Abstract
Purpose of Review
The objective of this review is to summarize the literature on the prevalence and diagnosis of obesity and its metabolic profile, including bone metabolism, focusing on the main inflammatory and turnover bone mediators that better characterize metabolically healthy obesity phenotype, and to summarize the therapeutic interventions for obesity with their effects on bone health.
Recent Findings
Osteoporosis and fracture risk not only increase with age and menopause but also with metabolic diseases, such as diabetes mellitus. Thus, patients with high BMI may have a higher bone fragility and fracture risk. However, some obese individuals with healthy metabolic profiles seem to be less at risk of bone fracture.
Summary
Obesity has become an alarming disease with growing prevalence and multiple metabolic comorbidities, resulting in a significant burden on healthcare and increased mortality. The imbalance between increased food ingestion and decreased energy expenditure leads to pathological adipose tissue distribution and function, with increased secretion of proinflammatory markers and harmful consequences for body tissues, including bone tissue. However, some obese individuals seem to have a healthy metabolic profile and may not develop cardiometabolic disease during their lives. This healthy metabolic profile also benefits bone turnover and is associated with lower fracture risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become a major public health problem worldwide with a growing epidemiology, and increased burden from chronic diseases (including diabetes mellitus, hypertension, dyslipidemia, heart disease, stroke, sleep apnea, and cancer) and mortality [1]. Obesity also has economic impacts with considerable costs for health care systems and broader society [2]. Its prevalence has nearly tripled worldwide since the 1970s [3, 4, 5•] (5.5% of males and 9.8% of females) [6].
However, the relationship between obesity and cardiometabolic complications is not linear. More than 80% of individuals with type 2 diabetes are obese, but 10–30% of obese individuals have a metabolically healthy profile characterized by preserved insulin sensitivity and normal blood pressure and lipid profiles [7]. The latter are described as metabolically healthy obese (MHO). Conversely, 30% of normal-weight individuals will develop metabolic diseases during their lifetimes [8, 9•, 10].
Like obesity, osteoporosis is a major public health challenge due to its high prevalence and its association with morbidity, mortality, and healthcare costs. Osteoporosis has been diagnosed in more than 200 million people worldwide and is responsible for more than 8.9 million fractures annually, leading to an osteoporotic fracture every 3 s [11, 12]. The interaction between obesity and bone metabolism, particularly in the context of MHO, is complex and not completely understood; therefore, understanding the interaction between healthy and unhealthy fat and bone is an essential key to the optimal management of these two chronic conditions. This review summarizes current evidence on the metabolic and inflammatory profiles of MHO and metabolically unhealthy obese (MUHO) individuals, including their clinical phenotypes and the changes induced by these two profiles on bone. Finally, the impact of these profiles on osteoporosis treatment will be discussed.
Obesity and Cardiometabolic Risk
Obesity Definition
Obesity is an abnormal or excessive fat accumulation that presents a health risk [13]. The body mass index (BMI), a surrogate measure of body fat (BF) based on the person’s weight adjusted for height, is typically used to categorize normal weight (< 25 kg/m2), overweight (25–29.9 kg/m2), and obesity (≥ 30 kg/m2) [14•] with morbid obesity defined as a BMI ≥ 40 kg/m2. However, it does not consider the large variability in body adiposity and fat distributions between individuals, partially related to age, sex, and ethnicity. For instance, Asians have a higher percentage of body fat than Caucasians for the same BMI [15].
Abdominal obesity has been associated with a greater cardiometabolic risk than other locations of fat mass. Many studies have suggested that waist circumference, or the waist-to-hip ratio, may better indicate abdominal obesity than BMI [16]. Indeed, guidelines recommend measuring waist circumference when BMI is between 25.0 and 34.9 kg/m2, with a cutoff point of 102 cm in men and 88 cm in women [17]. However, the fat distribution and its type are better prognostic health indicators than the above anthropometric measurements. Excess of visceral adipose tissue (VAT) may be more often associated with cardiovascular and metabolic disease, as well as colorectal cancer, than subcutaneous adipose tissue (SAT) [18, 19]. To date, the two gold-standard imaging methods for quantification of VAT and SAT are magnetic resonance imaging (MRI) and computed tomography (CT), which have limited use in research and clinical medicine given their cost and exposure to radiation (CT only) [20]. Dual-energy X-ray absorptiometry (DXA), initially used for osteoporosis diagnosis and monitoring, is also a validated technique to assess body composition, with lower cost and more availability [21]. These data support that there are heterogeneous obesity phenotypes among populations, depending on body fat distribution, each one with a different metabolic risk profile, leading to the concept of MHO and MUHO phenotypes [22•].
Metabolic Obesity: Pathophysiology and Definition
Adipose tissue is a dynamic organ with a major role in energy homeostasis, composed mainly of white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is involved in energy storage and includes VAT (the fat stored around internal organs), which is endocrinologically active, and SAT which is mainly metabolically inactive. In contrast, BAT is involved in energy expenditure and is mostly localized in the supraclavicular and paravertebral regions [23,24,25].
Accumulation of fat in visceral organs (VAT) and ectopic fat deposition (muscle, liver, heart) could lead to MUHO together with an increased risk of conditions associated with metabolic abnormalities such as hypertension, dyslipidemia, and impaired glucose metabolism, which are consequently associated with an increased risk of type 2 diabetes (T2DM) and cardiovascular disease (CVD) [26]. In addition, excess hepatic fat (NAFLD) is a potential predictor of the MUHO phenotype and subclinical atherosclerosis [27]. Failure adipose tissue expansion (hypertrophic fat cells), occurring after a positive energy balance, results in ectopic deposition of lipids with associated lipotoxicity, abnormal proinflammatory markers secretion, high immune cell infiltration, and consequently, insulin resistance in peripheral tissues [28].
Regarding MHO, there is no universal definition for this phenotype which can explain the large variability of its prevalence (6–40% of obese individuals) [29, 30]. This subgroup of people with obesity (BMI ≥ 30 kg/m2) is mainly characterized by the absence of insulin resistance, none of the criteria of metabolic syndrome (or some of its components) and no cardiovascular disease. Some studies also include a favorable inflammatory status according to C-reactive protein (CRP) levels [31,32,33]. Consequently, body composition, fat distribution, and function are critical in distinguishing metabolically healthy from metabolically unhealthy individuals.
Determinants of MHO/MUHO in the Genesis of CVD
Gender, age, genetic polymorphism, gut microbiota, and ethnicity are major etiologic factors leading to variation in fat visceral deposition, in addition to lifestyle factors [26, 34, 35••]. Despite the heterogeneity in classification, MHO individuals appear to have higher SAT levels and less visceral and ectopic fat deposition than MUHO individuals with the same BMI and usually do not develop cardiometabolic disease. Conversely, there are individuals with normal BMI and increased cardiometabolic risk36].
The quality of adipose tissue is equally important to explain the more favorable metabolic profile. MUHO might be the consequence of an impaired adipose tissue function after a chronic positive energy balance, leading to the inability of the subcutaneous adipose tissue to expand sufficiently to counter this long-term imbalance [7]. The inflammatory status also plays a key role field [37]: MHO phenotype is associated with lower proinflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and TNF-α, as well as lower levels of proinflammatory M1 macrophages and CD4 + T cells [37, 38].
Several adipokines produced by the adipose tissue are linked to the development of insulin resistance and T2DM [39,40,41]. Adiponectin, whose production is inversely correlated with adipose mass, and omentin-1 are both considered anti-inflammatory and cardioprotective adipokines and are higher in MHO subjects. Two non-cardioprotective adipokines, visfatin and resistin, are lower in MHO [42,43,44]. Leptin, expressed in levels proportionate to adiposity, is a product of the obesity gene with a pleiotropic effect on food intake, body weight, reproductive system, proinflammatory responses, and lipid metabolism [45]. It may act as a biomarker for cardiovascular diseases in obese individuals, as elevated plasma leptin levels are associated with proinflammatory effects, atherosclerosis, hypertension and metabolic syndrome [46]. Moreover, high serum leptin/adiponectin ratio may be a marker of “at risk” obesity, independent of waist circumference and BMI. In the literature, higher leptin levels among MUHO subjects have been found. However, some studies showed no difference between the two groups [47,48,49]. These few studies with contradictory results are likely secondary to other confounder parameters (differences in race, age, gender, definition of MHO used, or sample size).
In summary, the most important biological factors used to determine MHO profile are a lower amount of visceral and ectopic fat, a higher amount of subcutaneous adiposity, a decreased inflammation and fibrosis, specific adipokines secretion, and a preserved insulin sensitivity compared to MUHO profile.
Obesity and Bone Metabolism
Interaction Bone-Adipose Tissue
Osteoporosis is characterized by low bone mineral density (BMD), defined by a T-score 2.5 SD or more below the mean adult value with DXA (femoral neck measure), and progressive bone microarchitectural deterioration resulting in decreased bone strength and increased susceptibility to fractures [50]. Historically, the common belief was that obesity has a protective effect against osteoporosis [51]. Additionally, higher BMI results in lower fracture risk in the “fracture risk assessment tool (FRAX). Indeed, some studies showed a positive effect of fat on bone mass due to a higher BMD and local adipose padding in obese people compared to people with normal weight [52,53,54]. A low body weight is also a risk factor for fragility fracture [55]. Conversely, there is evidence that obesity has a harmful effect on bone mass [56•].
Several mechanical and biochemical mechanisms have been suggested to understand the complex communication between the adipose tissue and bone tissue. Higher body mass results in increased mechanical load on bone, leading to an increase in BMD to adapt to mechanical stress [57]. This hypothesis has been supported by precise quantitative methods (e.g., high-resolution peripheral quantitative CT) [58]. Moreover, fracture risk in obese people seems to be site-dependent: obesity is associated with higher fractures in the ankle, leg, humerus, and vertebral column, and lower fractures in the wrist, hip, and pelvis [59, 60].
Fat distribution also plays an important role. Each adipose tissue compartment has a specific metabolic profile and bone effect. VAT secretes more proinflammatory markers, negatively impacting BMD [61, 62]. Excess of SAT is associated with more leptin secretion and results in lower bone resorption and higher bone strength than excess VAT. Intramuscular fat leads to muscle performance impairment and myocyte insulin resistance [63, 64]. Additionally, brown fat (BAT) positively affects bone mass [65]. The bone marrow adipose tissue (BMAT), named yellow bone marrow, is another metabolically active adipose tissue involved in bone homeostasis and body energetic metabolism by direct or indirect effects.
Estrogens, synthesized from androgen precursors by aromatase in adipose tissue, have a crucial role in bone protection by promoting bone formation and reducing bone resorption. Obese post-menopausal women have been shown to have higher estrogen levels in blood compared with non-obese individuals [66]. Overproduction of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, results in osteoclast differentiation stimulation and bone resorption through activation of RANKL/RANK/osteoprotegerin (OPG) pathway [67, 68]. In addition, adipokines such as leptin, typically high in obesity, and adiponectin, low in obesity, as well as bone turnover markers (vitamin D, parathyroid hormone, calcium, osteocalcin), are other potential parameters involved in this interaction [69, 70].
Bone Metabolism Changes and Effects in MHO/MUHO
Bone Marrow Adipose Tissue (BMAT)
BMAT has a metabolically distinct phenotype with some similar WAT and BAT properties [71]. The degree of BMAT is not strictly related to BMI or body adipose tissue. To support these data, excess bone marrow is well described in patients with anorexia nervosa [72]. BMAT is considered a potential marker of compromised bone integrity and a major regulator of bone turnover with evidence showing an increased amount of bone marrow adipose tissue in osteoporosis, although it is unclear what is the cause and the consequence [73, 74••]. The hypothesis is that the higher amount of bone marrow fat may decrease bone mass in obesity due to aberrant differentiation of progenitor stem cells in the bone marrow. Indeed, osteoblasts and marrow adipocytes, come from the same progenitor cell, the mesenchymal stromal cell (MSC) ]. The ratio of bone marrow fat might increase with menopause, aging, and chronic renal failure, resulting in decreased bone density and enhanced fracture risk [76]. A high-fat diet might also increase BMAT in mice [77, 78]. In vitro 1,25(OH)2D treatment of pre-adipocytes in culture suppresses adipogenesis [79] and enhances MSC differentiation to osteoblasts [80]. In vivo, administration of 1,25(OH)2D in senescence-accelerated mice (SAM-P/6) was shown to inhibit adipogenesis and to accelerate differentiation of MSC into osteoblasts compared to placebo-treated animals [81]. This was accompanied by an increase in both cortical and trabecular bone strength [82].
The differentiation in osteoblast or adipocyte involves specific transcription factors (RUNX2 and Osterix for osteoblasts and PPARγ2 for adipocytes), and excess adipogenesis decreases bone formation [83,84,85]. Consequently, PPARγ suppression would result in bone formation and adipogenesis suppression [86]. Modified selection from the mesenchymal lineage to the adipogenic lineage rather than the osteogenic lineage might involve several mechanisms, such as oxidative stress, proinflammatory factors (TNF-α and IL-6) and adipokines [87, 88].
The only study that compared specific abdominal fat deposition, including lumbar bone marrow fat, between MHO/MUHO in 114 children showed no differences between the two groups [89]. Other studies showed a positive correlation between bone marrow fat and visceral fat [90,91,92]. Thus, MHO individuals, known to have lower visceral fat, might have lower bone marrow fat levels than MUHO individuals and, therefore, might be less at risk of osteoporosis.
Blood Biomarkers, Micronutrients, and Bone Mass
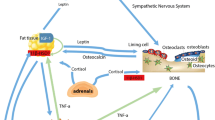
Adults with class III obesity are more at risk of having bone metabolism dysfunction, leading to an increase in bone turnover [93]. Consequently, even if there is limited literature on the bone metabolic changes in each phenotype, the detection of certain blood biomarkers and micronutrients specific to each metabolic profile would be a promising advance in the early screening of MHO/MUHO phenotypes and their bone risk fracture, in order to better target the indication for early BMD as well as therapeutic interventions (Fig. 1).
Bone regulatory factors associated in MHO versus MUHO: Metabolic healthy obesity is associated with lower insulin resistance (HOMA-IR), lower inflammatory markers (CRP, TNFα, IL-1, IL-6), lower leptin and higher adiponectin, potential higher estrogen and IGF1, higher vitamin D, calcium, phosphorus, osteocalcin, and lower alkaline phosphatase, parathyroid hormone, than metabolic unhealthy obesity. In addition, increased levels of marrow adiposity are observed in MUHO individuals (prepared using BioRender software)
Leptin directly and indirectly affects bone through central (hypothalamic) and peripheral pathways, possibly partially explaining the contradictory results reported in vivo and in vitro [94••]. In vitro, leptin activates the differentiation of MSC to osteoblasts, enhances osteoblast proliferation, and inhibits osteoclastogenesis through increasing OPG and the RANK/RANKL/OPG pathway [95, 96]. In vivo studies demonstrate that leptin has positive or negative effects on bone tissue, depending on its site and mode of action [97,98,99]. Human studies are limited and reported both positive [100, 101] and negative effects [102, 103] on bone mass. Furthermore, in the clinical field, an increased level of leptin in obese patients is usually associated with high bone mass, contrary to the decreased level seen in young women with anorexia nervosa and low BMD.
Adiponectin levels are inversely correlated with central and visceral fat volumes in MHO individuals. This has been demonstrated to stimulate both bone formation and bone resorption, but its action on bone mass is controversial [104,105,106,107,108]. In many studies, adiponectin is inversely correlated with bone mass, although its effects on bone are unclear. Nevertheless, obesity is an inflammatory state with the secretion of inflammatory mediators (CRP, IL-1, IL-6, and TNF-α), which might be inhibitors of adipokine expression, with a known negative impact on BMD [109]. A recent meta-analysis of the pooled correlations between adipokines and BMD included 47 studies and showed that leptin is correlated with BMD, adiponectin is inversely correlated with BMD, and patients with osteoporosis had lower leptin values and higher adiponectin values than patients with normal bone mass. Consequently, both adipokines might be promising blood biomarkers to predict bone fracture risk in obesity [110].
Multiple studies found an association between an increase in central adiposity, insulin resistance, and cardiometabolic disease with the menopause state [111,112,113]. Then, estrogen levels might be lower in MHO individuals than in MUHO individuals. Visceral obesity is associated with relative GH and IGF-1 deficiency [114]. IGF-1, a growth-promoting polypeptide, is an important determinant of cortical area in mice models [115]. Furthermore, it stimulates bone remodeling and has an anabolic effect on bone tissue. To support this finding, previous studies found a positive correlation between IGF-1 and bone mass and a negative correlation between IGF-1 and vertebral bone marrow fat in premenopausal women with or without obesity [62, 90]. These data could suggest a lower amount of IGF1 in MHO subjects.
Vitamin D plays a major role in mineral homeostasis through its essential ability to regulate calcium and phosphorus absorption, stimulating bone remodeling and modulating parathyroid hormone (PTH) and FGF-23 synthesis. Vitamin D insufficiency/deficiency is highly prevalent in the general population [116], and several studies have found a strong inverse correlation between weight and circulating vitamin D levels [117,118,119]. There are, however, substantial differences in the prevalence of vitamin D deficiency/insufficiency according to race/ethnic group, which is disproportionately more common in African Americans and Mexican Americans and in whom obesity is quite prevalent. Of particular interest was the finding that young Mexican American women with vitamin D insufficiency were significantly heavier and had 40% more subcutaneous and 80% more intra-abdominal fat than women with normal vitamin D levels [117]. It has been proposed that the association between obesity and vitamin D insufficiency/deficiency may be bidirectional through several potential pathophysiological mechanisms [120]. However, few studies have examined the effect of vitamin D supplementation on weight with diverging results. One study reported that high BMI might be associated with a modified response to vitamin D supplementation [121]. Another study by Ortega et al. found that baseline 25OHD levels predict the efficacy of the weight loss regimen and that the vitamin D status potentiates the effect of low caloric diet [122]. However, most studies found that in overweight and obese subjects, supplementation with vitamin D does not lead to a significant reduction in weight, percentage of fat mass, or change in fat distribution as evaluated by the waist-to-hip ratio [123,124,125]. Unfortunately, difficulties in controlling for the confounding effects of diet in vitamin D trials or weight reduction interventions have hindered the examination of the vitamin D/obesity link.
Moreover, since vitamin D is likely stored in body fat, simply increasing the vitamin D dosage may not be effective in overweight individuals. Nevertheless, overweight, or obese people tend to have secondary hyperparathyroidism and lower serum osteocalcin concentrations, which may, in part, be explained by low vitamin D levels leading to potential metabolic abnormalities [126, 127]. Significantly, vitamin D insufficiency/deficiency is also associated with the accumulation of muscle fat and reduction in muscle strength [128, 129], a known risk factor for falls and fractures. Overall, the combination of chronic vitamin D, calcium, and phosphorus deficiencies, particularly in obese people, enhances the risk of osteoporosis and fractures [130, 131].
Loureiro et al. [132] showed that the MHO profile in subjects with obesity class III does not protect against the abnormal secretion of bone biomarkers and micronutrients. However, among MUHO individuals, vitamin D levels are inversely correlated with BMI, and alkaline phosphatase, a marker of bone turnover regulated by vitamin D, is more concentrated. The lower vitamin D level could be partially explained by the higher abdominal fat responsible for vitamin D sequestration [133]. Moreover, there is an association between calcium, vitamin D and phosphorus in the MUHO phenotype. Thus, we can postulate that MUHO people with obesity class III have a higher risk of alterations to vitamin D, calcium, phosphorus, and PTH, promoting the development of bone disease. Sukumar et al. [134] showed that the MHO phenotype has higher serum concentrations of osteocalcin with lower serum PTH, and PTH positively correlates with body fat mass. Osteocalcin, a protein involved in bone mineralization, recently has been considered to regulate energy metabolism by increasing insulin secretion in β-cells and promoting insulin sensitivity.
In summary, early screening of these adipokines, hormones, and nutrient alterations, detected by simple blood tests, could help predict bone health in overweight or obese patients and lead to early and targeted management of bone disease.
Bone Structure and Fracture
Historically, obesity has been considered to protect against bone loss via mechanical loading under the influence of biomarkers secreted or regulated by adipocytes. Several studies tried to find an association between BMD and metabolic syndrome (MS) components, as seen in the MUHO profile, but the results have been discordant [135,136,137].
MHO profile is characterized by greater lean mass and lower fat mass, particularly visceral fat. Lean mass has been considered as the strongest predictor of BMD in premenopausal women. Yamaguchi et al. [138] found that VAT area measured by CT was positively correlated with BMD in a Japanese population with T2DM, but there was no longer a significant association after correction by BMI. The effect might be due to general obesity rather than visceral fat. Recent studies showed an inverse correlation between VAT and BMD, contrary to SAT, for which negative or no association was found [61, 139,140,141,142]
Currently, only a few studies have explored the specific bone phenotype in healthy/unhealthy obesity. Mirzababaei et al. [143••] found a potential link between BMD and metabolic healthy/unhealthy phenotypes among adult obese individuals. After adjusting for age, sex, and BMI, MUHO individuals had higher total BMD and positive correlation with hip BMD compared to MHO individuals. There was no statistical association with lumbar BMD. The BMI between the groups was the same, but fat percentage, fat spine, visceral fat, and trunk fat were higher in the MUHO group. Specific skeletal sites with local mechanical load on bone may explain the higher hip BMD seen in the MUHO group.
Wang et al. [144] described the relationship between metabolic obesity and forearm BMD in young, middle-aged Chinese people and found a lower forearm BMD in men with MUHO profile and women metabolically unhealthy but with normal weight than in the MHO group. They concluded that metabolic obesity might be a better predictor of bone health than BMI alone and showed that waist circumference, LDL-c concentration and insulin resistance might be negatively associated with bone health. Additionally, Ubago et al. [145] examined the association between areal BMD (aBMD) and metabolic obesity in overweight/obese children and the role of moderate-to-vigorous physical activity (MVPA) and cardiorespiratory fitness (CRF) in this association. A higher aBMD was found in TBLH (total body less head), trunk and pelvis in MHO compared to MUHO patients and was partially explained by MVPA and CRF. Confounding variables used were more reliable in this study (TBLH lean mass) than those used by Mirzababei (BMI) and could partially explain the different findings.
A meta-analysis of seven studies with 551,224 individuals conducted by Li et al. [146] found that waist circumference and waist-to-hip ratio positively correlate with an increased risk of hip fracture. Nguyen et al. [147] showed that the abdominal adipose tissue measured by DXA has a modest contribution to the risk of hip fracture in adults over 60 years old. However, this finding could be explained by the measure of abdominal obesity combining the sum of the differential effects of subcutaneous and visceral adipose tissues on bone health. Yamaguchi et al. [138], also evaluated the fracture risk and suggested a potential protective role of VAT on vertebral fractures in patients with T2DM.
Fracture risk may depend on BMD but also on applied loads experienced during activities (local higher body masses), explaining the fracture occurrence in specific skeletal sites in obese individuals [148]. A biomechanical study found a higher risk for low-trauma and compression fracture in the spine in obese patients with the same BMI but increased waist circumference, leading to increased local pressure on the spine [149]. Gandham et al. [150] found that obesity defined by BMI is associated with a lower risk of incident fracture mediated by higher BMD but associated with a higher risk if body fat percentage was used instead of BMI. MUHO profile is often associated with T2DM as part of metabolic syndrome. Evidence suggests that influence of advanced glycation end products (AGEs) on bone matrix, complications of diabetes and medication used (ex: thiazolidinediones) probably have a major impact on bone fragility and the increased fracture risk [151].
In summary, bone fracture risk, specifically in healthy/unhealthy obese individuals, has not been studied yet, but most available studies demonstrate a negative association between visceral adipose tissue or T2DM and BMD or fracture risk, suggesting that MUHO phenotype might be associated with a higher risk of osteoporosis, than MHO phenotype.
Intervention
In longitudinal and prospective studies, almost 50% of MHO patients progress to MUHO phenotypes within 10 years [152, 153]. Prevention and reversal of this transition should be considered in therapeutic management. Thus, the development of risk prediction tools is crucial. As seen above, several biomarkers could be helpful in characterizing the metabolic obesity profile and maybe predicting this transition (Fig. 1).
Lifestyle Intervention and Prevention of Osteoporosis
Guidelines recommend weight loss by lifestyle intervention first in all obese patients, without stratifying according to MHO and MUHO phenotypes. A low-fat, healthy diet with adequate dairy, calcium, and vitamin D intake, exercise, and smoking and alcohol intake cessation should be advised. Multiple studies suggested that weight loss is associated with loss of BMD in the hip and trabecular bone [154,155,156]. Other studies demonstrated that BMD is not decreased, and bone geometry is preserved with moderate weight loss (8 ± 4%) [157]. Conversely, a randomized clinical trial of 101 post-menopausal women with obesity showed that an intense energy restriction was associated with a higher loss of BMD in the hip BMD but not in the lumbar spine, compared to moderate energy restriction [158]. Clinical studies also showed a trend toward a reduction in BMAT with dietary-induced weight loss in obese patients [159••]. Given the potential role of hyperinsulinemia in converting MHO to MUHO, lower sugars and carbohydrate diet to avoid post-prandial insulin increments should also be suggested [160].
Regarding physical activity, studies showed that exercise training increases the probability of having MHO phenotype [161]. Resistance exercise programs, alone or combined with aerobic exercise programs, reduce frailty and attenuate bone mass and sarcopenia in the context of a weight loss program [162, 163]. In summary, a moderate weight loss program associated with resistance alone or with aerobic exercise training is currently the best lifestyle option advised in MHO to avoid the transition to MUHO and the harmful bone effects.
Treatment Options and Bone Health
In addition to its effect on appetite and food intake, evidence suggests that GLP-1 receptor agonists (GLP-1 RA) have positive effects on bone by promoting bone formation and inhibiting bone resorption [164]. Furthermore, several studies found an increase in BMD and a decreased risk of fracture in subjects with type 2 diabetes treated with GLP-1RA compared to placebo or other antihyperglycemic drugs [165, 166]. Another hormone secreted in the upper small intestine, a glucose-dependent insulinotropic peptide, GIP, when combined with GLP-1RA, named Tirzepatide, leads to significative weight loss and improvement of nonalcoholic steatohepatitis (NASH) biomarkers and fibrosis in patients with type 2 diabetes [167]. Overexpression of GIP has shown increased bone strength in experimental studies [168]. Consequently, GLP-1 RA and GIP, leading to the improvement of metabolic syndrome and potentially preventing progression to MUHO, could be beneficial treatments for bone health when osteoporosis is superimposed.
Bariatric surgery, such as Roux-en-Y gastric bypass surgery (RYGB), promotes sustained weight loss, with an improvement of metabolic profile and inflammatory profile, and could be, consequently, a benefic therapeutic option in obesity, including MHO [169]. Nevertheless, several effects on bone have been described in the literature: A decrease in BMD with a reduction of cortical load [170, 171], and an increase of bone turnover biomarkers, both resulting in increased risk of fracture, with increased incidence between 2 and 5 years after surgery, and mainly occurring in different sites than those associated with obesity [172, 173]. Interestingly, exercise could mitigate these negative bone effects [174].
Conclusion
Despite a higher BMD, accumulating data demonstrate a negative effect of obesity on bone tissue with a site-dependent fracture risk, secondary to increased mechanical loads on some bones, excess of visceral, ectopic (including hepatic adipose tissue) and BMAT, with a metabolic profile specific to each tissue, and secretion of inflammatory cytokines, adipokines, hormones and bone remodeling factors (Fig. 1). The MHO phenotype is characterized by a more favorable body composition, distribution (less visceral, ectopic and bone marrow fat), and function than MUHO phenotype, and thus, associated with less metabolism alterations.
Understanding the mechanisms underlying these different metabolic effects is paramount to help improve the indication for early bone densitometry, develop possible intervention targets against obesity and osteoporosis, and detect early metabolic complications, including bone disease. To date, the potential determinants of MHO are not clear, however, most studies found a positive or negative correlation with inflammatory markers (CRP, cytokines, adipokines), hormones (estrogen, IGF1), and bone turnover biomarkers (vitamin D, calcium, phosphorus, PTH, osteocalcin, alkaline phosphatase), which could be therefore detected in simple blood tests.
Few data comparing bone metabolism changes and healthy/unhealthy obesity are currently available, and comparability between the studies is difficult given the lack of a universal definition for metabolically healthy obesity. The main hypothesis is that MHO is defined by excess visceral fat and the absence of metabolic syndrome features, including T2DM, associated with a better bone structure and mass, and potentially a lower risk of fracture and osteoporosis than in unhealthy obese. However, other studies are needed to confirm this hypothesis. Patients with MHO still have an indication for lifestyle management (moderate weight loss and exercises) with or without medical or surgical treatments to avoid or delay the transition to the MUHO phenotype, which may lead to a higher risk of bone metabolism-related changes.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wang YC, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–25.
Okunogbe A, et al. Economic impacts of overweight and obesity: current and future estimates for eight countries. BMJ Glob Health. 2021;6(10):e006351.
Collaboration, N.C.D.R.F. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42.
Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 192 million participants. Lancet. 2016;387(10026):1377–96.
• Bluher M. Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–98. This paper summarizes evidence on the concept of metabolically healthy obesity.
Virani SS, et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation. 2020;141(9):e139–596.
Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41(3):bnaa004.
Ding C, Chan Z, Magkos F. Lean, but not healthy: the 'metabolically obese, normal-weight’ phenotype. Curr Opin Clin Nutr Metab Care. 2016;19(6):408–17.
• Wang B, et al. Prevalence of metabolically healthy obese and metabolically obese but normal weight in adults worldwide: A meta-analysis. Horm Metab Res. 2015;47(11):839–45. Summary of papers evaluating the specific characteristics of metabolically healthy obesity.
Loos RJF, Kilpelainen TO. Genes that make you fat, but keep you healthy. J Intern Med. 2018;284(5):450–63.
World Health Organization. Assessment of osteoporosis at the primary health care level. Summary report of a WHO scientific group. WHO, Geneva. (2007). www.who.int/chp/topics/rheumatic/en/index.html
Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33.
Obesity and Overweight. World Health Organization. Available at: www.who.int/mediacentre/factsheets/fs311/en/. Accessed 19 March 2020, 2016.
• Aung K, et al. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99(2):462–8. Report on the association between metabolically healthy obese individuals and osteoporotic fractures.
Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Pischon T, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–20.
American College of Cardiology/American Heart Association Task Force on Practice Guidelines, O.E.P. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring). 2014;22 Suppl 2:S5–39.
Britton KA, et al. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–5.
Yamaji T, et al. Visceral fat volume and the prevalence of colorectal adenoma. Am J Epidemiol. 2009;170(12):1502–11.
Shen W, Chen J. Application of imaging and other noninvasive techniques in determining adipose tissue mass. Methods Mol Biol. 2008;456:39–54.
Cheung AS, et al. Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. Int J Obes (Lond). 2016;40(8):1325–8.
• Vecchie A, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6–17. This paper describes how two types of obesity have different manifestations in the cardiovascular system.
Lee JH, et al. The role of adipose tissue mitochondria: Regulation of mitochondrial function for the treatment of metabolic diseases. Int J Mol Sci. 2019;20(19):4924.
Mittal B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J Med Res. 2019;149(5):571–3.
Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13.
Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404.
Zhang H, et al. Hepatic fat content is a determinant of metabolic phenotypes and increased carotid intima-media thickness in obese adults. Sci Rep. 2016;6:21894.
McMorrow AM, et al. Adipose tissue dysregulation and metabolic consequences in childhood and adolescent obesity: potential impact of dietary fat quality. Proc Nutr Soc. 2015;74(1):67–82.
Wildman RP, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168(15):1617–24.
van Vliet-Ostaptchouk JV, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9.
Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30(6):569–72.
Tsatsoulis A, Paschou SA. Metabolically healthy obesity: Criteria, epidemiology, controversies, and consequences. Curr Obes Rep. 2020;9(2):109–20.
Karelis AD, Rabasa-Lhoret R. Inclusion of C-reactive protein in the identification of metabolically healthy but obese (MHO) individuals. Diabetes Metab. 2008;34(2):183–4.
Li L, et al. Identification of genetic and environmental factors predicting metabolically healthy obesity in children: Data from the BCAMS study. J Clin Endocrinol Metab. 2016;101(4):1816–25.
•• Rask-Andersen M, Johansson A. Illuminating the “healthy obese” phenotype. Nat Metab. 2023;5(2):193–4. Presents evidence for the concept of "healthy obesity".
Phillips CM. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14(3):219–27.
Wentworth JM, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–56.
Fabbrini E, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145(2):366-74 e1-3.
Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98(10):E1610–9.
Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–53.
Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29(1):81–90.
Smekal A, Vaclavik J. Adipokines and cardiovascular disease: A comprehensive review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(1):31–40.
Aguilar-Salinas CA, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93(10):4075–9.
Christou KA, et al. The regulation of serum resistin levels in metabolically healthy and unhealthy obese individuals. Hormones (Athens). 2020;19(4):523–9.
Obradovic M, et al. Leptin and obesity: Role and clinical implication. Front Endocrinol (Lausanne). 2021;12: 585887.
Ghantous CM, et al. Differential role of leptin and adiponectin in cardiovascular system. Int J Endocrinol. 2015;2015: 534320.
Labruna G, et al. High leptin/adiponectin ratio and serum triglycerides are associated with an “at-risk” phenotype in young severely obese patients. Obesity (Silver Spring). 2011;19(7):1492–6.
Gomez-Ambrosi J, et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care. 2014;37(10):2813–21.
Jamar G, et al. Leptin as a cardiovascular risk marker in metabolically healthy obese: Hyperleptinemia in metabolically healthy obese. Appetite. 2017;108:477–82.
Kanis JA, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–41.
Scott D, Duque G, Ebeling PR. Does obesity reduce risk for osteoporosis and fractures in older adults? Intern Med J. 2018;48(1):104–5. https://doi.org/10.1111/imj.13655.
Cui LH, et al. Relative contribution of body composition to bone mineral density at different sites in men and women of South Korea. J Bone Miner Metab. 2007;25(3):165–71.
Lekamwasam S, et al. Association between bone mineral density, lean mass, and fat mass among healthy middle-aged premenopausal women: a cross-sectional study in southern Sri Lanka. J Bone Miner Metab. 2009;27(1):83–8.
Salamat MR, et al. Relationship between weight, body mass index, and bone mineral density in men referred for dual-energy X-ray absorptiometry scan in Isfahan. Iran J Osteoporos. 2013;2013: 205963.
De Laet C, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–8.
• Compston J. Obesity and fractures in postmenopausal women. Curr Opin Rheumatol. 2015;27(4):414–9. Overview of the association between obesity and fractures.
Felson DT, et al. Effects of weight and body mass index on bone mineral density in men and women: The Framingham study. J Bone Miner Res. 1993;8(5):567–73.
Berg RM, et al. Positive Association Between Adipose Tissue and Bone Stiffness. Calcif Tissue Int. 2015;97(1):40–9.
Compston JE, et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). J Bone Miner Res. 2014;29(2):487–93.
Pirro M, et al. High weight or body mass index increase the risk of vertebral fractures in postmenopausal osteoporotic women. J Bone Miner Metab. 2010;28(1):88–93.
Russell M, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95(3):1247–55.
Bredella MA, et al. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48(4):748–54.
Brumbaugh DE, et al. Intramyocellular lipid is associated with visceral adiposity, markers of insulin resistance, and cardiovascular risk in prepubertal children: The EPOCH study. J Clin Endocrinol Metab. 2012;97(7):E1099–105.
Ryan AS, et al. Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair. 2011;25(9):865–72.
Bredella MA, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97(4):E584–90.
Leeners B, et al. Ovarian hormones and obesity. Hum Reprod Update. 2017;23(3):300–21.
Khosla S. Minireview: The OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5.
Campos RM, et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: Effects of long-term interdisciplinary therapy. Endocrine. 2012;42(1):146–56.
Gkastaris K, et al. Obesity, osteoporosis and bone metabolism. J Musculoskelet Neuronal Interact. 2020;20(3):372–81.
de Paula FJ, Rosen CJ. Bone remodeling and energy metabolism: new perspectives. Bone Res. 2013;1(1):72–84.
Krings A, et al. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–52.
Bredella MA, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36.
Yeung DK, et al. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22(2):279–85.
•• Rosen CJ, Bouxsein ML. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. Presents current evidence on the role of marrow fat in osteoporosis.
Ko DS, et al. Altered physiology of mesenchymal stem cells in the pathogenesis of adolescent idiopathic scoliosis. World J Clin Cases. 2020;8(11):2102–10.
Moorthi RN, et al. Bone marrow fat is increased in chronic kidney disease by magnetic resonance spectroscopy. Osteoporos Int. 2015;26(6):1801–7.
Halade GV, et al. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21(12):1162–9.
Shu L, et al. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif Tissue Int. 2015;96(4):313–23.
Mutt SJ, et al. Vitamin D and adipose tissue-more than storage. Front Physiol. 2014;5:228.
Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151(1):14–22.
Duque G, Macoritto M, Kremer R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARgamma2). Exp Gerontol. 2004;39(3):333–8.
Duque G, et al. 1,25(OH)2D3 acts as a bone-forming agent in the hormone-independent senescence-accelerated mouse (SAM-P/6). Am J Physiol Endocrinol Metab. 2005;288(4):E723–30.
Shockley KR, et al. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106(2):232–46.
Ichida F, et al. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004;279(32):34015–22.
Ilich JZ, et al. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev. 2014;15:51–60.
Kang S, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(19):14515–24.
Horowitz MC, et al. Bone marrow adipocytes. Adipocyte. 2017;6(3):193–204.
Singh L, et al. Good, bad, or ugly: the biological roles of bone marrow fat. Curr Osteoporos Rep. 2018;16(2):130–7.
Cadenas-Sanchez C, et al. Differences in specific abdominal fat depots between metabolically healthy and unhealthy children with overweight/obesity: the role of cardiorespiratory fitness. Scand J Med Sci Sports. 2023;33(8):1462–72.
Bredella MA, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring). 2011;19(1):49–53.
Shen W, et al. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18(5):641–7.
Bredella MA, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97(11):4115–22.
Fischer V, et al. Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci Rep. 2017;7(1):7223.
•• Reid IR, Baldock PA, Cornish J. Effects of leptin on the skeleton. Endocr Rev. 2018;39(6):938–59. Presents mechanistic evidence for the impact of leptin on bone.
Cornish J, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405–15.
Thomas T, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–8.
Bjornholm M, et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117(5):1354–60.
Shi Y, et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105(51):20529–33.
Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207.
Pasco JA, et al. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab. 2001;86(5):1884–7.
Yamauchi M, et al. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf). 2001;55(3):341–7.
Ruhl CE, Everhart JE. Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res. 2002;17(10):1896–903.
Odabasi E, et al. Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol. 2000;142(2):170–3.
Jurimae J, Jurimae T. Adiponectin is a predictor of bone mineral density in middle-aged premenopausal women. Osteoporos Int. 2007;18(9):1253–9.
Jurimae J, et al. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26(6):618–23.
Zoico E, et al. Relation between adiponectin and bone mineral density in elderly post-menopausal women: role of body composition, leptin, insulin resistance, and dehydroepiandrosterone sulfate. J Endocrinol Invest. 2008;31(4):297–302.
Basurto L, et al. Adiponectin is associated with low bone mineral density in elderly men. Eur J Endocrinol. 2009;160(2):289–93.
Kontogianni MD, et al. Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J Bone Miner Res. 2004;19(4):546–51.
Fasshauer M, et al. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290(3):1084–9.
Lee S, et al. Effect of adipokine and ghrelin levels on BMD and fracture risk: an updated systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1044039.
Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–11.
Bailey CJ, Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion Diabetologia. 1980;19(5):475–81.
Suchacki KJ, Thomas BJ, Ikushima YM, Chen KC, Fyfe C, Tavares AAS, Sulston RJ, Lovdel A, Woodward HJ, Han X, Mattiucci D, Brain EJ, Alcaide-Corral CJ, Kobayashi H, Gray GA, Whitfield PD, Stimson RH, Morton NM, Johnstone AM, Cawthorn WP. The effects of caloric restriction on adipose tissue and metabolic health are sex- and age-dependent. Elife. 2023;12:e88080.
Miller KK, et al. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab. 2005;90(2):768–74.
Yakar S, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771–81.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Kremer R, et al. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009;94(1):67–73.
Arunabh S, et al. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88(1):157–61.
Parikh SJ, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–9.
Karampela I, et al. Vitamin D and obesity: current evidence and controversies. Curr Obes Rep. 2021;10(2):162–80.
Manson JE, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44.
Ortega RM, et al. Preliminary data about the influence of vitamin D status on the loss of body fat in young overweight/obese women following two types of hypocaloric diet. Br J Nutr. 2008;100(2):269–72.
Salehpour A, et al. A 12-week double-blind randomized clinical trial of vitamin D(3) supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78.
Wamberg L, et al. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels - results from a randomized trial. Eur J Intern Med. 2013;24(7):644–9.
Zhou J, et al. The effect of calcium and vitamin D supplementation on obesity in postmenopausal women: secondary analysis for a large-scale, placebo controlled, double-blind, 4-year longitudinal clinical trial. Nutr Metab (Lond). 2010;7:62.
Bolland MJ, et al. Testosterone levels following decreases in serum osteocalcin. Calcif Tissue Int. 2013;93(2):133–6.
Sukumar D, Shapses SA, Schneider SH. Vitamin D supplementation during short-term caloric restriction in healthy overweight/obese older women: effect on glycemic indices and serum osteocalcin levels. Mol Cell Endocrinol. 2015;410:73–7.
Gilsanz V, et al. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95(4):1595–601.
Li J, et al. Vitamin D prevents lipid accumulation in murine muscle through regulation of PPARgamma and perilipin-2 expression. J Steroid Biochem Mol Biol. 2018;177:116–24.
Shimizu Y, et al. Serum 25-hydroxyvitamin D level and risk of falls in Japanese community-dwelling elderly women: a 1-year follow-up study. Osteoporos Int. 2015;26(8):2185–92.
Aung K. Review: in postmenopausal women and older men, vitamin D plus calcium reduces some fractures. Ann Intern Med. 2014;161(6):JC5.
Marques Loureiro L, et al. Does the metabolically healthy obese phenotype protect adults with class III obesity from biochemical alterations related to bone metabolism? Nutrients. 2019;11(9):2125.
Wortsman J, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3.
Sukumar D, et al. Can bone-regulating hormones and nutrients help characterize the metabolically healthy obese phenotype. Nutr Health. 2018;24(3):153–62.
Wung CH, et al. Associations between metabolic syndrome and obesity-related indices and bone mineral density T-score in hemodialysis patients. J Pers Med. 2021;11(8):775.
Starup-Linde J, et al. Bone density and structure in overweight men with and without diabetes. Front Endocrinol (Lausanne). 2022;13: 837084.
Nobrega da Silva V, et al. Impact of metabolic syndrome and its components on bone remodeling in adolescents. PLoS One. 2021;16(7):e0253892.
Yamaguchi T, et al. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone. 2009;45(2):174–9.
Gilsanz V, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–93.
Janicka A, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92(1):143–7.
Choi HS, et al. Relationship between visceral adiposity and bone mineral density in Korean adults. Calcif Tissue Int. 2010;87(3):218–25.
Pollock NK, et al. Lower bone mass in prepubertal overweight children with prediabetes. J Bone Miner Res. 2010;25(12):2760–9.
•• Mirzababaei A, et al. Metabolically healthy/unhealthy components may modify bone mineral density in obese people. Arch Osteoporos. 2017;12(1):95. Presents the main components linking healthy and unhealthy obesity with bone.
Wang Y, et al. Association between forearm bone mineral density and metabolic obesity in a northern Chinese population. Metab Syndr Relat Disord. 2020;18(5):251–9.
Ubago-Guisado E, et al. Differences in areal bone mineral density between metabolically healthy and unhealthy overweight/obese children: the role of physical activity and cardiorespiratory fitness. Pediatr Res. 2020;87(7):1219–25.
Li X, Gong X, Jiang W. Abdominal obesity and risk of hip fracture: A meta-analysis of prospective studies. Osteoporos Int. 2017;28(10):2747–57.
Nguyen ND, et al. Abdominal fat and hip fracture risk in the elderly: The Dubbo Osteoporosis Epidemiology Study. BMC Musculoskelet Disord. 2005;6:11.
Bachmann KN, et al. Vertebral strength and estimated fracture risk across the BMI spectrum in women. J Bone Miner Res. 2016;31(2):281–8.
Ghezelbash F, et al. Obesity and obesity shape markedly influence spine biomechanics: a subject-specific risk assessment model. Ann Biomed Eng. 2017;45(10):2373–82.
Gandham A, et al. Incidence and predictors of fractures in older adults with and without obesity defined by body mass index versus body fat percentage. Bone. 2020;140: 115546.
Walsh JS, Vilaca T. Obesity, type 2 diabetes and bone in adults. Calcif Tissue Int. 2017;100(5):528–35.
Hamer M, et al. Stability of metabolically healthy obesity over 8 years: The English Longitudinal Study of Ageing. Eur J Endocrinol. 2015;173(5):703–8.
Schroder H, et al. Determinants of the transition from a cardiometabolic normal to abnormal overweight/obese phenotype in a Spanish population. Eur J Nutr. 2014;53(6):1345–53.
Langlois JA, et al. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: The NHANES I epidemiologic follow-up study. Osteoporos Int. 2001;12(9):763–8.
Ensrud KE, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90(4):1998–2004.
Bleicher K, et al. The role of fat and lean mass in bone loss in older men: findings from the CHAMP study. Bone. 2011;49(6):1299–305.
Pop LC, et al. Moderate weight loss in obese and overweight men preserves bone quality. Am J Clin Nutr. 2015;101(3):659–67.
Seimon RV, et al. Effect of weight loss via severe vs moderate energy restriction on lean mass and body composition among postmenopausal women with obesity: the TEMPO Diet randomized clinical trial. JAMA Netw Open. 2019;2(10): e1913733.
•• Tencerova M, Duque G, Beekman KM, Corsi A, Geurts J, Bisschop PH, Paccou J. The impact of interventional weight loss on bone marrow adipose tissue in people living with obesity and its connection to bone metabolism. Nutrients. 2023;15(21):4601. Comprehensive review on the impact of weight loss on marrow fat.
Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab Vasc Dis Res. 2019;16(2):118–27.
Velho S, et al. Metabolically healthy obesity: Different prevalences using different criteria. Eur J Clin Nutr. 2010;64(10):1043–51.
Shah K, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26(12):2851–9.
Emerenziani GP, et al. Effects of body weight loss program on parameters of muscle performance in female obese adults. J Sports Med Phys Fitness. 2019;59(4):624–31.
Xie B, et al. The impact of glucagon-like peptide 1 receptor agonists on bone metabolism and its possible mechanisms in osteoporosis treatment. Front Pharmacol. 2021;12: 697442.
Cai TT, et al. Effects of GLP-1 receptor agonists on bone mineral density in patients with type 2 diabetes mellitus: a 52-week clinical study. Biomed Res Int. 2021;2021:3361309.
Kong QX, et al. Evaluation of the risk of fracture in type 2 diabetes mellitus patients with incretins: an updated meta-analysis. Endokrynol Pol. 2021;72(4):319–28.
Hartman ML, et al. Effects of novel dual gip and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1352–5.
Ding KH, et al. Impact of glucose-dependent insulinotropic peptide on age-induced bone loss. J Bone Miner Res. 2008;23(4):536–43.
Felipe LA, et al. Effects of Roux-en-Y gastric bypass on the metabolic profile and systemic inflammatory status of women with metabolic syndrome: randomized controlled clinical trial. Diabetol Metab Syndr. 2023;15(1):19.
Yu EW, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100(4):1452–9.
Mele C, et al. Bone response to weight loss following bariatric surgery. Front Endocrinol (Lausanne). 2022;13: 921353.
Rousseau C, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354: i3794.
Yu EW, et al. Fracture risk after bariatric surgery: Roux-en-Y gastric bypass versus adjustable gastric banding. J Bone Miner Res. 2017;32(6):1229–36.
Murai IH, et al. Exercise mitigates bone loss in women with severe obesity after Roux-en-Y gastric bypass: a randomized controlled trial. J Clin Endocrinol Metab. 2019;104(10):4639–50.
Author information
Authors and Affiliations
Contributions
E.G., R.K., and G.D. wrote the main manuscript text and E.G. and G.D. prepared figure 1. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
EG, RK, and GD have no conflicts of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Approval
This review article does not present any previously unpublished original research, and ethical approval is therefore not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gruneisen, E., Kremer, R. & Duque, G. Fat as a Friend or Foe of the Bone. Curr Osteoporos Rep 22, 245–256 (2024). https://doi.org/10.1007/s11914-024-00864-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-024-00864-4