Abstract
Changes from the 1970s to 2000s in phosphorus and chlorophyll levels, water transparency, zooplankton, and benthic communities in the upper, International Section of the St. Lawrence River were evaluated using trend data from limnological surveys. The influence of Lake Ontario as a source for riverine production was evident in the upper river. Total phosphorus levels from 1976 to 1978 (average ~20 μg/l) sampled during a period of nutrient pollution declined (to 6–7 μg/l) following abatement. As expected, water transparency indicated by summer Secchi depths showed an opposite response increasing from ~3.5 m in the 1970s to >10 m by 2003 but declined to 6–7 m in more recent years. Zooplankton communities have experienced declines in overall densities, and the community has changed but its primary components, Bosmina and Diacyclops remain. Ceriodaphnia lacustris abundance declined substantially from the 1970s to the recent time period while Chydorus sphaericus increased and Eurytemora affinis and Cercopagis pengoi had first appearances. In contrast, benthic invertebrate biomass increased substantially between time periods. Increases in families and occurrence of gastropods were observed, but the primary components–chironomids, amphipods, and oligochaetes–were consistent between the periods with the exception of dreissenid mussels. Dreissena was dominated by D. bugensis, and both D. bugensis and D. polymorpha show evidence of recent declines. Conversely, high abundance of round goby (Apollonia melanostomus) has promoted new trophic pathways. Downstream attenuation in nutrients and chlorophyll, and increased transparency suggest continued strong effects of Lake Ontario on the downstream river environment, but increased energy in the benthos has likely promoted greater in situ production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The upper St. Lawrence River receives much of its plankton production from its source, Lake Ontario (Mills & Forney, 1982; Basu et al., 2000). A downstream attenuation in pelagic primary and secondary production along the River’s longitudinal gradient provides evidence of source-water dependency to fuel lower trophic levels. Declining productivity and recent ecological changes in Lake Ontario are expected to have similar effects in the River. The Lake Ontario food web has been altered by perturbations, including nonindigenous species (NIS) introductions such as zebra (Dreissena polymorpha) and quagga (D. bugensis) mussels (Mills et al., 1999), the more recent invasion by round goby (Apollonia melanstomus), and nutrient reduction associated with the Great Lakes Water Quality Agreement (GLWQA) phosphorus abatement initiative (Stevens & Neilson, 1987; Mills et al., 2003; Holeck et al., 2008a). Predatory pressures in Lake Ontario include changes in the fishery (Mills et al., 2003), and invasion by the spiny water flea Bythotrephes longimanus (Lange & Cap, 1986; Makarewicz & Jones, 1990) and the fish-hook water flea Cercopagis pengoi (Benoit et al., 2002). How these changes have influenced the aquatic ecosystem downstream in the St. Lawrence River needs to be addressed.
Crustacean zooplankton can be used as indicators of aquatic ecosystem functioning, since they are positioned in the food web to respond to bottom-up, top-down, and environmental pressures (Patalas, 1972; McQueen et al., 1986). While the effects of these pressures on Lake Ontario zooplankton have been examined (Johannsson, 1987; Johannsson et al., 1991; Hall et al., 2003), little attention has been given to lower trophic levels of the St. Lawrence River (Twiss, 2007). Examination of traditional limnological data including nutrients, chlorophyll a, and pelagic crustacean zooplankton and benthic communities are important to gauge secondary production changes in light of recent perturbations.
Early St. Lawrence River limnological surveys were conducted by Burkholder & Tressler (1932), but Mills et al. (1981) and Mills & Forney (1982) provide a detailed examination of the upper St. Lawrence beginning at its confluence with Lake Ontario. Our objective was to compare basic limnological parameters (i.e., total phosphorus, chlorophyll a, Secchi depth) and crustacean zooplankton and benthic communities of the St. Lawrence during a recent 5-year period, 2003–2007 with the earlier data from Mills and Forney serving as a baseline from 1976 to 1978. We use these data and comparisons to make inferences regarding ecological change among lower trophic levels in the context of downstream productivity attenuation and recent biotic perturbations.
Methods
Study area
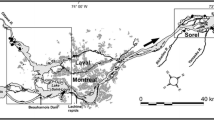
The St. Lawrence River, spanning 965 km from Lake Ontario to the Gulf of St. Lawrence, is the only natural outlet of the Great Lakes and drains an area of more than 764,000 sq km (Mills et al., 1981). The river had an average annual discharge at Cornwall, Ontario of 7,960 m3 s−1 between 1960 and 1990 (Water Survey of Canada, 1990) and ranks among the world’s largest rivers in terms of flow. Despite the lack of an obvious physical boundary between Lake Ontario and the St. Lawrence River, significant physical differences exist along the transition from Lake to River that separates them functionally. Aquatic habitats in the upper and mid-reaches of the St. Lawrence are highly heterogeneous compared to the open basins of the lake (Fig. 1). The upstream portion of the river, near its confluence with Lake Ontario, is the Thousand Islands archipelago, with over 1,800 islands and countless shoals, and coastal wetlands. Velocities in this area are varied with main channel current velocities of approximately 0.08 m/s. Velocity increases and maximum channel depth exceeds 70 m in the American Narrows near Alexandria Bay. Downstream from the islands the middle corridor portion of the river narrows again and velocities exceed 0.3–0.4 m/s (Mills & Forney, 1982). River currents promote mixing that prevent stable thermal stratification and formation of a strong hypolimnion and therefore downstream reaches are sometimes nearly homothermal. For this reason, native cool and warm water fish predators (percids and esocids, centrachids) predominate (Murry, 2006) and cold water species are typically absent during mid-summer.
Sampling stations
All variables were sampled along the International Section of the St. Lawrence River (Fig. 1) during the spring and summers of 1976–1978 and 2003–2007. Deep and shallow samples were collected monthly at fixed stations from Cape Vincent near Lake Ontario and 60 km downstream to Chippewa Bay in 1976 (June–September) and 1977 (March–July). In 1978, sampling frequency increased to bimonthly between May and September (Mills & Forney, 1982). Shallow samples were taken from each station between 2 and 3 m bottom depth, while deep samples were from fixed stations at bottom depths between 10 and 15 m. For the recent period, deep and shallow samples were collected at three stations including Clayton, and Chippewa Bay at the locations sampled by Mills in the 1970s and at the intermediate station, Clayton, NY, bimonthly from June to August. Shallow samples were taken from sites with bottom depths of 2–5 m, while deep samples were typically from sites with bottom depths of 10–20 m.
Transparency, TP, and chl a
Water transparency was measured using a Secchi disk during all years sampled. Water samples were taken using an integrated tube sampler (1.9 cm inside diameter Nalgene™ tube) from 0 to 10 m at deep stations and from 0 m to near bottom at shallow stations. Unfiltered water for total phosphorus (TP) analysis was frozen in Whirl–Pak bags, then thawed at a later date and analyzed with the persulfate oxidation method (Menzel & Corwin, 1965). Water for chlorophyll a (chl a) measurements was transported to the laboratory, filtered through Reeve Angel 934AH (1976–1978) or Whatman 934AH (2003–2007) glass fiber filters, then analyzed on the day of sampling using the acetone extraction method (Strickland & Parsons, 1968). All water variables indexed were compared using t tests and ANOVA and between sets of years for the recent period and the late 1970s.
Crustacean zooplankton and benthos
In all cases, zooplankton were collected by single vertical hauls with a 0.5 m diameter, 2 m long, and 153 μm mesh net. During 1976–1978, zooplankton were preserved in 100% isopropyl alcohol, subsampled with a Hensen-Stempel pipette, then identified and enumerated using an inverted microscope. Zooplankton biomass was estimated by multiplying species densities by the dry weight biomass conversion factors reported in Chamberlain (1975). Zooplankton were preserved in sugar formalin solution (Haney & Hall, 1973) in 2003 and in 70% ethyl alcohol in following years. Tow depths were used to calculate the volume of water strained for all years; therefore it was assumed that tows had similar efficiency from the 1970s to the recent period. Zooplankton were sub-sampled with a Hensen-Stemple pipette and a Sedgewick-Rafter counting cell. At least 100 individuals were identified and measured using a digitizing pad (CalComp Drawing Board III) interfaced with a compound microscope outfitted with a camera tube. Biomasses for each species were estimated using length-dry weight regressions derived from Lake Ontario specimens (Cornell Biological Field Station, unpublished data). All Cercopagis pengoi individuals were measured if fewer than 100 animals were present. Larger clumps (>100) were separated and at least 100 individuals were randomly subsampled. Total C. pengoi density and biomass was then estimated using the ratio of wet weight biomass subsampled versus the remainder of the sample (Warner et al., 2006). Statistical analyses for zooplankton were made using a t test comparing the two temporal periods.
Benthic samples were taken only at shallow stations using a 6″ × 6″ Ponar dredge. Excess mud and detritus were removed by sieving samples through a 250 μm mesh screen and contents were preserved in 70% ethyl alcohol. Organisms were identified under a dissecting scope to species level when possible. Following identification, samples were dried at 60°C for 48 h and weighed on a Mettler AE163 balance to determine dry weight biomass (grams). For 1978 only, winter benthic invertebrate species identification data were available for comparisons of community structure.
Results
Transparency, TP, and chl a
Seasonal mean Secchi depth (deep sites; June to September) in 1976 (mean = 3.4 m), 1977 (mean = 3.8 m), and 1978 (mean = 3.7 m) had increased to a mean of 10.2 m in 2003. Water transparency remained high (>9 m) in 2004 and 2005, but then declined in the last 2 years as evidenced by a mean Secchi depth of 6.3 m in 2006 and 7.4 m in 2007 (Fig. 2). An analysis comparing the early time period with the recent time period (and excluding the Clayton station which was not sampled in the early period) showed that Secchi depth in the recent period (8.9 m) was significantly higher than in early period (3.6 m; t test, P < 0.0001). A consistent spatial pattern emerged in the recent series, where Secchi measurements increased along the downstream gradient with lowest readings in Cape Vincent and highest at Chippewa Bay. Seasonal mean Secchi depth at Chippewa Bay (10.5 m) was significantly higher than at Clayton (8.4 m) and Cape Vincent (7.5 m; ANOVA F(2, 108) = 16.9, P < 0.0001, Tukey HSD). A maximum reading of 15.7 m Secchi depth was recorded at the Chippewa Bay station in September of 2004. Secchi depth during the 1970s did not show the same pattern; there was no significant difference between Chippewa Bay (3.8 m) and Cape Vincent (3.4 m; t test, df = 24, P > 0.05). As anticipated, total phosphorus (TP) levels demonstrated an inverse temporal pattern relative to that observed for Secchi depth (Fig. 3). Total phosphorus concentrations in the 1970s were significantly higher than in the 2000s at both deep and shallow stations (t test, P < 0.0001). In the 1970s, the mean TP concentration at deep stations was 19.9 μg/l (SD = 5.5), and this was reduced to an average of 6.2 μg/l (SD = 1.8) for 2003–2006. Similarly, mean TP concentration at shallow stations was 18.3 μg/l (SD = 7.6) for 1976–1978 compared to 7.2 μg/l (SD = 2.2) for 2003–2006. Phosphorus levels were significantly higher at shallow stations compared to deep stations during the recent period (t test, df = 38, P < 0.0001), but there was no significant difference between shallow and deep stations during the early period (t test, df = 38, P = 0.38). Along the longitudinal gradient, no consistent pattern in TP levels were apparent for either time period (ANOVA F(2, 181) = 1.08, P = 0.34). In the recent period, phosphorus levels were consistently higher at the middle station (Clayton) compared to either the upstream or downstream stations, but the difference was not significant. Chlorophyll a also declined significantly from the period of the 1970s to the recent period (Fig. 3). Deep (5.8 μg/l, SD = 3.3) and shallow stations (4.7 μg/l, SD = 2.3) during the early period had significantly higher chl a levels relative to the recent period levels of 1.2 (SD = 1.0 μg/l) for deep (t test, df = 97, P < 0.0001) and 2.0 (SD = 1.8 μg/l) for shallow (t test, df = 98, P < 0.0001). Along the longitudinal gradient, no pattern in chl a was detected in the early period; upstream deep chl a (6.7 μg/l, SD = 4.1) was not significantly different from downstream deep chl a (5.0 μg/l, SD = 2.3; t test, df = 24, P = 0.19), and upstream shallow chl a (4.7 μg/l, SD = 1.3) was not significantly different from downstream shallow chl a (4.7 μg/l, SD = 2.8; t test, df = 29, P = 0.99). For the recent period, seasonal mean deep chl a at Chippewa Bay (0.9 μg/l) was significantly lower than at Clayton (1.5 μg/l) and Cape Vincent (1.6 μg/l; ANOVA F(2, 109) = 8.8, P < 0.0003; Tukey HSD). In contrast, seasonal mean shallow chl a at Chippewa Bay (2.3 μg/l, SD = 0.3) was actually higher than at Clayton (1.7 μg/l, SD = 0.3) and Cape Vincent (1.8 μg/l, SD = 0.3), but the difference was not significant (ANOVA F(2, 105) = 1.4, P = 0.26).
Crustacean zooplankton and benthos
Summer crustacean zooplankton densities from the 1970s at deep stations were higher than at shallow stations, but the difference was not significant (t test, df = 26, P = 0.17; Fig. 4). In contrast, densities during the 2000s at shallow sites were significantly higher than at deep sites (t test, df = 58, P = 0.04). Contrary to the expected downstream productivity gradient, densities at Chippewa Bay were higher than those at Cape Vincent in the 2000s, but the difference was not significant (t test, df = 58, P = 0.30). In 2003 and 2004 mean zooplankton densities were depressed in both deep and shallow sites to ~10 individuals/l. For all sites combined, densities increased in 2005 and 2006, to levels exceeding 30 individuals/l, but only for the nearshore stations, while deep station levels remained relatively low compared to the 1970s (Fig. 5).
Changes were observed in zooplankton community composition between time periods, although Bosmina longirostris and Diacyclops thomasi were dominant in surveys both from the 1970s and the recent period, indicating stability in the core components of the community (Fig. 6). The cladoceran, Bosmina, represented greater than half of the community composition in both shallow and deep stations in the 1970s. Bosmina remained a dominant form in the recent period, but much greater relative abundance levels of nauplii were observed. Recent Diacyclops relative abundance was roughly one-half that observed in the 1970s. Species level changes in community composition occurred between the temporal periods and are highlighted by the appearance of Cercopagis pengoi in 2003. Ceriodaphnia lacustris previously represented 4–17% of relative abundance in the 1970s (Fig. 6), yet by the recent time period they accounted for less than 1%. In addition to the above compositional shifts, increased overall levels of Eurytemora affinis occurred in the recent period as did the abundances of Chydorus sphaericus in the shallow zone.
Changes in benthic invertebrate biomass were essentially inverse to those observed for density of crustacean zooplankton. The low biomass of benthic invertebrates in the 1970s (<5 g/m2) was replaced by high levels, exceeding 40 g/m2, observed for 2002–2005. A difference in biomass was also observed along the longitudinal gradient from upstream high levels in Cape Vincent of >40 g/m2 for 2002–2005 compared to the Chippewa Bay region downstream (<25 g/m2; Fig. 7). Interestingly, invertebrate biomass declined since 2005, and levels for the most recent samples in 2007 were lower than those observed in 1978. Invasive mussel abundance has been dominated by quagga mussels (D. bugensis) over zebra mussels (D. polymorpha) for most samples in the recent period (Fig. 8). Along the longitudinal gradient, the high levels of dreissenids observed in Cape Vincent in 2004 and 2005 have declined dramatically, while Clayton abundance has been generally intermediately high and consistent among years. At the downstream site, Chippewa Bay, dressenid abundance has been consistently low and absent from samples in both 2004 and 2007.
With the exception of gastropods, benthic invertebrate composition has remained remarkably stable over the temporal gradient despite the introduction of invasive dressenid mussels. The three primary components, oligochaetes, chironomids, and amphipods have consistently dominated benthic counts in samples over both time periods (Fig. 9). For these three groupings, certain years show an effect of the longitudinal gradient on their representation in the benthic community. In 2006 and 2007, oligochaetes declined in representation from Cape Vincent to Chippewa Bay with Clayton intermediate. The inverse trend was observed for amphipods during that same period. Chironomids show some evidence for a longitudinal attenuation on representation in samples, especially in 1978 and 2006. Concomitant to the recent period introduction of dressenids, families of gastropods increased in number and were consistently represented in the benthic community (Fig. 9). In 1978 nematodes were well represented but were absent from recent period samples, whereas hirudinae had an opposite response. Also in the recent period, tricopterans are more consistently represented at low levels.
Benthic invertebrate relative abundance in ponar grabs sampled along the longitudinal river gradient from Cape Vincent, NY to Chippewa Bay, NY in winter of 1978 (Mills et al., 1981) and from Cape Vincent to Clayton, NY to Chippewa Bay, NY for 2004–2007
Discussion
Rapid and dramatic changes including increased water clarity, declining total phosphorus, and declines in pelagic production were evidenced by our comparisons of the upper St. Lawrence River from the 1970s to the recent period. These changes were documented by Nicholls et al. (1999) and Mills et al. (2003) for Lake Ontario over a similar temporal period to our study. New energy pathways have likely resulted in response to the physical and chemical changes (Mills et al., 2003; Hecky et al., 2004) and along with proliferation of invasive dressenid mussels, both Lake Ontario and the upper River have experienced increased benthic production and reductions to pelagic production in response to these relatively new environmental conditions.
Despite changes associated with recent perturbations (water transparency, nutrient decline, and species introductions) that occurred since the 1970s, the dominant forms of crustacean zooplankton (e.g., Bosmina and Diacyclops) and primary benthic invertebrate taxa (chironomids, amphipods, and oligochaetes) remained important. The contemporary zooplankton community is similar to the one documented in the earliest survey of St. Lawrence River zooplankton. Burkholder and Tressler (1932) indicated a summer community dominated by cyclopoid copepods (Cyclops and Diaptomus) and Bosmina as the predominant cladoceran. Similarly, during the period of eutrophication of the Great Lakes, Mills et al. (1981) surveyed St. Lawrence zooplankton in the winter of 1978 and found low species diversity and biomass, with Cyclops bicuspidatus (Diacyclops thomasi) predominating. Mills & Forney (1982) estimated spring through summer St. Lawrence zooplankton stocks between the outlet of Lake Ontario at Cape Vincent and Massena, NY in 1976–1978, and dominant species were C. bicuspidatus, D. minutus, and B. longirostris. A recent data analysis (T. Stewart, submitted) found that Lake Ontario zooplankton species richness and diversity did not differ between the late 1980s and early 2000s, a time period encompassing the establishment of dreissenid mussels and the invasive predatory cladocerans, Cercopagis pengoi and Bythotrephes longimanus. These descriptions from the 1920s, the 1970s, the 1980s, and the recent period suggest resilience in basic zooplankton community structure, although significant community changes (e.g., compositional shifts, elimination of certain taxa and appearance of new taxa) have occurred over this period. Nauplii were the only dominant zooplankton form to increase in abundance between the survey periods. Declining abundance of other dominant species, Bosmina longirostris and Diacylops thomasi, mirrored the decrease in productivity between time periods. However, the increase in Chydorus sphaericus and Eurytemora affinis abundance may be indicative of a change in environmental conditions. C. sphaericus has been reported to be widely distributed through the Great Lakes and is known as an epibenthic species with a propensity to cling to filamentous algae (Fryer, 1968). This species functions as a scraper of epiphyton and also filters nanoplankton. Mills et al. (2003) reported declines in C. sphaericus in the 1990s during a period of phosphorus reduction in Lake Ontario, but the inverse response is observed here. Filamentous algal conditions reached nuisance levels in both the 1970s and 2000s, providing ample habitat for C. sphaericus. Phytoplankton abundance and biomass, which were not examined in this study, likely were reduced as evidenced by declines in chl a, increased light penetration, and declines in nutrient inputs from Lake Ontario where such reductions have been documented (Millard et al., 2003; Munawar & Munawar, 2003). These conditions may have favored epibenthic grazing of chydorids potentially fueling an increase in abundance.
Eurytemora affinis, an epibenthic species reported to prefer warm epilimnetic and littoral areas (Evans & Stewart, 1977), was not reported in the region until the 1960s, but was distributed in all the Great Lakes by 1968 (Balcer et al., 1984). The mean annual water temperature increase of 0.8°C between the study periods (Murry, 2006), along with ecological changes associated with benthification (Mills et al., 2003) may have benefited E. affinis and other epi-benthic species. Indicators of meso- to eutrophic conditions may be expected to have declined in response to nutrient reduction. Consistent with this idea was the decline in Ceriodaphnialacustris, a species known to be an efficient limnetic filter feeder of bacteria (Balcer et al., 1984) associated with more productive waters.
Cercopagispengoi, an invader from the Ponto-Caspian region of Eastern Europe introduced to Lake Ontario in 1998 (MacIsaac et al., 1999; Laxson et al., 2003), was present each year in the recent time period. Benoit et al. (2002) indicated C. pengoi fed upon Bosmina and copepods and affected their distribution. Cercopagis densities in the St. Lawrence River were comparable to those reported for Lake Ontario in recent years (Holeck et al., 2008b), but whether Cercopagis has affected the densities of Bosmina in the river is not known.
Contrary to descriptions by Mills & Forney (1982) and Basu et al. (2000), zooplankton density did not exhibit a downstream attenuation in recent years. Mills & Forney (1982) observed zooplankton biomass declines from Cape Vincent near the river confluence with Lake Ontario downstream to Massena, NY. Basu et al. (2000) showed evidence of decreasing zooplankton biomass down the St. Lawrence River from Lake Ontario to Trois-Rivières, Québec during the years just preceding our study. However, our study did show patterns in chlorophyll a and Secchi-depth that were consistent with the expected trend of downstream attenuation in productivity. It is possible that the lack of a similar pattern in zooplankton density was an artifact of the overall reduction in production observed between the periods. In Basu et al. ’s (2000) study, zooplankton biomass decreased most dramatically between Lake Ontario and the first St. Lawrence station at Brockville, NY, adjacent to Mills & Forney’s (1982) Ogdensburg-Prescott station, before decreasing more gradually with further movement downriver. Possibly production in the lateral dimension was more important in our study as the shallow stations revealed the greatest levels of production, a result inverse to that of the earlier period.
Further downriver, nutrient decline and attenuation of production along the longitudinal gradient of the St. Lawrence River has been shown to be largely unaffected by large fluvial lakes along the river and continues downstream to near Montreal, Quebec where cultural eutrophication elevates nutrient concentrations (Hudon et al., 1996). We also observed a longitudinal gradient in chl a, but only at deep stations. Notably, productivity declines from Cape Vincent to Chippewa Bay were observed in our benthos data for exotic dreissenids, with evidence of potential feedbacks for specific years for oligochaetes, and chironomids and an inverse response for amphipods. Deducing complex interactions in the benthos with single-site index data is severely limiting, yet hypotheses regarding dressenid facilitation and inhibition of specific functional groups should be examined in future research.
Native mussels (unionid species) have since been extirpated in the main stem of the St. Lawrence (J. M. Farrell, pers. observation) likely by invasive dreissenid mussels (Ricciardi et al., 1995), but populations exist in tributaries to the system (Strayer & Jirka, 1997). The current mussel biomass is dominated by D. bugensis, a shift first documented in the river in 1995 (Mills et al., 1999). Benthification is a term used to describe increased heterotrophic production in the Lake Ontario profundal zone associated with non-native mussel proliferation (Mills et al., 2003). Increases in benthic biomass and faunal representation in the 2000s provide evidence that similar processes have taken hold in the upper river.
Benthic production (e.g., 2006, 2007) observed from the stations shows evidence of substantial decline in recent years. This trend in declining is consistent with patterns of increase in invasive round goby in the upper St. Lawrence River (J. M. Farrell, unpublished data). Seining data for the upper river in adjacent nearshore littoral areas indicate increases in goby from 2006 to 2007 (Fig. 10). Densities in the habitat within profundal areas are much higher, and goby predation effects on dreissenids could affect population densities. A study of benthic invertebrate consumption by size cohorts of round goby in the Central basin of Lake Erie indicated zooplankton, chironomids, dreissenids and other species were eaten (Johnson et al., 2005). Interestingly, model expansion of these data indicated 57% of population consumption was by age-0 fish and 90% of this biomass was zooplankton. The authors conclude goby represent a new and significant energy pathway strongly influencing trophic dynamics through the food web. Round goby proliferation in the upper St. Lawrence appears to have taken hold in 2005, although the species had been in the system several years prior to their high abundance and widespread distribution (J. M. Farrell, unpublished data). Similar effects of round goby in conversion of benthic energy associated with dressenid facilitation up the food web are likely taking place. Smallmouth bass (Micropterus dolomieu), a top benthic predator, for example, has increased in the NYSDEC Thousand Islands Warmwater Assessment (R. Klindt & R. McCullough, pers. comm.) and have shifted their diet toward the round goby.
Round goby catch rates (catch per haul) in standardized seine hauls performed in the littoral zone along the longitudinal river gradient in bays from Cape Vincent to Chippewa Bay, NY. Farrell & Werner (1999) and Murry & Farrell (2007) given details on seining procedures and descriptions of study bays
Changes to the St. Lawrence River have likely taken the upper river from a fluctuating productivity state linked to Holocene climatic events such as those observed in Lake Ontario (McFadden et al., 2003) to one linked with anthropogenic nutrient loading during the 1970s (Mills & Forney, 1982). In the present, invasive filter feeders and nutrient abatement have created a new set of lower trophic conditions similar to those described for Lake Ontario by Mills et al. (2003) where benthic production predominates. These conditions in a lotic context may promote a greater influence of local conditions (both auto- and heterotrophic) on production, although the influence of downstream water movement on productivity is still apparent. With increasing mean annual temperature (Murry, 2006) and an apparent reduction in dressenid mussels with increasing abundance of round goby, the river appears to be going through a new set of adjustments that perhaps will lead it back toward reduced light transparency and increased pelagic production.
References
Balcer, M. D., N. L. Korda & S. I. Dodson, 1984. Zooplankton of the Great Lakes. University of Wisconsin Press, Madison, WI.
Basu, B. K., J. Kalff & B. Pinel-Alloul, 2000. Midsummer plankton development along a large temperate river: the St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 57(Suppl. 1): 7–15.
Benoit, H., O. E. Johannsson, D. M. Warner, W. G. Sprules & L. G. Rudstam, 2002. Assessing the impact of a recent predatory invader: the population dynamics, vertical distribution and potential prey of Cercopagis pengoi in Lake Ontario. Limnology and Oceanography 47: 626–635.
Burkholder, P. R. & W. L. Tressler, 1932. Plankton studies in some northern New York waters: 222–251. In A Biological Survey of the Oswegatchie and Black River Systems. Supplement of the 21st Annual Report, 1931. New York State Department of Conservation.
Chamberlain, H. D., 1975. A comparative study of the zooplankton communities of Skaneateles, Owasco, Hemlock, and Conesus lakes. Ph. D. Thesis, Cornell University. Ithaca, NY: 266 pp.
Evans, M. S. & J. A. Stewart, 1977. Epibenthic and benthic microcrustaceans (copepods, cladocerans, ostracods) from a nearshore area in Lake Michigan. Limnology and Oceanography 22(6): 1059–1066.
Farrell, J. M. & R. G. Werner, 1999. Abundance, distribution, and survival of age-0 muskellunge in Upper St. Lawrence River nursery embayments. North American Journal of Fisheries Management 19: 310–321.
Fryer, D. G., 1968. Evolution and adaptive radiation in the Chydoridae (Crustacea: Cladocera): a study in comparative morphology and ecology. Philosophical Transactions of the Royal Society Series B 254: 221–385.
Hall, S. R., N. K. Pauliukonis, E. L. Mills, L. G. Rudstam, C. P. Schneider, S. J. Lary & F. Arrhenius, 2003. A comparison of total phosphorus, chlorophyll a, and zooplankton in embayment, nearshore, and offshore habitats of Lake Ontario. Journal of Great Lakes Research 29: 54–69.
Haney, J. G. & D. J. Hall, 1973. Sugar-coated Daphnia: preservation technique for Cladocera. Limnology and Oceanography 18: 331–333.
Hecky, R. E., H. Smith, D. R. Barton, S. J. Guildford, W. D. Taylor, M. N. Charlton & T. Howell, 2004. The nearshore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 61: 1285–1293.
Holeck, K. T., J. M. Watkins, E. L. Mills, O. Johannsson, S. Millard, V. Richardson & K. Bowen, 2008a. Spatial and long-term temporal assessment of Lake Ontario water clarity, nutrients, chlorophyll a, and zooplankton. Aquatic Ecosystem Health and Management 11: 377–391.
Holeck, K. T., J. W. Swan, S. H. McConnachie, E. L. Mills, L. G. Rudstam, R. McCullough, D. Bishop, W. Pearsall, S. LaPan, B. Trometer, B. Lantry, R. O’Gorman & T. Schaner, 2008b. 2007 Status of the Lake Ontario Ecosystem. Section 19 in Lake Ontario Fisheries Unit Reports: 24 pp. http://www.dec.ny.gov/docs/fish_marine_pdf/lorpt07sec19.pdf.
Hudon, C., S. Paquet & V. Jarry, 1996. Downstream variations of phytoplankton in the St. Lawrence River (Québec, Canada). Hydrobiolgia 337: 11–26.
Johannsson, O. E., 1987. Comparison of Lake Ontario zooplankton communities between 1967 and 1985: before and after implementation of salmonid stocking and phosphorus control. Journal of Great Lakes Research 13: 328–339.
Johannsson, O. E., E. L. Mills & R. O’Gorman, 1991. Changes in the nearshore and offshore zooplankton communities in Lake Ontario: 1981–1988. Canadian Journal of Fisheries and Aquatic Sciences 48: 1546–1557.
Johnson, T. B., D. B. Bunnell & C. T. Knight, 2005. A potential new energy pathway in central Lake Erie: the round goby connection. Journal of Great Lakes Research 31(2): 238–251.
Lange, C. & R. Cap, 1986. Bythotrephes cederstromei (Schödler) (Cercopagidae: Cladocera): a new record for Lake Ontario. Journal of Great Lakes Research 12: 142–143.
Laxson, C. L., K. N. McPhedran, J. C. Makarewicz, I. V. Telesh & H. J. MacIsaac, 2003. Effects of the non-indigenous cladoceran Cercopagis pengoi on the lower food web of Lake Ontario. Freshwater Biology 48(12): 2094–2106.
MacIsaac, H. J., I. A. Grigorovich, J. A. Hoyle, N. D. Yan & V. E. Panov, 1999. Invasion of Lake Ontario by the Ponto-Caspian predatory cladoceran Cercopagis pengoi. Canadian Journal of Fisheries and Aquatic Sciences 56: 1–5.
Makarewicz, J. C. & D. Jones, 1990. The establishment of Bythotrephes cederstroemi in the offshore of Lake Ontario. Journal of Great Lakes Research 16: 143–147.
McFadden, M. A., H. T. Mullins, W. P. Patterson & W. T. Anderson, 2003. Paleoproductivity of eastern Lake Ontario over the past 10,000 years. Limnology and Oceanography 49(5): 1570–1581.
McQueen, D. J., J. R. Post & E. L. Mills, 1986. Trophic relationships in freshwater pelagic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences 43: 1571–1581.
Menzel, D. & N. Corwin, 1965. The measurement of total phosphorus on the liberation of the organically bound fraction of persulfate oxidation. Limnology and Oceanography 10: 280–282.
Millard, E. S., O. E. Johannsson, M. A. Neilson & A. H. El-Shaarawi, 2003. Long-term, seasonal and spatial trends in nutrients, chlorophyll a and light attenuation in Lake Ontario. In Munawar, M. (ed.), Ecovision World Monograph Series. Aquatic Ecosystem Health and Management Society, Burlington: 97–132.
Mills, E. L. & J. L. Forney, 1982. Response of Lake Ontario plankton entering the international section of the St. Lawrence River. Internationale Revue der Gesamten Hydrobiologie 67: 27–43.
Mills, E. L., S. B. Smith & J. L. Forney, 1981. The St. Lawrence River in winter: population structure, biomass, and pattern of its primary and secondary food web components. Hydrobiolgia 79: 65–75.
Mills, E. L., J. R. Chrisman, B. Baldwin, R. W. Owens, R. O’Gorman, T. Howell, E. F. Roseman & M. K. Raths, 1999. Changes in the Dreissenid Community in the Lower Great Lakes with Emphasis on Southern Lake Ontario. Journal of Great Lakes Research 25: 187–197.
Mills, E. L., J. M. Casselman, R. Dermott, J. D. Fitzsimons, G. Gal, K. T. Holeck, J. A. Hoyle, O. E. Johannsson, B. F. Lantry, J. C. Makarewicz, E. S. Millard, I. F. Munawar, M. Munawar, R. O’Gorman, R. W. Owens, L. G. Rudstam, T. Schaner & T. J. Stewart, 2003. Lake Ontario: food web dynamics in a changing ecosystem (1970–2000). Canadian Journal of Fisheries and Aquatic Sciences 60: 471–490.
Munawar, M. & I. F. Munawar, 2003. Changes in phytoplankton community structure and primary production of Lake Ontario. In Munawar, M. (ed.), Ecovision World Monograph Series. Aquatic Ecosystem Health and Management Society, Canada: 187–219.
Murry, B. A., 2006. Early life history and community structure of large river fishes. Doctoral dissertation, SUNY College of Environmental Science and Forestry, Syracuse, NY.
Murry, B. A. & J. M. Farrell, 2007. Quantification of native muskellunge nursery: influence of body size, fish community composition, and vegetation structure. Environmental Biology of Fishes 79: 37–47.
Nicholls, K. H., G. J. Hopkins & S. J. Standke, 1999. Reduced chlorophyll to phosphorus ratios in nearshore Great Lakes waters coincide with the establishment of dreissenid mussels. Canadian Journal of Fisheries and Aquatic Sciences 56: 153–161.
Patalas, K., 1972. Crustacean zooplankton and the eutrophication of St. Lawrence Great Lakes. Journal of the Fisheries Research Board of Canada 29: 1451–1462.
Ricciardi, A., F. G. Whoriskey & J. B. Rasmussen, 1995. Predicting the intensity and impact of Dreissena infestation on native unionid bivalves from Dreissena field density. Canadian Journal of Fisheries and Aquatic Sciences 52: 1149–1461.
Stevens, R. J. J. & M. A. Neilson, 1987. Response of Lake Ontario to reductions in phosphorus load, 1967–1982. Canadian Journal of Fisheries and Aquatic Sciences 44: 2059–5069.
Strayer, D. L. & K. L. Jirka, 1997. The pearly mussels of New York State. New York State Muesum Memoir No. 26, NYS Education Dept. Publication.
Strickland, J. D. H. & T. R. Parsons, 1968. A practical handbook of seawater analysis. Bulletin of the Fisheries Research Board of Canada, No. 167.
Twiss, M. R., 2007. Wither the St. Lawrence River? Journal of Great Lakes Research 33(3): 693–698.
Warner, D. M., L. G. Rudstam, H. Benoit, E. L. Mills & O. Johannsson, 2006. Changes in seasonal nearshore zooplankton abundance patterns in Lake Ontario following establishment of the exotic predator Cercopagis pengoi. Journal of Great Lakes Research 32: 531–542.
Water Survey of Canada, 1990. Historical Streamflow Summary, Ontario. Environment Canada, Water Survey of Canada, Ottawa, Ont.
Acknowledgments
We graciously thank those who participated in field data collection and sample processing including Kristin Hawley, Geof Eckerlin, Eric Reynolds, Sarah Walsh, Lea Calhoun, Brian Kelder and Katie Woodside. We are also indebted to Travis Spier, Jonathan Swan, Sarah McConnachie for laboratory processing of samples and John E. Cooper of Cooper Environmental Research for processing of benthic invertebrate samples and assistance with field collections. Financial support came indirectly through the Federal Aid in Sportfish Restoration Grant FA-5-R and Cornell University and to ESF through the Federal Work-Study Program. This research is a joint contribution between the Cornell Biological Field Station (publication #269) and the Thousand Islands Biological Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Power, J. Marty, M. R. Twiss, J. Ridal, Y. de Lafontaine, J. M. Farrell / St. Lawrence River—Great Lakes Ecosystems: An Ecological Overview
Rights and permissions
About this article
Cite this article
Farrell, J.M., Holeck, K.T., Mills, E.L. et al. Recent ecological trends in lower trophic levels of the international section of the St. Lawrence River: a comparison of the 1970s to the 2000s. Hydrobiologia 647, 21–33 (2010). https://doi.org/10.1007/s10750-009-0003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0003-7