Abstract
Monitoring of brain tissue oxygenation (PbtO2) is an important component of multimodal monitoring in traumatic brain injury. Over recent years, use of PbtO2 monitoring has also increased in patients with poor-grade subarachnoid hemorrhage (SAH), particularly in those with delayed cerebral ischemia. The aim of this scoping review was to summarize the current state of the art regarding the use of this invasive neuromonitoring tool in patients with SAH. Our results showed that PbtO2 monitoring is a safe and reliable method to assess regional cerebral tissue oxygenation and that PbtO2 represents the oxygen available in the brain interstitial space for aerobic energy production (i.e., the product of cerebral blood flow and the arterio-venous oxygen tension difference). The PbtO2 probe should be placed in the area at risk of ischemia (i.e., in the vascular territory in which cerebral vasospasm is expected to occur). The most widely used PbtO2 threshold to define brain tissue hypoxia and initiate specific treatment is between 15 and 20 mm Hg. PbtO2 values can help identify the need for or the effects of various therapies, such as hyperventilation, hyperoxia, induced hypothermia, induced hypertension, red blood cell transfusion, osmotic therapy, and decompressive craniectomy. Finally, a low PbtO2 value is associated with a worse prognosis, and an increase of the PbtO2 value in response to treatment is a marker of good outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nontraumatic subarachnoid hemorrhage (SAH), mostly secondary to aneurysmal rupture, accounts for 5% of all strokes [1] and is an important cause of morbidity and mortality and of potential years of life lost [1, 2]. About one quarter of patients admitted to the hospital after SAH will eventually die, and among survivors, half will have persistent severe neurological disability [3].
Immediately after blood enters the subarachnoid space, a complex pathophysiological process called early brain injury (EBI) leads to intracranial hypertension, cerebral edema, microcirculatory failure, neuroinflammation, and cerebral ischemia [4,5,6,7]. Patients with SAH are also susceptible to late ischemic complications, which can worsen prognosis, identified as delayed cerebral ischemia (DCI) [8,9,10,11].
Multimodal neuromonitoring, including brain tissue oxygenation (PbtO2) monitoring, has been recommended to identify patients with EBI and DCI and to optimize treatment [12], especially in those in whom neurological examination is difficult. Indeed, the primary goal of neuromonitoring is to enable the detection of secondary brain insults before they cause irreversible damage to the brain [13]. Because the final common pathway in acute brain injury is the failure of oxygen delivery [14], detecting low oxygen cerebral states is vital to reduce secondary brain damage [15], provide a better understanding of complex brain physiology, and help guide management [16]. Preliminary observational studies suggest that monitoring PbtO2 and treating patients with low PbtO2 values may be associated with improved outcomes after SAH [16, 17]. However, there are no randomized control trials (RCTs) specifically assessing the impact of PbtO2 monitoring and PbtO2-guided therapy on the outcome of patients with SAH. The aim of this scoping review was therefore to provide a summary of the role of PbtO2 in the management of adult patients with SAH by assessing the existing and emerging literature on this topic [28].
Methods
The review protocol was preregistered on April 11, 2019, on the Open Science Framework (https://osf.io/zyj7r/) and published in open access [18]; further details regarding the search strategy can be found in the Methods section of the Electronic Supplementary Material. This scoping review followed the five-stage framework proposed by Arksey and O’Malley [19], expanded by Peters et al. [20], and further developed by Levac et al. [21] and the Joanna Briggs Institute [22]. We also followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews guidelines [23].
The aims of the review were to describe in patients with SAH (1) the physiological bases of invasive brain oxygenation monitoring, (2) the technique of invasive brain oxygenation monitoring, (3) the indications and the utility of brain oxygenation monitoring, (4) the role of invasive brain oxygenation monitoring to guide medical and surgical therapy, and (5) the impact of PbtO2 monitoring and PbtO2-guided therapy on the outcome of these patients. We included all available scientific information from fully peer reviewed articles and gray literature that mentioned PbtO2 monitoring in the context of SAH in adult patients. We excluded studies that focused only on a pediatric population (patients < 18 years old) and experimental studies performed exclusively in animals. There were no language limitations or sample size restrictions.
The search was performed on August 1, 2022. Three authors (EGB, MF, and AM) screened different databases for relevant abstracts and studies in a two-phase process (see Methods section of the Electronic Supplementary Material). All disagreements were resolved by consensus. Data were extracted to predefined charts, including the following information: study population; type of probe used; technique and adverse events; the indication for PbtO2 monitoring, including to diagnose neurological complications such as EBI and DCI and to guide therapy; physiological determinants of PbtO2 (oxygen delivery, oxygen extraction, and oxygen consumption); the effect of different treatments and strategies (including but not limited to pharmacological, respiratory, and hemodynamic therapy) on PbtO2 values; outcomes (neurological outcomes and mortality).
Results
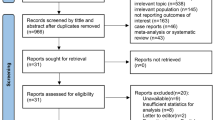
The initial search identified 21,550 references, of which 301 met our inclusion criteria (Fig. 1): 1 pilot RCT, 138 non-RCTs (prospective or retrospective), 1 cross-sectional study, 30 published abstracts, 16 case reports/case series, 20 book chapters, 70 reviews (5 of which were systematic reviews), 6 consensus statements, 6 editorials, 3 theses, 2 technical notes, 1 audit, 1 viewpoint, 6 conference statements/lectures, and 7 letters to the editor. A detailed description of all 301 references can be found in the Supplementary Tables S1, S2, and S3.
PbtO2 Monitoring: Physiological Bases
PbtO2 values represent the balance between oxygen delivery {DO2 = cardiac output × [1.39 × hemoglobin × oxygen arterial saturation SaO2 + (0.003 × PaO2)]}, determined by cerebral blood flow (CBF), hemoglobin, and arterial oxygenation and oxygen consumption [VO2 = (cardiac output + arterial content of oxygen) − (cardiac output − venous content of oxygen)], determined by brain metabolism, mitochondrial function, body temperature, and extraction (determined by blood–brain barrier and microcirculation) of brain cells [24, 25]. In this setting, the reasons for low PbtO2 values are often multifactorial (Fig. 2), and several interventions have the potential to correct brain hypoxia [26]. PbtO2 can be reduced because of a decrease in oxygen delivery either as a consequence of reduced CBF, leading to ischemia, or because of changes in the arterio-venous oxygen tension difference [27] caused by systemic hypoxemia secondary to impaired lung function [28, 29] (reduced arterial partial pressure of oxygen [PaO2]) and anemia [30]. PbtO2 can also be reduced because of increased oxygen consumption as a consequence of hypermetabolic states (e.g., seizures, fever, and shivering), because of mitochondrial dysfunction, and/or because of impaired oxygen extraction due to limited oxygen diffusion caused by brain edema and microvascular dysfunction [15, 31,32,33,34].
Physiological determinants of brain tissue oxygenation (PbtO2). PbtO2 represents a balance between oxygen delivery (DO2), oxygen extraction, and consumption (VO2) in the brain. Oxygen delivery to the brain is determined by the cerebral blood flow (CBF) and arterial content of O2. VO2 depends on brain metabolism, mitochondrial function, body temperature, and extraction (determined by blood–brain barrier and microcirculation) of brain cells. CaO2: arterial content of oxygen.
Normal PbtO2 values are highly variable but are generally defined as values between 23 and 35 mm Hg [35]. Several thresholds have been used to describe brain tissue hypoxia in this setting, ranging from 10 to 19 mm Hg for at least 5 min [36,37,38,39]; the most commonly used threshold is < 20 mm Hg [40, 41]. Tissue necrosis and cell death have been associated with PbtO2 values of < 10 and < 5 mm Hg, respectively [42,43,44,45]. A PbtO2 value of 0 mm Hg usually precedes diagnosis of brain death [46].
PbtO2 Monitoring: The Technique
Two methods are used to measure PbtO2 [47]: the luminescence quenching method used by the Neurovent-PTO (Raumedic AG, Munchberg, Germany), the Oxylab pO2 (Oxford Optronix Ltd, Oxford, United Kingdom) probes [16, 48], and the polarographic method used by the Licox brain oxygen monitor (Integra Neuroscience, Plainsboro, New Jersey) [49, 50]. Importantly, PbtO2 measurements from the two methods are not entirely interchangeable [47, 51,52,53,54].
PbtO2 monitoring involves the insertion of a probe into the brain parenchyma, ideally in the subcortical white matter, by using a bolt (single or multiple) or by tunneling, allowing continuous (every 2 s) PbtO2 monitoring [34, 40, 55]. PbtO2 is a local measurement [16, 56], reflecting the tissue oxygenation of an area of 2 mm to 22 mm2, depending on the device used, the type of probe, and the probe location (i.e., injured vs. noninjured area) [36]. Therefore, a cerebral computed tomography (CT) scan is required following the placement of the PbtO2 probe to confirm its adequate location [37, 57], as well as to exclude peri-procedural complications, which can include mispositioning or minor bleeding [33, 58,59,60,61].
Placing the PbtO2 catheter in areas where the ruptured aneurysm had been identified can provide reliable monitoring of the secondary ischemic insult (vasospasm and DCI), specially for aneurysms of the anterior circulation [62, 63]. For aneurysms of the posterior circulation, the optimal placement position is not well defined. Probe placement can potentially be guided by CT scan [64], Xenon CT [65], single photon emission CT [66], or transcranial Doppler [67] to increase the likelihood of monitoring the at-risk area or penumbra area of ischemia [68].
Importantly, probe placement causes microtrauma to the subcortical matter, making the first readings unreliable. It is recommended to wait at least 1 h before relying on the monitor [33, 59, 61, 69]. For further verification of the function and responsiveness of the catheter, an oxygen challenge should be performed [33, 59, 61, 69] by increasing inspired fraction of oxygen (FiO2) to 100% for 2–5 min [28, 33, 70,71,72]; if the probe is well-positioned and accurate, it will show an increase in the PbtO2 value of around two times baseline values [33, 59].
PbtO2 devices are considered safe and accurate with negligible zero drift [40, 61]. Hemorrhage and hematoma formation rates vary from 0 to 40%, including tract hemorrhages, although hematoma needing surgical intervention is rare [45, 61, 73,74,75,76]. Central nervous system infections are also rare [73, 75].
PbtO2 Monitoring: Indications and Uses
In general, patients are selected for PbtO2 monitoring when intracranial pressure (ICP) monitoring is required [40] and neurological evaluation is unreliable [77]. Criteria to initiate multimodal monitoring in patients with SAH include patients with Glasgow Coma Scale (GCS) < 9 who are unlikely to regain consciousness within the next 48 h and have a high probability of surviving for the next 48 h [78]. Rass et al. initiated multimodal monitoring in patients with SAH who required prolonged mechanical ventilation and/or had clinical or radiological signs suggestive of increased ICP [79]. Good-grade patients with delayed deterioration of their neurological status are also candidates for PbtO2 monitoring in the context of cerebral vasospasm and DCI [80].
PbtO2 monitoring has been employed for detecting ischemic events during aneurysm clipping [81, 82] and temporary artery occlusion [39, 83,84,85,86,87,88,89,90,91]. PbtO2 has also been used to direct therapy in the operating room [92,93,94]. In the first 72 h of SAH, PbtO2 monitoring is indicated to help detect microvascular injury [95] and ischemic events occurring in the context of EBI [96]. In fact, brain tissue hypoxia is common in the first 48 h after SAH [97]. PbtO2 can be used as a surrogate measure of regional CBF [98, 99], which can aid in the diagnosis of vasospasm and DCI [100, 101], especially in comatose patients with suboptimal clinical assessments who have angiographic vasospasm as well as in symptomatic patients who have suboptimal imaging [102]. In fact, several studies have focused on the use of PbtO2 to help detect DCI [77, 80, 103,104,105] because there appears to be a correlation between decreasing PbtO2 values and cerebral vasospasm detected by transcranial Doppler [85, 106, 107] when the PbtO2 probe is adequately placed in the at-risk area for vasospasm [108]. A PbtO2 threshold less than 20 mm Hg has a sensitivity of 71% and a specificity of 89% for prediction of vasospasm, with an area under the receiver operating characteristic curve of 0.90 [100]. Moreover, low PbtO2 values associated with signs of anaerobic metabolism assessed by cerebral microdialysis (CMD) can be present before visible CT scan infarction, enabling an early diagnosis of silent ischemia [78]. In fact, in patients with good-grade and poor-grade SAH, introduction of multimodal monitoring, including of PbtO2, resulted in earlier detection and earlier treatment of DCI, thus reducing DCI-related infarction [80, 109].
PbtO2 also has a role in assessing autoregulation. Impaired cerebral autoregulation is an important pathophysiological pathway of acute brain injury [110, 111] and can represent an independent risk factor for poor outcome in patients with SAH [112]. The oxygen reactivity index (ORx) is expressed as the moving correlation coefficient between cerebral perfusion pressure (CPP) and PbtO2, calculated every 30 s; a high ORx indicates impaired autoregulation [32]. The ORx has been proposed as a better predictor of cerebral hypoperfusion, DCI, and outcome than PbtO2 in patients with SAH [32, 113]. Interestingly, Jaeger et al. found that both ORx and PbtO2 values were lower in a group of patients with poor functional outcome [112]. Other studies have failed to show an association between ORx and cerebral ischemia or neurological outcome [114, 115].
PbtO2 can also be used as an adjunct monitor to assess the impact of ictal discharges and seizures in patients with SAH [116,117,118] and to monitor changes in brain oxygenation during mobilization of the patients [119, 120] and during transportation [121,122,123].
The PbtO2 probe should be kept in situ for a maximum of 7 to 10 days [40, 124] and can be removed if the patient is awake (motor GCS of 6 or motor GCS of 5 if patient is aphasic or unable to communicate) or if there is a medical indication for removal of the probe (such as infection or bleeding associated with the catheter) [33]; additionally, if ICP is normal (< 20 mm Hg) for 24 h without specific treatment and PbtO2 values are > 20 mm Hg for 48 h, it is also reasonable to remove multimodal neuromonitoring [33].
PbtO2 Monitoring: Assessing the Efficacy of Different Therapies
Numerous studies have been performed to monitor the efficacy of various therapies, especially focused on vasospasm and DCI. Regarding so-called triple H therapy (i.e., hypervolemia, hemodilution, and hypertension), Muench et al. [125] observed that vasopressor-induced hypertension, but not hypervolemia and hemodilution, could improve PbtO2 values in a population of patients with poor-grade SAH with cerebral vasospasm. Similarly, Raabe et al. noted that an increase in PbtO2 was far more frequent in patients who received induced hypertension compared with those who received hypervolemia [126]. These observations have helped shift treatment strategies from triple H therapy to induced hypertension alone.
Transluminal balloon angioplasty, used as rescue therapy to treat refractory vasospasm, can improve PbtO2 levels and reduce metabolic distress [103, 127]. In many studies, PbtO2 significantly improved during intermittent and continuous chemical spasmolysis with intra-arterial nimodipine (IAN), followed by resolution of vasospasm on angiography [128,129,130,131,132,133]. A recent observational study compared two strategies for treatment of vasospasm refractory to induced hypertension: one group of patients was treated with induced hypertension targeting a systolic blood pressure (SBP) of 180 mm Hg plus lower doses of continuous IAN, and the other group received higher doses of IAN without induced hypertension (SBP target = 120 mm Hg) [104]. Patients in the latter group had higher PbtO2 levels after IAN without relevant adverse events.
The effects of other vasodilatory agents on PbtO2 have also been studied in the context of refractory vasospasm: intra-arterial papaverine hydrochloride [134], intraventricular sodium nitroprusside [135], and intra-arterial verapamil [136, 137]. Only one studied showed a clear improvement in PbtO2 [136]. Inhaled nitric oxide (iNO) was also used in a pilot study to treat refractory DCI in seven patients; all patients experienced an increase of at least 5 mm Hg in PbtO2 after iNO [138]. Interestingly, the use of erythropoietin in patients with poor-grade SAH and vasospasm tended to increase PbtO2 [139].
PbtO2 can also be used to optimize CPP [126, 140,141,142,143,144,145,146,147] because higher CPP is associated with fewer episodes of brain tissue hypoxia and cerebral infarction [126, 142, 148]. In fact, strategies to increase mean arterial pressure (/CPP, such as fluid resuscitation [149], vasopressors [125], and the use of inotropes to augment cardiac output [150], can also promote an increase in PbtO2 levels, especially when accompanied by an increase in cardiac index [149, 151] in patients with low baseline PbtO2 [147, 151].
Osmotic therapy with hypertonic saline to treat intracranial hypertension has been shown to improve PbtO2 [152, 153]. On the other hand, mannitol may have no impact on PbtO2, especially if the baseline PbtO2 is > 20 mm Hg [154]. Decompressive craniectomy to treat refractory intracranial hypertension can also improve PbtO2 [155, 156], which typically decreases progressively before intervention [157]. The use of barbiturates to treat refractory intracranial hypertension may improve PbtO2 in some but not all patients with SAH [72].
Because brain temperature can influence CBF and PbtO2 measurements [158], some authors have investigated the effects of antipyretic drugs on PbtO2 [159] and have found that the degree of change in PbtO2 correlates with the reduction in core temperature. Mild hypothermia can also improve PbtO2 [160]. Moreover, in a cohort of patients with poor-grade SAH, higher PbtO2 measures were more frequently linked to normothermia (compared with fever) [161].
PbtO2 can also be used to monitor the effects of red blood cell (RBC) transfusion in optimizing oxygen delivery. Interestingly, PbtO2 response to RBC transfusion varies, with most patients showing an increase in PbtO2 (especially those with baseline hypoxia before transfusion and lower hemoglobin levels) and other patients having no change or even a decrease in PbtO2 [162,163,164].
In patients with acute brain injury and acute respiratory distress syndrome, PbtO2 may assist the clinician in assessing the effects of recruitment maneuvers [165] and the prone position [29, 166] on brain oxygenation. PbtO2 values can also be used to titrate FiO2 because normobaric hyperoxia usually results in increased PbtO2 [167,168,169]. During a hyperoxia challenge, smaller increases in PbtO2 are associated with higher CMD lactate and a higher risk of ischemia [170]. Importantly, the impact on outcome of improving PbtO2 by increasing FiO2 is still uncertain [171,172,173].
The impacts of different sedative drugs, such as propofol and dexmedetomidine, on PbtO2 were studied, and showed that for a similar Richmond Agitation and Sedation Scale, both drugs had a similar impact on cerebral oxygenation [174], usually leading to a modest elevation in PbtO2 [175]. PbtO2 has also been used to assess the success of a neurological wake-up test, in which reduction in PbtO2 was a criterion of test failure [176].
PbtO2 Monitoring: Impact on Outcome in Patients with SAH
Several studies found that patients with short- and long-term favorable neurological outcomes had higher PbtO2 values for longer periods of time than those with unfavorable outcome [85, 101, 133, 152, 177, 178]. The association between low PbtO2 values and unfavorable outcome is stronger when concomitant metabolic brain dysfunction is present [63]. However, other studies have failed to show an independent association between low PbtO2 levels and unfavorable outcome [63, 179].
Nonsurvivors have consistently lower PbtO2 levels during longer periods of time than survivors [148, 180,181,182]. In fact, low PbtO2 levels are independently associated with mortality, especially when accompanied with brain energetic dysfunction [28, 148, 183].
The rationale behind PbtO2 monitoring is that improving PbtO2 will translate into a better outcome [33]. However, studies that have investigated the impact of a PbtO2-guided therapy have yielded conflicting results. Bohman et al. analyzed patients with SAH managed with a goal-directed treatment aimed at maintaining a PbtO2 ≥ 20 mm Hg. The mean rate of response to the directed treatment was independently associated with a favorable functional outcome (defined as modified Rankin Scale < 4 and Glasgow Outcome Scale-Extended ≥ 3) [178]. Similarly, Al-Rawi et al. also found that a sustained increase in PbtO2 after treatment (hypertonic saline) was associated with a favorable outcome at 12 months in patients with SAH [152]. In a mixed cohort of patients with traumatic brain injury and SAH who underwent treatment when PbtO2 was 15 mm Hg for more than 10 min, patients had a decreased risk of unfavorable outcome and mortality [64]. In another mixed cohort of patients, Monteiro et al. found that multimodal monitoring (including of PbtO2) was associated with better short- and long-term neurological outcomes [184]. Additionally, Veldeman et al. found similar results for long-term outcomes [109].
Conversely, in patients with good-grade SAH who developed DCI, multimodal monitoring did not improve neurological outcome [80]. The lack of association between PbtO2-guided therapy and outcome can be explained by the results of a study conducted by Rass et al. in which, despite a protocolized PbtO2-guided therapy approach, episodes of cerebral hypoxia (PbtO2 < 20 mm Hg) still occurred in 81% of patients [179]. Similarly, Gouvea Bogossian et al. [185] did not find an association between ICP/PbtO2-guided therapy and outcomes compared with ICP-only guided therapy. Importantly, to date, no RCT has investigated the impact of PbtO2-guided therapy on outcome in patients with SAH.
Limitations
This review has some limitations that are inherent to scoping reviews: the findings are often broad, and synthetizing all the results can be challenging. In this review, we did not perform quality analysis, and all available data were charted and summarized. Because of the broad study period, some studies may be outdated and concepts may have changed. Therefore, this review provides only a summary of the available evidence regarding PbtO2 monitoring in patients with SAH; a systematic review and meta-analysis are needed to answer specific questions with a higher-quality standard. Moreover, RCTs in patients with SAH are needed to specifically assess if the use of PbtO2-guided therapy can improve outcome.
Conclusions
Maintenance of adequate brain oxygenation represents one of the primary objectives in neurocritical care, and the assessment of tissue oxygenation is important to patient management. Integration of PbtO2 into a multimodal neuromonitoring approach may help clinicians in the early detection of physiological derangements that can compromise oxygen supply to the brain, providing both a trigger and a target for interventions.
References
Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8(4):355–69.
Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50(5):1413–8.
Taufique Z, May T, Meyers E, Falo C, Mayer SA, Agarwal S, Park S, Connolly ES, Claassen J, Schmidt JM. Predictors of poor quality of life 1 year after subarachnoid hemorrhage. Neurosurgery. 2016;78(2):256–64.
Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22(4):654–61.
Hayman EG, Wessell A, Gerzanich V, Sheth KN, Simard JM. Mechanisms of global cerebral edema formation in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017;26(2):301–10.
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4(4):432–46.
de Oliveira Manoel AL, Goffi A, Marotta TR, Schweizer TA, Abrahamson S, Macdonald RL. The critical care management of poor-grade subarachnoid haemorrhage. Crit Care. 2016;20:21.
Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 2016;20(1):277.
Proust F, Hannequin D, Langlois O, Freger P, Creissard P. Causes of morbidity and mortality after ruptured aneurysm surgery in a series of 230 patients. Importance Control Angiogr Stroke. 1995;26(9):1553–7.
Lee H, Perry JJ, English SW, Alkherayf F, Joseph J, Nobile S, Zhou LL, Lesiuk H, Moulton R, Agbi C et al: Clinical prediction of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Neurosurg 2018:1–8.
Ropper AH, Zervas NT. Outcome 1 year after SAH from cerebral aneurysm. Management morbidity mortality and functional status in 112 consecutive good-risk patients. J Neurosurg. 1984;60(5):909–15.
Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, Diringer MN, Stocchetti N, Videtta W, Armonda R, et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care : a statement for healthcare professionals from the neurocritical care society and the European society of intensive care medicine. Intensive Care Med. 2014;40(9):1189–209.
Andrews PJ, Citerio G, Longhi L, Polderman K, Sahuquillo J, Vajkoczy P. Medicine N-ICaEMNSotESoIC: NICEM consensus on neurological monitoring in acute neurological disease. Intensive Care Med. 2008;34(8):1362–70.
Andrews PJ. Potential end points of treatment after acute brain injury: should we be using monitors of metabolism? Curr Opin Crit Care. 2003;9(2):83–5.
Bader MK. Recognizing and treating ischemic insults to the brain: the role of brain tissue oxygen monitoring. Crit Care Nurs Clin North Am. 2006;18(2):243–56.
De Georgia MA. Brain tissue oxygen monitoring in neurocritical care. J Intensive Care Med. 2015;30(8):473–83.
Stiefel MF, Spiotta A, Gracias VH, Garuffe AM, Guillamondegui O, Maloney-Wilensky E, Bloom S, Grady MS, LeRoux PD. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805–11.
Fiore M, Bogossian E, Creteur J, Oddo M, Taccone FS. Role of brain tissue oxygenation (PbtO2) in the management of subarachnoid haemorrhage: a scoping review protocol. BMJ Open. 2020;10(9):e035521.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–6.
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69.
Aromataris E, MZE: Joanna Briggs Institute Reviewer's Manual. . The Joanna Briggs Institute 2017 Available from https://reviewersmanual.joannabriggs.org/.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Soehle M, Jaeger M, Meixensberger J. Online assessment of brain tissue oxygen autoregulation in traumatic brain injury and subarachnoid hemorrhage. Neurol Res. 2003;25(4):411–7.
Rose JC, Neill TA, Hemphill JC. Continuous monitoring of the microcirculation in neurocritical care: an update on brain tissue oxygenation. Curr Opin Crit Care. 2006;12(2):97–102.
Bouzat P, Sala N, Payen JF, Oddo M. Beyond intracranial pressure: optimization of cerebral blood flow, oxygen, and substrate delivery after traumatic brain injury. Ann Intensive Care. 2013;3(1):23.
Rosenthal G, Hemphill JC, Sorani M, Martin C, Morabito D, Obrist WD, Manley GT. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008;36(6):1917–24.
Chen HI, Stiefel MF, Oddo M, Milby AH, Maloney-Wilensky E, Frangos S, Levine JM, Kofke WA, LeRoux PD. Detection of cerebral compromise with multimodality monitoring in patients with subarachnoid hemorrhage. Neurosurgery. 2011;69(1):53–63.
Reinprecht A, Greher M, Wolfsberger S, Dietrich W, Illievich UM, Gruber A. Prone position in subarachnoid hemorrhage patients with acute respiratory distress syndrome: effects on cerebral tissue oxygenation and intracranial pressure. Crit Care Med. 2003;31(6):1831–8.
Meixensberger J, Dings J, Kuhnigk H, Roosen K. Studies of tissue PO2 in normal and pathological human brain cortex. Acta Neurochir Suppl (Wien). 1993;59:58–63.
Menon DK, Coles JP, Gupta AK, Fryer TD, Smielewski P, Chatfield DA, Aigbirhio F, Skepper JN, Minhas PS, Hutchinson PJ, et al. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32(6):1384–90.
Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38(3):981–6.
Maloney-Wilensky E, Le Roux P. The physiology behind direct brain oxygen monitors and practical aspects of their use. Childs Nerv Syst. 2010;26(4):419–30.
Haitsma IK, Maas AI. Advanced monitoring in the intensive care unit: brain tissue oxygen tension. Curr Opin Crit Care. 2002;8(2):115–20.
Pennings FA, Schuurman PR, van den Munckhof P, Bouma GJ. Brain tissue oxygen pressure monitoring in awake patients during functional neurosurgery: the assessment of normal values. J Neurotrauma. 2008;25(10):1173–7.
Sarrafzadeh AS, Kiening KL, Bardt TF, Schneider GH, Unterberg AW, Lanksch WR. Cerebral oxygenation in contusioned vs. nonlesioned brain tissue: monitoring of PtiO2 with Licox and Paratrend. Acta Neurochir Suppl. 1998;71:186–9.
Doppenberg EM, Zauner A, Watson JC, Bullock R. Determination of the ischemic threshold for brain oxygen tension. Acta Neurochir Suppl. 1998;71:166–9.
Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26(9):1576–81.
Kett-White R, Hutchinson PJ, Al-Rawi PG, Czosnyka M, Gupta AK, Pickard JD, Kirkpatrick PJ. Cerebral oxygen and microdialysis monitoring during aneurysm surgery: effects of blood pressure, cerebrospinal fluid drainage, and temporary clipping on infarction. J Neurosurg. 2002;96(6):1013–9.
Oddo M, Bosel J. Participants in the international multidisciplinary consensus conference on multimodality M: Monitoring of brain and systemic oxygenation in neurocritical care patients. Neurocrit Care. 2014;21(2):103–20.
Sandsmark DK, Kumar MA, Park S, Levine JM. Multimodal monitoring in subarachnoid hemorrhage. Stroke. 2012;43(5):1440–5.
Vath A, Kunze E, Roosen K, Meixensberger J. Therapeutic aspects of brain tissue pO2 monitoring after subarachnoid hemorrhage. Acta Neurochir Suppl. 2002;81:307–9.
Scheufler KM, Lehnert A, Rohrborn HJ, Nadstawek J, Thees C. Individual value of brain tissue oxygen pressure, microvascular oxygen saturation, cytochrome redox level, and energy metabolites in detecting critically reduced cerebral energy state during acute changes in global cerebral perfusion. J Neurosurg Anesthesiol. 2004;16(3):210–9.
Valadka AB, Goodman JC, Gopinath SP, Uzura M, Robertson CS. Comparison of brain tissue oxygen tension to microdialysis-based measures of cerebral ischemia in fatally head-injured humans. J Neurotrauma. 1998;15(7):509–19.
Meixensberger J, Kunze E, Barcsay E, Vaeth A, Roosen K. Clinical cerebral microdialysis: brain metabolism and brain tissue oxygenation after acute brain injury. Neurol Res. 2001;23(8):801–6.
Palmer S, Bader MK. Brain tissue oxygenation in brain death. Neurocrit Care. 2005;2(1):17–22.
Dengl M, Jaeger M, Renner C, Meixensberger J. Comparing brain tissue oxygen measurements and derived autoregulation parameters from different probes (Licox vs. Raumedic). Acta Neurochir Suppl. 2012;114:165–8.
Neurovent-PTO™ system - Measurement of Oxygen Partial Pressure in the Brain [https://www.raumedic.com/neuromonitoring/neuro-icu/oxygen-partial-pressure]
Stewart C, Haitsma I, Zador Z, Hemphill JC 3rd, Morabito D, Manley G 3rd, Rosenthal G. The new Licox combined brain tissue oxygen and brain temperature monitor: assessment of in vitro accuracy and clinical experience in severe traumatic brain injury. Neurosurgery. 2008;63(6):1159–64 (discussion 1164-1155).
Hoelper BM, Alessandri B, Heimann A, Behr R, Kempski O. Brain oxygen monitoring: in-vitro accuracy, long-term drift and response-time of Licox- and Neurotrend sensors. Acta Neurochir (Wien). 2005;147(7):767–74 (discussion 774).
Jaeger M, Soehle M, Meixensberger J. Brain tissue oxygen (PtiO2): a clinical comparison of two monitoring devices. Acta Neurochir Suppl. 2005;95:79–81.
Purins K, Enblad P, Sandhagen B, Lewen A. Brain tissue oxygen monitoring: a study of in vitro accuracy and stability of Neurovent-PTO and Licox sensors. Acta Neurochir (Wien). 2010;152(4):681–8.
Morgalla MH, Haas R, Grozinger G, Thiel C, Thiel K, Schuhmann MU, Schenk M. Experimental comparison of the measurement accuracy of the Licox((R)) and Raumedic ((R)) Neurovent-PTO brain tissue oxygen monitors. Acta Neurochir Suppl. 2012;114:169–72.
Wolf S, Horn P, Frenzel C, Schurer L, Vajkoczy P, Dengler J. Comparison of a new brain tissue oxygenation probe with the established standard. Acta Neurochir Suppl. 2012;114:161–4.
Bhatia A, Gupta AK. Neuromonitoring in the intensive care unit. II. Cerebral oxygenation monitoring and microdialysis. Intensive Care Med. 2007;33(8):1322–8.
Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23(3):507–38.
Ponce LL, Pillai S, Cruz J, Li X, Julia H, Gopinath S, Robertson CS. Position of probe determines prognostic information of brain tissue PO2 in severe traumatic brain injury. Neurosurgery. 2012;70(6):1492–502 (discussion 1502-1493).
Ansgar M, Brambrink JK. Essentials of neurosurgical anesthesia & critical care - strategies for prevention, early detection, and successful management of perioperative complications. 2nd ed. Berlin: Springer International Publishing; 2020.
Geukens P, Oddo M. Brain tissue oxygen monitoring in neurocritical care. In: Vincent JL, editor. Annual update in intensive care and emergency medicine 2012 annual update in intensive care and emergency medicine, vol. 2012. Springer. Heidelberg: Berlin; 2012. p. 735–45.
Brawanski A, Faltermeier R, Rothoerl RD, Woertgen C. Comparison of near-infrared spectroscopy and tissue p(O2) time series in patients after severe head injury and aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2002;22(5):605–11.
Dings J, Meixensberger J, Jager A, Roosen K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery. 1998;43(5):1082–95.
Ulrich CT, Fung C, Vatter H, Setzer M, Gueresir E, Seifert V, Beck J, Raabe A. Occurrence of vasospasm and infarction in relation to a focal monitoring sensor in patients after SAH: placing a bet when placing a probe? PLoS ONE. 2013;8(5):e62754.
Tholance Y, Barcelos GK, Perret-Liaudet A, Omar E, Carrillon R, Grousson S, Lieutaud T, Dailler F, Marinesco S. Placing intracerebral probes to optimise detection of delayed cerebral ischemia and allow for the prediction of patient outcome in aneurysmal subarachnoid haemorrhage. J Cereb Blood Flow Metab. 2017;37(8):2820–32.
Hani L, Ropelato MD, Wagner F, Nowacki A, Soll N, Haenggi M, Raabe A, Z’Graggen WJ. Individualized brain tissue oxygen-monitoring probe placement helps to guide therapy and optimizes outcome in neurocritical care. Neurocrit Care. 2021;35(1):197–209.
Gupta AK, Hutchinson PJ, Fryer T, Al-Rawi PG, Parry DA, Minhas PS, Kett-White R, Kirkpatrick PJ, Mathews JC, Downey S, et al. Measurement of brain tissue oxygenation performed using positron emission tomography scanning to validate a novel monitoring method. J Neurosurg. 2002;96(2):263–8.
De Deyne CS, Struys MR. New developments in cerebral monitoring. Curr Opin Anaesthesiol. 2000;13(5):517–21.
Stiefel MF, Spiotta AM, Udoetuk JD, Maloney-Wilensky E, Weigele JB, Hurst RW, LeRoux PD. Intra-arterial papaverine used to treat cerebral vasospasm reduces brain oxygen. Neurocrit Care. 2006;4(2):113–8.
Robba C, Taccone FS, Citerio G: Monitoring cerebral oxygenation in acute brain-injured patients. Intensive Care Med 2022.
Integra LifeSciences Corp: Integra LICOX PtO2 monitor user’s manual oxygen partial pressure and temperature monitor for neurosurgical application. In. Plainsboro, NJ: Integra Life Sciences Corp; 2013.
Dominguez-Roldan JM, Lubillo S, Videtta W, Llompart-Pou JA, Badenes R, Rivas JM, Ibanez J, Godoy DA, Murillo-Cabezas F, Grupo de expertos en la monitorizacion del paciente neurologico c et al: International consensus on the monitoring of cerebral oxygen tissue pressure in neurocritical patients. Neurocirugia (Astur: Engl Ed) 2020, 31(1):24–36.
Oddo M, Milby A, Chen I, Frangos S, MacMurtrie E, Maloney-Wilensky E, Stiefel M, Kofke WA, Levine JM, Le Roux PD. Hemoglobin concentration and cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2009;40(4):1275–81.
Chen HI, Malhotra NR, Oddo M, Heuer GG, Levine JM, LeRoux PD. Barbiturate infusion for intractable intracranial hypertension and its effect on brain oxygenation. Neurosurgery. 2008;63(5):880–6 (discussion 886-887).
Bailey RL, Quattrone F, Curtin C, Frangos S, Maloney-Wilensky E, Levine JM, LeRoux PD. The safety of multimodality monitoring using a triple-lumen bolt in severe acute brain injury. World Neurosurg. 2019;130:e62–7.
Al Barajraji M, Bogossian E, Dewitte O, Gaspard N, El Hadwe S, Minini A, Andre J, Taccone FS, Schuind S, Barrit S: Safety profile of an intracranial multimodal monitoring bolt system for neurocritical care: a single-center experience. Acta Neurochir (Wien) 2021.
Stuart RM, Schmidt M, Kurtz P, Waziri A, Helbok R, Mayer SA, Lee K, Badjatia N, Hirsch LJ, Connolly ES, et al. Intracranial multimodal monitoring for acute brain injury: a single institution review of current practices. Neurocrit Care. 2010;12(2):188–98.
Charbel FT, Hoffman WE, Misra M, Hannigan K, Ausman JI. Cerebral interstitial tissue oxygen tension, pH, HCO3, CO2. Surg Neurol. 1997;48(4):414–7.
Diringer MN, Bleck TP, Claude Hemphill J, Menon D, Shutter L, Vespa P, Bruder N, Connolly ES, Citerio G, Gress D, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211–40.
Helbok R, Madineni RC, Schmidt MJ, Kurtz P, Fernandez L, Ko SB, Choi A, Stuart MR, Connolly ES, Lee K, et al. Intracerebral monitoring of silent infarcts after subarachnoid hemorrhage. Neurocrit Care. 2011;14(2):162–7.
Rass V, Solari D, Ianosi B, Gaasch M, Kofler M, Schiefecker AJ, Miroz J-P, Morelli P, Thomé C, Beer R, et al. Protocolized brain oxygen optimization in subarachnoid hemorrhage. Neurocrit Care. 2019;31(2):263–72.
Veldeman M, Albanna W, Weiss M, Conzen C, Schmidt TP, Clusmann H, Schulze-Steinen H, Nikoubashman O, Temel Y, Schubert GA. Treatment of delayed cerebral ischemia in good-grade subarachnoid hemorrhage: Any role for invasive neuromonitoring? Neurocrit Care. 2021;35(1):172–83.
Jodicke A, Hubner F, Boker DK. Monitoring of brain tissue oxygenation during aneurysm surgery: prediction of procedure-related ischemic events. J Neurosurg. 2003;98(3):515–23.
Hoffman WE, Wheeler P, Edelman G, Charbel FT, Torres NJ, Ausman JI. Hypoxic brain tissue following subarachnoid hemorrhage. Anesthesiology. 2000;92(2):442–6.
Hoffman WE, Charbel FT, Gonzalez-Portillo G, Ausman JI. Measurement of ischemia by changes in tissue oxygen, carbon dioxide, and pH. Surg Neurol. 1999;51(6):654–8.
Kett-White R, Hutchinson PJ, al-Rawi PG, Gupta AK, O’Connell MT, Pickard JD, Kirkpatrick PJ. Extracellular lactate/pyruvate and glutamate changes in patients during per-operative episodes of cerebral ischaemia. Acta Neurochir Suppl. 2002;81:363–5.
Kett-White R, Hutchinson PJ, Al-Rawi PG, Gupta AK, Pickard JD, Kirkpatrick PJ. Adverse cerebral events detected after subarachnoid hemorrhage using brain oxygen and microdialysis probes. Neurosurgery. 2002;50(6):1213–21 (discussion 1221-1212).
Kett-White R, Hutchinson PJ, Czosnyka M, al-Rawi P, Gupta A, Pickard JD, Kirkpatrick PJ. Effects of variation in cerebral haemodynamics during aneurysm surgery on brain tissue oxygen and metabolism. Acta Neurochir Suppl. 2002;81:327–9.
Gelabert-Gonzalez M, Fernandez-Villa JM, Ginesta-Galan V. Intra-operative monitoring of brain tissue O2 (PtiO2) during aneurysm surgery. Acta Neurochir (Wien). 2002;144(9):863–6 (discussion 866-867).
Hutchinson PJ, Al-Rawi PG, O’Connell MT, Gupta AK, Maskell LB, Hutchinson DB, Pickard JD, Kirkpatrick PJ. Monitoring of brain metabolism during aneurysm surgery using microdialysis and brain multiparameter sensors. Neurol Res. 1999;21(4):352–8.
Silva PA, Dias C, Vilarinho A, Cerejo A, Vaz R. Effects of temporary clipping as an expression of circulatory individuality: online measurement of temporal lobe oxygen levels during surgery for middle cerebral artery aneurysms. World Neurosurg. 2021;152:e765–75.
Chan MT, Boet R, Ng SC, Poon WS, Gin T. Effect of ischemic preconditioning on brain tissue gases and pH during temporary cerebral artery occlusion. Acta Neurochir Suppl. 2005;95:93–6.
Chan MT, Boet R, Ng SC, Poon WS, Gin T. Magnesium sulfate for brain protection during temporary cerebral artery occlusion. Acta Neurochir Suppl. 2005;95:107–11.
Critchley GR, O’Neill KS, Bell BA. Cerebral blood flow and tissue oxygenation monitoring during aneurysm surgery. Neurol Res. 1998;20(Suppl 1):S44-47.
Hoffman William E, Wheeler P, Edelman G, Charbel Fady T, Torres Norman J, Ausman James I. Hypoxic Brain Tissue following Subarachnoid Hemorrhage. Anesthesiology. 2000;92(2):442–442.
Hoffman WE, Charbel FT, Edelman G. Desflurane increases brain tissue oxygenation and pH. Acta Anaesthesiol Scand. 1997;41(9):1162–6.
Carvi y Nievas M, Toktamis S, Hollerhage HG, Haas E. Hyperacute measurement of brain-tissue oxygen, carbon dioxide, pH, and intracranial pressure before, during, and after cerebral angiography in patients with aneurysmatic subarachnoid hemorrhage in poor condition. Surg Neurol. 2005;64(4):362–7.
Narotam PK, Garton A, Morrison J, Nathoo N, Narotam N: Brain Oxygen-Directed Management (BOP) of aneurysmal Subarachnoid Hemorrhage. Temporal patterns of cerebral ischemia during acute brain attack (ABA), early brain injury (EBI) and territorial sonographic vasospasm (TSV). World Neurosurg 2022.
Helbok R, Schiefecker AJ, Beer R, Dietmann A, Antunes AP, Sohm F, Fischer M, Hackl WO, Rhomberg P, Lackner P, et al. Early brain injury after aneurysmal subarachnoid hemorrhage: a multimodal neuromonitoring study. Crit Care. 2015;19:75.
Veldeman M, Albanna W, Weiss M, Park S, Hoellig A, Clusmann H, Helbok R, Temel Y, Alexander Schubert G. Invasive multimodal neuromonitoring in aneurysmal subarachnoid hemorrhage: a systematic review. Stroke. 2021;52(11):3624–32.
Seule M, Sikorski C, Sakowitz O, von Campe G, Santos E, Orakcioglu B, Unterberg A, Keller E. Evaluation of a new brain tissue probe for intracranial pressure, temperature, and cerebral blood flow monitoring in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2016;25(2):193–200.
Khatibi K, Szeder V, Blanco MB, Tateshima S, Jahan R, Duckwiler G, Vespa P. Role of bedside multimodality monitoring in the detection of cerebral vasospasm following subarachnoid hemorrhage. Acta Neurochir Suppl. 2020;127:141–4.
Zauner A, Doppenberg E, Woodward JJ, Allen C, Jebraili S, Young HF, Bullock R. Multiparametric continuous monitoring of brain metabolism and substrate delivery in neurosurgical patients. Neurol Res. 1997;19(3):265–73.
Sanelli PC, Kishore S, Gupta A, Mangat H, Rosengart A, Kamel H, Segal A. Delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: proposal of an evidence-based combined clinical and imaging reference standard. AJNR Am J Neuroradiol. 2014;35(12):2209–14.
Hoelper BM, Hofmann E, Sporleder R, Soldner F, Behr R. Transluminal balloon angioplasty improves brain tissue oxygenation and metabolism in severe vasospasm after aneurysmal subarachnoid hemorrhage: case report. Neurosurgery. 2003;52(4):970–4 (discussion 974-976).
Weiss M, Albanna W, Conzen-Dilger C, Kastenholz N, Seyfried K, Ridwan H, Wiesmann M, Veldeman M, Schmidt TP, Megjhani M, et al. Intraarterial nimodipine versus induced hypertension for delayed cerebral ischemia: a modified treatment protocol. Stroke. 2022;53(8):2607–16.
Megjhani M, Weiss M, Kwon SB, Ford J, Nametz D, Kastenholz N, Fogel H, Velazquez A, Roh D, Agarwal S, et al. Vector angle analysis of multimodal neuromonitoring data for continuous prediction of delayed cerebral ischemia. Neurocrit Care. 2022;37(Suppl 2):230–6.
Cerejo A, Silva PA, Vilarinho A, Dias C, Vaz R. Intraoperative brain oxygenation monitoring and vasospasm in aneurysmal subarachnoid hemorrhage. Neurol Res. 2012;34(2):181–6.
Charbel FT, Du X, Hoffman WE, Ausman JI. Brain tissue PO2, PCO2, and pH during cerebral vasospasm. Surg Neurol. 2000;54(6):432–7.
Dhawan V, DeGeorgia M. Neurointensive care biophysiological monitoring. J Neurointerv Surg. 2012;4(6):407–13.
Veldeman M, Albanna W, Weiss M, Conzen C, Schmidt TP, Schulze-Steinen H, Wiesmann M, Clusmann H, Schubert GA. Invasive neuromonitoring with an extended definition of delayed cerebral ischemia is associated with improved outcome after poor-grade subarachnoid hemorrhage. J Neurosurg. 2020;134(5):1527–34.
Klein SP, Depreitere B, Meyfroidt G. How I monitor cerebral autoregulation. Crit Care. 2019;23(1):160.
Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5(5):347–60.
Jaeger M, Soehle M, Schuhmann MU, Meixensberger J. Clinical significance of impaired cerebrovascular autoregulation after severe aneurysmal subarachnoid hemorrhage. Stroke. 2012;43(8):2097–101.
Fritch C, Owen B, Shuttleworth CW, Carlson AP, The Ruptured Aneurysm Patient's Disability Correlation with Autoregulatory Measures, Spreading Depolarization, and Vasospasm. Neurosurgery 2020, 67(Supplement_1).
Barth M, Woitzik J, Weiss C, Muench E, Diepers M, Schmiedek P, Kasuya H, Vajkoczy P. Correlation of clinical outcome with pressure-, oxygen-, and flow-related indices of cerebrovascular reactivity in patients following aneurysmal SAH. Neurocrit Care. 2010;12(2):234–43.
Gaasch M, Schiefecker AJ, Kofler M, Beer R, Rass V, Pfausler B, Thome C, Schmutzhard E, Helbok R. Cerebral autoregulation in the prediction of delayed cerebral ischemia and clinical outcome in poor-grade aneurysmal subarachnoid hemorrhage patients. Crit Care Med. 2018;46(5):774–80.
Appavu BL, Doyle K, Velazquez A, Park S, Roh D, Agarwal S, Couch C, Matory A, Rohaut B, J C: Effect of duration of stimulus-induced rhythmic, periodic or ictal discharges on brain tissue oxygenation in aneurysmal subarachnoid hemorrhage patients. Abstracts presented at the neurocritical care society (NCS) 15th Annual Meeting. Neurocrit Care 2017;1–491.
Witsch J, Frey HP, Schmidt JM, Velazquez A, Falo CM, Reznik M, Roh D, Agarwal S, Park S, Connolly ES, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74(3):301–9.
Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt JM, Tu B, Badjatia N, Lantigua H, Hirsch LJ, Mayer SA, et al. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Ann Neurol. 2013;74(1):53–64.
Burnol L, Payen JF, Francony G, Skaare K, Manet R, Morel J, Bosson JL, Gergele L. Impact of head-of-bed posture on brain oxygenation in patients with acute brain injury: a prospective cohort study. Neurocrit Care. 2021;35(3):662–8.
Ledwith MB, Bloom S, Maloney-Wilensky E, Coyle B, Polomano RC, Le Roux PD. Effect of body position on cerebral oxygenation and physiologic parameters in patients with acute neurological conditions. J Neurosci Nurs. 2010;42(5):280–7.
Swanson EW, Mascitelli J, Stiefel M, MacMurtrie E, Levine J, Kofke WA, Yang W, Le Roux PD. Patient transport and brain oxygen in comatose patients. Neurosurgery. 2010;66(5):925–31 (discussion 931-922).
Kuchler J, Tronnier F, Smith E, Gliemroth J, Tronnier VM, Ditz C. The impact of intrahospital transports on brain tissue metabolism in patients with acute brain injury. Neurocrit Care. 2019;30(1):216–23.
Hosmann A, Angelmayr C, Hopf A, Rauscher S, Brugger J, Ritscher L, Bohl I, Schnackenburg P, Engel A, Plochl W et al: Detrimental effects of intrahospital transport on cerebral metabolism in patients suffering severe aneurysmal subarachnoid hemorrhage. J Neurosurg 2021:1–8.
Citerio G, Oddo M, Taccone FS. Recommendations for the use of multimodal monitoring in the neurointensive care unit. Curr Opin Crit Care. 2015;21(2):113–9.
Muench E, Horn P, Bauhuf C, Roth H, Philipps M, Hermann P, Quintel M, Schmiedek P, Vajkoczy P. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Critical care Med. 2007;35(8):1844–51.
Raabe A, Beck J, Keller M, Vatter H, Zimmermann M, Seifert V. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 2005;103(6):974–81.
Albanna W, Weiss M, Muller M, Brockmann MA, Rieg A, Conzen C, Clusmann H, Hollig A, Schubert GA. Endovascular rescue therapies for refractory vasospasm after subarachnoid hemorrhage: a prospective evaluation study using multimodal continuous event neuromonitoring. Neurosurgery. 2017;80(6):942–9.
Weiss M, Conzen C, Mueller M, Wiesmann M, Clusmann H, Albanna W, Schubert GA: Endovascular rescue treatment for delayed cerebral ischemia after subarachnoid hemorrhage is safe and effective. Front Neurol 2019;10(136).
von der Brelie C, Doukas A, Stopfer A, Larsen N, Mehdorn M, Synowitz M, Jansen O. Clinical course and monitoring parameters after continuous interventional intra-arterial treatment in patients with refractory cerebral vasospasm. World Neurosurg. 2017;100:504–13.
Hockel K, Diedler J, Steiner J, Birkenhauer U, Ernemann U, Schuhmann MU. Effect of intra-arterial and intravenous nimodipine therapy of cerebral vasospasm after subarachnoid hemorrhage on cerebrovascular reactivity and oxygenation. World neurosurgery. 2017;101:372–8.
Deshaies EM, Jacobsen W, Singla A, Li F, Gorji R. Brain tissue oxygen monitoring to assess reperfusion after intra-arterial treatment of aneurysmal subarachnoid hemorrhage-induced cerebral vasospasm: a retrospective study. AJNR Am J Neuroradiol. 2012;33(7):1411–5.
Wolf S, Martin H, Landscheidt JF, Rodiek SO, Schurer L, Lumenta CB. Continuous selective intraarterial infusion of nimodipine for therapy of refractory cerebral vasospasm. Neurocrit Care. 2010;12(3):346–51.
Ott S, Jedlicka S, Wolf S, Peter M, Pudenz C, Merker P, Schurer L, Lumenta CB. Continuous selective intra-arterial application of nimodipine in refractory cerebral vasospasm due to aneurysmal subarachnoid hemorrhage. Biomed Res Int. 2014;2014:970741.
Hosmann A, Wang W-t, Dodier P, Bavinzski G, Engel A, Herta J, Plöchl W, Reinprecht A, Gruber A. The impact of intra-arterial papaverine-hydrochloride on cerebral metabolism and oxygenation for treatment of delayed-onset post-subarachnoid hemorrhage vasospasm. Neurosurgery. 2019;87(4):712–9.
Raabe A, Zimmermann M, Setzer M, Vatter H, Berkefeld J, Seifert V. Effect of intraventricular sodium nitroprusside on cerebral hemodynamics and oxygenation in poor-grade aneurysm patients with severe, medically refractory vasospasm. Neurosurgery. 2002;50(5):1006–13 (discussion 1013-1004).
Craven CL, Reddy U, Asif H, Watkins LD, Toma AK. Brain parenchymal oxygen monitoring in delayed cerebral ischaemia. J Neurol Neurosurg Psychiatry. 2019;90(3): e2.
Stuart RM, Helbok R, Kurtz P, Schmidt M, Fernandez L, Lee K, Badjatia N, Mayer SA, Lavine S, Meyers P, et al. High-dose intra-arterial verapamil for the treatment of cerebral vasospasm after subarachnoid hemorrhage: prolonged effects on hemodynamic parameters and brain metabolism. Neurosurgery. 2011;68(2):337–45 (discussion 345).
Fung C, Z’Graggen WJ, Jakob SM, Gralla J, Haenggi M, Rothen HU, Mordasini P, Lensch M, Soll N, Terpolilli N, et al. Inhaled nitric oxide treatment for aneurysmal sah patients with delayed cerebral ischemia. Front Neurol. 2022;13:817072.
Helbok R, Shaker E, Beer R, Chemelli A, Sojer M, Sohm F, Broessner G, Lackner P, Beck M, Zangerle A, et al. High dose erythropoietin increases brain tissue oxygen tension in severe vasospasm after subarachnoid hemorrhage. BMC Neurol. 2012;12(1):32.
Nortje J, Gupta AK. The role of tissue oxygen monitoring in patients with acute brain injury. Br J Anaesth. 2006;97(1):95–106.
Schmitt J, Aries P, Danguy Des Deserts M, Giacardi C. Precise clinical outcome in high-grade aneurysmal subarachnoid hemorrhage: brain oxygenation matters. Neurocritical Care. 2021;34(3):1108–9.
Väth A, Kunze E, Roosen K, Meixensberger J. Therapeutic aspects of brain tissue pO2 monitoring after subarachnoid hemorrhage. Acta Neurochir Suppl. 2002;81:307–9.
Ford J, Park S, Boehme A, Megjhani M, Terilli K. Brain tissue oxygenation as an adjunctive monitor for determining optimal cerebral perfusion pressure in subarachnoid hemorrhage patients (P4.297). Neurology. 2018;90(15 Supplement):P4.297.
Johnston AJ, Steiner LA, Coles JP, Chatfield DA, Fryer TD, Smielewski P, Hutchinson PJ, O’Connell MT, Al-Rawi PG, Aigbirihio FI, et al. Effect of cerebral perfusion pressure augmentation on regional oxygenation and metabolism after head injury. Critical Care Med. 2005;33(1):189–95 (discussion 255-187).
Helbok R, Schiefecker A, Beer R, Dietmann A, Antunes A, Sohm F, Fischer M, Hackl W, Rhomberg P, Lackner P et al: Early brain injury after aneurysmal subarachnoid hemorrhage: A multimodal neuromonitoring study. Critical Care 2015, 19.
Stocchetti N, Chieregato A, De Marchi M, Croci M, Benti R, Grimoldi N. High cerebral perfusion pressure improves low values of local brain tissue O2 tension (PtiO2) in focal lesions. Acta Neurochir Suppl. 1998;71:162–5.
Kovacs M, Peluso L, Njimi H, De Witte O, Gouvea Bogossian E, Quispe Cornejo A, Creteur J, Schuind S, Taccone FS. Optimal cerebral perfusion pressure guided by brain oxygen pressure measurement. Front Neurol. 2021;12:732830.
Schmidt JM, Ko SB, Helbok R, Kurtz P, Stuart RM, Presciutti M, Fernandez L, Lee K, Badjatia N, Connolly ES, et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke. 2011;42(5):1351–6.
Kurtz P, Helbok R, Ko SB, Claassen J, Schmidt JM, Fernandez L, Stuart RM, Connolly ES, Badjatia N, Mayer SA, et al. Fluid responsiveness and brain tissue oxygen augmentation after subarachnoid hemorrhage. Neurocrit Care. 2014;20(2):247–54.
Coppalini G, Duvigneaud E, Diosdado A, Migliorino E, Schuind S, Creteur J, Taccone FS, Gouvea Bogossian E. Effect of inotropic agents on oxygenation and cerebral perfusion in acute brain injury. Front Neurol. 2022;13:963562.
Rass V, Bogossian EG, Ianosi BA, Peluso L, Kofler M, Lindner A, Schiefecker AJ, Putnina L, Gaasch M, Hackl WO, et al. The effect of the volemic and cardiac status on brain oxygenation in patients with subarachnoid hemorrhage: a bi-center cohort study. Ann Intensive Care. 2021;11(1):176.
Al-Rawi PG, Tseng MY, Richards HK, Nortje J, Timofeev I, Matta BF, Hutchinson PJ, Kirkpatrick PJ. Hypertonic saline in patients with poor-grade subarachnoid hemorrhage improves cerebral blood flow, brain tissue oxygen, and pH. Stroke. 2010;41(1):122–8.
Al-Rawi PG, Zygun D, Tseng MY, Hutchinson PJ, Matta BF, Kirkpatrick PJ. Cerebral blood flow augmentation in patients with severe subarachnoid haemorrhage. Acta Neurochir Suppl. 2005;95:123–7.
Helbok R, Kurtz P, Schmidt JM, Stuart RM, Fernandez L, Malhotra R, Presciutti M, Ostapkovich ND, Connolly ES, Lee K, et al. Effect of mannitol on brain metabolism and tissue oxygenation in severe haemorrhagic stroke. J Neurol Neurosurg Psychiatry. 2011;82(4):378–83.
Stiefel MF, Heuer GG, Smith MJ, Bloom S, Maloney-Wilensky E, Gracias VH, Grady MS, LeRoux PD. Cerebral oxygenation following decompressive hemicraniectomy for the treatment of refractory intracranial hypertension. J Neurosurg. 2004;101(2):241–7.
Jaeger M, Soehle M, Meixensberger J. Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J Neurol Neurosurg Psychiatry. 2003;74(4):513–5.
Veldeman M, Weiss M, Daleiden L, Albanna W, Schulze-Steinen H, Nikoubashman O, Clusmann H, Hoellig A, Schubert GA. Decompressive hemicraniectomy after aneurysmal subarachnoid hemorrhage-justifiable in light of long-term outcome? Acta Neurochir (Wien). 2022;164(7):1815–26.
Stocchetti N, Protti A, Lattuada M, Magnoni S, Longhi L, Ghisoni L, Egidi M, Zanier ER. Impact of pyrexia on neurochemistry and cerebral oxygenation after acute brain injury. J Neurol Neurosurg Psychiatry. 2005;76(8):1135–9.
Ianosi B, Rass V, Gaasch M, Huber L, Lindner A, Hackl WO, Kofler M, Schiefecker AJ, Almashad S, Beer R, et al. An observational study on the use of intravenous non-opioid analgesics and antipyretics in poor-grade subarachnoid hemorrhage: effects on hemodynamics and systemic and brain temperature. Ther Hypother Temp Manag. 2020;10(1):27–36.
Chen H, Zhi D, Zhang S, Changes of cerebral oxygen metabolism during mild hypothermia treatment of severe brain injury. Chinese J Eng Res 2005:142–144.
Claassen J, Rahman SA, Huang Y, Frey HP, Schmidt JM, Albers D, Falo CM, Park S, Agarwal S, Connolly ES, et al. Causal structure of brain physiology after brain injury from subarachnoid hemorrhage. PLoS ONE. 2016;11(4):e0149878.
Kurtz P, Helbok R, Claassen J, Schmidt JM, Fernandez L, Stuart RM, Connolly ES, Lee K, Mayer SA, Badjatia N. The effect of packed red blood cell transfusion on cerebral oxygenation and metabolism after subarachnoid hemorrhage. Neurocrit Care. 2016;24(1):118–21.
Smith MJ, Stiefel MF, Magge S, Frangos S, Bloom S, Gracias V, Le Roux PD. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33(5):1104–8.
Gouvea Bogossian E, Rass V, Lindner A, Iaquaniello C, Miroz JP, Cavalcante Dos Santos E, Njimi H, Creteur J, Oddo M, Helbok R et al: Factors Associated With Brain Tissue Oxygenation Changes After RBC Transfusion in Acute Brain Injury Patients. Crit Care Med 2022.
Wolf S, Plev DV, Trost HA, Lumenta CB. Open lung ventilation in neurosurgery: an update on brain tissue oxygenation. Acta Neurochir Suppl. 2005;95:103–5.
Bernon P, Mrozek S, Dupont G, Dailler F, Lukaszewicz A-C, Balança B. Can prone positioning be a safe procedure in patients with acute brain injury and moderate-to-severe acute respiratory distress syndrome? Crit Care. 2021;25(1):30.
Stambolija V, Miklić Bublić M, Lozić M, Nemir J, Ščap M. PbtO(2) monitoring in normobaric hyperoxia targeted therapy in acute subarachnoidal hemorrhage. Surg Neurol Int. 2018;9:46.
Longhi L, Valeriani V, Rossi S, De Marchi M, Egidi M, Stocchetti N. Effects of hyperoxia on brain tissue oxygen tension in cerebral focal lesions. Acta Neurochir Suppl. 2002;81:315–7.
Ghosh A, Highton D, Kolyva C, Tachtsidis I, Elwell CE, Smith M. Hyperoxia results in increased aerobic metabolism following acute brain injury. J Cereb Blood Flow Metab. 2017;37(8):2910–20.
Hosmann A, Schnackenburg P, Rauscher S, Hopf A, Bohl I, Engel A, Brugger J, Graf A, Plochl W, Reinprecht A et al: Brain Tissue Oxygen Response as Indicator for Cerebral Lactate Levels in Aneurysmal Subarachnoid Hemorrhage Patients. J Neurosurg Anesthesiol 2020.
Smrčka M, Neuman E, Ďuriš K, Svoboda T, Duba M: Monitoring PtiO2 and Changes in Oxygen Fraction in the Breathed Mixture after Severe Subarachnoid Haemorrhage. Cesk Slov Neurol 2010, 73/106(6):649–700.
Stambolija V, Miklic Bublic M, Lozic M, Nemir J, Scap M. PbtO2 monitoring in normobaric hyperoxia targeted therapy in acute subarachnoidal hemorrhage. Surg Neurol Int. 2018;9:46.
Singh V, Cheng R. Neurovascular physiology and neurocritical care. Handb Clin Neurol. 2021;176:71–80.
James ML, Olson DM, Graffagnino C. A pilot study of cerebral and haemodynamic physiological changes during sedation with dexmedetomidine or propofol in patients with acute brain injury. Anaesth Intensive Care. 2012;40(6):949–57.
Drummond JC, Sturaitis MK. Brain tissue oxygenation during dexmedetomidine administration in surgical patients with neurovascular injuries. J Neurosurg Anesthesiol. 2010;22(4):336–41.
Helbok R, Kurtz P, Schmidt MJ, Stuart MR, Fernandez L, Connolly SE, Lee K, Schmutzhard E, Mayer SA, Claassen J, et al. Effects of the neurological wake-up test on clinical examination, intracranial pressure, brain metabolism and brain tissue oxygenation in severely brain-injured patients. Crit Care. 2012;16(6):R226.
Winkler MK, Dengler N, Hecht N, Hartings JA, Kang EJ, Major S, Martus P, Vajkoczy P, Woitzik J, Dreier JP. Oxygen availability and spreading depolarizations provide complementary prognostic information in neuromonitoring of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2017;37(5):1841–56.
Bohman LE, Pisapia JM, Sanborn MR, Frangos S, Lin E, Kumar M, Park S, Kofke WA, Stiefel MF, LeRoux PD, et al. Response of brain oxygen to therapy correlates with long-term outcome after subarachnoid hemorrhage. Neurocrit Care. 2013;19(3):320–8.
Rass V, Solari D, Ianosi B, Gaasch M, Kofler M, Schiefecker AJ, Miroz JP, Morelli P, Thome C, Beer R, et al. Protocolized Brain Oxygen Optimization in Subarachnoid Hemorrhage. Neurocrit Care. 2019;31(2):263–72.
Meixensberger J, Vath A, Jaeger M, Kunze E, Dings J, Roosen K. Monitoring of brain tissue oxygenation following severe subarachnoid hemorrhage. Neurol Res. 2003;25(5):445–50.
Ramakrishna R, Stiefel M, Udoetuk J, Udoteuk J, Spiotta A, Levine JM, Kofke WA, Zager E, Yang W, Leroux P. Brain oxygen tension and outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;109(6):1075–82.
Ramakrishna R, Stiefel M, Udoetuk J, Spiotta A, Levine JM, Kofke WA, Zager E, Yang W, Leroux P. Brain oxygen tension and outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;109(6):1075–82.
Oddo M, Levine JM, Frangos S, Maloney-Wilensky E, Carrera E, Daniel RT, Levivier M, Magistretti PJ, LeRoux PD. Brain lactate metabolism in humans with subarachnoid hemorrhage. Stroke. 2012;43(5):1418–21.
Monteiro E, Ferreira A, Mendes E, Dias CC, Czosnyka M, Paiva JA, Dias C. Brain multimodal monitoring in severe acute brain injury: is it relevant to patient outcome and mortality? Acta Neurochir Suppl. 2021;131:83–6.
Gouvea Bogossian E, Diaferia D, Ndieugnou Djangang N, Menozzi M, Vincent J-L, Talamonti M, Dewitte O, Peluso L, Barrit S, Al Barajraji M, et al. Brain tissue oxygenation guided therapy and outcome in non-traumatic subarachnoid hemorrhage. Sci Rep. 2021;11(1):16235.
Funding
The authors received only institutional funding (Erasme Hospital - Université Libre de Bruxelles) for this work.
Author information
Authors and Affiliations
Contributions
MF, EGB, and FST conceived the study. AM, MF, and EGB performed the screening and selected the articles for the review. EGB, SF, AM, and GG extracted the data from the articles. SF, AM, GG, and EGB wrote the first draft. DB, MF, CR, and FST revised the text for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gouvea Bogossian, E., Battaglini, D., Fratino, S. et al. The Role of Brain Tissue Oxygenation Monitoring in the Management of Subarachnoid Hemorrhage: A Scoping Review. Neurocrit Care 39, 229–240 (2023). https://doi.org/10.1007/s12028-023-01680-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01680-x