Abstract
There is a general consensus that breast cancer is a rising trend disease in the world. It is one of the most common cancer types and is the leading cause of death among women’s cancers. There are several reasons for this high rate of mortality including metastasis which is responsible for about 90 % of cancer-related mortality. Therefore, recognition and understanding of metastatic process is important, and by considering the key role of pathophysiological route in metastasis as a multistep cascade of “invasion–metastasis,” it might modify and improve our insight toward this complex phenomenon. Moreover, it can provide novel approaches for designing advanced targeted therapies. The present work aimed to review the published papers regarding molecular basis of metastatic process of breast cancer to brain metastasis, especially related genes and signaling network. Furthermore, the use of molecular aspects of metastatic breast cancer to brain was discussed in horizon of future treatment of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis is responsible for more than 90 % of cancer-related mortality [1, 2]. The process of tumor metastasis is highly selective and consists of a series of sequential, interrelated steps [3]. During this process, cancer cells disseminate from the primary tumor to distant organs, or to other regions of the same organ to form secondary metastatic tumor(s), and establish long distant or local metastases, respectively [4]. Breast cancer (BC) is the most common malignancy and one of the major causes of cancer-related mortality among women [5]. The majority of deaths from breast cancer are due to metastasis [6]. Breast cancer has great economic and psychological burdens upon healthcare systems of patients, especially when metastasized to the brain [7]. Brain is the fourth most common site of breast cancer distant metastases, after the bone, lung, and liver [8]. Approximately 10–15 % of patients with breast cancer develop brain metastasis (BM), even though autopsy studies have shown much higher incidence [9]. There are many reports indicating the increasing rate of BM within recent years [7].
Recent increase in frequency and incidence of brain metastasis may be due to several factors, including increased aging population [10, 11] and increased awareness of the warning signs and risk factors [11]; however, improvement in treatment and advances in diagnostic methods are raising the chance of early detection [12]. Prognostic factors of breast cancer brain metastasis (BCBM) in the domain of central nervous system, focus on different insights including identification of high-risk individuals, and evaluation of therapeutic- programming and outcomes [13–16]; higher tumor size and grade [8, 9]; luminal B, triple-negative [17] and HER2-positive tumors [8, 9, 14, 15, 17]; overexpression of p53, p63, and Ki67 [8, 18]; multiple distant metastases [9, 17]; presence of lung metastases [14, 15, 18, 19]; lymph node involvement [8, 14]; and Karnofsky performance status (KPS) [16] (Fig. 1). Identification of risk factors and prognostic markers would provide specific surveillance, management of patients at risk [20], and designing therapeutic modalities to reduce the chance of metastasis [21].
Cancer Stem Cells and Concept of Metastasis Cascade
Most tumors are heterogeneous and appeared to harbor a slight population of self-renewing and expanding stem-like cells, known as cancer stem cells (CSCs) [22–24]. CSCs share self-renewal, differentiation, organogenesis, and features of normal stem cells [24, 25]. Recent studies have shown that differentiated cancer cells can be induced into a CSC-like state through a biologic multistep process entitled epithelial–mesenchymal transition (EMT) [26]. In addition to tumor progression and metastasis, EMT is a main and essential part of embryogenesis and tissue regeneration [27]. EMT consists of a series of radical changes in cell phenotype, during which epithelial cells lose their cell–cell adhesion structures and therefore their polarity and rearrange their cytoskeleton. EMT is a critical pathway in the mesenchymal movement of single migratory cells, and therefore, cells that undergo EMT acquire mesenchymal phenotype and become isolated, motile, and resistant to apoptosis [26].
The EMT programs and early stages of cancer are regulated by several key signaling pathways and transcription factors, that lead to deregulation of the expression of epithelial markers (e.g., E-cadherin), and enhancement in the expression of mesenchymal markers (e.g., vimentin) [28]. SNAIL, TWIST, and ZEB families of transcription factors are master regulators of EMT [29]. On the other hand, TGF-β, Wnt, and Notch signaling pathways are among the most critical regulators of this process. Indeed, interactions between key transcription factors and signaling pathways form an autoregulatory network are believed to regulate different steps of metastasis [30]. The CSCs are capable to continue the metastatic dissemination process, known as metastatic cascade (3).
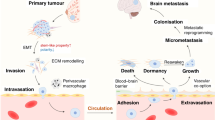
The metastatic cascade is a complex network of biological events [31, 32]. The most accepted model for metastasis, “seed and soil” theory, indicates that disseminated cancer cells (the seed) can thrive only in permissive tissues (the soil) [33, 34]. Looking at the pathophysiological process of metastasis as a series of distinguished steps, called “invasion–metastasis cascade,” has increased our understanding of this complex phenomenon [35]. On the formation of metastases, tumor cell growth and secretion of angiogenic factors lead to an extensive vascularization within primary tumors [34]. Thereupon, cells locally invade through the surrounding extracellular matrix (ECM) and stromal cell layers, via induction of EMT, enabling them to leave the primary site [36]. Locally invasive tumor cells intravasate and enter into the lumina of blood vessels or lymphatic system to be disseminated [37]. Tumor cells must evade the host’s immune system and apoptotic signals at the same time to survive. Henceforth, tumor cells must reach and attach to the vasculature of the brain, extravasate into the parenchyma, and pass through the blood–brain barrier (BBB) [38]. Interestingly, only a few tumor cells have a chance to survive [39] and reinitiate their proliferative program in foreign microenvironments and therefore form micrometastases. Next, tumor cells may further proliferate at metastatic site(s) and form secondary tumors. The latter step, called “metastatic colonization,” is the most rate-limiting step of metastasis [40]. In almost all steps, dynamic interactions of tumor cells with their specialized niche have a profound effect and govern metastasis [30].
In stem cell biology, the tumor microenvironment, or niche, is a specialized network [41] that supports stem cell induction and maintenance and actively controls cell function and proliferation. The niche consists of various elements such as nutrients, soluble factors, vascular networks, stromal cells, and ECM architecture [42]. The sophisticated patterns of interactions between different cell populations determine tumor behavior and subsequently the outcome of the disease [43]. A favorable microenvironment known as “pre-metastatic niche” (PMN) is required to evolve in order to support the tumor cells for development of macrometastasis from micrometastasis [42]. Understanding the molecular aspects of the “pre-metastasis” niche generation and its role in supporting the organ-specific metastasis may open new avenues toward achieving novel prognostic and therapeutic approaches in breast cancer management [44].
Metastasis Organotropism of Breast Cancer
Studies have approved that distribution of metastases is a disproportional highly selective process, in which each primary tumor metastasize to a number of distinct organs [45]. This sophisticated phenomenon in metastasis, called organotropism (organ-specific metastasis) [46], has been shown in breast tumors with remarkable trend in metastasis to the bone, lung, liver, and brain [40]. Indeed, human breast tumors are heterogeneous and are classified according to the diverse gene-expression patterns. These molecular subtypes include luminal A and luminal B as the estrogen-receptor- positive (ER+) tumors, and types of estrogen-receptor-negative (ER−) including basal-like, human epidermal growth factor receptor 2 (HER2+/ER− or Erbb2) [47–51]. Sometimes, normal breast-like [52, 53] and luminal C [52] groups have been described as other molecular subtypes. This classification has a considerable clinical value, since some of the molecular subtypes show aggressiveness and poor prognosis such as HER2 and basal-like [49–52]. These subtypes are characterized with differential statue and overlapped gene expression that might determine the preferential site of relapse. Bone metastases which are the most common type of metastasis of breast cancer is more frequently originated from the luminal subtypes and are found less frequently in the basal subtype. Lung metastases are found less frequent in the luminal A subtype [48] but are common in basal-like and Erbb2 tumors [54]. The highest number of liver metastases was observed in the Erbb2 group and has been found with less frequency in luminal B subtype [48]. Erbb2, basal-like subtype or triple-negative breast cancer (TNBC) have higher risk of developing brain metastases among patients affected with breast cancer [55, 56]. The architecture of the vascular and/or lymphatic system also has key role in the dissemination pattern of circulating tumor cells (CTCs) and intricate tumor–stroma interactions at the target organ. Therefore, both the intrinsic features of cancer cells and the distant organ microenvironment play critical roles in determining the efficiency of organ-specific metastasis [45].
Molecular Portraits of Breast Cancer Metastasis to Brain
Breast cancer brain metastasis is influenced by several genes and signaling pathways. Genes that mediate brain metastases may be excellent markers to predict the site of recurrence and afford targeted treatment for an individual patient [57].
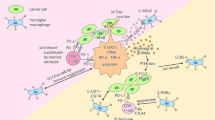
Chemokine signaling plays important roles in cancer metastasis [58, 59] and seems to be a worthy biological support for the seed and soil theory [60]. Migration, which is one of the most pivotal involved mechanisms in metastasis, is controlled by chemokines [61–63]. The chemokines that are expressed at specific organs determine the metastatic tropism by promoting tumor cell adhesion to microvessels and facilitating angiogenesis, extravasation, tumor proliferation, survival, and subsequently metastatic colonization, through key signaling pathways such as PI3K-Akt [64]. Chemokines such as stromal-derived factor-1 (SDF-1α, also called CXCL12) and C-C motif chemokine ligand 21 (CCL21) and their corresponding receptors CXCR4 and CCR7 play pivotal roles in homing, motility, and proliferation of tumor cells at distinct sites of metastasis [65]. It may be possible to predict the site of metastasis by evaluating the expression pattern of chemokine receptors in primary breast cancers [60]. It was reported that CXCR4 had significantly higher expression in primary breast cancer compared to normal breast tissues. CXCR4 is the outmost chemokine receptor expressed in most cancers, while SDF-1α has revealed to be highly expressed in common metastatic sites of breast cancer [66]. There is compelling evidence that CXCR4 may be one of the critical mediators of metastatic breast cancer [65, 67]. Besides, by binding to CXCR4, SDF-1α could activate multiple signaling pathways, including phosphatidylinositol-3 kinase (PI-3K/AKT), mitogen-activated protein kinase (MAPK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), as well as Crk [66, 68]. It has been demonstrated that PI-3K/AKT signaling pathway activation via CXCR4/SDF-1α is indispensable for breast cancer cell migration through the BBB barrier [68]. AKT, which is a downstream target of PI-3K, plays a critical role in promoting tumor cell survival by inactivating the apoptotic machinery [66] and chemotherapy resistance [69].
Direct contact between astrocytes, protective cells of BBB, and tumor cells induces calcium sequestration [69] and subsequently activate the AKT/MAPK signaling pathways [70]. These pathways stimulates upregulation of interleukin six (IL-6), IL-8 [69], BCL2L1, TWIST1, and GSTA5. In fact, these anti-apoptotic genes are responsible for breast cancer metastases to the brain and chemotherapy resistance in tumor cells [70, 69]. In addition to calcium, phospholipid-binding proteins such as annexin A1 (ANXA1 or lipocortin) ignite CXCR4-mediated migration of breast cancer cells in response to SDF-1α [71]. Experimental studies have shown that cooperation of SDF-1α with CXCR4 leads to penetration of breast cancer cells into human brain microvascular endothelial cells (HBMEC). Suppressing the CXCR4/ SDF-1α-mediated signaling pathway can be considered as a therapeutic approach for inhibition of breast cancer invasion and vascular permeability [66, 68]. The Cxcr4 and Cxcl12 signaling axis can be blocked by Slit family of secreted proteins (Slit1, 2, and 3) and their corresponding receptors (Robo1, 2, 3, and 4) [72]. Slits and Robos have critical roles in neuronal development and migration [73] and are candidate as tumor suppressor genes that are silenced in approximately 50 % of human breast tumors [72].
NF-κB regulates the motility of breast cancer cells through direct upregulation of CXCR4 expression. This complex, upregulates the expression of several prometastatic and proangiogenic genes including IL-6, IL-8, vascular endothelial growth factor (VEGF), and urokinase-type plasminogen activator (uPA) [65]. uPA convert plasminogen into plasmin which in turn inhibits the L1 cell adhesion molecule (L1CAM). L1CAM is an essential molecule for infiltration of metastatic breast cancer cells into brain capillaries and has a pivotal role in metastatic outgrowth of cancer cells. Furthermore, overexpression of anti-PA serpins (including neuroserpin and serpin B2), a family of protease-enzyme inhibitors, as brain metastatic cells originated from breast cancer tissues, can suppress plasmin and as a result provoke the metastatic process [39]. The uPA also degrades matrix components and activates matrix metalloproteinases (MMPs) through NF-κB activation [6, 74]. As one of the important biological markers in breast cancer [75], MMPs, which belong to a zinc-dependent endopeptidase family, are involved in different steps of tumor progression and facilitate cancer cell invasion and metastasis. These proteins act as enzymes that degrade structural components of the extracellular matrix. They are divided into two major types, soluble and membrane-MMP types. Based on substrate specificities and structural similarities, 28 human MMPs have been identified and categorized so far [76]. MMPs have also been broadly studied in context of breast cancer prognosis [77]. The mean messenger RNA expression of MMP-2, MMP-7, MMP-9, MMP14 genes besides tissue inhibitors of metalloproteinase-1 (TIMP-1) and TIMP-2 have shown to be significantly higher in breast cancer compared to normal tissue. This expression profile would be important in predicting the aggressive behavior of breast cancer cells [75].
There are many researches that highlighted the upregulation of MMPs in normal breast epithelium which was associated with invasive tumor formation through increase in genomic instability and EMT. Moreover, MMPs have critical roles in creating the pre-metastatic niche [74], and they induce growth factor signaling as well as TGF-β, FGF-2, and VEGF-A through enhancing their availability to corresponding receptors. It results in tumor evolution through stimulation of tumor fibroblasts and angiogenesis [74]. Among MMPs, MMP-2 and MMP-9 are known as type IV collagenases, or as an alternative gelatinase A and B, respectively [78]. Upon their function, MMP2/9 degrade type IV collagen, which is believed to be involved as a main component of the vascular basement membrane structure [75, 79]. Besides, MMP-2 is capable to hydrolyze other constituents of connective tissues such as elastin, laminin, fibronectin, proteoglycans, and fibrillin [79]. MMP-7 is upstream of MMP-2 and MMP-9 and turns them on to be critically involved in the degradation of the ECM components including type IV collagen [75]. Animal model studies have demonstrated that the MMP-2, MMP-3, and MMP-9 proteins expression is meaningfully higher in neoplastic compared to normal brain tissue. It has been proposed that MMP-2 [77, 80], MMP-3, and MMP-9 [77] might be active in the process of metastasis of breast cancer to the brain. It was confirmed that there is an association between MAPK pathway elements such as extracellular-signal-regulated kinase1/2 (ERK1/2), MMP expression, and/or astrocyte activity. It is assumed that astrocyte factors and the ERK1/2 signaling pathway may be associated with the development of BCBM. Animal studies have shown that ERK1/2 modulate the MMP2 to be modified by astrocyte factors [80].
Joyce and his colleagues confirmed the role of cathepsin S (CTSS) which encodes a lysosomal cysteine protease playing crucial role in metastatic seeding and outgrowth. They also demonstrated that CTSS modulates the organotropism and regulates BCBM through facilitating the transmigration of CSCs into BBB [81]. It was described that cyclooxygenase-2 (COX-2, also known as PTGS2) alters membrane arachidonic acid into prostaglandins and is able to upregulate the MT1-MMP, which itself activates MMP-2 that may provoke angiogenesis. COX-2 is well known to be involved in precursor lesions of various solid tumors and contributes to tumorigenesis by hindering the signaling pathways of the pro- and anti-apoptotic proteins [76]. COX2 accompanies the epidermal growth factor receptor (EGFR) and the alpha-2,6-sialyltransferase (ST6GALNAC5), which are highly distinguished genes among involved genes in breast cancer brain metastasis [82]. These genes are active when the tumor cells enter into the brain through the BBB (extravasation). The MMP1 and angiopoietin-like four (ANGPTL4) [82] are other involved proteins in this way which play pivotal role in driving the TGF-β and Notch signaling [32] and they thus mediate intravasation and extravasation processes [40]. The latent TGF-β-binding protein (LTBP1) as a major modulator of TGF-β activation, fascin-1 or FSCN1, and retinoic acid receptor responder protein3 (RARRES3) are other involved proteins [82]. Except ST6GALNAC5, they are linked to breast cancer infiltration of the lungs, suggesting that they have these mediators in common with cerebral and pulmonary metastases [82]. Moreover, eukaryotic translation initiation factor two (EIF2S3), FABP7, NMDA receptor regulated 1(NARG1), zinc finger proteins [involved in transcription and translation], aldehyde dehydrogenase 1 family, member A1 (ALDH1A1) [involved in metabolism], EGFR [involved in signal transduction], integrin alpha-6 (ITGA6), integrin, and laminin [involved in adhesion], ERGIC [involved in transportation] make a long list of proteins which have been shown to be expressed in breast cancer brain metastases [57].
Angiotensin II upregulates MMP2/MMP9 through which the sequential steps of cancer metastasis would be motivated. This progressive/ programming cascade includes promoting cancer cell adhesion to endothelial cells, transendothelial migration, and subsequently tumor cell migration across ECM, and subsequently facilitating the formation of metastatic foci at secondary sites [83]. MMP-9 is known as a major modulator of HER2/neu expression in human mammary epithelial cells. HER2/neu proto-oncogene or erbB-2 belongs to the ErbB protein family and is a cell surface receptor tyrosine kinase (RTK) that is principally contributed in cell growth and differentiation [83, 84]. ErbB-2 has been shown to be upregulated in 20–30 % of human breast cancers [85], while 34 % of HER2-positive breast tumors have led to brain metastases. HER2 pathway starts to relay the signals of several signaling proteins and pathways including PI3K/Akt, when it is activated [86]. Overexpression of HER2 upregulates the expression of MMP-9 and MMP-2 proteases [87], transmembrane proteins as well as plexin-B1 [86], and cluster of differentiation CD151 [88]. Accordingly, it was demonstrated that the CD151 and plexin-B1 play major roles in motility, invasion, and metastasis of cancer cells [88]. Of note, estrogen receptor beta (eRβ) and pea3 are among other proteins whose expression has been increased in response to HER2 overexpression. Importantly, they lead to IL-8 upregulation, which belongs to the superfamily of CXC chemokines and becomes overexpressed when its promoter is bound with the latter proteins [89]. IL-8 is a major mediator of angiogenesis and is capable to induce this process through stimulating the proliferation and sprouting of endothelial cells [40, 90]. In addition, the potential effects of TGF-β on HER2 signaling have been demonstrated. It was shown that IL-6, TGF-β, and IGF receptors are actively involved in the progression of breast cancer cells to the brain [91]. Therefore, by blocking the TGF-β, HER2 crosstalk may restrain breast cancer cells from progression and metastasis [92].
ErbB2 overexpression can also be associated with over expression of VEGF in breast cancer cells [85, 87]. It is also known as a vascular permeability factor (VPF) and major regulator of new blood vessel formation (angiogenesis) during tumor development [93]. It was described that it stimulates the proliferation and transendothelial migration of tumor cells, which is a key event in cancer metastasis inducing the expression of metalloproteinases and plasminogen proteins [38]. Potential of breast cancer cells to form brain metastases is a main consequence of the latter mentioned inductions [90]. VEGF also promotes the growth of BM induced by breast cancer in nude mice, and targeting endothelial cells with a VEGF receptor-specific tyrosine kinase inhibitor can reduce angiogenesis and restrict the growth of brain metastases [90] (Fig. 2).
Multistep process and signaling network of BCBM. From the figure, we can see that BCBM is influenced by several genes and signaling pathways. Potentially, inhibition of these pathways can be a valuable therapeutic approach, especially early signaling pathways, because of this approach leads to inhibit more of accessory signaling pathways involved in progression of metastasis
Therapeutic Approach
BM represents a significant healthcare concern and has a drastic deleterious impact on patient mortality [94]. Of note, not all the brain lesions are considered as primary tumor and the possibility of metastasis from other organs especially breast should always be noted [95]. The biology of the primary tumor, the number and location of metastatic lesions, and the phase of systemic disease are important considerations for the treatment of brain metastasis [34].
Appropriate diagnostic approaches are presently available such as computed tomography (CT) and contrast-enhanced magnetic resonance imaging (MRI). When it comes to the diagnosis of brain metastases, MRI is the ideal test [95]. Molecular diagnosis may also be useful, for example, miR-205 that is negatively regulated by HER2/neu overexpression and miR-342 that is involved in the breast tumor’s invasive behavior, these may be used as potential biomarkers for diagnosis of triple-negative breast cancer [96]. Treatment options of brain metastasis for diagnosed patients include surgery, whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS) [97], and chemotherapy that among them WBRT is the most common selected choice [12, 98]. In patients with several brain metastases, studies have identified that the use of surgical resection simultaneously with radiotherapy is highly preferred to using of radiotherapy solely. Additionally, patients who take systemic chemotherapy after brain radiotherapy show significant higher curative outcome [99]. The other commonly used method, chemotherapy, has played trivial roles in the treatment of BM; however, the intact BBB blocks the passage of many chemotherapeutic drugs into the brain [16].
Targeted therapies are the most attractive molecular therapeutic approaches that have shown to be promising. In these methods, certain proteins and signal transduction pathways that are involved in BCBM are specifically targeted, for instance targeting the angiogenesis by anti-VEGF agent [100]. Targeting poly-adenosine diphosphate ribose polymerase (PARP), which plays an important role in DNA damage repair, and its corresponding pathway using specific inhibitors such as iniparib, olaparib, and veliparib may have a critical role in increasing the responses of tumor cells to chemotherapy and radiotherapy [94]. In addition, targeting HER2 tyrosine kinase by their specific inhibitors such as gefitinib, erlotinib, lapatinib, and trastuzumab constitute one of the effective strategies of targeted therapeutic approaches [12]. Notably, trastuzumab is a humanized monoclonal antibody that binds to a specific epitope of the HER-2/neu (c-erbB-2) protein. This interaction suppresses signal transduction pathways that regulate cell growth, survival, migration, differentiation, and angiogenesis, thereby decreases malignancy [16, 101] and also increases the sensitivity of tumor cells to both endocrine therapy and certain chemotherapeutic agents [16]. Lapatinib inhibits the dual epidermal growth factor receptor and HER2 tyrosine kinase [95] and corresponding downstream signaling proteins and therefore cell proliferation and migration [102]. In targeted therapy of metastasis, inhibition of early signaling pathways is important, because this approach would be associated with inhibition of more accessory signaling pathways involved in progression of metastasis. Certainly, inhibition of EMT leads to the prevention of early steps of metastasis. Recent studies on stem cell showed that gene expression profiling of cancer stem cells is similar to gene expression profiling of induced pluripotent stem cells. These cells can be changed into mesenchymal-like phenotype by enhanced gene expression including insulin-like growth factor (IGF) or its binding protein as transferrin (IGFBP) [103]. It is possible to hypothesize that EMT and generation of mesenchymal-like cells are inhibited by expression inhibition of IGF and IGFBP genes, and potentially, these genes can be a more practical target therapy. However, many experimental researches are warranted to confirm this hypothesis.
BM as one of the metastatic organotropism of breast cancer has a great unpleasant effect upon patients and their families. Hence, a more comprehensive understanding of molecular aspects of metastatic cascade is essential to achieve an appropriate strategy in accurate diagnosis and novel methods of therapeutics. Using this viewpoint, many results have been reported in studies of involved signaling pathways in BCBM, ranging from CXCR4/ SDF-1α, PI-3K/AKT, MAPK, NF-κb. Comparison between pathways involved in BCBM with other pathways leading to metastasis of breast cancer cells to other organs as well as lung can shed further light on a new set of genes that play critical role in BCBM, as well. These findings could be important because these may lead to provide a new target-based therapy. Beyond question, further research is required to explore the unknown aspects of signaling network in BCBM. As a final word, the main concern is whether the results achieved in vitro and/or in vivo are translatable in human.
References
Fidler IJ, Balasubramanian K, Lin Q, Kim SW, Kim S-J (2010) The brain microenvironment and cancer metastasis. Mol Cell 30(2):93–98
Nguyen DX, Massagué J (2007) Genetic determinants of cancer metastasis. Nat Rev Genet 8(5):341–352
Fazilaty H, Mehdipour P (2014) Genetics of breast cancer bone metastasis: a sequential multistep pattern. Clin Exp Metastasis 31(5):595–612
Fidler IJ, Poste G (2008) The “seed and soil” hypothesis revisited. Lancet Oncol 9(8):808
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30
Weigelt B, Peterse JL, van’t Veer LJ (2005) Breast cancer metastasis: markers and models. Nat Rev Cancer 5(8):591–602
Pelletier EM, Shim B, Goodman S, Amonkar MM (2008) Epidemiology and economic burden of brain metastases among patients with primary breast cancer: results from a US claims data analysis. Breast Cancer Res Treat 108(2):297–305
M-m S, Liu J, Vong JS, Niu Y, Germin B, Tang P, Chan AW, Lui PC, Law BK, Tan P-H (2011) A subset of breast cancer predisposes to brain metastasis. Med Mol Morphol 44(1):15–20
Miller K, Weathers T, Haney L, Timmerman R, Dickler M, Shen J, Sledge G Jr (2003) Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol 14(7):1072–1077
Aragon-Ching JB, Zujewski JA (2007) CNS metastasis: an old problem in a new guise. Clin Cancer Res 13(6):1644–1647
Gril B, Evans L, Palmieri D, Steeg PS (2010) Translational research in brain metastasis is identifying molecular pathways that may lead to the development of new therapeutic strategies. Eur J Cancer 46(7):1204–1210
Stemmler H-J, Heinemann V (2008) Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: a treatment challenge. Oncologist 13(7):739–750
Evans A, James J, Cornford E, Chan S, Burrell H, Pinder S, Gutteridge E, Robertson J, Hornbuckle J, Cheung K (2004) Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol 16(5):345–349
Pestalozzi B, Zahrieh D, Price K, Holmberg S, Lindtner J, Collins J, Crivellari D, Fey M, Murray E, Pagani O (2006) Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 17(6):935–944
Park B-B, Uhm JE, Cho EY, La Choi Y, Ji SH, Lee JI, Park W, Huh SJ, Park YH, Ahn JS (2009) Prognostic factor analysis in patients with brain metastases from breast cancer: how can we improve the treatment outcomes? Cancer Chemother Pharmacol 63(4):627–633
Liu M-T, Hsieh C-Y, Wang A-Y, Chang T-H, Pi C-P, Huang C-C, Huang C-Y, Liou C-H (2006) Prognostic factors affecting the outcome of brain metastases from breast cancer. Support Care Cancer 14(9):936–942
YAN M, H-m LÜ, Z-z LIU, LIU H, M-w ZHANG, X-b SUN, S-d CUI (2013) High risk factors of brain metastases in 295 patients with advanced breast cancer. Chin Med J (Engl) 126(7):1269–1275
Sezgin C, Gokmen E, Esassolak M, Ozdemir N, Goker E (2007) Risk factors for central nervous system metastasis in patients with metastatic breast cancer. Med Oncol 24(2):155–161
Slimane K, Andre F, Delaloge S, Dunant A, Perez A, Grenier J, Massard C, Spielmann M (2004) Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol 15(11):1640–1644
Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, Christgen M, von Wasielewski R, Kreipe HH (2007) Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol 20(8):864–870
Khaitan D, Sankpal UT, Weksler B, Meister EA, Romero IA, Couraud P-O, Ningaraj NS (2009) Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC Cancer 9(1):258
Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF (2007) The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 356(3):217–226
Alison MR, Lin W-R, Lim SM, Nicholson LJ (2012) Cancer stem cells: in the line of fire. Cancer Treat Rev 38(6):589–598
Clarke MF, Fuller M (2006) Stem cells and cancer: two faces of eve. Cell 124(6):1111–1115
Wicha MS, Liu S, Dontu G (2006) Cancer stem cells: an old idea—a paradigm shift. Cancer Res 66(4):1883–1890
Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
Nakaya Y, Sheng G (2013) EMT in developmental morphogenesis. Cancer Lett
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
De Craene B, Berx G (2013) Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 13(2):97–110
Fazilaty H, Gardaneh M, Bahrami T, Salmaninejad A, Behnam B (2013) Crosstalk between breast cancer stem cells and metastatic niche: emerging molecular metastasis pathway? Tumor Biol:1–12
Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4(6):448–456
Spano D, Heck C, De Antonellis P, Christofori G, Zollo M (2012) Molecular networks that regulate cancer metastasis. In: Semin Cancer Biol. vol 3. Elsevier, pp 234–249
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133(3421):571–573
Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited—the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 128(11):2527–2535
Scheel C, Weinberg RA (2012) Cancer stem cells and epithelial–mesenchymal transition: concepts and molecular links. In: Semin Cancer Biol. vol 5. Elsevier, pp 396–403
Sarrió D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J (2008) Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 68(4):989–997
Thiery JP (2002) Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454
Fidler IJ, Yano S, R-d Z, Fujimaki T, Bucana CD (2002) The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol 3(1):53–57
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH-F, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E (2014) Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156(5):1002–1016
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292
Spano D, Zollo M (2012) Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis 29(4):381–395
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293
Tarin D (2012) Clinical and biological implications of the tumor microenvironment. Cancer Microenviron 5(2):95–112
Lu X, Kang Y (2007) Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia 12(2–3):153–162
Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL (2006) Metastatic patterns in adenocarcinoma. Cancer 106(7):1624–1633
Hu G, Kang Y, Wang X-F (2009) From breast to the brain: unraveling the puzzle of metastasis organotropism. J Mol Cell Biol 1(1):3–5
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW (2008) Subtypes of breast cancer show preferential site of relapse. Cancer Res 68(9):3108–3114
Sørlie T, Tibshirani R, Parker J, Hastie T, Marron J, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci 100(14):8418–8423
Nam B-H, Kim SY, Han H-S, Kwon Y, Lee KS, Kim TH, Ro J (2008) Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 10(1):R20
Schnitt SJ (2010) Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol 23:S60–S64
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci 98(19):10869–10874
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360(8):790–800
Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Sanna G, Franceschelli L, Rotmensz N, Botteri E, Adamoli L, Marenghi C, Munzone E, Rocca MC, Verri E, Minchella I (2007) Brain metastases in patients with advanced breast cancer. Anticancer Res 27(4C):2865–2869
Bollig-Fischer A, Michelhaugh S, Ali-Fehmi R, Mittal S The molecular genomics of metastatic brain tumours
Klein A, Olendrowitz C, Schmutzler R, Hampl J, Schlag PM, Maass N, Arnold N, Wessel R, Ramser J, Meindl A (2009) Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett 276(2):212–220
Tanaka T, Bai Z, Srinoulprasert Y, Yang B, Hayasaka H, Miyasaka M (2005) Chemokines in tumor progression and metastasis. Cancer Sci 96(6):317–322
Zlotnik A (2014) Chemokines in neoplastic progression. In: Semin Cancer Biol. vol 3. Elsevier, pp 181–185
Andre F, Cabioglu N, Assi H, Sabourin J, Delaloge S, Sahin A, Broglio K, Spano J, Combadiere C, Bucana C (2006) Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol 17(6):945–951
SALCEDO R, RESAU JH, HALVERSON D, HUDSON EA, DAMBACH M, POWELL D, Wasserman K, OPPENHEIM JJ (2000) Differential expression and responsiveness of chemokine receptors (CXCR1–3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J 14(13):2055–2064
Kakinuma T, Hwang ST (2006) Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol 79(4):639–651
Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56
Ben-Baruch A (2008) Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis 25(4):345–356
Helbig G, Christopherson KW, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H (2003) NF-κB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 278(24):21631–21638
Lee B-C, Lee T-H, Avraham S, Avraham HK (2004) Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial Cells1 1NIH grant NS39558 (S. Avraham), the Susan G. Komen Fellowship (S. Avraham), the Milheim Foundation (S. Avraham), CA97153 (H. Avraham), and K18 PAR-02-069 (H. Avraham). Note: this work was done during the term of an established investigatorship from the American Heart Association (H. Avraham). This article is dedicated to Charlene Engelhard for her continuing friendship and support for our research program. Mol Cancer Res 2(6):327–338
Ali S, Lazennec G (2007) Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev 26(3–4):401–420
Hinton CV, Avraham S, Avraham HK (2010) Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis 27(2):97–105
Kim SW, Choi HJ, Lee H-J, He J, Wu Q, Langley RR, Fidler IJ, Kim S-J (2014) Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro-oncol:nou128
Kim S-J, Kim J-S, Park ES, Lee J-S, Lin Q, Langley RR, Maya M, He J, Kim S-W, Weihua Z (2011) Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 13(3):286–298
Kang H, Ko J, Jang S-W (2012) The role of annexin A1 in expression of matrix metalloproteinase-9 and invasion of breast cancer cells. Biochem Biophys Res Commun 423(1):188–194
Marlow R, Strickland P, Lee JS, Wu X, PeBenito M, Binnewies M, Le EK, Moran A, Macias H, Cardiff RD (2008) SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4 within breast epithelium. Cancer Res 68(19):7819–7827
Schmid BC, Rezniczek GA, Fabjani G, Yoneda T, Leodolter S, Zeillinger R (2007) The neuronal guidance cue Slit2 induces targeted migration and may play a role in brain metastasis of breast cancer cells. Breast Cancer Res Treat 106(3):333–342
Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG (2012) Matrix metalloproteinases: changing roles in tumor progression and metastasis. The American journal of pathology
Zhang M, X-d T, X-x G, Z-g L, J-g H, Yao L (2013) Expression of tissue levels of matrix metalloproteinases and their inhibitors in breast cancer. Breast 22(3):330–334
Mohammad MA, Zeeneldin AA, Elmageed ZYA, Khalil EH, Mahdy SM, Sharada HM, Sharawy SK, Abdel-Wahab A-HA (2012) Clinical relevance of cyclooxygenase-2 and matrix metalloproteinases (MMP-2 and MT1-MMP) in human breast cancer tissue. Mol Cell Biochem 366(1–2):269–275
Mendes O, Kim H-T, Stoica G (2005) Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis 22(3):237–246
Vasaturo F, Solai F, Malacrino C, Nardo T, Vincenzi B, Modesti M, Scarpa S (2013) Plasma levels of matrix metalloproteinases 2 and 9 correlate with histological grade in breast cancer patients. Oncol Lett 5(1):316–320
Tester AM, Waltham M, Oh S-J, Bae S-N, Bills MM, Walker EC, Kern FG, Stetler-Stevenson WG, Lippman ME, Thompson EW (2004) Pro-matrix metalloproteinase-2 transfection increases orthotopic primary growth and experimental metastasis of MDA-MB-231 human breast cancer cells in nude mice. Cancer Res 64(2):652–658
Mendes O, Kim H-T, Lungu G, Stoica G (2007) MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metastasis 24(5):341–351
Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ (2014) Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol
Bos PD, Zhang XH-F, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459(7249):1005–1009
Rodrigues-Ferreira S, Abdelkarim M, Dillenburg-Pilla P, Luissint A-C, di-Tommaso A, Deshayes F, Pontes CLS, Molina A, Cagnard N, Letourneur F (2012) Angiotensin II facilitates breast cancer cell migration and metastasis. PLoS One 7(4):e35667
Fatunmbi M, Shelton J, Aronica SM (2012) MMP-9 increases HER2/neu expression and alters apoptosis levels in human mammary epithelial cells (HMEC). Breast Cancer Res Treat 135(2):519–530
Liu T, Sun B, Zhao X, Gu Q, Dong X, Yao Z, Zhao N, Chi J, Liu N, Sun R (2013) HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. J Cell Mol Med 17(1):116–122
Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, Offermanns S (2012) ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Invest 122(4):1296
Yu D, Hung M-C (2000) Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 19(53):6115
Kwon M, Park S, Choi J, Oh E, Kim Y, Park Y, Cho E, Nam S, Im Y, Shin Y (2012) Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br J Cancer 106(5):923–930
Chen Y, Chen L, Li J-Y, Mukaida N, Wang Q, Yang C, Yin W-J, X-h Z, Jin W, Z-m S (2011) ERβ and PEA3 co-activate IL-8 expression and promote the invasion of breast cancer cells. Cancer Biol Ther 11(5):497–511
Kim LS, Huang S, Lu W, Lev DC, Price JE (2004) Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis 21(2):107–118
Nishizuka I, Ishikawa T, Hamaguchi Y, Kamiyama M, Ichikawa Y, Kadota K, Miki R, Tomaru Y, Mizuno Y, Tominaga N (2002) Analysis of gene expression involved in brain metastasis from breast cancer using cDNA microarray. Breast Cancer 9(1):26–32
Chow A, Arteaga CL, Wang SE (2011) When tumor suppressor TGFβ meets the HER2 (ERBB2) oncogene. J Mammary Gland Biol Neoplasia 16(2):81–88
Lee T-H, Avraham HK, Jiang S, Avraham S (2003) Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 278(7):5277–5284
Fokas E, Steinbach JP, Rödel C (2012) Biology of brain metastases and novel targeted therapies: time to translate the research. Biochim Biophys Acta (BBA) Rev Cancer
Marques JB (2009) Treatment of brain metastases in patients with HER2+ breast cancer. Adv Ther 26(1):18–26
Savad S, Mehdipour P, Miryounesi M, Shirkoohi R, Fereidooni F, Mansouri F, Modarressi MH (2012) Expression analysis of MiR-21, MiR-205, and MiR-342 in breast cancer in Iran. Asian Pac J Cancer Prev 13:873–877
Smith ML, Lee JY (2007) Stereotactic radiosurgery in the management of brain metastasis. Neurosurg Focus 22(3):1–8
Khuntia D, Brown P, Li J, Mehta MP (2006) Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol 24(8):1295–1304
Ogawa K, Yoshii Y, Nishimaki T, Tamaki N, Miyaguni T, Tsuchida Y, Kamada Y, Toita T, Kakinohana Y, Tamaki W (2008) Treatment and prognosis of brain metastases from breast cancer. J Neurooncol 86(2):231–238
Soffietti R, Trevisan E, Rudà R (2012) Targeted therapy in brain metastasis. Curr Opin Oncol 24(6):679–686
Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B (2006) Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 24(36):5658–5663
Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD (2008) Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst 100(15):1092–1103
Williams L, Davis-Dusenbery B, Eggan K (2012) SnapShot: directed differentiation of pluripotent stem cells. Cell 149(5):1174
Acknowledgments
We would like to thank the colleagues whose works fill the gaps in metastatic processes.
Conflict of Interest
Both authors have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tayyeb, B., Parvin, M. Pathogenesis of Breast Cancer Metastasis to Brain: a Comprehensive Approach to the Signaling Network. Mol Neurobiol 53, 446–454 (2016). https://doi.org/10.1007/s12035-014-9023-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9023-z