Selectivity coefficients were compared with neutral selection (1/m) to test for significance using a one-sample t-test (α = 0.05).
Results
CPUE and Percent Survival
The present study was conducted over an 18-day period in 2016 (June 13 – July 1), and while there were numerous sites available, 16 were used. Excavated channels and pools were created between 2010 and 2013, and 8 excavated (5 pools and 3 channels) and 8 non-excavated composed the study. A total of 148 juvenile pike were caught; 22 at non-excavated, 79 at pools, and 47 at channels. Sixty of the 121 (~50%) retained were determined to be stocked (Table 1). Length at emigration was not significantly different between stocked and wild fish (t(107) = 0.66, p = 0.51), and averaged 84.8 mm (sd = 14.6; Fig. 3). Date of emigration was not significantly different between stocked and wild fish (t(110) = 0.21, p = 0.83), and emigration was temporally-distributed across the sampling period (Fig. 4). CPUE was significantly different among site types (F(2,276) = 9.13, p < 0.001) and post hoc Tukey comparisons indicated CPUE was lower at non-excavated sites (mean ± 95% confidence interval = 0.13 ± 0.07) than pools or channels (Table 2). CPUE was not different between excavated site types and averaged 0.43 ± 0.18 at pools and 0.51 ± 0.2 at channels (Table 2).
Stocked pike were recovered at all pool and channel sites (Table 1) whereas stocked pike were recovered at only half of the non-excavated sites and no YOY pike (wild or stocked) were collected at two of the non-excavated sites (Table 1). No difference in mean percent survival (%) was observed between channels (2.36% ± 3.11) and pools (2.41% ± 2.45; t(4) = 0.02, p = 0.98). When pools and channel estimates were combined to represent “excavated sites”, percent survival at excavated sites was determined to be higher (2.39% ± 1.79) than at non-excavated sites (0.13% ± 0.11; t(7) = 2.47, p = 0.04). One pool (SP-4) and one channel (CH-2) had much higher percent survival than other sites of that type. Percent survival was 7.3% (179 fish) at SP-4 and 5.46% (34 fish) at CH-2.
Water Quality Factors
Differences in water temperature among site types were detected (F(2,941) = 139.6, p < 0.001) and post-hoc Tukey comparisons revealed that water temperatures were highest in pools (mean ± 95% confidence = 21.7 °C ± 0.29), followed by channels (19.9 °C ± 0.37), and lastly non-excavated sites (17.8 °C ± 0.27). The mean water temperature from May 17 to July 1 at SP-4 was lower than all other pools, averaging 19.2 °C. The next lowest mean water temperature among pools was recorded at SP-3 (20.3 °C), more than 1 °C warmer than the mean at SP-4. SP-4 also had the lowest minimum (11.4 °C) and the lowest maximum (25.9 °C) temperatures on record for the sampling period. In contrast, SP-5 had the next lowest minimum temperature (12.2 °C) and SP-3 had the next lowest maximum temperature (27.1 °C), a 1.2 °C difference. Overall SP-4 was cooler than the other pool sites. The mean water temperature at CH-2 was 18.8 °C, between the means at CH-3 (20.1 °C) and CH-1 (18.2 °C). Lowest minimum and maximum temperatures at CH-2 were also in-between those values at CH-1 and CH-3, where CH-1 was the coolest channel and CH-3 was the warmest.
Significant differences in dissolved-oxygen concentrations were detected among site types (F(2,258) = 147.5, p < 0.001), and post-hoc Tukey tests revealed that dissolved oxygen was highest in pools (6.15 mg/L ± 0.36) followed by non-excavated sites (4.36 mg/L ± 0.35), and lastly channels (1.15 mg/L ± 0.33). Further comparisons of dissolved oxygen among pools and among channels did not explain among differences in survival, possibly due to logger malfunction and stationary position of the loggers.
Larval Diets and Prey Selection
A total of 56 pike larvae were captured for diet analysis, 25 from non-excavated sites, 11 from channels, and 20 from pools. Zooplankton densities (number per liter) were not different among site types (F(2,29) = 0.71, p = 0.50), and ranged from 78 to 122 per liter. The cluster analysis identified three distinct zooplankton assemblages existing among larval diets and the environment (Fig. 5). The assemblage in the environment (Cluster A) was not different at any of the sites, suggesting there was no difference in the zooplankton available to larvae despite differences in stocking locations. Cluster A was dominated by Bosmina longirostris (55.1%) and copepod nauplii (30.5%; Fig. 5). The diets of larvae recovered in non-excavated and pool sites (Cluster B) were not different and were dominated by Simocephalus spp. (29.1%) and cyclopoid copepods (28.6%; Fig. 5). The diets of larvae recovered in channels (Cluster C) were dominated by Ceriodaphnia species (62.9%; Fig. 5).
Significant assemblages identified from grab samples and larval diets using the Bray-Curtis similarity index and UPGMA linkage method with 1000 random bootstrap samples. The dendrogram (a) displays the classification of assemblages, which are indicated with a unique letter and an asterisk (*). The stacked bar chart (b) displays the percent contribution of each prey type to the assemblage. Prey taxa codes are as follows: AL, Alonella spp.; AM, Amphipoda; BO, B. longirostris; CA, Calanoida; CE, Ceriodaphnia spp.; CH, Chydoridae; CS, C. sphaericus; CY, Cyclopoida; DI, Diaphanosoma spp.; GA, Gastropoda; HY, Hydrachnida; NU, nauplii; OS, Ostracoda; PO, P. pediculus; SC, Scapholeberis spp.; SD, S. crystallina; SI, Simocephalus species
Differences in the zooplankton assemblage available to larvae (Cluster A) and the larval diets (Clusters B and C) were observed. Selection results indicated larvae recovered in non-excavated sites avoided B. longirostris, Chydorus sphaericus, and cyclopoid copepods (p < 0.05; Table 3) whereas selection of Diaphanosoma spp., Scapholeberis spp., and Simocephalus spp. did not differ from neutral (p > 0.05). Similarly, B. longirostris, C. sphaericus, and cyclopoid copepods were avoided by larvae from pools (p < 0.05; Table 3). Positive selection of Simocephalus spp. by larvae recovered in pools did occur (t(9) = 3.49, p = 0.008; Table 3). Mean selection of Simocephalus spp. by larvae recovered in non-excavated sites was relatively high (0.15; sd = 0.05), but not statistically different from neutral, possibly due to a low sample size. Larvae recovered in channels avoided chydorids, cyclopoid copepods, and Polyphemus pediculus (p < 0.05; Table 3). Mean selection of Ceriodaphnia spp. was positive (0.076; sd = 0.03) but did not differ from neutral (p > 0.05). Due to the nature of the index, selectivity coefficients could not be calculated for taxa not found in grab samples. For example, Simocephalus spp. was found in the stomachs of 5 of the 11 larvae recovered in channels (45%) but was not found in any grab samples. Similarly, Simocephalus spp. was found in the stomachs of 19 of the 25 larvae from non-excavated sites (76%), but only 2 of the 8 grab samples.
Discussion
Our results support the creation of connectivity enhancements as viable techniques for increasing survival of pike larvae in Great Lakes drowned river mouth wetlands as also suggested by Mathers and Hartley (1995) and Vincent (1995). In the present study, the creation of spawning-pool and channel-connectivity sites resulted in an increase of hatchery-reared YOY pike survival from 0.13% (non-excavated sites) to 2.39% (excavated sites). Excavations were rapidly colonized by spawners, as evident by the presence of wild YOY in emigration traps, suggesting excavations fulfilled spawning habitat requirements. The low abundance or complete absence of YOY pike at several of the non-excavated sites provided empirical data supporting concerns for low YOY production in cattail-dominated habitats. Zooplankton also displayed rapid colonization and have been documented elsewhere to quickly colonize following restoration projects (Dodson and Lillie 2001; Badosa et al. 2010). This study highlighted differences in some water quality factors, including temperature and dissolved-oxygen concentrations (summarized in Table 2), which may in part explain the variation in survival.
There are factors that were not addressed which could have affected survival, including location within the watershed, water depth, presence of predators, and other factors that varied longitudinally along the creek. For example, channels were located further upstream than pools, whereas non-excavated sites were located throughout the drainage. Most studies describing the longitudinal variation in physical, chemical, and biological factors of riverine systems have focused on large rivers such as the St. Lawrence River (Basu et al. 2000), versus the roughly 8-km long French Creek. Differences in water temperatures among site types did not seem to display a relationship to longitudinal gradient, as highest water temperatures were recorded in pools (downstream) followed by channels (upstream), both of which were warmer than non-excavated sites (throughout the drainage). Dissolved oxygen may have displayed a longitudinal pattern where highest concentrations were recorded in pools (downstream) followed by non-excavated (throughout), and lastly channels (upstream), but this pattern may have also been driven by differences in water depths. We describe our observations and acknowledge the limitations in determining the specific mechanisms responsible for the differences in survival.
Mean percent survival of larvae was similar in channels and pools, despite the lower temperatures and dissolved oxygen observed in channels. Dissolved oxygen is higher during the day because oxygen is quickly depleted in shallow, productive wetlands after nightfall, when photosynthesis is no longer occurring, and respiration continues. Channels were shallower than both non-excavated or pool sites and did experience anoxia (0 mg/L) overnight later in the survey. Dense sedge vegetation in channels may have also reduced photosynthesis via shading, and the upslope position of the channels may have prevented mixing between water in the site and more oxygenated water from the mainstem of French Creek. Oxygen steadily lowered throughout the sampling period but did increase to above 0 mg/L at all sites, each day. Northern pike are extremely tolerant of low-oxygen conditions, surviving in concentrations as low as 0.3 mg/L for short periods of time (Casselman 1978). Some St. Lawrence River wetlands are known to be devoid of oxygen by early summer (Farrell et al. 2014), and some sites in this study approached anoxia. Five juvenile pike recovered during this survey were dead upon arrival with gaping mouths and no signs of predation or disease, suggesting hypoxia occurred, although the reason for mortality is unknown. Pike in channels may have located an oxygenated refuge overnight and during other periods of low oxygen. Data loggers were only set at single locations and therefore could not effectively sample oxygen throughout the entire site. Nevertheless, low dissolved oxygen was a concern at channels. Future channel excavations should focus on creating habitat complexity and connectivity to increase water flow through channels and improve dissolved oxygen.
Temperature is an important factor influencing growth (Farrell et al. 2006; Oele et al. 2019) and year-class strength of YOY pike (Smith et al. 2007). Growth is a key determinant of YOY survival (Houde 1987) and a principal predictor of recruitment for pike, more so than either YOY or adult abundance (Oele et al. 2019). Optimum temperatures for linear growth of pike are between 22 and 23 °C for YOY and 19 °C for juveniles (Casselman 1978) and although degree differences may seem insignificant, modest shifts in temperature may reduce growth efficiencies, resulting in recruitment variability (Houde 1987; Oele et al. 2019). For example, the highest percent survival among pools occurred at SP-4, where the lowest average, minimum, and maximum water temperatures were also recorded. Temperatures at SP-4 may have been closest to optimal among the pools investigated, resulting in improved growth and survival. Warmer temperatures recorded at the other pools may have exceeded optimal, resulting in lower survival. There were no obvious explanations for the higher survival documented at CH-2 compared with the other channels gleaned from the temperature data. Nevertheless, warm temperatures observed in pools and channels may have promoted faster growth for YOY than cooler conditions in non-excavated sites.
Prey selection and avoidance occurred in all of the examined site types. Small zooplankton taxa such as Chydorus sphaericus and Bosmina longirostris were avoided by larvae, possibly due to their inadequate sizes. Pike larvae are gape-limited but prefer large prey (Massa and Farrell 2019) and likely consume small-sized taxa opportunistically rather than by active selection to conserve energy. Of particular interest was the discrepancy in larval diets originating from non-excavated and pool sites versus channels. Larvae from non-excavated and pool sites primarily consumed Simocephalus spp., whereas larvae from channels consumed Ceriodaphnia spp., although significant selection was only detected for larvae from pools. Selectivity coefficients for Simocephalus spp. could only be calculated for two non-excavated sites where Simocephalus spp. was represented in grab samples, and the detection of positive selection might have been impeded by a small sample size. A larger grab sample may have resulted in the inclusion of Simocephalus spp. and Ceriodaphnia spp., and is recommended for future studies. Simocephalus spp. are typically found in or around vegetated areas (Thorp and Covich 2001), which may have provided shelter during grab sampling, although the samples were taken throughout the entire water column, in locations that were representative of the site. The diet differences of larvae in channels and larvae in non-excavated and pool sites may in-part be explained by the low dissolved-oxygen concentrations recorded in channels, as feeding behaviors and conversion efficiency of pike are altered in low oxygen environments (Adelman and Smith 1970). Overall, Simocephalus spp. and Ceriodaphnia spp. were the dominant taxa in larval diets and possess several similarities. Both are large in size (0.5–3.0 mm lengths) and have large black eyes, which are likely very noticeable by predators. Simocephalus spp. and Ceriodaphnia spp. are both filter-feeders belonging to the Daphniidae family, which inhabit littoral zones, likely as a feeding grounds and for protection from predators (Amoros 1984). Simocephalus spp. can reach larger total lengths than Ceriodaphnia spp. (Thorp and Covich 2001), and are more closely associated with macrophytes whereas Ceriodaphnia spp. are more likely to be “free-swimming” (Fairchild 1981). Despite these few differences, these taxa are essentially analogous in their functional role as food for larval fish.
Zooplankton food limitation is thought to contribute to recruitment failure of YOY pike in the Baltic Sea (Ljunggren et al. 2010), which guided the inclusion of the prey selection examination in this study. Zooplankton densities, however, were not significantly different among site types, similar to previous findings in French Creek (Farrell et al. 2014). Wetlands in the upper St. Lawrence River do not appear to be nutrient-limited and are much more productive than the main river (Farrell et al. 2010a, 2014). Zooplankton limitation to pike is more likely to occur in deeper offshore sites associated with protracted spawning (Massa and Farrell 2019). Discrepancies in taxa consumed at channels versus pools and non-excavated sites did not mimic differences in survival, suggesting larvae in French Creek were not prey-limited.
Farrell (2001) estimated pike survival to be 84.1% from spawning to egg hatch and 0.00008–0.0001% from egg to fall juvenile in one St. Lawrence River bay, whereas Forney (1968) estimated 77% of northern pike eggs were viable following spawning but only 0.03% of eggs survived to the post-larval stage (exogenous feeding; Dahlberg 1979). Mean percent survival estimates in the current study were greater than those of Forney (1968) or Farrell (2001), and ranged from 0.13% - 2.39% from larvae to fall juvenile. Larvae in this study were stocked following the transition to exogenous feeding, whereas Forney (1968) and Farrell (2001) observed naturally spawned eggs and larvae. The transition to exogenous feeding is thought to be a critical period for fishes, typified by high mortality (Hjort 1914; Bry et al. 1995), and larvae in the present study likely displayed higher survival because they were stocked following this critical period.
Density dependence affects pike growth rates and influences cannibalism (Skov et al. 2003; Grønkjær et al. 2004) and while we were unable to quantify density dependence, we controlled for it by stocking larvae at similar densities at all sites. This would have allowed stocked pike to exist in similar densities to other stocked pike. Several studies have suggested that stocking of pike where natural reproduction occurs is not successful (Skov and Nilsson 2007; Hühn et al. 2014), because of low genetic fitness and lasting hatchery effects (Skov et al. 2011). Adult spawners from the French Creek drainage were used as brood stock in this study, therefore progeny would have been spawned somewhere within the watershed where they were stocked. The French Creek pike population exhibits local genetic differences from other populations in the St. Lawrence River, possibly due to well-suited habitat and spatial isolation (Bosworth and Farrell 2006). Lab-reared pike were stocked early in the larval stage and at comparable sizes (~13 mm), which provided a long period of growth, lowering risks of cannibalism among stocked individuals (Grønkjær et al. 2004). The similar temporal distribution of emigration and similar total lengths of stocked and wild pike suggest hatchery effects were not detrimental to some stocked larvae in this study. Stocking of pike larvae at locations without a sustaining population has shown success (Vuorinen et al. 1998; Sutela et al. 2004), and we demonstrated successful survival of hatchery larvae to the fall juvenile stage. However, the presence of wild juvenile pike indicated spawners rapidly colonized the newly-created habitats.
Excavations fostered favorable conditions for larval pike survival, but entry into spawning and nursery habitats remains water-level dependent. Connectivity between rivers and ephemeral wetlands is created during spring flooding and naturally occurring water-level fluctuations enhance the abundance and quality of pike spawning habitat (Mingelbier et al. 2008). High spring water levels are positively associated with pike year-class strength (Johnson 1957; Smith et al. 2007) and YOY pike production (Vuorinen et al. 1998; Cucherousset et al. 2009). The YOY pike production in French Creek observed in 2016 was low when compared with historic numbers (Farrell et al. 2015) and water levels of the St. Lawrence River measured during 2016 at the Alexandria Bay, NY gauging station (NOAA buoy station ID: 8311062, http://tidesandcurrents.noaa.gov) were well below average. The abundance of adult pike in the upper St. Lawrence River has been declining since the 1980s (McCullough and Gordon 2015), and lower water-levels and degraded spawning habitat are believed to be contributors (Farrell 2001; Farrell et al. 2006; Smith et al. 2007). Furthermore, stabilized water levels may increase expansion rates of T. x. glauca (Boers and Zedler 2008). Vegetation serves as critical protection for pike during the larval and juvenile stages (Bry 1996; Skov and Berg 1999). Larval and juvenile pike will likely use any available vegetation for cover and may not prefer or avoid specific taxa but catches of larvae have been shown to be lower in areas where T. x. glauca was present (Timm and Pierce 2015). More natural water-level fluctuations, including high water in spring to increase connectivity and declining water levels throughout the growing season to reduce Typha (Farrell et al. 2010b), coupled with wetland excavations may provide the necessary environment for YOY northern pike production to increase.
Improved larval survival and CPUE observed at excavated sites demonstrate channel-connectivity and spawning pool sites were viable nursery habitats for northern pike during their early life stages. This study describes broad patterns observed in these excavation sites but fails to identify specific mechanisms promoting pike survival. Additional research is needed to identify which environmental factors have the greatest impact on larval survival in both channels and pools, and whether those same factors influence survival in non-excavated sites. Empirical results from monitoring of excavated sites is needed to adapt management recommendations. This study quantifies the success of wetland excavations in increasing percent survival of larval pike and the investigators recommend the continuation of wetland restoration and enhancement work in the upper St. Lawrence River.
References
Adelman JR, Smith LL (1970) Effect of oxygen on growth and food conversion efficiency of northern pike. Progressive Fish-Culturist 32:93–96
Amoros C (1984) Introduction pratique à la systématique des organismes des eaux continentales françaises - 5. Crustacés Cladocères. Bulletin Mensuel de La Société Linnéenne de Lyon 53:72–107
Badosa A, Frisch D, Arechederra A, Serrano L, Green AJ (2010) Recovery of zooplankton diversity in a restored Mediterranean temporary marsh in Doñana National Park (SW Spain). Hydrobiologia 654:67–82
Basu BK, Kalff J, Pinel-Alloul B (2000) Mid-summer plankton development along a large temperature river: the St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 57:7–15
Boers AM, Zedler JB (2008) Stabilized water levels and Typha invasiveness. Wetlands 28:676–685
Bosworth A, Farrell JM (2006) Genetic divergence among northern pike from spawning locations in the upper St. Lawrence River. North American Journal of Fisheries Management 26:676–684
Bry C (1996) Role of vegetation in the life cycle of pike. In: Craig JF (ed) Pike: biology and exploitation. Chapman and Hall, London, pp 45–67
Bry C, Bonamy F, Manelphe J, Duranthon B (1995) Early life characteristics of pike, Esox lucius, in rearing ponds: temporal survival pattern and ontogenetic diet shifts. Journal of Fish Biology 46:99–113
Burrow JF, Horwood JW, Pitchford JW (2011) The importance of variable timing and abundance of prey for fish larval recruitment. Journal of Plankton Research 33:1153–1162
Casselman JM (1978) Effects of environmental factors on growth, survival, activity, and exploitation of northern pike. American Fisheries Society Special Publication 11:114–128
Casselman JM, Lewis CA (1996) Habitat requirements of northern pike (Esox lucius). Canadian Journal of Fisheries and Aquatic Sciences 53:161–174
Chesson J (1978) Measuring preference in selective predation. Ecology 59:211–215
Chesson J (1983) The estimation and analysis of preference and its relationship to foraging models. Ecology 64:1297–1304
Cooper JE, Mead JV, Farrell JM, Werner RG (2008) Potential effects of spawning habitat changes on the segregation of northern pike (Esox lucius) and muskellunge (E. masquinongy) in the upper St. Lawrence River. Hydrobiologia 601:41–53
Cucherousset J, Paillisson JM, Cuzol A, Roussel JM (2009) Spatial behaviour of young-of-the-year northern pike (Esox lucius L.) in a temporarily flooded nursery area. Ecology of Freshwater Fish 18:314–322
Cushing DH (1974) The natural regulation of fish populations. In: Harden-Jones FR (ed) Sea fisheries research. John Wiley and Sons, New York, pp 399–412
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. In: Larson SE, Lowry D (eds) Advances in marine biology. Academic Press, San Diego, pp 249–293
Dahlberg MD (1979) A review of survival rates of fish eggs and larvae in relation to impact assessments. Marine Fisheries Review 41:1–12
Dodson SI, Lillie RA (2001) Zooplankton communities of restored depressional wetlands in Wisconsin, USA. Wetlands 21:292–300
Fairchild GW (1981) Movement and microdistribution of Sida crystallina and other littoral microcrustacea. Ecology 62:1341–1352
Farrell JM (2001) Reproductive success of sympatric northern pike and muskellunge in an upper St. Lawrence River bay. Transactions of the American Fisheries Society 130:796–808
Farrell JM, Werner RG (1999) Distribution, abundance, and survival of age-0 muskellunge in upper St. Lawrence River nursery bays. North American Journal of Fisheries Management 19:309–320
Farrell JM, Mead JV, Murry BA (2006) Protracted spawning of St Lawrence river northern pike (Esox lucius): simulated effects on survival, growth, and production. Ecology of Freshwater Fish 15:169–179
Farrell JM, Holeck KT, Mills EL, Hoffman CE, Patil VJ (2010a) Recent ecological trends in lower trophic levels of the international section of the St. Lawrence River: a comparison of the 1970s to the 2000s. Hydrobiologia 647:21–33
Farrell JM, Murry BA, Leopold DJ, Halpern A, Rippke MB, Godwin KS, Hafner SD (2010b) Water-level regulation and coastal wetland vegetation in the upper St. Lawrence River: inferences from historical aerial imagery, seed banks, and Typha dynamics. Hydrobiologia 647:127–144
Farrell JM, Bachman C, Looi A, LaPan S, Regan M, Runner J, Schulz K, Mitchell MJ, Gibbs J, Leopold DJ (2014) Coastal fisheries habitat restoration in the St. Lawrence River: documenting project efficacy. Final Report Submitted to Ducks Unlimited and the National Oceanographic and Atmospheric Administration (NOAA) Coastal Fisheries Habitat Restoration Program
Farrell JM, Satre N, Augustyn EA (2015) Northern pike research, monitoring, and management in the Thousand Islands section of the St. Lawrence River. In 2015 NYSDEC Annual Report, Bureau of Fisheries Lake Ontario Unit and St. Lawrence River Unit to the Great Lakes Fishery Commission’s Lake Ontario Committee
Fielder DG (2002) Methodology for immersion marking walleye fry and fingerlings in oxytetracycline hydrochloride and its detection with fluorescence microscopy. Michigan Department of Natural Resources Fisheries Technical Report 2002-01: 1–21
Forney JL (1968) Production of young northern pike in a regulated marsh. New York Fish and Game Journal 15:143–154
Franklin DR, Smith LL Jr (1963) Early life history of the northern pike, Esox lucius L., with special reference to the factors influencing the numerical strength of year classes. Transactions of the American Fisheries Society 92:91–110
Grønkjær P, Skov C, Berg S (2004) Otolith-based analysis of survival and size-selective mortality of stocked 0+ year pike related to time of stocking. Journal of Fish Biology 64:1625–1637
Gutreuter S, Bartels AD, Irons K, Sandheinrich MB (1999) Evaluation of the flood-pulse concept based on statistical models of growth of selected fishes of the upper Mississippi River system. Canadian Journal of Fisheries and Aquatic Sciences 56:2282–2291
Hjort J (1914) Fluctuations in the great fisheries of northern Europe, viewed in the light of biological research. Rapports et Procès-Verbaux des Réunions du Conseil Permanent International pour l'Exploration de la Mer 20:1–228
Houde ED (1987) Fish early life dynamics and recruitment variability. American Fisheries Society Symposium 2:17–29
Hühn D, Lübke K, Skov C, Arlinghaus R, Taylor E (2014) Natural recruitment, density-dependent juvenile survival, and the potential for additive effects of stock enhancement: an experimental evaluation of stocking northern pike (Esox lucius) fry. Canadian Journal of Fisheries and Aquatic Sciences 71:1508–1519
Johnson FH (1957) Northern pike year-class strength and spring water levels. Transactions of the American Fisheries Society 86:285–293
Jude DJ, Pappas J (1992) Fish utilization of Great Lakes coastal wetlands. Journal of Great Lakes Research 18:651–672
Klingbiel J (1986) Culture of purebred muskellunge. American Fisheries Society Special Publication 15:273–378
Ljunggren L, Sandström A, Bergström U, Mattila J, Lappalainen A, Johansson G, Sundblad G, Casini M, Kaljuste O, Eriksson BK (2010) Recruitment failure of coastal predatory fish in the Baltic Sea is related to an offshore system shift. ICES Journal of Marine Science 67:1587–1595
Manly BFJ (1974) A model for certain types of selection experiments. Biometrics 30:281–294
Massa EA, Farrell JM (2019) Prey selection by larval northern pike (Esox lucius) exposed to different zooplankton assemblages representing seasonally-flooded wetland and nearshore bay habitats. Limnol Oceanogr 64:1200-1213
Mathers A, Hartley K (1995) Sawquin Creek marsh channel creation project. In: Kelso J, Hartig, J (eds) Methods of modifying habitat to benefit the Great Lakes ecosystem. CISTI (Canadian Institute of Science Technical Information) Occasional Paper No. 1, pp 197–199
Mayer CM, Wahl DH (1997) The relationship between prey selectivity and growth and survival in a larval fish. Canadian Journal of Fisheries and Aquatic Sciences 54:1504–1512
McCullough RD, Gordon DJ (2015) Thousand Islands warmwater fish stock assessment. In: New York State Department of Environmental Conservation. Lake Ontario Annual Report 2015 (St. Lawrence River), Section 6, pp 1–19
McKenna JE (2003a) An enhanced cluster analysis program with bootstrap significance testing for ecological community analysis. Environmental Modelling and Software 18:205–220
McKenna JE (2003b) Community metabolism during early development of a restored wetland. Wetlands 23:35–50
Mingelbier M, Brodeur P, Morin J (2008) Spatially explicit model predicting the spawning habitat and early stage mortality of northern pike (Esox lucius) in a large system: the St. Lawrence River between 1960 and 2000. Hydrobiologia 601:55–69
Neveldine BL, Leblanc JP, Farrell JM (2019). Vegetation response and juvenile northern pike (Esox lucius) outmigration following connectivity enhancement of a Typha dominated coastal wetland. Wetlands https://doi.org/10.1007/s13157-019-01145-y
Nilsson PA, Brönmark C (2000) Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88:539–546
Nilsson J, Engstedt O, Larsson P (2014) Wetlands for northern pike (Esox lucius L.) recruitment in the Baltic Sea. Hydrobiologia 721:145–154
Nunn AD, Harvey JP, Cowx IG (2007) Benefits to 0+ fishes of connecting man-made waterbodies to the lower river Trent, England. River Research and Applications 23:361–376
Oele DL, Gaeta JW, Rypel AL, McIntyre PB (2019) Growth and recruitment dynamics of young-of-year northern pike: implications for habitat conservation and management. Ecology of Freshwater Fish 28:285–301
Raat, AJP (1988) Synopsis of biological data on the northern pike Esox lucius Linnaeus, 1758. FAO Fisheries Synopsis No. 30: 178 pp
Rippke MB, Distler MT, Farrell JM (2010) Holocene vegetation dynamics of an upper St. Lawrence River wetland: Paleoecological evidence for a recent increase in cattail (Typha). Wetlands 30:805–816
Skov C, Berg S (1999) Utilization of natural and artificial habitats by YOY pike in a biomanipulated lake. Hydrobiologia 408/409:115–122
Skov C, Nilsson PA (2007) Evaluating stocking of YOY pike Esox lucius as a tool in the restoration of shallow lakes. Freshwater Biology 52:1834–1845
Skov C, Jacobsen L, Berg S (2003) Post-stocking survival of 0+ year pike in ponds as a function of water transparency, habitat complexity, prey availability and size heterogeneity. Journal of Fish Biology 62:311–322
Skov C, Koed A, Baastrup-Spohr L, Arlinghaus R (2011) Dispersal, growth, and diet of stocked and wild northern pike fry in a shallow natural lake, with implications for the management of stocking programs. North American Journal of Fisheries Management 31:1177–1186
Smith BM, Farrell JM, Underwood HB, Smith SJ (2007) Year-class formation of upper St. Lawrence River northern pike. North American Journal of Fisheries Management 27:481–491
Sorenson L, Buss K, Bradford AD (1966) The artificial propagation of esocid fishes in Pennsylvania. Progressive Fish Culturist 28:133–141
Sutela T, Korhonen P, Nyberg K (2004) Stocking success of newly hatched pike evaluated by radioactive strontium (85Sr) marking. Journal of Fish Biology 64:653–664
Thorp JH, Covich AP (2001) Ecology and classification of North American freshwater invertebrates, 2nd edn. Academic Press, San Diego
Timm AL, Pierce RB (2015) Vegetative substrates used by larval northern pike in rainy and Kabetogama Lakes, Minnesota. Ecology of Freshwater Fish 24:225–233
Vincent J (1995) Toronto Islands northern pike spawning habitat. In: Kelso J, Hartig, J (eds) Methods of modifying habitat to benefit the Great Lakes ecosystem. CISTI (Canadian Institute of Science Technical Information) Occasional Paper No. 1, pp 139–144
Vuorinen P, Nyberg K, Lehtonen H (1998) Radioactive strontium (85Sr) in marking newly hatched pike and success of stocking. Journal of Fish Biology 52:268–280
Ward HB, Whipple GC (1959) Freshwater biology, 2nd edn. Wiley, New York
Wilcox DA, Xie Y (2007) Predicting wetland plant community responses to proposed water-level-regulation plans for Lake Ontario: GIS-based modeling. Journal of Great Lakes Research 33:751–773
Acknowledgments
Funding was provided by the Fish Enhancement, Mitigation, and Research Fund administered by the U.S. Fish and Wildlife Service (FWS), New York Field Office, Cortland, NY and the Environmental Protection Fund (contract # AM10165) administered by the NYSDEC. Excavations were funded by the FWS and Ducks Unlimited. We thank the 2016 TIBS crew for their hard work during the field season. We are grateful especially to Jay Palumbo who assisted in otolith dissection, preparation, and viewing. This research is a contribution of the Thousand Islands Biological Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Massa, E.A., Farrell, J.M. Improving Habitat Connectivity in a Typha-Dominated Wetland Shows Increased Larval Northern Pike Survival. Wetlands 40, 273–286 (2020). https://doi.org/10.1007/s13157-019-01177-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01177-4