Abstract
Bacterial infection is one of the vital reasons of morbidity and mortality, especially in developing countries. It appears silently without bothering the geological borders and imposes a grave threat to humanity. Nuclear medicine technique has an important role in helping early diagnosis of deep-seated infections. The aim of this study was to develop a new radiopharmaceutical 99mTc-labeling sulfadiazine as an infection imaging agent. Radiolabeling of sulfadiazine with technetium-99m (99mTc) was carried out using stannous tartrate as a reducing agent in the presence of gentistic acid at pH = 5. The quality control tests revealed ~98% labeling efficiency. Paper chromatographic (PC) and instant thin-layer chromatographic (ITLC) techniques were used to analyze radiochemical yield. Biodistribution and infection specificity of the radiotracer were performed with Escherichia coli (E. coli) infection-induced rats. Scintigraphy and glomerular filtration rate (GFR) study was performed in E. coli-infected rabbits. Scintigraphy indicated E. coli infection targeting potential of 99mTc-SDZ, while biodistribution study showed minimal uptake of 99mTc-SDZ in non-targeted tissues. The uptake in the kidneys was found 2.56 ± 0.06, 2.09 ± 0.10, and 1.68 ± 0.09% at 30 min, 1 h, and 4 h, respectively. The infected muscle (target) to non-infected muscle (non-target) ratio (T/NT) was found 4.49 ± 0.04, 6.78 ± 0.07, and 5.59 ± 0.08 at 30 min, 1 h, and 4 h, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection is explicitly a major cause of morbidity and mortality, and the symptoms of infection include fever, swelling, and pain. The diagnosis of infection is frequently based on clinical, pathological, and microbiological results. Despite the enhanced adroitness of human understanding of the mechanism of bacterial proliferation and action, medical science and world health organization still declare bacterial infection a bigger threat to humanity owing to the substantial diagnostic dilemma involved in bacterial infection [1, 2]. Molecular biology, immunology, and medical biotechnology offer various approaches for infection and inflammation imaging involving radiological standard imaging techniques such as computerized tomography (CT), magnetic resonance imaging (MRI), and radiolabeled leukocytes scintigraphy can pinpoint the site of infection and inflammation, but fail to discriminate between them. Compared with these two standard imaging techniques, 99mTc-labeled antibiotics (e.g., 99mTc-ciprofloxacin) scintigraphy is clinically more effective for bacterial infection and revealed discrimination between bacterial infection and inflammation [3]. In subsequent years, this approach has gained ample attention and other members of fluoroquinolone class and sister antibiotic classes have been labeled with 99mTc, and tested against different infections such as 99mTc-enorfloxacin [4], 99mTc-sitafloxacin [5], 99mTc-cefepime [6], and 99mTc-cefzolin [7] which showed promising diagnostic results as compared to 99mTc-leukocytes and 99mTc-ciprofloxacin [8–10]. Sulfonamide is the main functional group present in majority of drugs showing important biological properties such as antimicrobial, antifungal [11], antidiabetic, diuretics, anticonvulsants, dermatological, antiretroviral, hepatitis C antivirals, stimulant, and inhibitors of different enzymes could be an attractive choice of labeling with technetium-99m. Sulfadiazine (SDZ) is an active antibacterial agent of a sulfonamide family, which works by inhibiting the dihydropteroate synthetase enzyme. Dihydropteroate synthetase produces dihydropteroate in bacteria which is an important intermediate in folate synthesis. Folate further helps in DNA and RNA synthesis, required for cell division. Therefore, dihydropteroate synthetase enzyme could be a good target for 99mTc-SDZ to diagnose bacterial infection. In present study, sulfadiazine was labeled with technetium-99m to assess the imaging potential of 99mTc-SDZ in Escherichia coli bacteria-induced infected rat and rabbit models.
Experimental
Materials
Sulfadiazine was obtained from Neo Quimica, Brazil. A locally produced fission-based PAKGEN 99Mo/99mTc generator was used to elute pertechnetate (99mTcO4 −) in saline. Whatman No. 3 chromatographic paper (Maidstone, UK) and ITLC-SG (Gelman sciences Institute, USA) were used for radiochemical analysis. Ammonium hydroxide (NH4OH), sodium hydroxide (NaOH), gentistic acid, and all other chemicals used were of analytical grade and purchased from Merck, Germany. E. coli bacterial strain (ATCC 25923) was obtained from the Department of Biological Sciences, Quaid-e-Azam University, Islamabad. The animal study was properly approved by the animal ethics committee of the institute. Sprague-Dawley (SD) rats and New Zealand white rabbit were obtained from the National Institute of Health (NIH), Islamabad, for the purpose of biodistribution and scintigraphy, respectively. The animals were kept under standard conditions with free ingress to food and water.
Labeling of Sulfadiazine with Technetium-99m
Direct labeling of SDZ with technetitium-99m was carried out at room temperature. The influence of different parameters such as ligand concentration, reducing agent, radioactivity, and pH was assessed by serial changes with regular defined intervals. Typically, sulfadiazine (0.2 to 2.0 mg) and 4 μL (8.3 mg/mL) gentistic acid were taken into the sterilized glass vial followed by the addition of stannous tartrate (60–150 μg) as a reducing agent. The pH of solution was tested from 2 to 11 using 0.1 N NaOH or 0.1 N HCl. One milliliter of freshly eluted pertechnetate (60 to 200 MBq) solution was added to the above mixture in the vial. The volume of all experiments was adjusted to 2 mL using distilled water. The reaction mixture was vigorously shaken and quality control analysis was performed at different intervals from 10 to 110 min.
Radiochemical Purity of 99mTc-Sulfadiazine Analysis
Percent formation of 99mTc-SDZ, free 99mTcO4 −, and reduced 99mTc (colloids) was determined by using PC (Whatman No. 3) and ITLC-SG analysis.
Paper Chromatography
In order to measure the percentage of the free 99mTcO4 −1, a drop of radiochemical reaction sample (~2 μL) was spotted at the baseline of chromatogram (size 2 × 14 cm) and allowed to run with acetone as mobile phase. In this system, free 99mTcO4 − moves along with mobile phase and bound and hydrolyzed 99mTc remain at baseline. The strip for radioactivity counts was analyzed with NaI scintillation well-type γ-counter and radiometric 2π-scanner.
Instant Thin-Layer Chromatography
The percentage of hydrolyzed 99mTc was determined using ITLC-SG analysis with saline as mobile solvent system. In order to do this, a small drop of reaction mixture sample (~2 μL) was spotted at baseline of ITLC strip (size 2 × 14 cm) and allowed to run in saline as mobile phase. In this system, the 99mTc-SDZ and free 99mTcO4 − move along with solvent front while reduced 99mTc remains at baseline. The strip was then cut into 0.25-cm patches, and the counts were measured using NaI scintillation well-type γ-counter and radiometric 2π-scanner. The percentage of free 99mTcO4 −1, colloids, and 99mTc-SDZ was determined by using the following formula;
Biodistribution
For biodistribution study, three healthy SD rats (weighing approximately 50–70 g) were used for each time point. The infection was introduced into right thigh muscles of all rats by injecting 100 μL saline suspension of 1 × 108 CFU (colony forming units) of E. coli. After 30 h, when visible swelling appeared in the infected thighs, 99mTc-SDZ was administrated after anesthetizing the animals with chloroform. The rats were sacrificed at 30 min, 60 min, and 4 h time points; different body organs such as the heart, liver, kidney, urinary bladder, and right and left thigh muscles (normal and infected) were separated and washed with saline and weighed. The distribution of 99mTc-SDZ activity in different organs was measured with NaI gamma scintillation counter in terms of percent injected dose per gram body organ.
Scintigraphic Study
The scintigraphic study was performed by using healthy male New Zealand white rabbits (~1.0–1.5 kg weight). To introduce infection, 300 μL saline solution of E. coli having 1 × 108 CFU was injected into the right thigh flank muscles, while 300 μL saline was injected in the left thigh flank muscles as control. After 30 h, visible swelling appeared in the infected thigh muscles, whereas minute swelling also appeared in negative control thigh muscles. The rabbits were then anesthetized by IV injection of diazepam followed by placing the rabbit under a dual-headed SPECT gamma camera (connected to an on-line dedicated computer system) by stretching the fore and rear legs out with polythene tape. Then, 99mTc-SDZ (185 MBq) was administrated through the rear ear vein of the rabbit and scintigraphic images were taken at 30 and 60 min.
Glomerular Filtration Rate Study
Glomerular filtration rate study was performed following the previously published protocol [12]. On the day of GFR study, the E. coli-infected rabbits were kept at fasting for about 6 h, with the exception of water intake to increase the urine flow. Then, 1 mL of 99mTc-SDZ was injected. Filtration rate through the kidney and collection of the urine activity in the urinary bladder was measured continuously in the form of GFR data using a dual-headed SPECT gamma camera connected to GFR software-installed computer system.
Results and Discussion
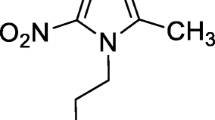
Reduced 99mTc can make complex with electron donor ligands. Fluoroquinolones and cephalosporin have certain electron donor groups which make complex with 99mTc. In all proposed structures of 99mTc-flouroquinolones, 99mTc uses two-electron donor oxygen atoms, i.e., carboxylic acid oxygen and carbonyl oxygen for complex formation [13]. However, other electron donor groups such as –S–, –NH–, –NH2, and –COOH make coordinate covalent bond with 99mTc [6, 14, 15]. Sulfadiazine (Fig. 1) has =N–, –NH–, –NH2, and –SO2– electron donor groups which facilitate stable complex chemistry of 99mTc-SDZ.
99mTc-SDZ purity, stability, formation of colloid, and free 99mTc were assessed by PC and ITLC methods. In paper chromatography using acetone as a mobile phase, the free 99mTcO4 −1 migrated along with solvent front (R f = 1), while 99mTc-SDZ and reduced 99mTc remained at the point of spotting (R f = 0) (Fig. 2a). Using ITLC with saline solvent system, the 99mTc-SDZ and free 99mTcO4 −1 moved to the solvent front (R f = 1), while the reduced 99mTc remained at spotting point (R f = 0) (Fig. 2b). The both radiochromatograms were analyzed with radiometric 2π-scanner and well-type NaI scintillation gamma counter.
Radiochromatogram profile of 99mTc-SDZ reaction mixture with paper chromatography a) The 99mTc-SDZ and colloid remained at radiochemical spotting point (R f = 0) while free 99mTcO4 − migrated along with solvent front (R f = 1), and ITLC b) The colloid at radiochemical remained at spotting point (R f = 0) while free 99mTcO4 − and 99mTc-SDZ migrated along solvent front (R f = 1)
The maximum labeling yield (~98%) was achieved by reducing185 MBq NaTcO4 with 120 μg stannous tartrate in the presence of 1.5 mg sulfadiazine and 4 μL (8.3 mg/mL) of gentistic acid at pH 5. The labeling reaction was carried out for 45 min at room temperature.
Effect of Labeling Parameters on Labeling Yield
The labeling yield of the 99mTc-SDZ was assessed by varying the labeling conditions; initially through hit-and-trail ways then after obtaining some pattern, the labeling was assessed through a regular variation in parameters.
Effect of Sulfadiazine Amount
It is noticed that 1.5 mg SDZ is stoichiometrically equal to 185 MBq 99mTcO4 − because at these concentrations, obtained maximum radiochemical and remained almost constant by subsequent increase in SDZ concentration (Fig. 3). By using 0.5 or 1 mg SDZ, the labeling yield remained below the maximum value. However, free and colloid impurities appeared in high percentage because below the stoichiometric amount of SDZ, most of the reduced 99mTcO4 − gets hydrolyzed. A similar interaction between ligand concentration and labeling yield was also reported by other authors [6, 16].
Effect of Reducing Agent Quantity
Optimal quantity of the reducing agent in 99mTc complex chemistry offers high radiochemical yield. Technetium-99m in its +7 oxidation state does not make a complex. Reduction of 99mTcO4 − offers maximum opportunity of making complex which is carried out with some appropriate reducing agent. The 120 μg of stannous tartrate was found to be optimal quantity to reduce maximum number of 99mTcO4 − ions to lower oxidation state. At 120 μg quantity of reducing agent, 185 MBq 99mTcO4 − provided maximum labeling yield; however, at low quantities of stannous tartrate such as 60, 90, and 110 μg, 45, 25, and 9% free 99mTcO4 − were obtained, respectively (Fig. 4). Similarly, a subsequent increase in stannous tartrate than the optimal quantity resulted in an increased concentration of colloids. The results revealed that to perform maximum suppression of free 99mTcO4 −1 and colloid formation, the optimal amount of reducing agent should be opted.
Effect of pH
The sensitivity of radiochemical formation at different pH is obvious from Fig. 5. The pH 5 was found to be the optimal value to obtain maximum labeling efficiency (>98%) at 120 μg stannous tartrate, 1.5 mg SDZ, and 185 MBq 99mTcO4 −. At these conditions, other impurities (free 99mTcO4 − and colloids) decreased to less than 2%. Figure 5 shows a sharp increase and decrease in complex formation of 99mTc-SDZ which indicated the pronounced effect of pH on radiochemical complex formation and also showed the agreement with previously reported data which revealed the dominant effect of pH on 99mTc-labeling chemistry [6, 7, 16].
Effect of Radiochemical Reaction Time
After addition of all the radiochemical reaction components, the progress of reaction was monitored with an interval of 10 min. At 10 min of reaction, 35% reaction was completed leaving 72% free 99mTcO4 −1 which increased to 45 and 85% at 20 and 30 min, respectively. At 40 min reaction period, the maximum radiochemical yield (~98%) was obtained (Fig. 6). Further, incubation of radiochemical up to 4 h at room temperature revealed that the complex is sufficiently stable (~97%) and could be safely administrated up to 4 h after its development.
Biodistribution and Scintigraphic Study
Table 1 shows the biodistribution results of 99mTc-SDZ in infected SD rats. The infected thigh (targeted organ) showed 4.27 ± 0.05%ID/g organ uptake at 1 h post injection, which washed out up to 2.09% at 4 h time point. In contrast to the infected thigh, the normal thigh (non-targeted organ) which was injected with saline as control showed 0.37 ± 0.06% at 30 min and 0.63 ± 0.02% at 1 h post injection. The T/NT ratio at 30 min, 1 h, and 4 h post injection was found 4.49 ± 0.04, 6.78 ± 0.07, and 5.59 ± 0.08, respectively; which is higher than the commercially marketed 99mTc-ciprofloxacin (infecton®; T/NT = 3.6 ± 0.4) [4]. Previous report on the assessment of 99mTc-SDZ (development using SnCl2·2H2O as reducing agent) in Staphylococcus aureus infection showed T/NT ratio 3.6 [17] which is almost 50% lesser than we noticed in this study by using stannous tartrate as a reducing agent in E. coli infection-induced animals. Further, the radiochemical was found more bacterial infection-specific agent in terms of T/NT ratio, as compared to many reported 99mTc-labeled antibiotics for infection imaging such as 99mTc-gemifloxacin (T/NT = 3.5 and 1.89) [13, 18], 99mTc-sulfadimidine (T/NT = 2.25) [19], 99mTc-cefuroxime (T/NT = 1.8) [20], 99mTc-ceftriaxone (T/NT = 1.5) [21], 99mTc-cefotaxime (T/NT = 2.89) [16], 99mTc-cefprozil (T/NT = 5.5) [22], 99mTc-clindamycine (T/NT = 3.1) [23], and 99mTc-azithromycin (T/NT = 6.20) [24]. The nature of radiochemical may pose the accumulation effect; least accumulation in the normal muscles might be due to its low lipophilicity. Other non-targeted organs (the liver, heart, lungs, spleen, and kidneys) showed uptake of activity at 30 min post injection time point however declined to minimal quantity at 4 h post injection time point. The kidney showed the appreciable activity (2.64 ± 0.04%) at 30 min post injection and almost maintained up to 1 h post injection, which later on decline to 1.85 ± 0.11% at 4 h post injection. The continuous renal filtration of radiochemical indicated the absence of glomerular reabsorption which consequently omitted the chance of nephrotoxicity. The higher uptake of activity at 30 min post injection in the liver is due to the metabolic pathway of drug; while blood pool circulation through the heart was the cause of accumulation in the heart. The typical scintigrams of the rabbit with E. coli-infected thigh muscle at 30 min and 1 h post injection are shown in Fig. 7. The scintigram demonstrates mild uptake of activity in infected muscles at 30 min post injection which increased to significant quantity at 1 h time point. However, the non-infected thigh muscle remained cold. The trend of the activity uptake in the infected muscle and other organs were found to be almost similar to that of biodistribution data.
Scintigraphy of E. coli infection-induced rabbit models at 30 min (a) and 1 h (b) post injection. a Visible uptake of 99mTc-SDZ but saline-injected thigh muscles (normal muscles) showing no uptake of activity. b Clear uptake in promising quantity and normal muscles showing minute uptake ( T/NT ratio 6.78)
Glomerular Filtration Rate Study
Glomerular filtration rate study of 99mTc-SDZ revealed the maximum blood pool concentration was obtained within 1 min and 50% of the injected 99mTc-SDZ radiopharmaceutical excreted within 7 min as shown in Fig. 8. Both kidneys showed (blue and green lines) rapid decay toward horizontal axis as a consequence of renal filtration of 99mTc-SDZ which resulted in continuous increase in the bladder activity as indicated by bladder activity line in Fig. 8. Comparing with the gold standard GFR agent (99mTc-DTPA) which takes 11.4 min for 50% renal excretion and does not show nephrotoxicity, we argue the non-radionephrotoxicity of 99mTc-SDZ [25].
Conclusion
In this work, direct labeling of sulfadiazine with technetium-99m was carried out in the presence of stannous tartrate as reducing agent. The maximum radiochemical yield was obtained in a 40-min reaction at room temperature by subsequent addition of 185 MBq 99mTcO4 − freshly eluted from 99Mo/99mTc generator, 120 μg stannous tartrate, 1.5 mg SDZ, 4 μL (8.3 mg/mL) of gentistic acid, and 0.1 N NaOH/HCl to achieve reaction pH 5. The T/NT ratio at 1 h post injection in E. coli infection-induced animal model was found 6.78 ± 0.07, which is higher than the commercially marketed infection imaging agent 99mTc-ciprofloxacin (infecton®, T/NT = 3.6 ± 0.4) [4] and also 50% higher than 99mTc-SDZ investigated for S. aureus infection imaging (T/NT = 3.6) [17]. On the basis of all these facts and the promising GFR value, the newly developed 99mTc-SDZ could be a suitable candidate for selective E. coli infection imaging in nuclear medicine technique.
References
Al Amiry, A. (2015). Methicillin-resistant Staphylococcus aureus: an occupational health hazard in the prehospital setting. J. Acute. Dis., 4, 274–276.
Morens, D. M., & Fauci, A. S. (2013). Emerging infectious diseases: threats to human health and global stability. PLoS Pathogens, 9, e1003467.
De Winter, F., Van de Wiele, C., Dumont, F., Van Durme, J., Solanki, K., Britton, K., Slegers, G., Dierckx, R. A., & Thierens, H. (2001). Biodistribution and dosimetry of 99mTc-ciprofloxacin, a promising agent for the diagnosis of bacterial infection. European Journal of Nuclear Medicine, 28, 570–574.
Siaens, R. H., Rennen, H. J., Boerman, O. C., Dierckx, R., & Slegers, G. (2004). Synthesis and comparison of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. Journal of Nuclear Medicine, 45, 2088–2094.
Qaiser, S. S., Khan, A. U., & Khan, M. R. (2010). Synthesis, biodistribution and evaluation of 99mTc-sitafloxacin kit: a novel infection imaging agent. Journal of Radioanalytical and Nuclear Chemistry, 284, 189–193.
Motaleb, M. A., El-Kolaly, M. T., Ibrahim, A. B., & Abd El-Bary, A. (2011). Study on the preparation and biological evaluation of 99mTc–gatifloxacin and 99mTc–cefepime complexes. Journal of Radioanalytical and Nuclear Chemistry, 289, 57–65.
El-Tawoosy, M. (2013). Preparation and biological distribution of 99mTc-cefazolin complex, a novel agent for detecting sites of infection. Journal of Radioanalytical and Nuclear Chemistry, 298, 1215–1220.
Mirshojaei, S. F. (2015). Advances in infectious foci imaging using 99mTc radiolabelled antibiotics. Journal of Radioanalytical and Nuclear Chemistry, 304, 975–988.
Auletta, S., Galli, F., Lauri, C., Martinelli, D., Santino, I., & Signore, A. (2016). Imaging bacteria with radiolabelled quinolones, cephalosporins and siderophores for imaging infection: a systematic review. Clin. Transl. Imaging., 4, 229–252.
Akbar, M. U., Ahmad, M. R., Shaheen, A., & Mushtaq, S. (2016). A review on evaluation of technetium-99m labeled radiopharmaceuticals. Journal of Radioanalytical and Nuclear Chemistry, 310, 477–493.
Sharma, P., Thakur, S., & Awasthi, P. (2015). Synthesis, characterization, biological evaluation and docking study of heterocyclic-based synthetic sulfonamides as potential pesticide against G. mellonella. Applied Biochemistry and Biotechnology, 176, 125–139.
Levey, A. S., Greene, T., Schluchter, M. D., Cleary, P. A., Teschan, P. E., Lorenz, R. A., Molitch, M. E., Mitch, W. E., Siebert, C., Hall, P. M., Steffes, M. W., for the Modification of Diet in Renal Disease Study, G, & the Diabetes, C and Complications Trial Research, G. (1993). Glomerular filtration rate measurements in clinical trials. Journal of the American Society of Nephrology, 4, 1159–1171.
Shah, S. Q., & Khan, M. R. (2011). Radiolabeling of gemifloxacin with technetium-99m and biological evaluation in artificially Streptococcus pneumoniae infected rats. Journal of Radioanalytical and Nuclear Chemistry, 288, 307–312.
Iglesias, F., Roca, M., Martín-Comín, J., Tubau, F., & Barreto, V. G. (2000). Marcaje de ceftizoxima con 99mTc. Revista Española de Medicina Nuclear., 19, 479–483.
Roohi, S., Mushtaq, A., Jehangir, M., & Malik, S. A. (2006). Synthesis, quality control and biodistribution of 99mTc-kanamycin. Journal of Radioanalytical and Nuclear Chemistry, 267, 561–566.
Mirshojaei, S. F., Gandomkar, M., Najafi, R., Sadat Ebrahimi, S. E., Babaei, M. H., Shafiei, A., & Talebi, M. H. (2011). Radio labeling, quality control and biodistribution of 99mTc-cefotaxime as an infection imaging agent. Journal of Radioanalytical and Nuclear Chemistry, 287, 21–25.
Essouissi, I., Darghoutha, F., Saied, N. M., Saidi, M., Kanoun, A., & Saidi, M. (2015). Radiolabeling, quality control, and biodistribution of 99mTc-sulfadiazine as an infection imaging agent. Radiochemistry, 57, 307–311.
Erfani, M., Rekabgardan, M., Mortazavi, P., & Shafiei, M. (2016). Radiocomplexation and evaluation of the 99mTc-gemifloxacin in artificially Escherichia coli infected mice. Journal of Radioanalytical and Nuclear Chemistry, 308, 825–833.
Amin, A. M., Ibrahim, I. T., Attallah, K. M., & Ali, S. M. (2014). 99mTc-sulfadimidine as a potential radioligand for differentiation between septic and aseptic inflammations. Radiochemistry, 56, 72–75.
Chattopadhyay, S., Ghosh, M., Sett, S., Das, M. K., Chandra, S., De, K., Mishra, M., Sinha, S., Ranjan Sarkar, B., & Ganguly, S. (2012). Preparation and evaluation of 99mTc-cefuroxime, a potential infection specific imaging agent: a reliable thin layer chromatographic system to delineate impurities from the 99mTc-antibiotic. App. Rad. Isotop., 70, 2384–2387.
Fazli, A., Salouti, M., Ahmadi, G., Mirshojaei, F., Mazidi, M., & Heydari, Z. (2012). Radiolabeling of ceftriaxone with 99mTc as a targeting radiopharmaceutical for Staphylococcus aureus detection in mouse model. Iran. J. Med. Phy., 9, 103–110.
Sanad, M. H., & Borai, E. (2014). Performance characteristics of biodistribution of 99mTc-cefprozil for in vivo infection imaging. J. Anal. Sci. Tech., 5, 32.
Hina, S., Rajoka, M. I., Roohi, S., Haque, A., & Qasim, M. (2014). Preparation, biodistribution, and scintigraphic evaluation of 99mTc-clindamycin: an infection imaging agent. Applied Biochemistry and Biotechnology, 174, 1420–1433.
Sanad, M. H. (2013). Labeling and biological evaluation of 99mTc-azithromycin for infective inflammation diagnosis. Radiochemistry, 55, 539–544.
Rasheed, R., Tariq, S., Naqvi, S. A. R., Gillani, S. J. H., Rizvi, F. A., Sajid, M., & Rasheed, S. (2016). 177Lu-5-fluorouracil a potential theranostic radiopharmaceutical: radiosynthesis, quality control, biodistribution, and scintigraphy. J. Label. Comp. Radiopharm., 59, 398–403.
Acknowledgements
The authors are grateful to the Higher Education Commission (HEC), Islamabad, Pakistan, for providing funds (No. 20-3413/NRPU/R&D/HEC/2014/702) for the development of radiopharmaceuticals and promoting the research culture in Pakistan. The authors are also thankful to Director INOR, Abbottabad for providing the lab and SPECT camera facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahmed, M.T., Naqvi, S.A.R., Rasheed, R. et al. Technetium-99m-Labeled Sulfadiazine: a Targeting Radiopharmaceutical for Scintigraphic Imaging of Infectious Foci Due To Escherichia coli in Mouse and Rabbit Models. Appl Biochem Biotechnol 183, 374–384 (2017). https://doi.org/10.1007/s12010-017-2451-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2451-2