Abstract
In recent decades, wetlands have been constructed or restored around the Baltic sea to counteract the eutrophication of its coastal waters. Some of these wetlands could also be suitable spawning and nursery areas for anadromous northern pike (Esox lucius L.). We studied juvenile pike production in three coastal wetlands along the south-eastern coast of Sweden that were restored in different ways. Where terrestrial vegetation was temporarily flooded, pike larval/juvenile emigration increased from a few thousand individuals before restoration to over a hundred thousand afterwards. We suggest that vegetation was the key to this successful reproduction, as wetlands where vegetation was removed or reduced saw no similar increase in pike production. Flooded vegetation in shallow waters offers optimal spawning conditions, increased food resources, and refuge from predation. The growth and emigration of larvae and juveniles were followed over time, revealing that 80–95% of individuals left the wetlands within 1 month (at a size <6 cm). This emigration probably represents an adaption to seasonally decreasing water levels but may also be a way to avoid cannibalism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The historical wetland drainage in Europe has resulted in the loss of anadromous northern pike (Esox lucius L.) recruitment areas. In the most exploited areas in Sweden, such as Skåne and Mälardalen, it is estimated that approximately 90% of the wetland area has been lost (Hagerberg et al., 2004; Alström & Krook, 2008). The loss or alteration of wetland habitats may negatively affect pike production (Casselman & Lewis, 1996) and, as a large proportion of pike in the Baltic sea are of freshwater origin (Engstedt et al., 2010), lead to decreasing coastal stocks (Nilsson et al., 2004). This change of the landscape is also a key contributor to the eutrophication of Baltic sea coastal areas (Larsson et al., 1985). The general decrease in retention time for Baltic sea tributaries as a result of drainage, channelization, and decreased total wetland area has resulted in the loss of nutrients from freshwater systems to the sea (Hoffmann et al., 2000). Nutrients that otherwise would have been denitrified or incorporated into freshwater primary production are instead causing the excess growth of, for example, opportunistic filamentous macro algae in the coastal zone (e.g., Vahteri et al., 2000).
In Sweden and other countries around the Baltic, wetlands have been constructed or restored over the last two decades with the primary goal of reducing agricultural nutrient loads, thereby counteracting eutrophication (e.g., Paludan et al., 2002). Some wetlands are also being restored with the aim of increasing biodiversity (Hansson et al., 2005). Along the south-eastern coast of Sweden, new wetlands are often situated in streams where anadromous pike are present, but the design of these wetlands does not always meet the criteria for optimal spawning and nursery habitat for northern pike (Casselman & Lewis, 1996). These wetlands, for example, often lack shallow vegetated spawning grounds and the passage of pike is often physically obstructed.
Populations of northern pike in the Baltic Sea use two different reproductive strategies, spawning in either shallow brackish waters or coastal freshwater streams (Müller, 1986; Westin & Limburg, 2002; Nilsson, 2006). Engstedt et al. (2010) show that 46% of the Baltic Sea pike caught in their study was of freshwater origin. In spring, adult pike migrate from foraging areas in the sea to freshwater spawning areas (Müller, 1986; Nilsson, 2006; Engstedt, 2011). The freshwater habitats used for spawning include streams and ditches sometimes connected to wetlands and flooded grasslands. The spawning migration is extensive, and we have recorded >1,000 adult individuals in small streams. The mature pike migrate upstream in these streams to find suitable spawning sites. Spawning may last for a month, after which the adult fish return to the sea (Müller, 1986; Engstedt, 2011).
The considerable importance of vegetation in the reproduction and early life of pike has long been recognized (Bry, 1996), and the presence of live or decaying vegetation is crucial for successful spawning. Numerous plant taxa have been observed in pike spawning grounds, ranging from partially submerged terrestrial vegetation to completely submerged aquatic vegetation (Bry, 1996; Grimm & Klinge, 1996). The pike eggs are scattered in small clutches in shallow water among vegetation, to which they immediately adhere due to their sticky outer layer. Over approximately 2–5 days, a single female may lay 8,000–100,000 eggs, depending on her size and health. The eggs are left unattended and hatch in approximately 120° days. The yolk-sac larvae remain attached to the vegetation by means of adhesive papillae for some days. When almost all of the yolk sac has been absorbed, at a larva size of 11–13 mm, the larvae start exogenous feeding (Raat, 1988; Craig, 1996), mainly on zooplankton during the first weeks (Wright & Giles, 1987). The larvae may remain near the spawning site for several weeks, after which they gradually disperse to nearby areas (Raat, 1988). Most juveniles migrate to the sea during their first summer (Müller, 1986).
We examined three small coastal freshwater streams along the south-eastern coast of Sweden, all known for having populations of homing anadromous pike (Engstedt, 2011). A wetland was restored or constructed within each drainage system. Two of the wetlands (Törnebybäcken and Lervik) were designed mainly to increase the retention of nutrients by increasing the residence time, without any particular consideration given to creating new vegetated spawning grounds for pike. The third wetland (Kronobäck) was also designed to reduce nutrient discharge, but was mainly intended to create new pike spawning grounds by flooding terrestrial vegetation.
We evaluated the production of pike larvae and juveniles before and after the measures, hypothesizing that the flooded system would produce more pike larvae and juveniles. This flooded wetland was examined in detail over the season, by following pike larvae and zooplankton distribution, and the connection to various types of vegetation (this was not possible for the other two wetlands as they were newly excavated). The objectives were to study the growth and abundance of pike larvae, and their migration pattern, in order to give preliminary fish management advice, i.e., how to construct a wetland that provides improved spawning and nursery habitats for pike.
Materials and methods
Studied wetlands
In 2007–2008, wetlands were restored or established within 200–700 m of the sea in association with three coastal freshwater streams, i.e., Törnebybäcken, Lervik, and Kronobäck, in south-eastern Sweden (Fig. 1). The streams are situated within 40 km of each other. All three streams are known for the spawning migration of anadromous pike in numbers ranging from several hundred to over a thousand adult fish (Engstedt, 2011). Each stream is characterized by a mean annual water discharge of less than 0.5 m3 s−1, and the discharge is normally significantly reduced in summer months. The streams are no more than 2–3-m wide and are nutrient enriched by agricultural activities.
The Baltic Sea showing the study area. The three wetlands are depicted in photographs a Lervik, where open water surface was created by widening the existing stream. b Kronobäck, where grasslands were flooded; and c Törnebybäcken, where a dense bed of reeds (Phragmites australis) was removed and the open water surface was extended
In Törnebybäcken, an existing wetland, mainly covered with dense stands of common reed (Phragmites australis), was restored. The reeds were removed together with the root mat and approximately 0.2 m of sediment; the sediment was deposited in the wetland in the form of small islands. After restoration, the total area available for fish increased from 1.5 to 3.1 ha and the average depth was about 1 m. Outside the reed communities, submerged plants such as Elodea canadensis and Myriophyllum spicatum dominated the wetland. The year after the restoration, the total cover of submerged macrophytes decreased by more than 90% and no new vegetation became established during our study of the former reed area. The measures thus increased the potential spawning area, though the loss of vegetation would initially not favour recruitment (Casselman & Lewis, 1996).
The stream at Lervik was widened to approximately 75 m by digging a shallow pond of 1.5 ha and removing 200 m of the northern bank. The new wetland had an average water depth of approximately 0.5 m, with gently sloping littoral areas, but almost completely lacked vegetation. As in Törnebybäcken, the measures increased the potential spawning area, though the loss of vegetation would initially not favour recruitment (Casselman & Lewis, 1996).
In Kronobäck, formerly flooded grasslands of approximately 3.2 ha were restored by constructing an adjustable embankment near the outlet to the sea. Before the measure was implemented, about 0.5 ha of the area was flooded annually. Digging was restricted to a new streambed that meandered through the restored wetland, and the grasslands, consisting mainly of grasses and sedges, were inundated by directing approximately 50% of the stream water into the wetland. The depth was 0.2–0.5 m in the flooded areas and 0.5–1.5 m in the streambed. To maintain the dominant terrestrial vegetation, the wetland was flooded only from the beginning of March to the beginning of June; in the last 2 weeks of the flooding period, the wetland was slowly drained to become completely dry by mid June. Maintaining the grassland and the flooding would probably favour spawning and recruitment (Casselman & Lewis, 1996).
Pike larvae and zooplankton inventory in the Kronobäck wetland
We used the white plate method to estimate the number of pike larvae in the restored wetlands (Lappalainen et al., 2008). A white disc (0.3 m in diameter), fixed to a 1.5-m-long arm, was slowly moved over the bottom, fixed, and the number of typically 13–25-mm-long larvae was easily observed by naked eyes against the white background. Pike larval abundance was estimated in four different habitats: flooded terrestrial vegetation (grasses and sedges), Phragmites belts, submerged macrophytes, and non-vegetated areas. The inventory was taken at eight sites in each habitat and on three different occasions (i.e., April 12, 22, and 29). The first occasion was when the larvae still were in the yolk-sac stage, and the second and third were when most larvae had become free swimming, i.e., at mean lengths of approximately 14 and 19 mm, respectively. The larval food supply was studied by collecting zooplankton samples from each site on each occasion. An ordinary 2-l white water scoop was used with a swift movement to collect water from each sampled habitat. Samples were taken at a depth of 0.1–0.5 m. The water samples were filtered through a 50-μm net and preserved in Lugol’s solution. Zooplankton were counted under a microscope at 63× magnification and separated into two categories: cladocerans and copepods. The water temperature in the stream of Kronobäck, in the flooded wetland, and in an adjacent brackish bay, was continuously registered at 0.5 m depth using automatic loggers (Tinytag Aquatic 2, Gemini Data Loggers [UK] Ltd.).
Larvae and juvenile emigration
After spawning had taken place and free-swimming larvae were observed, a downstream trap was placed below each wetland to collect emigrating pike juveniles. Depending on the water discharge, 25–100% of the overflow from the wetlands was directed through the trap. The trap had a conical net with a rectangular entrance opening. The net initially had a mesh size of 1 mm, fine enough to retain the smallest larvae, and as the emigrating pike grew, the net was replaced with one of 2-mm mesh size. The downstream traps were emptied daily, or twice a day in periods of more extensive emigration, i.e., the first week. The collected pike were counted and thereafter immediately released. Pike were measured about once a week to determine growth rate and length distribution.
Statistical analyses
The statistics were calculated using the software IBM SPSS Statistics 20. To meet the basic assumptions of an ANOVA length data were log transformed before the analysis and the homogeneity of variances for all data sets was tested with Cochran’s test. The estimates of juvenile growth were based on the length measurements of emigrants trapped at the outlets of the wetlands. A repeated measures analyses of variance (RM ANOVA) were used to test differences in juvenile length between study sites and years. RM ANOVA was used; as the pike juvenile populations are sampled repeatedly during the season.
Results
Adult pike spawning behaviour
The first spawning pike were sighted at the end of March in the flooded grasslands at Kronobäck. In this wetland, the spawning peaked in the first week of April, at daily mean temperatures of 8.0–11.0°C. In Törnebybäcken and Lervik, the spawning peaked 2–4 weeks later at temperatures of 8.9–13.8°C. The temperature in the flooded areas of Kronobäck was >3°C higher than in the stream when the pike spawning peaked and >4°C higher during juvenile pike development (Fig. 2).
The water temperature in the stream of Kronobäck, in the flooded wetland, and in an adjacent brackish bay, April 2009. Shaded bars indicate a where spawning peaked, b sampling of zooplankton and yolk-sac larvae, c sampling of zooplankton and pike larvae (14-mm mean length), and d sampling of zooplankton and pike larvae (19-mm mean length)
Abundance and distribution of larvae by habitat type
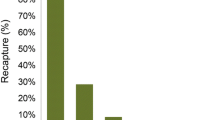
In general, most of the yolk-sac larvae were found in the shallowest areas (0.1–0.4-m deep) in all investigated vegetation habitats. Yolk-sac larvae were most abundant and most frequently recorded among the flooded terrestrial vegetation (grasses and sedges; Fig. 3; ANOVA F 3,28 = 35, P < 0.001). The relative abundance of yolk-sac larvae in the Phragmites belts and in the submerged vegetation was approximately 45 and 10%, respectively, of that found in the flooded terrestrial vegetation. No yolk-sac larvae were found in the non-vegetated areas. When the larvae were free swimming at mean lengths of 14 and 19 mm in the flooded vegetation and Phragmites belts, respectively, there were no longer any significant differences in abundance between the two vegetated habitats (ANOVA, P > 0.5). The abundance of free-swimming larvae was significantly higher in these two habitats than in areas with submerged vegetation (ANOVA, Tukey post hoc, F 3,28 = 92, P < 0.001) or in areas lacking vegetation (ANOVA, Tukey post hoc, F 3,28 = 99, P < 0.001).
Zooplankton
The community of zooplankton (>50 μm) at the study sites consisted of cladocerans and various stages of copepods (i.e., nauplii, copepodites, and adults). In general, copepods were most abundant on the two first sampling occasions and cladocerans on the third (Fig. 4). Differences in the average densities of copepods and cladocerans, the prey items preferred by larval pike, were significant among the four habitats studied (ANOVA F 3,28 = 35, P = 0.005); averages were significantly higher in areas with than without vegetation. Of the vegetated habitats, zooplankton were most abundant in the flooded terrestrial vegetation and in the Phragmites belts. As temperatures increased in the wetlands, the total abundance of zooplankton increased (Fig. 4).
Number of cladoceran zooplankton (a) and number of copepod zooplankton (b) in habitats dominated by flooded vegetation (mainly grasses and half grasses), P. australis, and submerged vegetation and in non-vegetated areas. Mean ± SD, n = 8. Sampling dates were the same as those used for pike larvae sampling, i.e., April 12, 22, and 29
Emigration and growth of juveniles
The emigration of pike larvae started in the last week in April at Kronobäck and in the first half of May in Törnebybäcken and Lervik. The emigration was most intense in the first 3 weeks (Fig. 5), and after 1 month, 80–95% of the larvae and juveniles (<6 cm) had left the wetlands. It was only the restoration of the wetland at Kronobäck that led to an increase in the number of emigrating pike from approximately 3,000 to more than one hundred thousand. The total number of emigrating pike was approximately 3,100 in Törnebybäcken and 4,600 in Lervik the year before restoration. At both these two wetlands the number of emigrants decreased with about 70% the 2 years after restoration.
Number and mean length (±SD) of emigrating pike juveniles caught in downstream traps in three wetlands in 3 successive years: Kronobäck, 2007–2009; Törnebybäcken, 2006–2008; and Lervik, 2007–2009. Note that catch data from Kronobäck in 2008 and 2009 should be multiplied by 10. Time of restoration of each wetland is indicated in the figure
The estimates of juvenile growth were based on the length measurements of emigrants trapped at the outlets of the wetlands. The first trapped pike larvae were 13–17-mm long. In the first month, growth appeared to be linear and the daily length increment was approximately 1.3–1.8 mm (Fig. 5). After wetland restoration, the juveniles were significantly longer as of mid June in the wetlands at Lervik and Törnebybäcken (Repeated Measures ANOVA, F 8,402 = 36, P < 0.005) but not at Kronobäck (RM ANOVA, F 8,402 = 36, P = 0.86). The total mean length of emigrating juveniles in June increased from 43.7 to 56.8 mm at Törnebybäcken, from 42.7 to 64.9 mm at Lervik and from 63.5 to 69.3 mm at Kronobäck. Comparing all three wetlands, the pike juveniles at Kronobäck achieved significantly higher growth in all 3 years (Tukey post hoc, P < 0.01).
Discussion
It has been suggested that the main reason for the anadromous behaviour of pike in the Baltic Sea is that the water in coastal freshwater streams warms up more rapidly than do adjacent brackish areas (Müller, 1986). It seems however not to be the temperature in the coastal streams that trigger the pike to migrate in our study area in the southern Baltic, as the water in the streams was no warmer than in adjacent coastal bays (Ljunggren et al., 2011). The wetlands were warmer, however, and pike could migrate into the streams to seek shallow vegetated areas which warm up most rapidly.
Interestingly, the increase in juveniles was not constant between the three wetlands, suggesting that variation in wetland construction could play a role in pike production. The number of spawning adult pike was about equal the years before and after wetland restoration (Engstedt, 2011). The more than 30-fold increase in juvenile pike production in the flooded grasslands at Kronobäck was immediate, indicating that production of juveniles was not limited by the number of spawning pike. The production increase was not only a result of two extremely good years as no increase was confirmed in the other two restored wetlands or in other regional areas (Ljunggren et al. 2011). The production increase at Kronobäck likely resulted from the enlarged spawning and nursery area (Forney, 1968; Casselman & Lewis, 1996). The estimated average areal production of pike juveniles in this wetland varied between 2.2 and 3.0 per square meter in the 2 years after restoration. These values are in the same order of magnitude as the highest production figures compiled in Raat (1988). A similar increase in pike production was not observed at Lervik or Törnebybäcken, suggesting that the measures undertaken at these two wetlands did not favour recruitment. The estimated average areal production of pike juveniles in these wetlands varied between 0.1 and 0.2 per square meter.
Vegetation is likely the key to explaining why only one of three restored wetlands increased the production of pike juveniles (Casselman & Lewis, 1996). The increase was likely due to the new areas of flooded vegetation suitable for pike spawning. We studied the distribution of pike larvae in the wetlands over time. The larvae displayed a clear distribution pattern after hatching: their preferred habitat was near flooded or emergent vegetation, while areas without vegetation were avoided. Areas with submerged vegetation were not used to any great extent by the small larvae. That the abundance of larvae is high in areas used as spawning places by the adult fish is not surprising, as the deposited eggs often attach to flooded emergent vegetation (Bry, 1996). High production of pike in other temporarily flooded areas has previously been described (e.g., Threinen et al., 1966), and such areas are referred to as pike factories. The lack of positive effects in terms of increased production at the two other wetlands is probably because these new habitats were almost completely lacking in vegetation.
Vegetation plays a crucial role in the reproduction and early life of pike, not only as spawning substrate (Bry, 1996), but also as habitat of high complexity suitable for young pike and their prey (Franklin & Smith, 1963). The food availability in the vegetated areas at Kronobäck could also explain the increased pike production. After the yolk-sac stage, the smallest larvae forage on zooplankton (e.g., Wright & Giles, 1987; Skov et al., 2003a). The distribution of larvae in the wetland followed the abundance pattern of zooplankton, measured as density of cladocerans and copepods. Zooplankton abundance was high in areas with emergent vegetation and low in areas lacking any type of vegetation. This is because shallow vegetated areas offer more favourable conditions, such as a higher water temperature, more nutrients, and more substrate, for the early development of zooplankton and invertebrates (Montén, 1948; Jeppesen et al., 1997). The pike larvae start foraging shortly before they become completely free swimming, which is a few days before full yolk resorption (Billard, 1996). The high production of pike larvae at Kronobäck is likely an effect of increased food resources and an appropriate matching with the zooplankton community; the smallest larvae, for example, prefer copepods and cladocerans (Montén, 1948; Wright & Giles, 1987; Skov et al., 2003a; Lehtiniemi et al., 2007).
Vegetation also plays an important role as refuge from predation (e.g., Rozas & Odum 1988). The difference in juvenile pike production between the three restored wetlands could partly be explained by higher cannibalism in the two wetlands (ponds) where a less complex unvegetated habitat dominated (Grimm & Klinge, 1996). Skov and Koed (2004) demonstrated that bigger pike, the potential cannibals, pushed smaller pike out of structured vegetated habitats into suboptimal habitats in open water. Increased cannibalism leading to fewer but faster growing individuals could also support the increased growth of emigrating pike after restoration of these two wetlands (Raat, 1988; Skov et al., 2003b). However, cannibalism need not be the single explanation of the increased growth. The construction of the new ponds at Lervik and Törnebybäcken increased the water residence time resulting in faster warming of the water in the upper areas of the ponds. Data from the years after restoration indicated that spawning occurred about 2 weeks earlier, likely due to the higher water temperature. Earlier hatching could result in an increased length of emigrating juveniles in June.
For several reasons, 80–95% of the juveniles emigrated from the wetlands at a size <60 mm and by 1 month of age. We think that the major reason for this early migration concerns a selection process. Early emigration may be an adaption to the naturally decreasing water levels in the coastal streams in spring. Early emigration when water levels are still high increases the possibility of survival, allowing the pike to spread to new nursery habitats in the coastal waters. Juvenile pike are cannibalistic, and a reduced nursery area or inadequate food availability means that cannibalism increases (Franklin & Smith, 1963; Skov et al., 2003b). Thus, avoidance of cannibalism could be another factor affecting the emigration process, at least after a few weeks when the juveniles are more size separated. A trap situated upstream of the wetland in Kronobäck caught juveniles originating from adults that had spawned in the margins of the stream. These juveniles were considerably smaller than those emigrating from the wetland (Nilsson, unpublished data). The juveniles that hatched in the colder stream water with practically no existing nursery areas probably have a lower chance of survival. Intraspecific competition among the juveniles is a third possible explanation (Farrell et al., 2006). It should, however, be noted that this early migration takes place when appropriate food items are still abundant in the wetland, so food limitation is probably not a major cause.
Conclusion
To conclude, and giving fish management advice on how to construct pike factories, our results indicate that an optimized wetland for juvenile pike production should be shallow (0.2–0.5 m) and contain temporarily flooded terrestrial vegetation. The water level should be stable for at least 2 months after spawning. Flooded grasslands give favourable conditions for spawning and early zooplankton development; providing suitable food for pike larvae after hatching and following natural water regimes that almost dry out in summer can produce a large number of juveniles that contribute significantly to the coastal stock.
It is also relevant to emphasize that wetland restoration probably works best if there already is a population of anadromous pike in the area. If one starts from scratch in an area where there are no pike, it could be difficult to induce pike to use the new wetland habitat as they seem to have high site fidelity and do not switch to new areas (Engstedt, 2011). If the wetlands are receiving water from nutrient-enriched streams, they can function both as nutrient traps and recruitment areas for pike.
References
Alström, T. & J. Krook, 2008. Att återskapa historiska våtmarker i Kävlingeåns avrinningsområde: möjligheter, hinder och praktiska erfarenheter. Ekologgruppen, Landskrona: 34 (in Swedish).
Billard, R., 1996. Reproduction of pike: gametogenesis, gamete biology and early development. In Craig, J. F. (ed.), Pike: biology and exploitation. Chapman & Hall, London: 13–43.
Bry, C., 1996. Role of vegetation in the life cycle of pike. In Craig, J. F. (ed.), Pike: biology and exploitation. Chapman & Hall, London: 45–67.
Casselman, J. M. & C. A. Lewis, 1996. Habitat requirements of northern pike (Esox lucius). Canadian Journal of Fisheries and Aquatic Sciences 53: 161–174.
Craig, J. F., 1996. Population dynamics, predation and role in the community. In Craig, J. F. (ed.), Pike: biology and exploitation. Chapman & Hall, London: 201–217.
Engstedt, O., 2011. Anadromous pike in the Baltic Sea. Doctoral thesis, Linnaeus University, Kalmar, 46 pp.
Engstedt, O., P. Stenroth, P. Larsson, L. Ljunggren & M. Elfman, 2010. Assessment of natal origin of pike (Esox lucius) in the Baltic Sea using Sr:Ca in otoliths. Environmental Biology of Fishes 89: 547–555.
Farrell, J. M., J. V. Mead & B. A. Murry, 2006. Protracted spawning of St Lawrence River northern pike (Esox lucius): simulated effects on survival, growth, and production. Ecology of Freshwater Fish 15: 169–179.
Forney, J. L., 1968. Production of young northern pike in a regulated marsh. New York Fish and Game Journal 15: 143–154.
Franklin, D. R. & L. L. Smith, 1963. Early life history of the northern pike (Esox lucius L.) with special reference to the factors influencing the numerical strength of year classes. Transactions of the American Fisheries Society 92: 91–110.
Grimm, M. P. & M. Klinge, 1996. Pike and some aspects of its dependence on vegetation. In Craig, J. F. (ed.), Pike: biology and exploitation. Chapman & Hall, London: 125–156.
Hagerberg, A., J. Krook & D. Reuterskiöld, 2004. Åmansboken: vård, skötsel och restaurering av åar i jordbruksbygd. Saxån–Braåns vattenvårdskomm, Landskrona (in Swedish).
Hansson, L.-A., C. Brönmark, P. A. Nilsson & K. Åbjörnsson, 2005. Conflicting demands on wetland ecosystem services: nutrient retention, biodiversity or both? Freshwater Biology 50: 705–714.
Hoffmann, M., H. Johnsson, A. Gustafson & A. Grimvall, 2000. Leaching of nitrogen in Swedish agriculture: a historical perspective. Agriculture, Ecosystems and Environment 80: 277–290.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top–down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342(343): 151–164.
Lappalainen, A., M. Härmä, S. Kuningas & L. Urho, 2008. Reproduction of pike (Esox lucius) in reed belt shores of the SW coast of Finland, Baltic Sea: a new survey approach. Boreal Environment Research 13: 370–380.
Larsson, U., R. Elmgren & F. Wulff, 1985. Eutrophication and the Baltic Sea: causes and consequences. Ambio 14: 9–14.
Lehtiniemi, M., T. Hakala, S. Saesmaa & M. Viitasalo, 2007. Prey selection by the larvae of three species of littoral fishes on natural zooplankton assemblages. Aquatic Ecology 41: 85–94.
Ljunggren, L., J. Olsson, J. Nilsson, P. Stenroth, P. Larsson, O. Engstedt, T. Borger & O. Sandström, 2011. Våtmarker som rekryteringsområden för gädda i Östersjön. Report, Finfo no. 1. Fiskeriverket, Kustlaboratoriet, Öregrund: 63 (in Swedish with English summary).
Montén, E., 1948. Research on the biology of northern pike larvae and some related problems. Skrifter utgivna av Södra Sveriges Fiskeriförening 1: 3–38 (in Swedish with English summary).
Müller, K., 1986. Seasonal anadromous migration of the pike (Esox lucius L.) in coastal areas of the northern Bothnian Sea. Archive für Hydrobiologie 107: 315–330.
Nilsson, J., 2006. Predation of northern pike (Esox lucius L.) eggs: a possible cause of regionally poor recruitment in the Baltic Sea. Hydrobiologia 553: 161–169.
Nilsson, J., J. Andersson, P. Karås & O. Sandström, 2004. Recruitment failure and decreasing catches of perch (Perca fluviatilis L.) and pike (Esox lucius L.) in the coastal waters of southeast Sweden. Boreal Environment Research 9: 295–306.
Paludan, C., F. E. Alexeyev, H. Drews, S. Fleischer, A. Fuglsang, T. Kindt, P. Kowalski, M. Moos, A. Radlowki, G. Stromfors, V. Westberg & K. Wolter, 2002. Wetland management to reduce Baltic Sea eutrophication. Water Science Technology 45: 87–94.
Raat, A. J. P., 1988. Synopsis of biological data on the northern pike, Esox lucius Linnaeus, 1758. FAO, Rome: 178.
Rozas, L. P. & W. E. Odum, 1988. Occupation of submerged aquatic vegetation by fishes: testing the roles of food and refuge. Oecologia 77: 101–106.
Skov, C., O. Lousdal, P. H. Johansen & S. Berg, 2003a. Piscivory of 0+ pike (Esox lucius L.) in a small eutrophic lake and its implication for biomanipulation. Hydrobiologia 506–509: 481–487.
Skov, C., L. Jacobsen & S. Berg, 2003b. Post-stocking survival of 0+ year pike in ponds as a function of water transparency, habitat complexity, prey availability and size heterogeneity. Journal of Fish Biology 62: 311–322.
Skov, C. & A. Koed, 2004. Habitat use of 0+ year pike in experimental ponds in relation to cannibalism, zooplankton, water transparency and habitat complexity. Journal of Fish Biology 64: 448–459.
Threinen, C. W., C. Wistrom, B. Apelgren & H. Snow, 1966. The northern pike, life history, ecology, and management. Wisconsin Conservation Department Publication No. 235, 16 pp.
Vahteri, P., A. Mokinen, S. Salovius & I. Vuorinen, 2000. Are drifting algal mats conquering the bottom of the Archipelago Sea, SW Finland? Ambio 29: 337–338.
Westin, L. & K. E. Limburg, 2002. Newly discovered reproductive isolation reveals sympatric populations of Esox lucius in the Baltic. Journal of Fish Biology 61: 1647–1652.
Wright, R. M. & N. Giles, 1987. The survival, growth and diet of pike fry, Esox lucius L., stocked at different densities in experimental ponds. Journal of Fish Biology 30: 617–629.
Acknowledgments
Financial support was provided by the Faculty of Natural Sciences, Engineering and Technology at Linnaeus University and the Swedish National Board of Fisheries. We also thank Christian Skov and two anonymous reviewers for constructive input regarding previous versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: M. Power
Rights and permissions
About this article
Cite this article
Nilsson, J., Engstedt, O. & Larsson, P. Wetlands for northern pike (Esox lucius L.) recruitment in the Baltic Sea. Hydrobiologia 721, 145–154 (2014). https://doi.org/10.1007/s10750-013-1656-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1656-9