Abstract
Cattails (Typha latifolia L., Typha angustifolia L., and Typha x glauca Godr.) are the predominant emergent vegetation of upper St. Lawrence River coastal wetlands. We sought to describe Holocene vegetation in a St. Lawrence River wetland to assess patterns of succession and examine the timing and potential causes of a historic cattail invasion. Paleoecological analysis indicated presence of four distinct wetland vegetation stages, including a shallow water marsh (8240 YBP to 5160 YBP), a variable-depth aquatic plant community with adjacent alder (5160 YBP to 1610 YBP), a shallow sedge community (1610 YBP to 100 YBP), and a robust emergent marsh (100 YBP to present). The record of pollen tetrads demonstrated cattail presence throughout the history of the marsh, but a rapid increase in relative abundance of Typha cf. angustifolia/Sparganium monads indicated major expansion of robust emergent plants beginning near the peak of agricultural activity (ca. 1880 AD) and reaching modern levels around 1940 AD. Increase in the abundance of robust emergents in this wetland occurred decades before regulation of St. Lawrence River water levels and were contemporary with increased sedimentation and changes associated with the early agricultural period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cattails (Typha latifolia L., Typha angustifolia L., and Typha x glauca Godr.) are considered robust emergent species, and the latter two forms are considered invasive taxa (Grace and Harrison 1986) that can displace sedge-dominated wetlands following alteration of hydrologic regimes (Wilcox et al. 1984), addition of nutrients (Woo and Zedler 2002), or the deforestation of wetlands (Janssen 1967). Extensive homogenous stands of cattail dominate most upper St. Lawrence River wetlands (Geis and Kee 1977). Several robust emergent species of the genus Sparganium are native to the St. Lawrence River ecosystem and also often occupy an ecological niche similar to that of Typha species. Recent photointerpretation of wetlands in the region indicated a significant shift (since the late 1950s) in vegetation community composition from sedge meadow to dominance of the invasive cattails, T. angustifolia and T. x glauca (Cooper et al. 2008; Wilcox et al. 2008; Farrell et al. 2010).
Short-term hydrologic changes, from interannual variation to decadal cycles, have a major effect on the vegetation of Great Lakes coastal wetlands (Keddy 1983; Keddy and Reznicek 1986; Wilcox and Meeker 1991; Wilcox 1995; Chow-Fraser et al. 1998). Reducing hydrological variability of lakes and rivers decreases sedge dominated meadow marsh areas, resulting in more homogenous vegetation communities (Jansson et al. 2000; Keddy 2000), a trend which was observed in wetlands of the Upper St. Lawrence River and Lake Ontario following the completion of the St. Lawrence Seaway project in 1958 (Patch and Busch 1984; Wilcox et al. 2008; Farrell et al. 2010). The interannual and decadal (30–35 years) water level cycling of the Upper St. Lawrence River and Lake Ontario was reduced after regulation (Wilcox et al. 2008), and models based on field analysis of wetland zonation predict that current regulation will continue to select against meadow marsh communities and promote expansion of invasive cattail (Wilcox and Xie 2007; Farrell et al. 2010).
While analysis of historical aerial photos can provide information on the relationship between human activities and vegetation change beginning in the 20th century AD (Gottgens et al. 1998; Wilcox et al. 2008), the effects of human disturbance to these wetlands before this time are more poorly documented and understood. Additionally, the role of water levels in controlling wetland vegetation succession on a temporal scale of millennia is variable and unclear. Long-term patterns of sea level rise (analogous to the isostatic water level rise in the Great Lakes) have been proposed as the primary cause of observed long-term vegetation community changes in tidal marshes (Clark and Patterson 1985; Thomas and Varecamp 1991). However, Orson and Howes (1992) suggested that tidal coastal wetlands with direct connections to open water bodies develop from autecological processes, while wetlands with restricted outflows change mainly in response to allogenic factors such as storm-related sedimentation events.
Paleoecological methods can provide a long-term context for recent changes by revealing the history of succession and climate-induced vegetation dynamics. The analysis of fossilized pollen grains preserved in sediment cores indicates the relative abundance of the vegetation at the time of deposition (Moore et al. 1994). Analysis of sediment TOC and TON can indicate processes of peat accumulation and nutrient dynamics, respectively, and bulk density is used to examine changes in sediment composition (mineral vs. organic). Sediment C:N ratios can reveal whether organic matter is derived from algae or from vascular plants (Talbot and Laerdal 2000). The pollen record, coupled with sediment chemistry, offers a long-term account of vegetation change that can describe trends in allogenic and autogenic wetland succession (Singer et al. 1996).
This paleoecological study focused on Holocene vegetation dynamics of St. Lawrence River non-tidal freshwater coastal wetland successional processes and the effects of anthropogenic disturbances. Our objectives were to 1) determine the chronology of vegetation change within the wetland throughout the Holocene and 2) interpret wetland responses to disturbance in the context of long-term successional processes, including possible perturbations that may have led to a cattail-dominated wetland.
Study Area
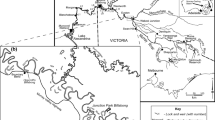
Goose Bay (44°21′ N, 75°52′ W) is located on the southern shore of the upper St. Lawrence River, Jefferson County, New York, USA. Cranberry Creek and Little Cranberry Creek watersheds drain into Goose Bay and have created 177 ha of emergent wetland (Fig. 1). The Goose Bay study site is protected from the direct river current by a bedrock peninsula and island up-river, creating a location suitable for the preservation of the stratigraphic sedimentary record.
Vegetation within Goose Bay marsh is similar to other wetlands in the Upper St. Lawrence River (Geis and Kee 1977). The wetland perimeter contains a narrow shrub layer typically composed of arrowood (Viburnum dentatum L.), dogwood (Cornus spp.), and meadowsweet (Spiraea alba Du Roi). Sedge (Cyperaceae) and grass (Poaceae) emergents are relatively uncommon in the wetland and occur in a narrow band down slope from the shrub layer. Cattail marsh composed primarily of T. x glauca and T. angustifolia is the principal vegetation type in Goose Bay, dominating up to the edge of the open water channel with an average density of 24 stems/m2 (Beland 2003). Along channel edges, vegetation consists of floating leaved plants including water lilies (Nuphar lutea (L.) Sm. and Nymphaea odorata Aiton) along with invasive European frog’s-bit (Hydrocharis morsus-ranae L.). Submersed vegetation includes Eurasian watermilfoil (Myriophyllum spicatum L.) and common bladderwort (Utricularia macrorhiza Leconte).
The Goose Bay wetland is largely surrounded by a substantial wooded buffer, consisting primarily of white pine (Pinus strobus L.), hemlock (Tsuga canadensis (L.) Carr.), and swamp white oak (Quercus bicolor Willd.). In the upland areas surrounding the wetland, gneiss bedrock outcrops and Rhinebeck soil in glacial lake sediments and Chatfield loam soils in glacial till predominate, with an average depth to bedrock of about 50 to 100 cm (McDowell 1989).
Methods
Paleoecological Reconstruction
Core Collection
To locate a coring location with both a long sedimentary record and a protected interior location within the cattail marsh, a sediment thickness map was created by probing peat depths with fiberglass extension rods along transects through the wetland. A 10 m long core was extracted from Goose Bay on 14 October 2002 with a 4 cm diameter square-rod piston corer (Fig. 1). Material from a second 1.5 m core, taken near the location of the first core, was used for all analyses to this depth, replacing near-surface sediments compressed during the extrusion of the 10 m core. The first meter of material (0–100 cm depth) was sliced into 1 cm sections (n = 100), and 1 cm thick sections were removed every 5 cm from core depths between 101 and 1,000 cm (n = 144).
Dating and Chronology
Sediments deposited within the past 150 years were dated using the radionuclide 210Pb abundance (Goldberg 1963). Core samples from the top meter were 210Pb dated by the Great Lakes Water Institute at the University of Wisconsin, Milwaukee. The Constant Rate of Supply Model (CRS) was used to calculate ages for sediments in 1-cm depth increments (Appleby and Oldfield 1978). This model is considered suitable for dating of peat profiles, particularly where the sediment deposition rate has varied over time (Appleby 2001; Turetsky et al. 2004).
Older sediments were radiocarbon-dated by The University of Arizona Accelerator Mass Spectrometry Laboratory. Ten 1-cm bulk sediment samples from the core, taken at 100 cm intervals between depths of 150 to 850 cm, as well as at 916 and 964 cm depths, were submitted for radiocarbon dating (Table 1). CALIB™ version 5.0.1 computer software was used to calibrate 14C dates to dendrocalendar years (Stuiver et al. 1998; Stuiver et al. 2005). Calibrated dates were corrected to represent years before 2002 AD (YBP) by adding 50 years to dates before interpolation, so that they would be comparable to and merge seamlessly with 210Pb dating. Calibrated dates from adjacent samples were linearly interpolated to approximate ages for depths 150–964 cm and to estimate sediment accumulation rates (Webb and Webb 1988). The oldest 210Pb age and the 150 cm-depth calibrated date were used for the interpolation of sediment age from depths 41–150 cm, and the ages of sediments from 964–1,000 cm depth were extrapolated.
Organic Matter, TOC, TON, and Bulk Density
Percent organic matter was estimated with loss-on-ignition following water content measurements (Bickelhaupt and White 1982). The oven-dried samples (n = 244) were placed in a muffle furnace and heated to 470°C for 14 h, placed in desiccators to cool for 30 min, and final weights were recorded to the nearest μg.
Sediment total organic carbon (TOC) and total organic nitrogen (TON) were analyzed at 5 cm intervals along the entire core (n = 165). Samples (0.5 cm3) were freeze-dried and ground to a uniform powder using a Wig-L-Bug™ ball-mill. The TOC and TON of ground samples were measured with a Carlo Erba™ NC2500 Elemental Analyzer. Simple linear regressions were performed using corrected sediment age versus percent organic matter, TOC, TON, and C:N ratios, to detect down-core trends.
Bulk density (g/cm3) was measured at depths 1–100 cm (n = 100) and 106–1,000 cm (n = 144) by weighing a known volume of sediment, obtained by pressing a plastic tube (1 cm diameter, 5 cm length) of known weight into the core to extract 5 cm3 of sediment, drying the sample at 60°C for 10 days, and weighing the sample and tube (Berglund 1986).
Peat Composition Analysis
The peat matrix was visually inspected for texture and plant fibers, and approximately every meter a ∼10 mL sample was dispersed in water and inspected under a dissecting microscope (40x) to provide a qualitative description of plant macrofossils.
Pollen Analysis
Sediment samples (1 cm3) were removed from the top 40 cm in 1 cm intervals (n = 40), and then every 10 cm to a depth of 896 cm (n = 69). The pollen grains and spores in the samples were concentrated using standard potassium hydroxide, acetolysis, and hydrofluoric acid techniques (Moore et al. 1994), and counted at 400x magnification to a sum of 300 grains, including upland, wetland, and aquatic types. Pollen was identified using keys (Kapp 1969; McAndrews et al. 1973; Moore et al. 1994) and reference pollen type slides. Upland pollen was presented as a percentage of the sum of total land plants (TLP) and wetland pollen was presented as a percentage of the sum of TLP, indeterminate-source taxa, and total wetland and aquatic plants (equal to the main pollen sum) following Bennett and Willis (2002).
Pollen from the cattail family (Typhaceae) occurs as monads, tetrads, and polyads. Nearly all pollen produced by T. angustifolia and the majority of pollen produced by T. x glauca consist of monads (single grains), which are very similar to Sparganium pollen (Finkelstein 2003). These taxa were not differentiated but were instead grouped as “Typha cf. angustifolia/Sparganium” pollen. Cattail pollen tetrads and polyads were identified as T. latifolia (Finkelstein 2003). To assist in defining wetland pollen zones, a stratigraphically constrained hierarchical cluster analysis was performed on the wetland pollen dataset using CONISS software (Grimm 1987, 2004). Results were presented using Tilia and TGview software (Grimm 2004).
Results
Dating (210Pb and 14C) and Chronology
A depth-age model was derived using the Constant Rate of Supply (CRS) model of 210Pb accumulation, dating the lowest sample with detectable unsupported 210Pb activity at 290 YBP (40 cm). The CRS model produced older dates for most depths than the alternative Constant Initial Conditions model (CIC), with a mean difference of 22 years (28%) for the 40 dated samples. Given the relatively non-linear relationship between log210Pb activity and depth, the CRS model was considered more reliable (Appleby 2001) and was used to derive the final age-depth model (Fig. 2).
Ages derived from 14C techniques on the Goose Bay core yielded no dating inversions and no unreasonable dates (Table 1). Long-term Goose Bay wetland vertical accretion rates, calculated from the calibrated 14C dates (YBP), ranged from a maximum of 0.31 cm/year between 1611 and 1289 YBP to a minimum of 0.04 cm/yr between 9707 and 8415 YBP (Fig. 3). Recent accumulation rates derived from 210Pb dates ranged from a low of 0.02 cm/yr in the early 1700s AD (39 to 40 cm depth) to an extreme high of 0.92 cm/yr in the early 1970s AD (9 cm depth). Accumulation rates generally increased up-core from the lowest 210Pb dates with a small spike to 0.22 cm/yr at 100 YBP (23 cm). Rates reached maximum in the 1970s AD and remained above 0.2 cm from the 1970s AD to the surface of the core.
Organic matter (% by weight), TOC (% by weight), TON (% by weight), C:N (atomic) and vertical accretion (cm/year). Regression lines and R2 values refer to linear regressions of sediment properties vs. depth. Horizontal lines delimit pollen zones illustrated in Fig. 4
Sediment Description
The Goose Bay sediment record began with a basal layer of a bluish gray (Munsell Color: Gley2 4/5B) clayey mineral matter from 1,000 to 974 cm depth. At 974 cm, the clay transitioned to a 28 cm thick sandy layer with bark, woody debris, Potamogeton leaf fragments, and seeds of Carex, Typha, Sagittaria, and Potamogeton. A radiocarbon date of material in this layer gave a calibrated date of 9707 YBP. The sandy/organic layer then transitioned to a second layer of dark bluish gray clay between 946 and 927 cm. From 928 cm depth to around 288 cm, the sediment was largely organic. At 725 cm, macrofossils included several Potamogeton seeds as well as seeds of Carex, Eleocharis, and Najas flexilis. Between 650 and 550 cm peat was sapric and silty, with abundant Potamogeton leaf fragments and Najas flexilis oospores present at 600 and 605 cm. Between 288 cm and 100 cm, the organic matter was largely undecomposed, and macrofossils consisted of graminoid culms, Carex achenes, and pteridophyte leaf and root fragments. Live roots were present in the core from 49 cm to the top of the core but were largely concentrated in the top 7 cm, including a dead cattail rhizome at 5 cm depth.
Sediment Composition
Strong overall down-core trends of decreasing organic matter (r2 = 0.89), total organic carbon (TOC, r2 = 0.84), and total organic nitrogen (TON, r2 = 0.73) content were observed (Fig. 3). A weak down-core trend was detected in the sediment C:N (r2 = 0.31). The content of organic matter (2–97%), TOC (1–52%), and TON (<1–3%) was highly variable along the core.
A major anomaly in the decreasing down-core pattern of organic matter, TOC, and TON occurred when organic matter content decreased from 45% at 5100 YBP (638 cm) to a low of around 27% at 4800 YBP (577 cm) then rebounded to its pre-decline content at 4600 YBP (550 cm) (Fig. 3). TOC and TON content were also depressed during this time, with a TOC minimum of 13%, and minimum TON levels of 1% at around 4800 YBP. In this same timeframe, sediment C:N ratios were depressed (to a minimum of 10.6 at 4940 YBP). While sediment organic matter content and C:N were depressed during this timeframe (5000–4500 YBP, 630–535 cm depth), the rate of substrate vertical accretion was increased. Increased mineral sediment was also observed during the analysis of the pollen slide preparations.
A rapid increase in sediment organic matter content began around 1900 YBP (282 cm) rising from approximately 60% to plateau around 93% after 1450 YBP (200 cm depth). Also around 1900 YBP, sediment vertical accretion rate temporarily quadrupled (from 0.1 to 0.4 cm/yr), percent organic matter and TOC increased, and C:N ratios increased from 14.9 ± 2.5 to 22.6 ± 3.4. Several sharp changes were observed between ∼200 YBP (1800 AD, 35 cm) and the present. Annual accumulation rates of inorganic material increased from around 1 mg/cm2/yr prior to 230 YBP (1770 AD, 38 cm) to a peaks of 7.5 and 6.2 mg/cm2/yr at 100 YBP (1900 AD, 23 cm) and 73 YBP (1927 AD, 19 cm) respectively. After a period of decrease, a third peak of 7.2 mg/cm/yr was observed at 30 YBP (1970 AD, 10 cm) corresponding with a spike in accretion rate, organic matter accumulation rate, and TON accumulation rate from 35–25 YBP (1965–1975 AD). Bulk density was elevated by about 0.04 g/cm3 between 190–54 YBP (1812–1948 AD, 34–16 cm). C:N ratio rose between approximately 50 and 30 YBP (1950–1970 AD), corresponding with a period of increased organic matter, TOC, and decreasing bulk density (Fig. 3).
Pollen Analysis
Cluster analysis of the wetland source pollen data using CONISS indicated four wetland pollen zones (W1–W4, Fig. 4).
Pollen diagram showing relative abundances of pollen (black curves) and 5 X exaggeration of relative pollen abundances (stippled curves) from selected wetland species plus Poaceae from indeterminate source. CONISS dendrogram and zonation are based on wetland pollen abundances (excluding Poaceae). Chronology is based on linear interpolation among 210Pb and 14C dates
Zone W1 (8240 to 5160 YBP) showed increasing dominance of Poaceae pollen of unknown source, reaching a maximum of 74% of the total pollen sum at 5450 YBP (674 cm). Wetland source pollen made up approximately 10% of pollen in this zone, and was characterized by Cyperaceae (5–10% of total sum), T. latifolia (<5%), and Typha cf. angustifolia/Sparganium type (<5%) pollen, as well as small proportions (<2%) of Alnus, Ilex and aquatic plants such as Nuphar and Nymphaea.
In zone W2 (5160 to 1610 YBP), Cyperaceae and Typha pollen continued to make up the bulk of the wetland pollen (<10% and <5% of main sum, respectively), but the increase of Alnus pollen (to 5%) represented the most significant change. In the lower portion of this zone, there was a decrease in frequency and abundance of Nymphaea (water lily), Nuphar (spatterdock), and Myriophyllum (water milfoil) pollen, all of which increased (to ∼1–2%) in the upper two-thirds of the zone. Poaceae pollen relative abundance generally increased from the beginning of the pollen record (8300 YBP) to 5700 YBP then declined rapidly over the next 700 years to a minimum of around 10% relative abundance. Around 1900 YBP, Cyperaceae pollen abundance increased to 11%. Typha cf. angustifolia/Sparganium pollen also increased, but only after around 1800 YBP, and the increase (around 2%) was smaller than that of Cyperaceae pollen.
Zone W3 (1610–100 YBP) was characterized by an elevated abundance of Cyperaceae pollen that increased throughout the stage (as high as 32%) and the near absence of floating-leaved macrophyte pollen. Equisetum (horsetail) spores, virtually absent during the shallow water period of Zone W2, appeared regularly as the sedge marsh developed, attaining up to 3% relative abundance. Typha cf. angustifolia/Sparganium pollen relative abundance increased slightly from Zones W1 and W2, but still remained low (2–6%) throughout the sedge marsh stage. Typha latifolia pollen was present only in small amounts (<2%) during the sedge marsh stage and was found in abundances similar to Zones W1 and W2. Pollen and spores from other herbaceous plants commonly found in modern sedge meadows were at peak abundance during this timeframe, including Equisetum, Polygonum amphibium L. (water smartweed), Potentilla palustris L. (marsh cinquefoil), and Polypodiophyta. Ambrosia pollen first increased significantly in this zone, rising to over 2% around 1710 AD (∼290 YBP, 39 cm depth) and rising sharply to above 10% after 1830 AD (∼170 YBP, 32 cm depth).
An abrupt increase in Typha cf. angustifolia/Sparganium pollen occurred in Zone W4 (100–0 YBP), indicating a rapid transition to a robust emergent marsh (Fig. 5). Typha latifolia pollen type increased from <1% to 16%, and Typha cf. angustifolia/Sparganium pollen increased from 11% to 80% relative abundance over a time span of only a century. Typha cf. angustifolia/Sparganium pollen abundance increased most rapidly from 1900 to 1940 AD (23–17 cm), after which the increase slowed and stabilized near modern high levels (between 67% and 80%), while the T. latifolia pollen type continued to increase to the surface (Fig. 5).
Discussion
Early Aquatic System
Increased organic content of the sediment indicates that a wetland or productive aquatic system developed at the study site around 9000 YBP. Entomophilous (insect-transported) pollen of Nuphar and Nymphaea throughout Zones W1 and W2 in combination with low levels of Cyperaceae and Typha pollen and a mixture of aquatic and emergent macrofossils indicates that the coring site was a shallow open water bay for most of the period between 8200–1900 YBP, with adjacent emergent wetland habitat characterized by sedges and cattails. Supporting this interpretation, sediment C:N ratios of 15–20 throughout most of this period suggest a mixture of algal and vascular plant tissues (Meyers and Lallier-Vergès 1999). The Poaceae pollen signal could represent either a strong regional signal from upland grass or a more local signal from nearby wetland grasses.
The decrease in TOC and percent organic matter from 5100 to 4200 YBP (640–480 cm) suggests an influx of inorganic sediment, and a decrease in C:N ratio to around 10 suggests higher algal or aquatic plant contribution to the organic fraction (Meyers and Lallier-Vergès 1999). Together with Potamogeton leaf fragments and Najas flexilis oospores and the lack of emergent species macrofossils in peat samples from 600 and 605 cm, these changes in sedimentary characteristics indicate deepening water during this period. In light of these sediment characteristics, decreased frequency of Nymphaea and Nuphar and increased Alnus and Sagittaria pollen may indicate that depth tolerances of Nymphaea and Nuphar were exceeded locally while Alder and Sagittaria colonized newly flooded habitats along the nearby upland margin. These changes in pollen and sedimentary characteristics correlate temporally with the Nipissing flood period (5728–4500 YBP) as described by Anderson and Lewis (1985), during which waters from the upper Great Lakes first began to drain through the lower Great Lakes, raising their water levels to 1 m above modern levels.
Frequent aquatic taxa pollen and abundant Poaceae in Zone W2 after the putative Nippissing period suggest that open water, possibly near an emergent zone dominated by grasses, appears to have characterized the study site until sedges increased around 1900 YBP. Macrofossils suggest varying depth or proximity to emergent habitat during this period, as the 382 cm sample (3000 YBP) consisted of mixed aquatic and emergent fossils while the 480 cm and 330 cm samples (4200 and 2300 YBP) consisted of fine aquatic sediments with Potamogeton leaves and seeds.
Transition to Sedge Wetland
Rapid increases in Cyperaceae pollen, organic matter content, TOC, vertical accretion rate, and C:N of the sediment at the transition from Zone W2 to W3 (∼1900 YBP) suggests a transition from a shallow aquatic sediments to sedge marsh peat. This interpretation is supported by the presence to fibric peat and emergent plant macrofossils (Carex and fern) in this zone, and the absence of aquatic plant macrofossils. The slowing rate of isostatic uplift may provide an explanation for the shift to a sedge dominated community at this time. Lake Ontario water levels have generally been increasing since deglaciation due to differentially greater isostatic rebound of the land surrounding the lake’s outlet in relation to the rest of the lake basin (Anderson and Lewis 1985; Reeder 1989). In the early to mid-Holocene, when rates of rebound were rapid, the rate of isostatic water level rise likely exceeded the rate of vertical accretion, slowing the process of infilling and prolonging the shallow water phase of the marsh. As rebound rates slowed in the late Holocene, sediment accumulation rates were able to surpass the rate of water level rise. Once sediments accumulated to near the water surface, around 1900 YBP, sedges were able to colonize. The high productivity of sedge communities (Bernard and Solsky 1977) and high C:N ratio of sedge litter (Enríquez et al. 1993) relative to shallow water aquatic sediments resulted in further increases in OM and TOC and further decreases in water depth.
Models of current isostatic rebound (Tushingham 1992; Koohzare et al. 2008) indicate that the difference in the rate of isostatic uplift between Goose Bay and the outlet of Lake Ontario, which should equal the rate of isostatic rebound-driven water level rise at the study site, is less than 0.1 cm/yr. Because sediment accumulation rates (≥0.2 cm/yr) well exceed this rate of increase, we suggest that climate-related hydrologic changes have been occuring since ∼1,900 years ago and will continue to be the primary abiotic factors influencing vertical accretion rates and thus vegetation succession at this site.
Post-Settlement: Anthropogenic Disturbance
While the transitions from shallow water marsh in Zones W1 and W2 to sedge marsh of Zone W3 occurred over several hundred years, the transition from sedge to a robust emergent dominated marsh began ca. 1880 AD (W4) and occurred on the scale of decades. Based on the low level of Sparganium (particularly around the coring site) and dominance of Typha in the Goose Bay area (Beland 2003), we suggest that Typha cf. angustifolia/Sparganium pollen in surface sediments most likely originates from T. angustifolia and T. x glauca. For deeper sediments, however, we cannot infer which taxon is the source of the pollen. The rapid transition to robust emergent marsh correlates temporally with the peak of Ambrosia (ragweed), considered an indicator of increased regional agriculture (Clark et al. 1996). 210Pb dating, which places the beginning of Ambrosia rise at 1830 AD and the peak at 1900 AD, agrees closely with historical records of deforestation and peak agriculture in Jefferson County (Hough 1854; Bond 1954; USDA National Agricultural Statistics Service 1997). Due to the temporal correlation of agricultural activity with the rise in Typha cf. angustifolia/Sparganium and T. latifolia pollen, anthropogenic disturbance associated with deforestation and the increase of agriculture in the region likely played a part in the shift to a robust emergent community.
While we cannot evaluate all of the disturbances that might have affected wetland vegetation during the period of agricultural development (e.g., re-introduction of beaver (Castor canadensis Kuhl.), livestock grazing in wetlands, changes in fire frequency), the paleoecological data presented here provide some insight into the importance of the most commonly observed drivers of robust emergent invasion of sedge marshes: sedimentation, eutrophication, introduction of invasive Typha, and hydrological changes (Werner and Zedler 2002; Woo and Zedler 2002; Wilcox et al. 2008).
Increased Sedimentation
Rising mineral sediment accumulation rates, relatively low organic matter content, high bulk density values, and pollen slide clay particles in the Goose Bay core between 34 and 19 cm (1810–1930 AD) indicate an influx of mineral soil corresponding temporally with the deforestation and agricultural period of the study area (Fig. 5). Similar layers of mineral sediment have been correlated with anthropogenic land-clearing events in coastal salt-marshes (McCaffrey and Thomson 1980), as well as in Lake Ontario embayments (Warwick 1980). The close temporal correlation between the peak mineral sediment accumulation rates in Goose Bay around 1900 AD (at the time of peak agriculture), Carex pollen decline and accelerated increases in Typha-type pollen suggests that sedimentation may have been a factor in the vegetation change in Goose Bay. Ecological literature also suggests that high rates of inorganic sediment capture and retention in wetlands can lead to vegetation change (Keddy 2000), either directly through burial of plants and seeds or indirectly through changing soil properties and topography (Werner and Zedler 2002). Field and mesocosm studies have shown that sedimentation can be one factor leading to dominance of wetlands by robust clonal species (Kercher and Zedler 2004) including Typha spp. (Rice et al. 2000; Werner and Zedler 2002; Byrd and Kelly 2006).
Studies on prairie wetland seed banks have shown that sediment loads as low as 0.25 cm can lower the number of species and the number of seedlings germinated (Jurik et al. 1994). Sedimentation negatively affects density and cover of at least some Carex species (Ewing 1996; Werner and Zedler 2002), but it appears to have no negative effect on mature cattail (Wang et al. 1994), which have significant underground rhizome reserves and pointed shoots thought to be specifically adapted to piercing sediment after burial (Keddy 2000). Similarly, Sparganium spp. have the potential to resprout from rhizomes, and their large seeds have been shown to be capable of germination after moderate burial (Gleason et al. 2003).
Eutrophication
Another possible mechanism for conversion to a robust emergent dominated marsh is the large influx of nutrients commonly associated with deforestation, agriculture, and human waste. Both Typha and Sparganium spp. are highly competitive taxa and respond vigorously to increased nutrients (Neely and Davis 1985; Woo and Zedler 2002). Nutrient concentrations, particularly phosphorus, in the Great Lakes and St. Lawrence River water began to increase slowly with deforestation and increased rapidly between 1940s and the 1970s (Schelske and Hodell 1995). Despite this record of regional eutrophication and the close correlation between agricultural land use and increased stream nutrient levels (Chen and Driscoll 2009), annual accumulation rates of peat and N in Goose Bay show only slight increases during the period of intense agricultural development (Fig. 5), providing little evidence for a substantial increase in nutrient availability in Goose Bay during that period. The increase in accretion rates and accompanying increase in N accumulation rates between 35 and 25 YBP (1965–1975 AD) could be a result of increased Typha biomass production driven by the mid 20th century eutrophication of the Lake Ontario and St. Lawrence system.
Invasive Typha
A third factor that may have caused changes in cattail dominance in this area is the arrival of T. angustifolia, concurrent with the increase in agriculture. Typha angustifolia is thought to have migrated into the Great Lakes region along canals in the late 1800s, breeding with the native T. latifolia as it moved west, resulting in the hybrid T. x glauca (Grace and Harrison 1986). The increase in Typha tetrads with rapid increases in Typha cf. angustifolia/Sparganium monads during the agricultural period could have been due to concurrent expansion of T. latifolia and T. angustifolia, or it might be primarily due to increases in T. x glauca, which produces up to 14% tetrad pollen along with abundant monads (Finkelstein 2003). Typha x glauca in particular could have increased partly in response to agricultural or other disturbances, as it responds vigorously to eutrophication (Woo and Zedler 2002) or hydrological stabilization (Shay et al. 1999). However, recent research suggests that it can invade and dominate even in relatively pristine habitats (Tuchman et al. 2009).
Water Level Regulation
Based on the timing of the increase of cattail pollen types in the Goose Bay core, Lake Ontario water level regulation beginning in the late 1950s could not have initiated the increase of robust emergents at this site. Around 1900 AD, when dramatic increase in cattail pollen began in Goose Bay, Lake Ontario/St. Lawrence water levels were rising from several years of low waters. Cattail pollen continued to increase through three high and two low water cycles between 1900 and 1950 AD, after which pollen levels stabilized. Cattail expansion during such a period of apparently natural hydrological cycling suggests that water levels were probably not the driving factor in the cattail increase at this site. However, dramatic increases in peat accretion rates between 1965 and 1975 AD do suggest that the stabilization of water levels after 1960 AD may have increased Typha dominance and biomass production at that time. A slight decrease in percent organic matter and an increase in inorganic deposition during this period suggest that disturbances in the watershed may also have played a part in this event.
Conclusions
Based on the gradual vegetation transitions seen in Goose Bay prior to the increase in robust emergents, vegetation changes prior to European settlement were probably not disturbance-driven. The long-term vegetation succession of Goose Bay wetland demonstrates an overall progression to shallower water depths representing succession slowed by isostatic rebound-driven water level rise. The timing of this expansion in relation to European settlement and land use changes suggests that sedimentation or other secondary effects of land-clearing and agriculture may have played a part in promoting a shift to cattail dominance in this St. Lawrence River marsh. Because the cattail expansion into the sedge marsh occurred decades before water level regulation in the Lake Ontario/St. Lawrence River system, regulation could not have caused the initial invasion.
References
Anderson TW, Lewis CFM (1985) Postglacial water-level history on the Lake Ontario Basin. In: Karrow PF, Calkin PE (eds) Quaternary evolution of the great lakes. Geological Association of Canada Special Paper, 30, St. John’s, Newfoundland, pp 231–253
Appleby PG (2001) Chronostratigraphic techniques in recent sediments. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments, volume 3: terrestrial, algal, and siliceous indicators. Kluwer Academic, Dordrecht, pp 171–203
Appleby PG, Oldfield F (1978) The calculation of lead-210 dates assuming a constant rate of supply of unsupported lead-210 to the sediment. Catena 5:1–8
Beland M (2003) Holocene vegetation dynamics of an upper St. Lawrence River coastal wetland and surrounding uplands: effects of climate change and anthropogenic disturbance. M.S. Thesis, State University of New York, College of Environmental Science and Forestry
Bennett KD, Willis KJ (2002) Pollen. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments, volume 3: terrestrial, algal, and siliceous indicators. Kluwer Academic, Dordrecht, pp 5–32
Berglund BE (1986) Handbook of holocene palaeoecology and palaeohydrology. Wiley, Chinchester
Bernard JM, Solsky BA (1977) Nutrient cycling in a Carex lacustris wetland. Canadian Journal of Botany 55:630–638
Bickelhaupt DH, White EH (1982) Laboratory manual for soil and plant tissue analysis. State University of New York College of Environmental Science and Forestry, Syracuse
Bond MC (1954) Changes in New York State Agriculture, 1850–1950, as indicated by state and federal censuses. Department of Agricultural Economics, Cornell University, Ithaca
Byrd K, Kelly M (2006) Salt marsh vegetation response to edaphic and topographical changes from upland sedimentation in a Pacific estuary. Wetlands 26:813–829
Chen X, Driscoll CT (2009) Watershed land use controls on chemical inputs to Lake Ontario embayments. Journal of Environmental Quality 38:2084–2095
Chow-Fraser P, Lougheed V, Le Thiec V, Crosbie B, Simser L, Lord J (1998) Long-term response of the biotic community to fluctuating water levels and changes in water quality in Cootes Paradise Marsh, a degraded coastal wetland of Lake Ontario. Wetlands Ecology and Management 6:19–42
Clark JS, Patterson WA (1985) The development of a tidal marsh: upland and oceanic influences. Ecological Monographs 55:189–217
Clark JS, Royall PD, Chumbley C (1996) The role of fire during climate change in an eastern deciduous forest at Devil’s Bathtub, New York. Ecology 77:2148–2166
Cooper JE, Mead JV, Farrell JM, Werner RG (2008) Coexistence of pike (Esox lucius) and muskellunge (E. masquinongy) during early life and the implications of habitat change. Hydrobiologia 601:41–53
Enríquez S, Duarte CM, Sand-Jensen K (1993) Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia 94:457–471
Ewing K (1996) Tolerance of four wetland plant species to flooding and sediment deposition. Environmental and Experimental Botany 36:131–146
Farrell JM, Murry BA, Leopold DJ, Halpern A, Rippke M, Godwin KS, Hafner SD (2010) Water-level regulation and coastal wetland vegetation in the upper St. Lawrence River: inferences from historical aerial imagery, seed banks, and Typha dynamics. Hydrobiologia 647:127–144
Finkelstein SA (2003) Identifying pollen grains of Typha latifolia, Typha angustifolia, and Typha × glauca. Canadian Journal of Botany 81:985–990
Geis JW, Kee JL (1977) Coastal wetlands along Lake Ontario and St. Lawrence River in Jefferson County, New York. State University of New York College of Environmental Science and Forestry, Syracuse
Gleason RA, Euliss NH Jr, Hubbard DE, Duffy WG (2003) Effects of sediment load on emergence of aquatic invertebrates and plants from wetland soil egg and seed banks. Wetlands 23:26–34
Goldberg ED (1963) Geochronology with 210Pb. Radioactive dating. International Atomic Energy Agency Symposium Proceedings, Vienna, pp 121–131
Gottgens JF, Swartz BP, Kroll RW, Eboch M (1998) Long-term GIS-based records of habitat changes in a Lake Erie coastal marsh. Wetlands Ecology and Management 6:5–17
Grace JB, Harrison JS (1986) The biology of Canadian weeds. 73. Typha latifolia L., Typha angustifolia L., and Typha x glauca Godr. Canadian Journal of Plant Science 66:361–379
Grimm EC (1987) CONISS: A FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Computers and Geosciences 13:13–35
Grimm EC (2004) Tilia (Version 2.0.b.4) and TGView (Version 2.0.2). Illinois State Museum, Springfield
Hough FB (1854) A history of Jefferson County. Sterling and Riddell, Watertown
Janssen CR (1967) Stevens pond: a postglacial pollen diagram from a small Typha swamp in northwestern Minnesota, interpreted from pollen indicator and surface samples. Ecological Monographs 37:145–172
Jansson R, Nilsson C, Dynesius M, Andersson E (2000) Effects of river regulation on river-margin vegetation: a comparison of eight boreal rivers. Ecological Applications 10:203–224
Jurik TW, Wang S, van der Valk AG (1994) Effects of sediment load on seedling emergence from wetland seed banks. Wetlands 14:159–165
Kapp RO (1969) How to know pollen and spores. WMC Brown Company, Dubuque
Keddy PA (1983) Shoreline vegetation in Axe Lake, Ontario: effects of exposure on zonation patterns. Ecology 64:331–344
Keddy PA (2000) Wetland ecology principles and conservation. Cambridge University Press, New York
Keddy PA, Reznicek AA (1986) Great Lakes vegetation dynamics: the role of fluctuating water levels and buried seeds. Journal of Great Lakes Research 12:25–36
Kercher SM, Zedler JB (2004) Multiple disturbances accelerate invasion of reed canary grass (Phalaris arundinacea L.) in a mesocosm study. Oecologia 138:455–464
Koohzare A, Vaníček P, Santos M (2008) Pattern of recent vertical crustal movements in Canada. Journal of Geodynamics 45:133–145
McAndrews JH, Berti AA, Noris G (1973) Key to the quaternary pollen and spores of the great lakes region. Toronto Royal Museum Miscellaneous Publications, Toronto
McCaffrey RJ, Thomson J (1980) A record of the accumulation of sediments and heavy metals in a Connecticut salt marsh. In: Saltzman B (ed) Estuarine physics and chemistry: studies in long island. Academic, New York, pp 165–236
McDowell L (1989) Soil survey of Jefferson County. U.S.D.A. Soil Conservation Service and Cornell University Agricultural Experiment Station, Ithaca
Meyers PA, Lallier-Vergès E (1999) Lacustrine sedimentary organic matter records of Late Quaternary paleoclimates. Journal of Paleolimnology 21:345–372
Moore PD, Collinson ME, Webb JA (1994) Pollen analysis. Blackwell Scientific Publications, Ltd, Oxford
Neely RK, Davis CB (1985) Nitrogen and phosphorus fertilization of Sparganium eurycarpum Engelm. and Typha glauca Godr. Stands. I. Emergent plant production. Aquatic Botany 22:347–361
Orson RA, Howes BL (1992) Salt marsh development studies at Waquoit Bay, Massachusetts: influence of geomorphology on long-term plant community structure. Estuarine, Coastal and Shelf Science 27:453–471
Patch SP, Busch WDN (1984) The St. Lawrence River—past and present. U.S. Fish and Wildlife Service, Cortland
Reeder BC (1989) Quaternary history of Old Woman Creek wetland. In: Mitsch WJ (ed) Wetlands of Ohio’s Coastal Lake Erie: a hierarchy of systems. Ohio State University Press, Columbus, pp 97–109
Rice D, Rooth J, Stevenson JC (2000) Colonization and expansion of Phragmites australis in upper Chesapeake Bay tidal marshes. Wetlands 20:280–299
Schelske CL, Hodell DA (1995) Using carbon isotopes of bulk sedimentary organic matter to reconstruct the history of nutrient loading and eutrophication in Lake Erie. Limnology and Oceanography 40:918–929
Shay JM, de Geus PMJ, Kapinga MRM (1999) Changes in shoreline vegetation over a 50-year period in the Delta Marsh, Manitoba in response to water levels. Wetlands 19:413–425
Singer DK, Jackson ST, Madsen BJ, Wilcox DA (1996) Differentiating climatic and successional influences on long-term development of a marsh. Ecology 77:1765–1778
Stuiver M, Reimer PJ, Bard E, Beck JW, Burr GS, Hughen KA, Kromer B, McCormac FG, Plicht J, Spurk M (1998) INTCL 98 radiocarbon age calibration, 24, 000–0 cal BP. Radiocarbon 40:1041–1083
Stuiver M, Reimer PJ, Reimer RW (2005) CALIB 5.0. 14CHRONO Centre, Queens University, Belfast. http://intcal.qub.ac.uk/calib. Accessed 5 Feb 2005
Talbot MR, Laerdal T (2000) The late Pleistocene-Holocene paleolimnology of Lake Victoria, East Africa, based upon elemental and isotopic analyses of sedimentary organic matter. Journal of Paleolimnology 23:141–164
Thomas E, Varecamp JC (1991) Paleo-environmental analyses of marsh sequences (Clinton, Connecticut): evidence for punctuated rise in relative sealevel during the latest Holocene. Journal of Coastal Research 11:125–158
Tuchman NC, Larkin DJ, Geddes P, Wildova R, Jankowski K, Goldberg DE (2009) Patterns of environmental change associated with Typha x glauca invasion in a Great Lakes coastal wetland. Wetlands 29:964–975
Turetsky MR, Manning SW, Wieder RK (2004) Dating recent peat deposits. Wetlands 24:324–356
Tushingham AM (1992) Postglacial uplift predictions and historical water levels of the Great Lakes. Journal of Great Lakes Research 18:440–455
USDA National Agricultural Statistics Service (1997) Census of agriculture summary for the State of New York. USDA, Washington DC
Wang S, Jurik TW, van der Valk AG (1994) Effects of sediment load on various stages in the life and death of cattail (Typha x glauca). Wetlands 14:166–173
Warwick WF (1980) Paleolimnology of the Bay of Quinte, Lake Ontario: 2800 years of cultural influence. Canadian Bulletin of Fisheries and Aquatic Science 206:1–118
Webb RS, Webb TI (1988) Rates of sediment accumulation in pollen cores from small lakes and mires of Eastern North America. Quaternary Research 30:284–297
Werner KJ, Zedler JB (2002) How sedge meadow soils, microtopography, and vegetation respond to sedimentation. Wetlands 22:451–466
Wilcox DA (1995) Wetland and aquatic macrophytes as indicators of anthropogenic hydrologic disturbance. Natural Areas Journal 15:240–248
Wilcox DA, Meeker JE (1991) Disturbance effects on aquatic vegetation in regulated and unregulated lakes in northern Minnesota. Canadian Journal of Botany 69:1542–1551
Wilcox DA, Xie Y (2007) Predicting wetland plant responses to proposed water-level-regulation plans for Lake Ontario: GIS-based modeling. Journal of Great Lakes Research 33:751–773
Wilcox DA, Apfelbaum SI, Hiebert RD (1984) Cattail invasion of sedge meadows following hydrologic disturbance in the Cowles Bog wetland complex, Indiana Dunes National Lakeshore. Wetlands 4:115–128
Wilcox DA, Kowalski KP, Hoare HL, Carlson ML, Morgan HN (2008) Cattail invasion of sedge/grass meadows in Lake Ontario: photointerpretation analysis of sixteen wetlands over five decades. Journal of Great Lakes Research 34:301–323
Woo I, Zedler JB (2002) Can nutrients alone shift a sedge meadow towards dominance by the invasive Typha x glauca? Wetlands 22:509–521
Acknowledgements
This project was in part funded by the Federal Aid in Sport Fish Restoration Program (#FA-5-R) administered by the New York Department of Environmental Conservation and the Great Lakes Protection Fund WR 537. We also extend our gratitude to Val Klump of the Great Lakes WATER Institute at the University of Wisconsin, Milwaukee for completing 210Pb dating and the University of Arizona, Atomic Mass Spectometry Laboratory for completing the carbon dating. We thank Henry Mullins of Syracuse University Geology for his assistance with coring and valuable advice, Mark Teece of the SUNY-ESF Chemistry for support of C:N analyses and Donald Leopold (SUNY-ESF Environmental and Forest Biology) for his assistance and support. This research is a contribution of the Thousand Island Biological Station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rippke, M.B., Distler, M.T. & Farrell, J.M. Holocene Vegetation Dynamics of an Upper St. Lawrence River Wetland: Paleoecological Evidence for a Recent Increase in Cattail (Typha). Wetlands 30, 805–816 (2010). https://doi.org/10.1007/s13157-010-0068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-010-0068-0