Abstract
Purpose
We conducted this study to analyze clinicopathologic features and treatment outcomes for various treatment modalities in breast cancer patients with brain metastases.
Patients and methods
Retrospective analysis was performed using medical records of patients who were diagnosed with metastatic brain tumors from breast cancer. The treatment modalities applied included whole-brain radiotherapy (WBRT), surgical resection, stereotactic radiosurgery (SRS) and systemic treatments such as chemotherapy and endocrine therapy.
Results
Among 125 female breast cancer patients with brain metastases, 87.2% had Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2. The median overall survival (OS) was 6.6 months (95% CI 3.9–9.2). A multivariate analysis using the Cox-regression test identified three risk factors; poor PS (P = 0.023), HER2 positivity (P = 0.013), and no additional systemic treatment (P = 0.006). Those patients who had no risk factors showed outstanding outcome (median OS 49 months). On the contrary, the patients who had all risk factors (poor PS with HER2 positive and did not receive additional systemic chemotherapy) showed dismal prognosis (median OS 2 months).
Conclusions
Our new classification according to the suggested risk factors for patients with metastatic brain tumor from breast cancer reflects particular characteristics of each subset of the patients with good prognostic capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the second most common cause of brain metastases, after lung cancer, and represents 14–20% of all cases [1, 2]. Overall incidence of symptomatic brain metastases in patients with breast cancer ranges from 4 to 10%, but the incidence of metastases reported in autopsy studies ranges from 18 to 30% [3, 4].
Patients with breast cancer rarely present with manifestations of brain metastasis before detection of the primary breast cancer. On average, the median latency between the initial diagnosis of breast cancer and the onset of brain metastasis is 2–3 years [4], suggesting that central nervous system (CNS) involvement typically occurs late in the course of metastatic breast cancer. In most cases, involvement of the lungs, liver, or bone precedes the diagnosis of CNS metastasis [3, 5].
Several risk factors for brain metastases have been reported. Brain metastasis is usually associated with aggressive tumor behavior, a negative hormone receptor status, relatively young women, c-erbB-2 overexpression or the presence of lung and liver metastases [1, 3, 6–8].
In contrast to patients with brain metastasis from other solid tumors, who usually die from progression of systemic disease, about half of the patients with brain metastasis from breast cancer die from their neurological disease [9]. Therefore, long-term survival in breast cancer cases with brain metastasis depends more on local control of brain metastasis than on control of other metastatic sites [10]. However, there is considerable controversy about the optimal treatment of brain metastases.
As systemic therapy improves, control of extracranial disease may no longer be the limiting factor determining outcomes in patients with breast cancer metastatic to the brain, and the issue of CNS metastases will likely become more problematic [8].
The present study was conducted to analyze the treatment outcomes for clinicopathologic characteristics and various treatment modalities in breast cancer patients with brain metastases and to identify independent prognostic factors.
Patients and methods
We conducted a retrospective analysis using the medical records of patients who were diagnosed with metastatic brain tumor from breast cancer between January 1995 and June 2006 at Samsung Medical Center. The study protocol was approved by the institutional review board.
All patients were histologically confirmed as having infiltrative ductal or lobular adenocarcinoma of the breast. Brain metastases were diagnosed by brain magnetic resonance imaging (MRI) with or without cytologic and/or pathological confirmation.
All symptomatic patients with surrounding brain edema received dexamethasone intravenously following the detection of brain metastases. Their treatment modalities included whole-brain radiotherapy (WBRT), surgical resection, stereotactic radiosurgery (SRS) including gamma-knife surgery (GKS), and systemic treatments such as chemotherapy and endocrine therapy. The treatments for brain metastasis consisted of either single or combined modalities. The patients were diagnosed with leptomeningeal carcinomatosis if malignant cells were found in the cerebrospinal fluid or if findings compatible with leptomeningeal seeding on brain MRI were obtained. In these cases, the patients were treated with intrathecal methotrexate via Ommaya reservoir.
Statistical analysis
OS was measured from the date of diagnosis of brain metastasis to death or to the last follow-up date. The date of diagnosis of brain metastasis was defined as the day when brain metastasis was confirmed by imaging or by pathological examination. The following data were analyzed: age, performance status at the time of diagnosis of brain metastasis, hormonal receptor status with erbB2 expression profile, RPA class [11], interval between diagnosis of primary tumor and detection of brain metastasis, treatment modalities such as brain surgery, WBRT, SRS, and systemic treatments. OS was estimated by the Kaplan–Meier product limit method. The log-rank test was used to compare survival rates. Differences were considered as statistically significant when P value was less than 0.05. Multivariate analysis was performed with Cox regression analysis.
Results
Patient characteristics
Medical records of 125 female breast cancer patients with brain metastases were available for review. The median interval between the diagnoses of primary breast cancer and brain metastasis was 34.9 months (range 1–206 months), and the median age at the time of brain metastasis was 47 years (range 23–72 years). The majority of the patients (87.2%) had Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2. The brain was the first site of metastasis in eighteen patients (14.4%), with brain metastases in 107 patients (85.6%) occurring after other systemic metastases. The major sites of systemic disease preceding brain metastases were lung (61.6%), bone (53.6%), and liver (24.8%). At the initial presentation of brain metastasis, more than three brain metastatic lesions were observed in 89 patients (71.2%), and 109 patients (87.2%) belonged to RPA class I–II (Table 1).
Treatment of brain metastasis
All patients received at least one local treatment, which consisted of WBRT, SRS, and/or surgery. Most patients (95.2%) were treated with WBRT. Table 2 summarizes the various treatment modalities delivered to these patients. Four treatment groups received different treatment modalities, with the single local modality group accounting for 36.6% of all patients and the combined local modalities followed by systemic treatments group included 19.2% (Table 2). Additional systemic treatment following at least one local treatment for brain metastasis was performed in 68 patients (54.4%) and the median number of treatment chemotherapeutic regimens was 2 (range 1–9). Table 3 summarizes the treatment agents which were used as systemic treatments following local control of brain metastases.
Treatment outcomes
With a median follow-up duration of 50.8 months, the median overall survival (OS) was 6.6 months (95% CI 3.9–9.2) from the date of diagnosis of brain metastasis (Fig. 1). The 1- and 2-year overall survival rates were 32.5 and 15.0%, respectively. The most common cause of death was neurological problems (60.0%) due to progression of brain lesions.
Prognostic factor analysis
The clinicopathologic factors predicting survival at univariate analysis by log-rank test are shown in Table 4. Hepatic metastases (P = 0.010), number of CNS lesions (P = 0.007), RPA class (P < 0.001), short disease free survival (DFS) less than 1 year (P = 0.0448), treatment modalities (P < 0.001), and additional systemic treatments after local control (P < 0.001) were statistically significant. To identify independent prognostic factors, we conducted a multivariate analysis using the Cox-regression test. However, contrary to the univariate analysis, identified risk factors were poor PS (P = 0.023), HER2 positivity (P = 0.013), and no additional systemic treatment (P = 0.006) (Table 5).
Suggested prognostic model
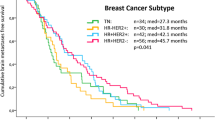
According to the number of identified risk factors, we divided the patients into four groups: group 1, no risk factor; group 2, one risk factor; group 3, two risk factors; group 4, three risk factors (Table 6). The survival curves according to the suggested prognostic index for CNS metastasis from breast cancer are shown in Fig. 2. The uppermost line, in descending order, represents group 1; second, group 2; third, group 3; and the last, group 4. The survival curves of the four groups showed significantly different survival outcomes (median OS; group 1, 49.4 months; group 2, 10.6 months; group 3, 4.4 months; group 4, 2.2 months, P < 0.0001).
Discussion
The survival of breast cancer patients has been prolonged with advanced systemic treatment. However, the incidence of brain metastases has also increased in these patients, suggesting that systemic agents may penetrate poorly into the CNS under physiologic conditions [11–13]. Apparently, the development of brain metastasis showed higher incidence in HER-2 positive breast cancer [9, 10]. However, some other studies did not show any increased risk in this subgroups [14]. Recently, a few reports also suggested that CNS metastases tended to occur in patients who were doing well on trastuzumab therapy in HER-2 overexpressing metastatic breast cancer [9, 15]. Moreover, a majority of patients with brain metastases share a poor prognosis related to progressive neurological disability [16]. Therefore, the control of brain metastasis is a critical treatment for prolonged survival in this population.
Neurosurgery followed by WBRT as a combined local modality has been shown in previous randomized studies to improve survival over WBRT alone [17–19]. However, in this study, we confirmed that the benefit of this combined local modality has been observed only in patients with controllable extracranial disease, and the importance of surgery in the treatment of multiple lesions is still controversial [20]. Although no randomized trial has compared SRS to surgery for the treatment of brain metastases, SRS has emerged as an appealing alternative to neurosurgery during the last decade, even for multiple brain lesions [21, 22]. In a randomized phase III study, a combination of WBRT and SRS boosted survival in patients with a single unresectable brain metastasis [23]. However, in another study, a survival advantage for a combined local modality with SRS followed by WBRT was not shown, although additional WBRT decreased intracranial tumor recurrence [24]. These studies included many kinds of primary malignancies, including breast cancer, which are known to have different radio-sensitivities. Because of this, the survival benefit of combined local modalities for brain metastasis from breast cancer can be accurately determined only in breast cancer patients.
The present study identified three independent prognostic factors for survival of the patients with brain metastases from breast cancer. Even if our study might have many limitations that a retrospective study could have, our suggested prognostic factors have their clinical relevance. Although single-lesion metastasis, low RPA class, absence of hepatic metastasis, combined modalities for the treatment of brain metastasis and long DFS correlate with improved survival by univariate analysis, these factors were not identified as independent factors by multivariate analysis. Conversely, HER2 positivity, poor PS, and absence of additional chemotherapy appeared as independent poor prognostic factors in this analysis. It suggests that good therapeutic outcomes of active combined modalities might result from good PS and less aggressive clinical features. Clinical relevance of brain metastasis from breast cancer may depend mainly on its biologic characteristics. In our analysis, combined treatment modalities for brain metastasis could not overcome underlying biologic behavior. Besides, additional systemic treatment was identified as an independent prognostic factor. The metastasis of breast cancer to the CNS is generally a late feature of metastatic disease and is thought to be hematogenous in origin [15]. As shown in our analysis, the importance of systemic treatment should be emphasized even though many of the patients die from neurologic complications. It also suggests that systemic treatment in this setting can be mainstay as well as other systemic metastasis.
The most important finding from our study is that the patients with brain metastasis from breast cancer can be divided with four prognostic groups according to the number of suggested independent risk factors. To improve therapeutic strategies, according to the risk for this distinctive subset of the patients with brain metastasis from breast cancer, we attempted to identify specific risk factors. As shown in Fig. 1, median OS of all patients is merely 6.6 months. However, median OS of group 1, which has no risk factor, extends up to 49 months. On the contrary to this, median OS of group 4, which has all three risk factors, was no more than 3 months. Considering median OS of all patients in this study was 6.6 months and clinical course of group 1 showed completely different features. This group of patients should be regarded as a particular subset which has indolent clinical behavior. This finding is quite different to the clinical course of the patients of group 3 or 4. Excluding this particular subset, our results suggest that the patients who have risk factor 2 or more should be considered for new treatment strategies. Individualized treatment according to the risk stratification should be adapted, especially in this setting. Lapatinib is an oral small-molecular, reversible dual inhibitor of EGFR and HER-2 tyrosine kinases, which have recently been approved in combination with capecitabine for treating advanced-stage HER-2-overexpressing (HER-2) breast cancers that have progressed during prior anthracycline, taxane, and trastuzumab therapies [25, 26]. This new agent might be a good therapeutic option for HER-2 positive patients with brain metastasis who had shown resistant to trastuzumab and other combination chemotherapies.
Among our three independent risk factors, additional systemic treatment is treatment factor, not tumor nor host factor. This fact suggests that clinical outcomes of some portion of group 2, 3, 4 patients can be improved. In addition, there are some reports which showed the activity of systemic chemotherapy in this setting. In the largest previous study including breast cancer patients with mestastasis to the brain, reported by Rosner et al. [27], CMF (cyclophosphamide, fluorouracil, and methotrexate)-like regimens were delivered to 100 female patients. Although none of these agents could penetrate into the normal CNS to a significant degree, the treatment produced a 50% response rate in the CNS. In another small study, Boogerd et al. [28] treated 22 patients with CMF or CAF (cyclophosphamide, doxorubicin, and fluorouracil) in a similar setting and observed a response rate for CNS lesions of 59%. These results strongly suggested that chemosensitivity of the primary tumor may be important in determining the response of CNS lesions.
In conclusion, our new classification according to the suggested risk factors for patients with metastatic brain tumor from breast cancer reflects particular characteristics of each subset of the patients with good prognostic capacity.
References
Boogerd W, Vos VW, Hart AA, Baris G (1993) Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol 15:165–174
Lohr F, Pirzkall A, Hof H, Fleckenstein K, Debus J (2001) Adjuvant treatment of brain metastases. Semin Surg Oncol 20:50–56
Tsukada Y, Fouad A, Pickren JW, Lane WW (1983) Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 52:2349–2354
Chang EL, Lo S (2003) Diagnosis and management of central nervous system metastases from breast cancer. Oncologist 8:398–410
Patanaphan V, Salazar OM, Risco R (1988) Breast cancer: metastatic patterns and their prognosis. South Med J 81:1109–1112
Sparrow G, Rubens R (1981) Brain metastases from breast cancer: clinical course, prognosis and influence of treatment. Clin Oncol 7:291–301
Stewart JF, King RJ, Sexton SA, Millis RR, Rubens RD, Hayward JL (1981) Oestrogen receptors, sites of metastatic disease and survival in recurrent breast cancer. Eur J Cancer 17:449–453
Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97:2972–2977
Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, Wilkinson PM, Welch RS, Magee B, Wilson G, Howell A, Wardley AM (2004) Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 91:639–643
Lin NU, Winer EP (2007) Brain metastases: the HER2 paradigm. Clin Cancer Res 13:1648–1655
Saip P, Cicin I, Eralp Y, Kucucuk S, Tuzlali S, Karagol H, Aslay I, Topuz E (2008) Factors affecting the prognosis of breast cancer patients with brain metastases. Breast (Epub ahead of print)
Calyton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, Wilkinson PM, Welch RS, Magee B, Wilson G, Howell A, Wardley AM (2004) Incidence of cerebral metastses in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 91:639–643
Shmueli E, Wigler N, Inbar M (2004) Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer 40:379–382
Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R (2006) Primary breast cancer phenotype associated with propensity for central nervous systmen metastses. Cancer 107:696–704
Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS (2005) Breast cancer metastasis to the central nervous system. Am J Pathol 167:913–920
Engel J, Eckel R, Aydemir U, Aydemir S, Kerr J, Schlesinger-Raab A, Dirschedl P, Hölzel D (2003) Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys 55:1186–1195
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583–590
Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR (1994) The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 29:711–717
Lin NU, Bellon JR, Winer EP (2004) CNS metastases in breast cancer. J Clin Oncol 22:3608–3617
Nieder C, Nestle U, Motaref B, Walter K, Niewald M, Schnabel K (2000) Prognostic factors in brain metastases: should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int J Radiat Oncol Biol Phys 46:297–302
Pollock BE, Brown PD, Foote RL, Stafford SL, Schomberg PJ (2003) Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J Neurooncol 61:73–80
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Peacock KH, Lesser GJ (2006) Current therapeutic approaches in patients with brain metastases. Curr Treat Options Oncol 7:479–489
Cockeril S, Stubberfied C, Stables J, Carter M, Guntrip S, Smith K, McKeown S, Shaw R, Topley P, Thomsen L, Affleck K, Jowett A, Hayes D, Willson M, Woollard P, Spalding D (2001) Indazolylamino quinazolines and pyridopyrimidines as inhibitors of the EGFR and C-erb-B-2. Bioorg Med Chem Lett 11:1401–1405
Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, Lombardi DP, ahmed SB, Citrin DL, DeSilvio ML, Harris J, Westlund RE, Salazar V, Zaks TZ, Spector NL (2007) Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammtory breast cancer with lapatinib monotherapy. J Clin Oncol 26:1–7
Rosner D, Nemoto T, Lane WW (1986) Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer 58:832–839
Boogerd W, Dalesio O, Bais EM, van der Sande JJ (1992) Response of brain metastases from breast cancer to systemic chemotherapy. Cancer 69:972–980
Acknowledgments
Supported by a grant of the Korean Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (0412-CR01-0704-0001).
Author information
Authors and Affiliations
Corresponding author
Additional information
B.-B. Park and J. E. Uhm contributed equally to the work.
Rights and permissions
About this article
Cite this article
Park, BB., Uhm, J.E., Cho, E.Y. et al. Prognostic factor analysis in patients with brain metastases from breast cancer: how can we improve the treatment outcomes?. Cancer Chemother Pharmacol 63, 627–633 (2009). https://doi.org/10.1007/s00280-008-0779-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0779-6