Abstract
Purpose
There is limited prospective evidence at population scale of the impacts of lifestyle and surrogate measures of general and abdominal adiposity on the transition of a metabolically healthy (absence of a metabolic disorder) overweight/obese (MHOO) phenotype to a metabolically abnormal overweight/obese (MAOO) phenotype. Therefore, we determined the relationship between 10-year body mass index (BMI), waist circumferences (WC), waist to height ratio (WHtR), and lifestyle changes and the transition of the MHOO phenotype.
Methods
We conducted a prospective population-based study of 3,052 male and female Spaniards aged 25–74 years who were followed from 2000 through 2009. Diet and leisure-time physical activity were recorded on validated questionnaires. Weight, height, WC, blood lipids, glycemia, and blood pressure were measured. All variables were obtained at baseline (BL) and follow-up (FL). Participants with a BMI ≥ 25 kg/m2 and free from hypercholesterolemia, hypertriglyceridemia, diabetes, hypertension, and low HDL and high LDL cholesterol levels were characterized as the MHOO phenotype. A composite healthy lifestyle index (HLI) was constructed by including temporary changes in 3 lifestyle variables (diet, leisure-time physical activity, and smoking).
Results
Initially, 20.8 % of subjects had the MHOO phenotype; 49.2 % of these shifted to MAOO phenotype. In multivariate analysis, changes in BMI, WC, WHtR were positively associated (p = 0.004, p = 0.018, and p = 0.016, respectively) with this transition. One unit increase in the HLI was associated with a 33 % lower risk (p = 0.025) to the MAOO phenotype transition after adjusting for age, sex, educational level, and baseline energy intake, BMI, WC, and WHtR.
Conclusions
The presence of metabolic disorders in the MHOO phenotype is predicted by an increase in anthropometric surrogate measures of general and abdominal adiposity. In contrast, a healthy lifestyle protects against a transition to the MAOO phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excessive weight, defined as a body mass index (BMI) ≥ 25 (weight in kilograms divided by the square of the height in meters), is associated with an increased risk of metabolic complications such as dyslipidemia, hypertension, and diabetes [1–3]. However, not all overweight and obese subjects present an at-risk metabolic profile. Indeed, recent evidence suggests that a considerable number of overweight and obese subjects are free of metabolic complications [4, 5]. The prevalence of this metabolically healthy obese/overweight (MHOO) phenotype varied between 18 and 44 % [4]. Little is known about the magnitude of the prospective manifestation of cardiometabolic complications in the MHOO phenotype [4–8]. Furthermore, it is unknown whether weight or WC gain or both are predictors for a shift from the MHOO to the MAOO phenotype.
A healthy lifestyle is associated with a favorable cardiometabolic risk profile [9–12]. Therefore, it is likely that a healthy diet, not smoking, and/or physical activity counterbalance the adverse effects of excessive weight. So far, evidence on the impact of behavioral factors on the MHOO phenotype is scarce [13–16].
We undertook this study with the aim to determine 10-year changes in anthropometric variables and lifestyle associated with the transition of the MHOO to MAOO phenotype in a representative Spanish population.
Subjects and methods
Study population
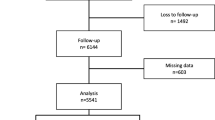
Data were obtained from a population-based survey conducted in Girona (Spain) in 2000 and 2009. The baseline survey in 2000 examined a random population-based sample of 3058 men and women aged 25–74 years (participation rate: 71.0 %). In 2009, the 2,715 noninstitutionalized participants still residing in the catchment area were invited for re-examination and 2,181 attended (participation rate of follow-up: 80.3 %). For the purpose of the present study, participants with a baseline BMI ≥ 25.0 were included for cross-sectional analysis (n = 1,445). Prospective analysis was performed with the baseline MHOO phenotype group (n = 301). All measurements were performed at baseline and at follow-up. Participants were duly informed and signed their consent to participate in the study. The project was approved by the local ethics committee (CEIC—PSMAR, Barcelona, Spain).
Definition of the MHOO phenotype
In the present study, we were interested in the predictive value of lifestyle on the maintenance of metabolic health in subjects with abnormal fat accumulation. The WHO had defined overweight and obesity as “abnormal or excessive fat accumulation that presents a risk to health” [17]. Therefore, we decided to combine the two categories characterized by excessive accumulation of fat in adipose tissue in our analysis. The outcome of interest was the transition of the MHOO to the MAOO phenotype. For this purpose, we defined the MHOO phenotype as participants with a BMI ≥ 25.0 and free from the following common cardiometabolic risk factors: hypercholesterolemia, hypertriglyceridemia, diabetes, hypertension, and low HDL and high LDL cholesterol levels as defined in laboratory measurements. In contrast, the presence of one or more of these conditions defined the MAOO phenotype.
Laboratory measurements
Blood was withdrawn after 10–14 h fasting. Serum-sample aliquots were stored at −80 °C. Glycemia was measured in an aliquot of serum. Total cholesterol and triglyceride concentrations were determined enzymatically (Roche Diagnostics, Basel, Switzerland). High-density lipoprotein (HDL) cholesterol was measured after precipitation of apoprotein B-containing lipoproteins with phosphotungstic-Mg++ (Boehringher, Mannheim, Germany). Analyses were performed in a Cobas Mira Plus autoanalyzer (Roche Diagnostics, Basel, Switzerland). Quality control was performed with External Quality Assessment-WHO Lipid Program (World Health Organization, Prague, Czech Republic) and Monitrol-Quality Control Program (Baxter Diagnostics, Dudingen, Switzerland). Low-density lipoprotein (LDL) cholesterol was calculated by the Friedewald equation whenever triglycerides were <300 mg/dL. Cut-points for abnormal lipid levels were total cholesterol ≥ 240 mg/dL, LDL ≥ 160 mg/dL, triglycerides ≥ 200 mg/dL, and HDL ≤ 40 mg/dL for men and HDL ≤ 50 mg/dL for women [18]. Diabetes was defined as use of insulin or hypoglycemic agents or fasting blood glucose >125 mg/dL or previous diagnosis of diabetes by a physician [19].
Blood pressure measurement
Blood pressure was measured with a periodically calibrated mercury sphygmomanometer. A cuff adapted to upper arm perimeter (young, adult, and obese) was selected for each participant. Measurements were performed after a 5-minute rest. Two measurements were taken, and the lower value was recorded for the study. Participants were considered hypertensive if previously diagnosed by a physician, under treatment, or presenting systolic blood pressure (SBP) ≥ 140 or diastolic blood pressure (DBP) ≥ 90 mmHg [18].
Anthropometrics
A calibrated precision scale was used for weight measurement. Readings were rounded up to 200 g. Height was measured in the standing position and rounded up to the nearest 0.5 cm. Weight was divided by height squared (kg/m2) to establish the BMI.
Measurement of waist circumference was performed midway between the lowest rib and the iliac crest in the horizontal positions. The measurement, taken in cm, was rounded to the nearest 0.5 cm.
Dietary assessment
Food consumption was determined using a validated [20, 21] food frequency questionnaire (FFQ) administered by a trained interviewer at baseline and at follow-up. In a 166-item food list including alcoholic and nonalcoholic beverages, participants indicated their usual consumption and chose from 10 frequency categories ranging from never or less than once per month to 6 or more times per day.
The Mediterranean-like dietary score (MLDS) was calculated according to the tertile distribution of energy-adjusted food intake [21]. For cereals, fruits, vegetables, legumes, fish, olive oil, low-fat dairy products, and nuts, the lowest tertile is coded as 1, medium as 2, and highest as 3. For meat (including red meat and sausages), sugar-sweetened carbonated beverages and added sugars, pastries, and fast food, the score was inverted, with the highest tertile coded as 1 and lowest as 3.
Other variables
Leisure-time physical activity (LTPA) was measured by the validated Minnesota LTPA questionnaire administered by a trained interviewer [22, 23]. Measurements of smoking habits and demographic and socioeconomic variables were obtained from structured standard questionnaires administered by trained personnel. Participants were categorized as nonsmokers (never smokers and ex-smokers with more than 1 year of smoking cessation) and current smokers. Maximum education level attained was elicited and recorded for analysis as primary school versus secondary school or university.
Lifestyle changes
We hypothesized that changes or maintenance of several lifestyle variables together will have an additive effect on the transition away from the MHOO phenotype. For this analysis, we created a joint variable composed of secular trends of diet quality, LTPA, and smoking. Unhealthy behaviors were coded 0 and defined as follows: low LTPA (below median) at baseline and follow-up; low adherence to the MLDS (below median) at baseline and follow-up; smoking at baseline and follow-up; high LTPA at baseline and low LTPA at follow-up; high adherence to the MLDS and low adherence to the MLDS at follow-up. Healthy behaviors were coded 1 and defined as follows: high LTPA (above median) at baseline and follow-up; high adherence to the MLDS (above median) at baseline and follow-up; nonsmoking at baseline and follow-up; low LTPA at baseline and high LTPA at follow-up; low adherence to the MLDS and high adherence to the MLDS at follow-up; smoking at baseline and cessation of smoking at follow-up.
The sum of scores of the dichotomized lifestyle variables LTPA, smoking, and diet quality yielded healthy lifestyle index (HLI) scores ranging from 0 to 7.
Statistical analysis
Differences in continuous variables were compared using the Student t test. The chi-square test was used for categorical variables. Multiple logistic regression models were fitted to determine the confounder-controlled association of metabolic phenotypes and changes in BMI, WC, WHtR, and lifestyle. Additionally, we compared the magnitude of the associations between metabolic phenotypes and changes in these anthropometric measures. For this purpose, anthropometric variables were standardized as z values. To explore effect modification according to sex, lifestyle, and baseline weight and WC, we modeled interaction terms for sex/weight change, sex/WC change, sex/HLI index, sex/BMI change, and sex/WHtR change. Differences were considered significant if p < 0.05. Statistical analysis was performed using SPSS version 18.0. (SPSS Inc. Chicago, IL).
Results
The prevalence of the MHOO phenotype was 20.8 (24.2 % in overweight and 16.1 % in obese participants) at baseline. At 10-year follow-up, 49.3 % of the initial MHOO phenotype had transitioned to the MAOO phenotype (48.4 % in overweight and 57.0 % in obese participants).
No significant interactions between sex/weight change, sex/WC change, sex/HLI index, sex/BMI change, and sex/WHtR change were observed.
At baseline, the MHOO phenotype group (n = 301) was younger, more highly educated, smoke more, adhered to a less healthy diet, and had higher energy intake, lower BMI, lower WC, and lower WHtR than the MAOO phenotype group (n = 1,144) (Table 1). Participants who remained MHOO after 10 years of follow-up (n = 153) were younger and had a higher energy intake at baseline compared with those who became MAOO (n = 148) (Table 1). At follow-up, higher WC and WHtR were found in participants who became MAOO. Blood lipids, fasting glucose, and systolic and diastolic blood pressures were significantly different between the MHOO and MAOO phenotype at all measurement points with the exception of follow-up diastolic blood pressure of participants who remained MHOO and those who became MAOO after 10 years of follow-up (Table 1). The incidence rate of diabetes in the initial MHOO phenotype was 9.3 %.
At follow-up, 16.4 % (n = 187) of the metabolically abnormal healthy overweight/obese phenotype [19.4 % overweight (n = 137) and 11.5 % obese (n = 50)] at baseline had transitioned to the MHOO phenotype. This phenotype was significantly younger, with lower BMI and WC at baseline than the stable MAOO phenotype. Stratified analysis by overweight and obesity categories revealed the same results.
A central question of the present study was to determine the effect of changes in BMI, WHtR, WC, and lifestyle variables on the shift from the MHOO to MAOO phenotype. For this purpose, we focused our analysis on participants who presented the MHOO phenotype at baseline. An increase in BMI, WC, and WHtR was a positive predictor for the transition to the MAOO phenotype in sex- and age-adjusted models (Table 2). Further adjusting for the HLI, educational level, alcohol consumption, and energy intake at baseline did not meaningfully affect the direction and magnitude of the associations. The magnitude of the associations was similar among anthropometric measures.
An increase in 1 unit of the HLI score was associated with a 33.0 % lower risk of transition to the MAOO phenotype (Table 3). This association was independent of baseline values of educational level, alcohol consumption, smoking, energy intake, BMI, WC, and WHtR (Table 3). None of the possible combinations of lifestyle changes, with the exception of the concomitant presence of all three healthy lifestyle changes, were significantly associated with the transition of the MHOO to the MAOO phenotype (Table 4).
Age- and sex-adjusted linear regression models, stratified by the overweight and obese phenotypes, revealed that a 1 point increase in the HLI was associated with a 39 % [OR 0.61 (CI 0.39;0.94), p = 0.026] and 18 % [OR 0.82 (CI 0.46;1.44), p = 0.494] decrease in risk in the transition from the healthy to the unhealthy metabolically overweight and obese phenotype, respectively.
Changes in BMI, WC, and WHtR were predictive, albeit not significant in all cases, for the transition from the healthy to the unhealthy metabolically overweight [∆BMI = OR 1.35 (CI 1.11;1.47), p = 0.002; ∆WC = OR 1.05 (CI 1.00; 1.10), p = 0.066; WHtR = OR 2.53 (CI 1.10; 5.83), p = 0.029] and obese phenotype [∆BMI = OR 1.05 (CI 0.90;1.23), p = 0.525; ∆WC = OR 1.05 (CI 0.99;1.12), p = 0.11; WHtR = 2.47 (CI 0.87;7.00), p = 0.089]. All models were adjusted for sex, age, and the corresponding anthropometric variable at baseline.
Discussion
An increase in surrogate measures of general and abdominal adiposity was positively associated with the shift from the MHOO to the MAOO phenotype. These associations were not independent of weight gain. In contrast, a high HLI score was negatively associated with this transition.
The prevalence of the MHOO phenotype was 20.8 % in this overweight and obese population, which is considerably less than that reported by another large population-based study [24]. However, this finding is not surprising because prevalence rates of the MHOO phenotype vary greatly between studies, mainly due to the lack of a unique definition of the phenotype [4, 13]. Additionally, most of the studies included small, nonrepresentative populations, making it difficult to compare results.
It has been shown that the MHOO phenotype is more prevalent in younger people, [24, 25] which is in line with results from this study. Moreover, our prospective analysis revealed that a shift of the MHOO phenotype is more probable in older age groups. Of course, this finding is not surprising if we consider the strong positive association between age and cardiometabolic risk [26]. On the other hand, little is known about socioeconomic and lifestyle determinants of the MHOO phenotype. Wildman et al. [24] reported a higher degree of physical activity and a higher educational level in the MHOO phenotype. However, this finding was not confirmed by other authors [27]. Obese black women with the metabolically healthy obese (MHO) phenotype were more educated and had lower unemployment than their metabolically unhealthy obese peers [27]. The MHOO phenotype in the present study was characterized by a higher educational level, a higher smoking prevalence, and a less healthy diet. However, these associations were strongly age dependent. Furthermore, none of these variables significantly predicted the transition from the MHOO to MAOO phenotype. This finding is in line with a recently published report showing no significant prospective association between baseline physical activity, alcohol consumption, or smoking and the transition to the metabolically unhealthy obese phenotype [16]. A few longitudinal studies have addressed the impact on health outcomes of obesity without metabolic complications [5–8, 16]. The evidence from these studies is inconsistent; therefore, whether healthy obese individuals are at lower risk of degenerative changes remains an open question. A recent publication by Appleton et al. [16] was the first to report on the association between the stability of the MHO phenotype and incidence of diabetes and cardiovascular disease (CVD). They found that the risk of developing diabetes, but not CVD, was significantly higher for the transition of the MHO phenotype to the metabolically unhealthy obese phenotype compared with the stable or improved MHO. It is important to note that controlling for cardiovascular risk factors strongly attenuates the impact of overweight and obesity on cardiovascular events [28].
A key question is whether the MHOO phenotype is time-related or represents a constant characteristic. In this study, nearly half of the initially MHOO subjects shifted to the MAOO phenotype after 10 years. Recently published data show a positive association between WC and the MHOO phenotype at population scale [25]. Indeed, WC increase, and weight gain has been associated with the presentation of cardiometabolic abnormalities [29–31]. Therefore, it is reasonable to assume that gaining weight and increasing WC will increase the risk of a shift from the MHOO to the MAOO phenotype. Our results showed that an increase in 1 standard deviation of BMI, WC, and WHtR increased the risk of the transition to the MAOO phenotype by 49, 41, and 43 %, respectively. This increase in general and abdominal adiposity predicted the transition to the MAOO phenotype independently of lifestyle changes.
Both maintaining a healthy lifestyle and making healthful lifestyle changes have been associated with a favorable cardiometabolic profile [9–12]. In this study, the adherence to a healthy diet and not smoking were nonsignificant predictors for the stability of the MHOO phenotype. The strongest although not significant association was observed for diet quality measured by the MLDS. A large body of literature demonstrates protective effects of the Mediterranean diet on cardiovascular health in different populations [32–35]. An interesting finding of this study is the additive effect of healthy lifestyle factors on cardiovascular risk factors in subjects at cardiometabolic risk. A previous report from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam cohort demonstrated progressive decline of the incidence of chronic disease as the number of healthy lifestyle factors increased [9], in line with findings from other cohort studies indicating a favorable and additive effect of lifestyle variables on disease outcomes [10–12]. Kantartzis et al. [36] reported a significant increase in insulin sensitivity in obese insulin resistant but not in MHO patients after a 9 months lifestyle intervention program. However, follow-up insulin sensitivity was nearly twice as high in the MHO group compared with insulin-resistant obese group. In the present study, we found an independent association of anthropometric measures of adiposity and lifestyle changes on the transition of the MHOO phenotype. This finding indicates that anthropometric and lifestyle changes are linked to this transition by different pathophysiological pathways.
The underlying mechanisms linked to the preservation of a favorable cardiometabolic profile in at-risk individuals are poorly understood. It has been shown that visceral fat mass accumulation is strongly linked with a cardiometabolic risk profile. Furthermore, excessive fat mass is associated with a chronic inflammatory state and rise in oxidative stress, which are in turn mediators for the development of cardiometabolic disorders [37, 38]. However, not all individuals with elevated fat mass suffer from a chronic inflammatory state and a rise in oxidative stress [39, 40]. In this context, it is of interest to note that intervention and observational studies provide evidence for a protective role of diet and physical activity on inflammation and oxidative stress [41–43]. The impact of ectopic fat for the metabolic status of the obese has been highlighted by Stefan and colleagues [44]. They found that lower ectopic fat accumulation in the liver and skeletal muscle is predictive for the MHO phenotype.
The strengths of this study are the population-based design and the measurement of anthropometric, cardiovascular risk, and lifestyle variables at baseline and at 10-year follow-up. Furthermore, dietary and LTPA data were recorded using validated questionnaires. One limitation of this study is the relatively modest sample size that makes it impossible to perform stratified analysis by BMI class.
In conclusion, our results indicate that an increase in anthropometric measures of adiposity heightens the risk of transition from the MHOO phenotype to the MAOO phenotype. In contrast, a healthy lifestyle increases the probability of maintaining a favorable cardiometabolic profile and thus counterbalances the risk of a transition away from the MHOO phenotype.
References
Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89:2595–2600
Dorresteijn JA, Visseren FL, Spiering W (2012) Mechanisms linking obesity to hypertension. Obes Rev 13:17–26
Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H (2006) Body mass index history and risk of type 2 diabetes: results from the European prospective investigation into cancer and nutrition (EPIC)-potsdam study. Am J Clin Nutr 84:427–433
Primeau V, Coderre L, Karelis AD, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R (2011) Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 35:971–981
Stefan N, Häring HU, Hu FB, Schulze MB (2013) Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 1:152–162
Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D’Agostino RB (2006) Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 91:2906–2912
Chang Y, Ryu S, Suh BS, Yun KE, Kim CW, Cho SI (2012) Impact of BMI on the incidence of metabolic abnormalities in metabolically healthy men. Int J Obes (Lond) 36:1187–1194
Kuk JL, Ardern CI (2009) Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 32:2297–2299
Ford ES, Bergmann MM, Kröger J, Schienkiewitz A, Weikert C, Boeing H (2009) Healthy living is the best revenge: findings from the European prospective investigation into cancer and nutrition-potsdam study. Arch Intern Med 169:1355–1362
Chiuve SE, McCullough ML, Sacks FM, Rimm EB (2006) Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation 114:160–167
Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N (2008) Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med 5:e12
Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR (2010) Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med 170:711–718
Pataky Z, Makoundou V, Nilsson P, Gabriel RS, Lalic K, Muscelli I, Casolaro A, Golay A, Bobbioni-Harsch E (2011) Metabolic normality in overweight and obese subjects. Which parameters? Which risks? Int J Obes (Lond) 35:1208–1215
Wildman RP (2009) Healthy obesity. Curr Opin Clin Nutr Metab Care 12:438–443
Blüher M (2010) The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 21:38–43
Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, Adams RJ, North West Adelaide Health Study Team (2013) Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care 36:2388–2394
WHO Obesity and overweight (2013) Fact sheet N°311. http://www.who.int/mediacentre/factsheets/fs311/en/
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 285:2486–2497
The Expert Committee on the diagnosis and Classification of Diabetes Mellitus (1997) Report of the committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20:1183–1197
Schroder H, Covas MI, Marrugat J, Vila J, Pena A, Alcántara M, Masiá R (2001) Use of a three-day estimated food record, a 72-hour recall and a food-frequency questionnaire for dietary assessment in a Mediterranean Spanish population. Clin Nutr 20:429–437
Benítez-Arciniega AA, Mendez MA, Baena-Díez JM, Rovira Martori MA, Soler C, Marrugat J, Covas MI, Sanz H, Llopis A, Schröder H (2011) Concurrent and construct validity of Mediterranean diet scores as assessed by an FFQ. Public Health Nutr 14:2015–2021
Elosua R, Marrugat J, Molina L, Pons S, Pujol E (1994) Validation of the minnesota leisure time physical activity Questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol 139:1197–1209
Elosua R, Garcia M, Aguilar A, Molina L, Covas MI, Marrugat J (2000) Validation of the minnesota leisure time physical activity Questionnaire in Spanish women. Investigators of the MARATDON Group. Med Sci Sports Exerc 32:1431–1437
Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR (2008) The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168:1617–1624
Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L, Ruotolo G, Perseghin G (2011) Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the cremona study. Diabetes Care 34:210–215
Tuomilehto J (2004) Impact of age on cardiovascular risk: implications for cardiovascular disease management. Atheroscler Suppl 5:9–17
Jennings CL, Lambert EV, Collins M, Joffe Y, Levitt NS, Goedecke JH (2008) Determinants of insulin-resistant phenotypes in normal-weight and obese Black African women. Obesity (Silver Spring) 16:1602–1609
Emerging Risk Factors Collaboration, Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA et al (2011) Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 377:1085–1095
Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R (2007) Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for weight management and obesity prevention; NAASO, The obesity society; the American society for nutrition; and the American diabetes association. Am J Clin Nutr 85:1197–1202
Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J (2010) Body mass index, waist circumference and waist: hip ratio as predictors of cardiovascular risk–a review of the literature. Eur J Clin Nutr 64:16–22
Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB (2004) Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol 159:1150–1159
Lairon D (2007) Intervention studies on Mediterranean diet and cardiovascular risk. Mol Nutr Food Res 51:1209–1214
Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB (2011) The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol 57:1299–1313
Schröder H (2007) Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J Nutr Biochem 18:149–160
Mozaffarian D, Appel LJ, Van Horn L (2011) Components of a cardioprotective diet: new insights. Circulation 123:2870–2891
Kantartzis K, Machann J, Schick F, Rittig K, Machicao F, Fritsche A, Häring HU, Stefan N (2011) Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia 54:864–868
Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G (2008) Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem 19:491–504
Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83:456S–460S
Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, Rabasa-Lhoret R (2005) The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 90:4145–4150
Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH (2006) Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 30:1529–1534
Gaesser GA, Angadi SS, Sawyer BJ (2011) Exercise and diet, independent of weight loss, improve cardiometabolic risk profile in overweight and obese individuals. Phys Sportsmed 39:87–97
Bulló M, Casas-Agustench P, Amigó-Correig P, Aranceta J, Salas-Salvadó J (2007) Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr 10:1164–1172
Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, Folkerts G, Friedmann PS, Frost GS et al (2009) Inflammatory disease processes and interactions with nutrition. Br J Nutr 101(Suppl 1):S1–S45
Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU (2008) Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 168:1609–1616
Acknowledgments
The authors appreciate the English revision made by Elaine Lilly, PhD (Writers First Aid). This research was supported by grants from European Regional Development Found-EDRF (2FD097-0297-CO2-01), Instituto de Salud Carlos III-EDRF (PI11/01900; Red HERACLES RD06/0009), Agència de Gestió d’Ajuts Universitaris de Recerca (2009 SGR 1195) and by a joint contract of the Instituto de Salud Carlos III and the Health Department of the Catalan Government (Generalitat de Catalunya), CP 03/00115. The CIBEROBN and CIBERESP are initiatives of the Instituto de Salud Carlos III, Madrid, Spain.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schröder, H., Ramos, R., Baena-Díez, J.M. et al. Determinants of the transition from a cardiometabolic normal to abnormal overweight/obese phenotype in a Spanish population. Eur J Nutr 53, 1345–1353 (2014). https://doi.org/10.1007/s00394-013-0635-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0635-2