Abstract
Bacterial infection poses life-threatening challenge to humanity and stimulates to the researchers for developing better diagnostic and therapeutic agents complying with existing theranostic techniques. Nuclear medicine technique helps to visualize hard-to-diagnose deep-seated bacterial infections using radionuclide-labeled tracer agents. Metronidazole is an antiprotozoal antibiotic that serves as a preeminent anaerobic chemotherapeutic agent. The aim of this study was to develop technetium-99m-labeled metronidazole radiotracer for the detection of deep-seated bacterial infections. Radiosynthesis of 99mTc-metronidazole was carried by reacting reduced technetium-99m and metronidazole at neutral pH for 30 min. The stannous chloride dihydrate was used as the reducing agent. At optimum radiolabeling conditions, ~ 94% radiochemical was obtained. Quality control analysis was carried out with a chromatographic paper and instant thin-layer chromatographic analysis. The biodistribution study of radiochemical was performed using Escherichia coli bacterial infection-induced rat model. The scintigraphic study was performed using E. coli bacterial infection-induced rabbit model. The results showed promising accumulation at the site of infection and its rapid clearance from the body. The tracer showed target-to-non-target ratio 5.57 ± 0.04 at 1 h post-injection. The results showed that 99mTc-MNZ has promising potential to accumulate at E. coli bacterial infection that can be used for E. coli infection imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Targeted molecular imaging provides optimistic diagnostic procedure particularly in case of hard-to-diagnose deep-seated diseases. Bacterial infections are posing serious threat to humanity in recent years as compared to those in the past [1, 2]. Since the twentieth century, molecular biologist and immunologist had been struggling to reduce morbidity rate by introducing antibacterial agents and controlling over bacterial resistance, but bacterial resistance issue not only remained intact but also caused many infectious issues. The main reason is to prescribe antibiotics that are not in complying with the bacterial strain and load at the infectious area [3]. Nuclear medicine technique has played phenomenally a dynamic role in the management of clinical infection [4] by targeted diagnosis of infection/cancer place and volume in the human body. From the last decade, the nuclear medicine technique has been proven as a blessing for humanity because of its high accuracy and sensitivity. In the last decade, according to one survey, out of 1000 population, ~ 16 patients were subjected to nuclear medicine procedure [5]. Nuclear medicine technique is a non-invasive imaging technique and surpasses the invasive radiological anatomical imaging to discriminate the microbial infection from inflammation [6]. Manipulation of radiopharmaceutical probes has the key potentials of targeting the disease with good specificity and accuracy. The whole-body imaging determines the deep-seated infection and also has potential to discriminate it from sterile inflammation and tumors [7]. Radiological imaging techniques such as computed tomography scan and magnetic resonance imaging both offer morphological imaging procedures and do little at the molecular level that is the basis of the disease. Shifting toward positron emission tomography (PET) and single-photon emission computed tomography (SPECT) in compliance with radiopharmaceuticals provides imaging at the molecular level [8, 9]. The main advantage we observe during whole-body SPECT scan is when some hot dots appear instead of main diseased organ. This indicates in addition to the main diseased organ that there are seedlings of diseases in different areas of the body [10]. Reports show that the scintigraphic imaging of patients with 99mTc-ciprofloxacin (Infecton®) has shown 90% accuracy, 86% specificity, and 93% sensitivity while studying using > 800 multinational patients [11]. Today, the nuclear medicine technique is being well-developed to diagnose hard-to-diagnose diseases. Technetium-99m (99mTc)-labeled agents are used routinely in more than 90% nuclear medicine imaging procedures [5, 12, 13]. Nitroimidazoles is the class of antibiotics that is predominantly used to combat anaerobic bacteria, protozoal, and parasitic infections. This class of antibiotics found major applications against Giardia and Trichomonas. Metronidazole (MNZ) is a member of this family and could be developed as an anaerobic bacterial infection imaging agent by labeling it with 99mTc [14]. The aim of this study was to develop 99mTc-metronidazole as an Escherichia coli (E. coli) infection imaging agent.
Material and Method
Metronidazole was obtained from Sigma-Aldrich, Germany. Stannous chloride dihydrate (SnCl2.2H2O), sodium hydroxide (NaOH), and hydrochloric acid (HCl) were purchased from Merck, Germany. All these chemicals were of analytical grade. Whatman paper no. 2 and instant thin-layer chromatographic sheets (ITLC-SG) were purchased from Agilent Technology. Freshly prepared carrier-free 99mTc as Na99mTcO4 was obtained from Mo/99mTc generator of Pakistan Atomic Reactor-1 (PAR-1), PINSTECH, Islamabad, Pakistan. E. coli bacterial strain (ATCC 25923) was acquired from New England Biolabs, Abbottabad, Pakistan. Sprague-Dawley (SD) rats (120–150 g) and New Zealand white rabbits, weighing 1–1.5 kg, were obtained from the National Institute of Health (NIH), Islamabad, Pakistan for the purpose of biodistribution and scintigraphy, respectively. The biodistribution and scintigraphy studies were performed according to the Institute of Nuclear Medicine Oncology and Radiotherapy (INOR) guidelines and standards designed by the FELASA [15].
Radiosynthesis of 99mTc-Metronidazole
Metronidazole was radiolabeled with 99mTc using saline solution of 99mTcNaO4 by taking the initial guideline from reaction conditions; reported previously to achieve more stable radiochemical [16]. The amount of metronidazole ligand was studied from 50 to 150 μg, pH 4–12 (adjusted with 0.5 N NaOH and 0.1 N HCl). The reducing agent (SnCl2.2H2O) was studied from 60 to 120 μg. Volume of the reaction mixture was adjusted in all experiments to 2 ± 0.2 mL. After addition of all reagents, ~ 250–300 MBq of 99mTcNaO4 solution in saline solution was added into the reaction vial. Radiochemical purity of 99mTc-metronidazole was assessed by using Whatman No. 2 chromatographic paper and ITLC-SG strips.
Quality Control of 99mTc-Metronidazole
Paper Chromatography
In order to calculate free 99mTcO4 − formation, a small aliquot, about ~2 μL of sample was spotted at Whatman No. 2 chromatographic paper and then developed in acetone solution as an eluting solvent. Free 99mTcO4 − moved along with mobile phase, while hydrolyzed 99mTc remained at baseline. The radioactivity counts with other impurities on the strip were measured with gamma counter and scanned with 2π-scanner. The free 99mTcO4 −1 was then calculated by using the following expression:
Instant Thin-Layer Chromatography Analysis
In ITLC-SG analysis, the percentage of hydrolyzed 99mTc was determined. The elution of reaction components was eluted with sodium hydroxide (NaOH) solution. The ITLC-SG strip was spotted with 2 μL aliquot of reaction mixture sample at the baseline and allowed to run. In this system, 99mTc-MNZ and free 99mTcO4 − were moved along with solvent front, while hydrolyzed or reduced 99mTc remained at baseline. The radioactivity counts were measured by scanning with advanced TLC radiochromatographic scanner connected with radionuclidic and radiochemical purity determination software system after making the patches of strip of 0.25 cm. The percentage of colloid/hydrolyzed 99mTc and 99mTc-MNZ was determined by using the following expression:
Effect of Quality Control Parameters
Effect of the Amount of Metronidazole
The effect of amount of ligand was studied using 50–150 μg. The radiolabeling was studied with an increment of 10 μg metronidazole by varying the other parameters such as pH, reducing agent, and reaction time [17].
Effect of the Amount of Reducing Agent
The concentration of reducing agent plays a vital role to execute 99mTc radiolabeling. The effect of reducing agent on 99mTc radiolabeling was studied using 60–150 μg of stannous chloride varying the other reaction conditions. Each reaction was assessed with chromatographic procedures to measure radiochemical yield.
Effect of pH
The pH of the radiolabeling reaction was studied from 4 to 12 pH with an increment of 1 pH unit that was adjusted by using 0.5 N NaOH and 0.1 N HCl. The optimum pH was obtained by measuring the radiochemical yield using different sets of reaction conditions.
Effect of Incubation Time
Radiolabeling reactions were also studied at varied reaction times from 10 to 70 min with an increase of 10 min. Each reaction yield was measured with chromatographic analysis with respect to other reaction parameters.
Biodistribution in E. coli-Infected Rats
For in vivo biodistribution of freshly prepared 99mTc-metronidazole complex, three healthy SD rats (weighing 120–150 g) were injected intramuscularly at the right thigh with E. coli bacteria. The inflammation was induced chemically by injecting 0.2 mL sterile turpentine oil in the left thigh muscles. After 30 h, the swelling at both thigh muscles indicated the presence of infection and inflammation. The three rats per time point study were then subjected for biodistribution of 99mTc-MNZ. Prior to the administration of 99mTc-MNZ into the blood vein, the rats were anesthetized with chloroform. For biodistribution, 200 μL 99mTc-MNZ (185 MBq) was administrated to the anesthetized rats through tail blood vein. The three rats were sacrificed at 1, 4, and 24 h post-injection. The in vivo uptake by different organs was measured by removing body organs such as the heart, liver, brain, lungs, spleen, stomach, left kidney, right kidney, inflamed thigh muscles, and infected thigh muscles. The organs were then washed and stored into gamma counter tubes. The radioactivity was measured by placing the tubes into NaI gamma scintillation counter. From the measured radioactivity, the percent injected dose per gram (%ID/g) body organ was calculated.
Scintigraphic Study
Scintigraphy study of 99mTc-metronidazole was performed with E. coli bacterial infection-induced rabbit model. The infection was induced in the right thigh muscle of male New Zealand white rabbit (weighing 1.0–1.5 kg) by intramuscular injection of 300 μL saline solution of E. coli. The left thigh muscles were injected with 300 μL saline as control, and the rabbits were left for 30 h. After 30 h, slight swelling was observed in both thigh muscles; however, it was less as observed in rats. On the day of scintigraphy imaging, the rabbit was anesthetized with 2 mL diazepam injection, laid flat on a hard board with fore and hind legs spread out, and fixed with surgical tap under dual-headed SPECT gamma camera. An aliquot of 250 μL 99mTc-MNZ solution was injected through rear ear vein, and static scintigraphic images were recorded with on-line-dedicated computer at 5, 10, 15, and 20 min time point as per direction of the institutional animal ethical committee.
Glomerular Filtration Rate Study
The glomerular filtration rate (GFR) for 99mTc-metronidzole was determined according to the protocol previously published by Levey et al. [18]. Briefly, the rabbit was kept at fast overnight and served with water for frequent excretion of urine. The rabbit was then administrated with 250 μL of 99mTc-MNZ solution at the rear ear vein. The urine was collected at regular interval as well as the renal excretion into the bladder was recorded scintigraphically using GFR-dedicated computer software interfaced with SPECT gamma camera.
Results and Discussion
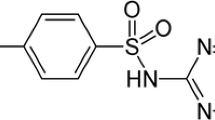
The antiprotozoal nitroimidazole antibiotic, 5-nitroimidazole, metronidazole (MNZ) possesses highly reducing nitro (–NO2 −) group which enables it to target successfully the bacterial infection both in vivo and in vitro environments under anaerobic conditions (Fig. 1). The radiolabeling of metronidazole with gamma emitter 99mTc radionuclide provides the opportunity to target bacterial infections. The 99mTc in its lower oxidation state (that is achieved reduction of 99mTc with appropriate reducing agent) can easily bind with the ligand molecules that can donate electrons to 99mTc [19]. Fluoroquinolones, cephalosporins, and other antibiotic members that possess electron-donating functional groups get attached to the 99mTc easily and have been reported for infection imaging successfully [17]. Metronidazole as shown in Fig. 1 contains nitro group (–NO2), hydroxyl group (–OH), and nitrogen (=N–) that are good electron donor groups to electron-deficient metal atoms. The presence of these electron donor groups enables metronidazole to form stable complexation with reduced 99mTc [20].
Optimized Radiochemical Synthesis Conditions and Yield
Radiosynthesis and quality control analysis reveals ~ 94% radiochemical yield at optimal reaction parameters [8]. The subsequent mixing of 100 μg metronidazole (ligand), 120 μg stannous chloride as reducing agent, 250 MBq 99mTcO4 −1, adjusting the pH to 7 with 0.5 N NaOH or 0.1 N HCl, and reaction time at 30 min was set as optimized conditions to achieve maximum radiochemical. At optimized conditions, the other impurities that are free 99mTcO4 −1 and colloids were obtained at 2.56 and 3.41%, respectively. The radiochromatogram scanned with 2π scanner at maximum labeling yield showed main single peak as shown in Fig. 2.
Effect of Radiolabeling Parameters on Labeling Yield
Effect of the Amount of Metronidazole
The stoichiometric amount of ligand is required for the maximum radiochemical yield. Different sets of radiolabeling experiments were tested with 50–150 μg metronidazole. A hundred microgram of metronidazole was found as the optimal amount for maximum radiochemical yield as shown in Fig. 3.
Effect of Reducing Agent
Freshly eluted 99mTcO4 −1 from 99Mo/99mTc generator exists in high oxidation state i.e. +7, and at this state, it cannot make complex with other ligand molecules. Reduction process in 99mTc-complex formation is a key step for this variety of reducing agent we tested, but stannous salts were found favorable in 99mTc-complex chemistry. In this study, SnCl2.2H2O was used as the reducing agent. Different quantities i.e. 60–150 μg were tested for maximum yield. The optimal amount of reducing agent is the amount of SnCl2.2H2O at which maximum 99mTcO4 −1 ions reduced to lower oxidation state that can react with ligand strongly. At 120 μg SnCl2.2H2O, the maximum (~ 94%) radiochemical was obtained. Radiochemical yield in different reactions with continuously increased amount of SnCl2.2H2O is shown in Fig. 4.
Effect of pH
In 99mTc radionuclide complexation chemistry, pH of the reaction mixture shows vital role in equilibrating the ionic strength to facilitate electron donation to radionuclide. Metronidazole as reported previously shows good stability with 99mTc radionuclide at 7–8 pH. We studied radiochemical yield by varying pH from 4 to 12 and found that at neutral pH maximum radiochemical was obtained. Other than neutral pH < 94% radiochemical yield was recorded as shown in Fig. 5.
Effect of Incubation Time
At optimized reaction conditions (as discussed in the “Optimized Radiochemical Synthesis Conditions and Yield” section), the effect of reaction time was monitored at room temperature with an interval of 10 min up to 70 min as shown in Fig. 6. It was found that 30 min is required to complete the radiolabeling reaction. However, at subsequent time points, fraction of complex was degraded that might be due to alteration in one or more reaction conditions.
Biodistribution Study and Scintigraphic Study
The in vivo biodistribution of 99mTc-MNZ was studied in E. coli infection-induced SD rats. The results of biodistribution in different body organs as %ID/g organ are shown in Table 1. The targeted organ (infected muscles) showed promising radiochemical uptake (1.84 ± 0.06%ID/g organ) as compared to inflamed muscles, which showed 0.32 ± 0.03%ID/g organ at 1 h post-injection. At subsequent time points, i.e. 4 and 24 h, infected and inflamed muscle showed 1.9 ± 0.02 and 0.2 ± 0.05 and 1.1 ± 0.04 and 0.2 ± 0.02%ID/g organ, respectively. The calculation of T/NT ratio (infected to inflamed muscle uptake ratio) reveals 5.57 ± 0.04, 6.55 ± 0.07, and 5.5 ± 0.08 at 1, 4, and 24 h post-injection, respectively. The obtained values of T/NT ratio are showing promising potential of 99mTc-MNZ to target the bacterial infection. However, it also reveals the tracer agent has week interaction with inflamed muscles—that is an indication of ability of 99mTc-MNZ to discriminate infection from inflammation. On comparison T/NT ratio of 99mTc-MNZ with other reported infection imaging radiopharmaceuticals, it appears that 99mTc-MNZ has better position as compared to 99mTc-ciprofloxacin (being marketed with trade name Infecton®) T/NT = 3.6 ± 0.4 [21], 99mTc-levofloxacin (T/NT = 3.57) [22], 99mTc-difloxacin (T/NT = 5.5 ± 0.5) [23], 99mTc-pefloxacin (T/NT = 4.9 ± 0.3) [23], 99mTc-ceftazidime (T/NT = 1.4 ± 0.2) [24], and 99mTc-meropenem (T/NT~ 4) [25]. If we see the uptake of 99mTc-MNZ in other body organs, it showed 1.47 ± 0.89 and 1.5 ± 0.07%ID/g organ uptake in lungs and liver, respectively, at 1 h post-injection. It also showed minimal uptake in stomach (0.146 ± 0.56%ID/g organ) at 1 h post-injection which represents minimum in vivo re-oxidation phenomenon. Scintigraphic results showed minimal uptake at infected thigh muscles as we recorded in mice. This was obvious because after 30 h induction of infection, a small area of muscles swelled, and we could not repeat the experiment due to limited permission from the institutional animal ethical committee. However, presence of small amount of activity at the infected site that remained for a long time while the activity at the inflamed thigh muscles appeared initially due to blood flow but later on no activity was found at the inflamed tissues as shown in Fig. 7.
Glomerular Filtration Rate Study
Glomerular filtration rate (GFR) study for 99mTc-metronidazole was performed to analyze kidney function, its clearance, and the dosage of radiopharmaceutical agent excreted from the kidney. The renal excretion graph (renogram) shows a constant filtration from kidneys, and no accumulation was found in kidneys (that is no rising trend in graph lines was observed), as shown in Fig. 8. Thus, there is no possibility of the 99mTc-MNZ nephrotoxicity [26].
Conclusion
The direct radiolabeling of metronidazole with 99mTc is a reliable and easy-to-conduct method that produces ~ 94% radiochemical yield with mild reaction conditions such as subsequent mixing of 100 μg metronidazole (ligand), 120 μg stannous chloride, 250 MBq 99mTcO4 −1, pH 7, and reaction time of 30 min at room temperature. Biodistribution study with E. coli infection-induced rat models showed T/NT value 5.57 ± 0.04 at 1 h post-injection, which is in sense of T/NT ratio that looks better than Infecton® (T/NT = 3.6 ± 0.4). Glomerular filtration rate showed the tracer agent is non-nephrotoxic. Keeping in mind the results of this study, 99mTc-MNZ might be a radiotracer of choice for infection imaging after proper preclinical studies.
References
Eggleston, H. & Panizzi, P. (2014) Molecular imaging of bacterial infections in vivo: the discrimination between infection and inflammation.
Wareham, D., Michael, J., & Das, S. (2005). Advances in bacterial specific imaging. Brazilian Archives of Biology and Technology, 48, 145–152.
Sharma, P., Thakur, S., & Awasthi, P. (2015). Synthesis, characterization, biological evaluation and docking study of heterocyclic-based synthetic sulfonamides as potential pesticide against G. mellonella. Applied Biochemistry and Biotechnology, 176, 125–139.
Becker, W., & Meller, J. (2001). The role of nuclear medicine in infection and inflammation. The Lancet Infectious Diseases, 1, 326–333.
Auletta, S., Galli, F., Lauri, C., Martinelli, D., Santino, I., & Signore, A. (2016). Imaging bacteria with radiolabelled quinolones, cephalosporins and siderophores for imaging infection: a systematic review. Clinical and Translational Imaging, 4, 229–252.
Boerman, O., Dams, E. T. M., Oyen, W., Corstens, F., & Storm, G. (2001). Radiopharmaceuticals for scintigraphic imaging of infection and inflammation. Inflammation Research, 50, 55–64.
Ady, J., & Fong, Y. (2014). Imaging for infection: from visualization of inflammation to visualization of microbes. Surgical Infections, 15, 700–707.
Kumar, S. N., Lankalapalli, R. S., & Kumar, B. D. (2014). In vitro antibacterial screening of six proline-based cyclic dipeptides in combination with Β-lactam antibiotics against medically important bacteria. Applied Biochemistry and Biotechnology, 173, 116–128.
Paul, T., Mandal, A., Mandal, S. M., Ghosh, K., Mandal, A. K., Halder, S. K., Das, A., Maji, S. K., Kati, A., & Mohapatra, P. K. D. (2015). Enzymatic hydrolyzed feather peptide, a welcoming drug for multiple-antibiotic-resistant Staphylococcus aureus: structural analysis and characterization. Applied Biochemistry and Biotechnology, 175, 3371–3386.
Amin, A., Ibrahim, I., Attallah, K., & Ali, S. (2014). 99mTc-sulfadimidine as a potential radioligand for differentiation between septic and aseptic inflammations. Radiochemistry, 56, 72–75.
Malamitsi, J., Giamarellou, H., Kanellakopoulou, K., Dounis, E., Grecka, V., Christakopoulos, J., Koratzanis, G., Antoniadou, A., Panoutsopoulos, G., & Batsakis, C. (2003). Infecton: a 99mTc-ciprofloxacin radiopharmaceutical for the detection of bone infection. Clin. microb. infect., 9, 101–109.
Akbar, M. U., Ahmad, M. R., Shaheen, A., & Mushtaq, S. (2016). A review on evaluation of technetium-99m labeled radiopharmaceuticals. Journal of Radioanalytical and Nuclear Chemistry, 310, 477–493.
Mirshojaei, S. F. (2015). Advances in infectious foci imaging using 99mTc radiolabelled antibiotics. Journal of Radioanalytical and Nuclear Chemistry, 304, 975–988.
Upcroft, J. A., Dunn, L. A., Wright, J. M., Benakli, K., Upcroft, P., & Vanelle, P. (2006). 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrobial Agents and Chemotherapy, 50, 344–347.
Guillen, J. (2012). Felasa guidelines and recommendations. Journal of the American Association for Laboratory Animal Science, 51, 311–321.
Ibrahim, I. T. (2009). Preparation of 99mTc-metronidazole as a model for tumor imaging. Journal of Radioanalytical and Nuclear Chemistry, 281, 669.
Motaleb, M., El-Kolaly, M., Ibrahim, A., & El-Bary, A. A. (2011). Study on the preparation and biological evaluation of 99mTc–gatifloxacin and 99mtc–cefepime complexes. Journal of Radioanalytical and Nuclear Chemistry, 289, 57–65.
Levey, A. S., Greene, T., Schluchter, M. D., Cleary, P. A., Teschan, P. E., Lorenz, R. A., Molitch, M. E., Mitch, W. E., Siebert, C., & Hall, P. M. (1993). Glomerular filtration rate measurements in clinical trials. Modification of diet in renal disease study group and the diabetes control and complications trial research group. Journal of the American Society of Nephrology, 4, 1159–1171.
Ahmed, M. T., Naqvi, S. A. R., Rasheed, R., Zahoor, A. F., Usman, M. & Hussain, Z. (2017). Technetium-99m-labeled sulfadiazine: a targeting radiopharmaceutical for scintigraphic imaging of infectious foci due to Escherichia coli in mouse and rabbit models. Applied Biochemistry and Biotechnology, 183, 374 –384.
Löfmark, S., Edlund, C., & Nord, C. E. (2010). Metronidazole is still the drug of choice for treatment of anaerobic infections. Clinical Infectious Diseases, 50, S16–S23.
Siaens, R. H., Rennen, H. J., Boerman, O. C., Dierckx, R., & Slegers, G. (2004). Synthesis and comparison of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. Journal of Nuclear Medicine, 45, 2088–2094.
El-Ghany, E., Amin, A., El-Kawy, O., & Amin, M. (2007). Technetium-99m labeling and freeze-dried kit formulation of levofloxacin (L-Flox): a novel agent for detecting sites of infection. Journal of Labelled Compounds and Radiopharmaceuticals, 50, 25–31.
Motaleb, M. (2010). Radiochemical and biological characteristics of 99mTc-difloxacin and 99mTc-pefloxacin for detecting sites of infection. Journal of Labelled Compounds and Radiopharmaceuticals, 53, 104–109.
Mirshojaei, S., Erfani, M., & Shafiei, M. (2013). Evaluation of 99mTc-ceftazidime as bacterial infection imaging agent. Journal of Radioanalytical and Nuclear Chemistry, 298, 19–24.
Sakr, T., Motaleb, M., & Ibrahim, I. (2012). 99mtc–meropenem as a potential Spect imaging probe for tumor hypoxia. Journal of Radioanalytical and Nuclear Chemistry, 292, 705–710.
Rasheed, R., Tariq, S., Naqvi, S. A., Gillani, S. J., Rizvi, F. A., Sajid, M., & Rasheed, S. (2016). (177) Lu-5-fluorouracil a potential theranostic radiopharmaceutical: radiosynthesis, quality control, biodistribution, and scintigraphy. J Labelled Comp Radiopharm, 59, 398–403.
Acknowledgements
The authors are thankful to the HEC, the Government College University, Faisalabad, and the director INOR Abbottabad (Dr. Syed Jawad Akhtar Hussain Gillani) for providing the platform to execute this piece of work. The authors are also thankful to the technical staff of the INOR and PINSTECH Islamabad for providing technical assistance.
Funding
This work is a part of the Higher Education Commission (HEC) funded project No. 5612/Punjab/R&D/HEC/2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure Statement
The authors declare that there are no any potential sources of conflict of interest.
Human and Animal Rights and Informed Consent
The biodistribution and scintigraphy studies were performed according to the Institute of Nuclear Medicine Oncology and Radiotherapy (INOR) guidelines and standards designed by the FELASA.
Rights and permissions
About this article
Cite this article
Iqbal, A., Naqvi, S.A.R., Rasheed, R. et al. Radiosynthesis and Biodistribution of 99mTc-Metronidazole as an Escherichia coli Infection Imaging Radiopharmaceutical. Appl Biochem Biotechnol 185, 127–139 (2018). https://doi.org/10.1007/s12010-017-2641-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2641-y