Abstract
In the general population, low body weight and body mass index (BMI) are significant risk factors for any fracture, but the specific association between body weight, BMI, and prevalence of vertebral fractures in osteoporotic women is not fully recognized. Hence, the association between body weight, BMI, and prevalent vertebral fractures was investigated in 362 women with never-treated postmenopausal osteoporosis. All participants underwent measurement of BMI, bone mineral density (BMD), and semiquantitative assessment of vertebral fractures. Thirty percent of participants had ≥1 vertebral fracture. Body weight and BMI were associated with L1–L4 BMD (R = 0.29, P < 0.001 and R = 0.17, P = 0.009, respectively). In logistic regression analysis, BMI was positively associated with the presence of vertebral fractures independent of age and other traditional risk factors for fractures. Including weight and height instead of BMI in the multivariate model, showed weight as a positive and significant covariate of the presence of vertebral fractures (OR = 1.045; P = 0.016; 95% CI 1.008–1.084). BMI was associated with the number of vertebral fractures (rho = 0.18; P = 0.001), this association being confirmed also in the multivariate analysis (β = 0.14; P = 0.03) after correction for smoking, early menopause, family history of fragility fractures and BMD. In conclusion, among postmenopausal women with osteoporosis, body weight and BMI are associated with a higher likelihood of having a vertebral fracture, irrespective of the positive association between weight and BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is characterized by compromised bone strength, which predisposes patients to increased risk of any bone fracture [1]. Osteoporotic or low trauma fractures are common, and it has been estimated that the lifetime risk of any fracture exceeds 50% at age 50 years among women, and 20% among men [2]. Vertebral, hip, and wrist fractures are the most typical osteoporotic fractures, even though vertebral fractures account for almost half of all fracture presentations because of osteoporosis [1, 3].
Bone mineral density (BMD) is the most important determinant of bone strength, accounting for approximately 60% to 80% of skeletal mechanical resistance [4]. Epidemiological data show that increased body weight and body mass index (BMI) are positively correlated with high BMD [5] and that weight reduction may contribute to bone loss [6, 7]. An inverse relationship between body weight, BMI, and the risk of any fracture has been also shown [8, 9]. Moreover, low body weight and BMI has been shown to be associated with an increased vertebral fracture risk in women above age 50 to 55 years [10, 11].
The protective effect of high body weight and BMI on osteoporosis and bone fractures has been explained by a combination of mechanical and hormonal factors [12]. It is accepted that a larger body mass imposes a greater mechanical loading on bone, and that bone mass increases to accommodate the greater load [13]. Furthermore, increased body weight is associated with endocrine changes that could positively affect bone metabolism either directly or indirectly [14].
On the other hand, studies examining the association between anthropometric measurements and the occurrence of future osteoporotic fractures have also been carried out showing an inconsistent association between BMI and fracture risk [15–18]. Recently, Zhao et al. [14] reviewed results of many of these studies and proposed mechanistic explanations to support the contrasting association between fat and bone. Importantly, none of the prospective studies showing an inverse or an inconsistent association between body weight, BMI, and fractures [8–11, 15–18] has been specifically performed in the setting of postmenopausal women with osteoporosis. Thus, data examining the relationship between BMI, body weight, and prevalent vertebral fractures in postmenopausal women with osteoporosis are lacking.
Although a number of studies suggest the presence of a prospective positive or even an inconsistent association between body weight, BMI, and risk of any fracture in mixed populations, a possible negative association between body weight, BMI, and the actual presence of vertebral fractures cannot be excluded among postmenopausal women with osteoporosis. Because high body weight involves a high mechanical load being exerted on weight bearing bones [19] and increasing fat mass may have a detrimental effect on the risk of vertebral fractures [18], it may be hypothesized that body weight might be associated positively with the presence of vertebral fractures at least in the presence of a decreased bone strength.
Hence, the aim of this study was to investigate the relationship between body weight, BMI, and the prevalence of osteoporotic vertebral fractures in women with postmenopausal osteoporosis.
Methods
Study group
Six-hundred fifty postmenopausal women were screened for study enrollment. Exclusion criteria included history of chronic disease, such as renal, hepatic, cardiac, and rheumatic disease, current or prior use of drugs that could interfere with bone mass (i.e., glucocorticoids, antiresorptive drugs, or hormonal replacement therapy), and history of traumatic vertebral fractures. A total of 238 subjects were not included because of the presence of 1 or more exclusion criteria or patient refusal to participate. The remaining 412 women underwent dual-energy X-ray absorptiometry (DEXA) and spine radiography. Among the 412 women, 362 were recruited because they fulfilled the following inclusion criteria: T-score ≤ −2.5 SD at either the lumbar spine, femoral neck, or total hip, or ≥1 osteoporotic vertebral fracture. Osteoporotic vertebral fractures were defined when as occurring without trauma or falling from a standing height or less.
Data collection
Data on patient demographics and risk factors for osteoporosis and fractures were collected for all participants. Anthropometric measures included weight, height, and BMI. Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, while subjects were wearing hospital gowns and had bare feet. BMI was calculated as weight in kilograms divided by height squared in meters. BMD was measured at the lumbar spine and hip (femoral neck and total hip) by DEXA scan with a bone densitometer (Hologic Discovery Bone Densitometer, Bedford, MA, USA). A standard phantom has been used for the correct calibration of every site of the exams [20]. T-score has been calculated as described in the third National Health and Nutrition Survey (NHANES III) [21]. The assessment of vertebral fractures was done with antero-posterior and latero-lateral X-ray projection of thoracic and lumbar spine from T1 to L5 and vertebral fractures were identified by direct visualisation using the Genant semiquantitative grading scale [22]. All spine radiographs were evaluated in the Institute of Radiology of our hospital by the same musculoskeletal radiologist who was blinded to the DEXA results.
Statistics
SPSS statistical package, release 13.0 (SPSS Inc, Chicago, Illinois, USA) was used for all statistical analyses. Data are presented as mean ± standard deviation. Independent sample t test or Mann–Whitney U test were used for comparisons of parametric and nonparametric variables between postmenopausal women with or without vertebral fractures. Chi-square statistics was used to compare smoking frequency between postmenopausal women with or without vertebral fractures. Pearson’s and Spearman’s correlation analyses were performed between parametric and nonparametric variables, respectively. A partial correlations procedure was computed to obtain partial correlation coefficients that describe the linear relationship between variables while controlling for the effects of confounders. Logistic regression analysis with presence of vertebral fractures (yes vs. no) as categorical dependent variable and multiple linear regression analysis with the number of vertebral fractures as continuous dependent variable were performed to evaluate the effect of body weight and BMI on the dependent variable by including univariate correlates of vertebral fractures as confounders. Regression analyses were also performed after including simultaneously the following traditional risk factors for fractures as independent variables: age, weight, height or BMI, smoking, early menopause, family history of fragility fractures, and BMD.
Results
The characteristics of 112 postmenopausal women with vertebral fractures and 250 postmenopausal women without vertebral fractures are summarized in Table 1. Patients with vertebral fractures were older, had higher BMI and weight and lower height compared to osteoporotic women without vertebral fractures. There was no difference in BMD and T-score at lumbar spine, total hip and femoral neck between the two groups (Table 1). Logistic regression analysis with the presence of vertebral fractures (yes vs no) as categorical dependent variable, showed BMI as a positive covariate of the presence of vertebral fractures (OR = 1.107, P = 0.022, 95% CI 1.015–1.207), independent of age and other traditional risk factors for fractures (smoking, early menopause, family history of fragility fractures and BMD). Including weight and height instead of BMI in the multivariate model, showed weight to be positively associated with the presence of vertebral fractures (OR = 1.045, P = 0.016, 95% CI 1.008–1.084).
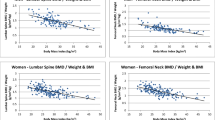
BMI was significantly correlated with BMD measured at spine (R = 0.17; P = 0.009), femoral neck (R = 0.24; P = 0.007), and total hip (R = 0.35; P < 0.001). Similarly, body weight was significantly correlated with BMD measured at spine (R = 0.29; P < 0.001), femoral neck (R = 0.42; P < 0.001), and total hip (R = 0.48; P < 0.001). Age was negatively associated with BMD at femoral neck (R = −0.27; P = 0.001) and total hip (R = −0.24; P = 0.003).
Frequency distributions of vertebral fractures in the entire sample are presented in Table 2. Significant correlates of the number of vertebral fractures were age (rho = 0.40; P < 0.001), BMI (rho = 0.18; P = 0.001), height (rho = −0.15; P = 0.007), but not weight (rho = 0.08; P = NS). Correlation between the number of vertebral fractures, BMI (R = 0.12; P = 0.036), and height (R = −0.15; P = 0.008), were still significant after controlling for the confounding effect of age. Multivariate regression analysis with the number of vertebral fractures as dependent variable and significant bivariate correlates of the number of vertebral fractures as independent variables showed that both age and BMI were independently associated with the number of vertebral fractures (Multivariate model R = 0.36; P < 0.001). After adjusting the multivariate model for additional risk factors for fractures, age (β = 0.24; P < 0.001) and BMI (β = 0.14; P = 0.03) both remained significantly associated with the prevalence of vertebral fractures, independent of smoking, early menopause, family history of fragility fractures, and BMD (Multivariate model R = 0.29; P = 0.005). After including weight and height instead of BMI in the multivariate model, age (β = 0.21; P = 0.003), height (β = −0.19; P = 0.01) but not weight (β = 0.10; P = NS) were significantly associated with the number of vertebral fractures (Multivariate model R = 0.31; P = 0.004).
Discussion
In our cross-sectional study of 362 women with postmenopausal osteoporosis, we confirmed a positive association between body weight, BMI, and bone density; however, we found that body weight and BMI were associated with a higher likelihood of having at least 1 vertebral fracture. Importantly, body weight fully explained the positive association between BMI and the presence of vertebral fractures, independent of traditional risk factors for fractures like age, smoking, early menopause, family history of fragility fractures, and also low BMD. The association between body weight and the presence of vertebral fractures was also independent from height, which is known to be generally reduced in subjects with vertebral fractures [23, 24]. Hence, irrespective of the consolidated positive association between body weight and bone density, our results suggest that looking at overweight and obesity as protective conditions for bone health may be not correct, at least in postmenopausal women with osteoporosis. Conversely, postmenopausal women with osteoporosis might have benefit from having low body weight in terms of vertebral fracture risk, even in the presence of traditional risk factors for fractures.
A number of prospective studies suggests that excessive weight and BMI may not always protect against osteoporotic fractures [15–18]. In particular, it has been found that fat mass was a positive predictor of osteoporotic fractures in a cohort of postmenopausal women [18]. Moreover, LaFleur et al. [15] underscored that the association between body weight, BMI, and the risk of fragility fractures in women with postmenopausal osteoporosis may be not always negative, but also null or even positive. Hence, although a substantial body of evidence indicates that low BMI and body weight is a risk factor for fractures in many studies performed in nonosteoporotic cohorts [8–11], we suggest that this may not be the case for postmenopausal women with reduced BMD for whom body weight and BMI are positively associated with prevalent vertebral fractures.
In the present study the association between weight, BMI, and prevalent vertebral fractures was weak, probably because it reflects the contrasting effects of weight on bone; the former increasing bone mass and possibly reducing bone quality. Although specific evidence of reduced bone quality in overweight or obese postmenopausal osteoporotic women is not inferable from the present study and the current literature, it is possible that reduced bone quality might have represented a determinant of reduced bone strength and increased prevalence of vertebral fractures in our study. Poor bone quality increases bone strain from mechanical loads, and impairment of bone quality could be compensated for by raising bone mass in weight-bearing bones [25]. An increased compensation mechanism due to poor bone quality and weight-induced bone strain could explain the positive association between body weight and BMD at the lumbar spine and femur. Hence, it could be hypothesized that among postmenopausal osteoporotic women, increasing body weight and BMI, by negatively affecting bone quality more than positively affecting bone mass, have the net effect to reduce bone strength.
A proven mechanism whereby increased body weight might contribute to vertebral fractures, despite being associated with increased BMD, is not inferable from the results of our study. However, possible hypotheses may be inferred from some lines of current evidence. While adiposity enhances BMD through increased mechanical loading and release of hormonal factors (estrogen, leptin, and adiponectin) that are anabolic on bone [12], increasing BMI has been also associated with a relevant rise in injury rates [26], which may predispose to fragility fractures especially in osteoporotic women. Thus, among postmenopausal osteoporotic women, a reduction in bone strength coupled with an increased BMI-related injury rate could overcome the mechanical and anabolic effect on bone of body weight.
Also, body weight gain after menopause might have a role in contributing to fracture risk. This speculative hypothesis might be supported by the observation that decreased endogenous estrogen levels in postmenopausal women were shown to be accompanied by an increase in body fat [27], decreased osteoblast count [28], and acceleration of bone loss [29]. Conversely, estrogen replacement therapy prevented menopause-induced gain in fat mass [27, 30] and reduced the incidence of osteoporotic fractures in postmenopausal women [31].
Finally, unfavourable postural changes at the spine level and altered mechanical forces in the spinal column [32, 33] might further contribute to fracture risk among obese osteoporotic women, although the latter and the previously proposed hypotheses remain unproven in our study and are not all derived from studies specifically performed in postmenopausal osteoporotic women.
Assuming that our cross-sectional results showing a positive association between body weight, BMI, and prevalent vertebral fractures in osteoporosis might prelude a prospective link between overweight to obesity and vertebral fracture risk, we should consider the possibility that increased body weight might have an opposite influence on vertebral fracture risk in healthy versus postmenopausal osteoporotic women. Thus, one could hypothesize a beneficial effect of weight loss on the vertebral fracture risk in postmenopausal osteoporotic women who are also overweight to obese. However, we should be cautious in suggesting weight loss to all overweight to obese postmenopausal osteoporotic women, because it is sufficiently demonstrated that weight loss is generally associated with BMD reduction, which may be only partially prevented by appropriate calcium supplementation [34]. Studies performed specifically in overweight to obese postmenopausal osteoporotic women with the aim to explore the association between caloric restriction, weight loss, and the future risk of vertebral fractures are still lacking and this is a relevant topic to be explored in future studies.
In the present study, other than the presence of prevalent vertebral fractures, even the number of osteoporotic vertebral fractures is positively associated with BMI levels. As expected, most of the association between BMI and the number of vertebral fractures was attributable to the negative association between height and vertebral fractures. In this regard, a loss of stature occurring in osteoporotic subjects with multiple vertebral fractures is largely proved [23, 24].
Limitations of our study have to be acknowledged. The results are based on cross-sectional data and prevalent vertebral fractures. Hence, no comments can be made about the incidence of new fractures and the ability of body weight and BMI to predict future vertebral fractures. Given the cross-sectional design of this study, a causative unidirectional relationship between body weight, BMI, and the presence vertebral fractures cannot be inferred with certainty. Studies with a larger group of osteoporotic patients and a higher number of collected parameters including localizations of other fractures as well as a prospective design need to be undertaken to provide a definitive answer to this issue. Finally, only weight and BMI, but not fat mass and other more accurate indices of adiposity, were considered in the present study. A more detailed assessment of fat presence and anatomical distribution would have given more clear information on the influence of adiposity on bone mass and the risk of vertebral fractures.
To summarize, we have shown that body weight and BMI are positively associated with the presence and the number of vertebral fractures in women with postmenopausal osteoporosis. Hence, the protective effect of body weight on osteoporosis can be partially overtaken by the negative effect of obesity on vertebral fractures, possibly as a consequence of the greater load that insists on the frail vertebral body of obese osteoporotic women. Further prospective studies need to be performed to confirm this results and eventually to investigate whether among obese-overweight postmenopausal osteoporotic women a controlled loosing weight strategy to be accompanied to calcium supplementation and/or antiresorptive treatment may beneficially affect bone quality and reduce the risk of vertebral fractures.
References
Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporos Int 16:3–7
van Staa TP, Dennison EM, Leufkens HG, Cooper C (2001) Epidemiology of fractures in England and Wales. Bone 29:517–522
Briggs AM, Greig AM, Wark JD (2007) The vertebral fracture cascade in osteoporosis: a review of aetiopathogenesis. Osteoporos Int 18:575–584
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, Therapy (2001) Osteoporosis prevention, diagnosis and therapy. JAMA 285:785–795
Felson DT, Zhang Y, Hannan MT, Anderson JJ (1993) Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 8:567–573
Svendsen OL, Hassager C, Christiansen C (1993) Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med 95:131–140
Compston JE, Laskey MA, Croucher PI, Coxon A, Kreitzman S (1992) Effect of diet-induced weight loss on total body bone mass. Clin Sci (Lond) 82:429–432
Espallargues M, Sampietro-Colom L, Estrada MD, Solà M, del Rio L, Setoain J, Granados A (2001) Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int 12:811–822
De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
van der Klift M, de Laet CE, McCloskey EV, Johnell O, Kanis JA, Hofman A, Pols HA (2004) Risk factors for incident vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res 19:1172–1180
Finigan J, Greenfield DM, Blumsohn A, Hannon RA, Peel NF, Jiang G, Eastell R (2008) Risk factors for vertebral and nonvertebral fracture over 10 years: a population-based study in women. J Bone Miner Res 23:75–85
Crepaldi G, Romanato G, Tonin P, Maggi S (2007) Osteoporosis and body composition. J Endocrinol Invest 30:42–47
Rubin CT, Lanyon LE (1985) Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37:411–417
Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW (2008) Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 23:17–29
LaFleur J, McAdam-Marx C, Kirkness C, Brixner DI (2008) Clinical risk factors for fracture in postmenopausal osteoporotic women: a review of the recent literature (March). Ann Pharmacother 29 (Epub ahead of print)
Papaioannou A, Joseph L, Ioannidis G, Berger C, Anastassiades T, Brown JP, Hanley DA, Hopman W, Josse RG, Kirkland S, Murray TM, Olszynski WP, Pickard L, Prior JC, Siminoski K, Adachi JD (2005) Risk factors associated with incident clinical vertebral and nonvertebral fractures in postmenopausal women: the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 16:568–578
Hollaender R, Hartl F, Krieg MA, Tyndall A, Geuckel C, Buitrago-Tellez C, Manghani M, Kraenzlin M, Theiler R, Hans D (2009) Prospective evaluation of risk of vertebral fractures using quantitative ultrasound measurements and bone mineral density in a population-based sample of postmenopausal women: results of the Basel Osteoporosis Study. Ann Rheum Dis 68:391–396
Albrand G, Munoz F, Sornay-Rendu E, DuBoeuf F, Delmas PD (2003) Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone 32:78–85
Takata S, Yasui N (2002) Effects of constitution, atraumatic vertebral fracture and aging on bone mineral density and soft tissue composition in women. J Med Invest 49:18–24
Genant HK, Grampp S, Glüer CC, Faulkner KG, Jergas M, Engelke K, Hagiwara S, Van Kuijk C (1994) Universal standardization for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 9:1503–1514
Looker AC, Orwoll ES, Johnston CC Jr, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP (1997) Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 12:1761–1768
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Huang C, Ross PD, Lydick E, Davis JW, Wasnich RD (1996) Contributions of vertebral fractures to stature loss among elderly Japanese–American women in Hawaii. J Bone Miner Res 11:408–411
Siminoski K, Jiang G, Adachi JD, Hanley DA, Cline G, Ioannidis G, Hodsman A, Josse RG, Kendler D, Olszynski WP, Ste Marie LG, Eastell R (2005) Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos Int 16:403–410
Sugiyama T, Yamaguchi A, Kawai S (2002) Effects of skeletal loading on bone mass and compensation mechanism in bone: a new insight into the “mechanostat” theory. J Bone Miner Metab 20:196–200
Finkelstein EA, Chen H, Prabhu M, Trogdon JG, Corso PS (2007) The relationship between obesity and injuries among U.S. adults. Am J Health Promot 21:460–468
Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET (1998) Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coron Artery Dis 9:503–511
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171
Riggs BL, Khosla S, Melton LJ 3rd (1998) A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 13:763–773
Jensen LB, Vestergaard P, Hermann AP, Gram J, Eiken P, Abrahamsen B, Brot C, Kolthoff N, Sørensen OH, Beck-Nielsen H, Nielsen SP, Charles P, Mosekilde L (2003) Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res 8:333–342
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Fabris de Souza SA, Faintuch J, Valezi AC, Sant’Anna AF, Gama-Rodrigues JJ, de Batista Fonseca IC, de Melo RD (2005) Postural changes in morbidly obese patients. Obes Surg 15:1013–1016
Keller TS, Harrison DE, Colloca CJ, Harrison DD, Janik TJ (2003) Prediction of osteoporotic spinal deformity. Spine 28:455–462
Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA (2005) Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res 2005:455–463
Acknowledgments
The authors thank Iliana Lega, MD, for her assistance in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pirro, M., Fabbriciani, G., Leli, C. et al. High weight or body mass index increase the risk of vertebral fractures in postmenopausal osteoporotic women. J Bone Miner Metab 28, 88–93 (2010). https://doi.org/10.1007/s00774-009-0108-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0108-0