Abstract
Background
Marginal ulcer (MU) is an uncommon but significant complication following one-anastomosis gastric bypass (OAGB). Our study aims to understand the incidence rates, risk factors, and management of MU following OAGB.
Methods
MEDLINE, Scopus, and Cochrane Library databases were examined to identify all studies on OAGB where authors had reported on MU. Data were collected on basic demographics, incidence rates, risk factors, and management of this condition.
Results
Thirty-two studies involving 8868 patients were analysed. The mean age and body mass index (BMI) of patients in these studies were 40.9 ± 4.5 years and 47.6 ± 5.6 kg/m2, respectively. Among the patient cohort, approximately 72% were female, and 20.6% had preoperative gastroesophageal reflux disease (GERD). The authors described prescribing proton-pump inhibitors (PPI) prophylaxis to 14.1% of patients after surgery. Two hundred twenty-eight patients were reported to have MU. The incidence of MU was 2.59% (95% CI 1.89–3.52), of which 53 patients presented within 12 months, 24 patients presented after 31 months, and five patients after 6 years. One hundred forty-six patients did not have presentation time documented. Sixty-five patients were described to have MU diagnosed on endoscopy, of which 54 were symptomatic and 11 were asymptomatic. The authors were, however, not specific on the choice of investigation for the remaining 163 patients. Of patients, 89.7% were treated conservatively with PPIs, whilst 10.3% had surgery to treat MU.
Conclusions
Marginal ulcer is an uncommon complication following OAGB. The majority of patients are treated conservatively with PPIs. Larger, well-designed studies reporting on risk factors, investigation, and management of MU following OAGB are warranted.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Health Survey for England 2019 estimated that the proportion of the adult UK population living with obesity has risen from 14.9% in 1993 to 28.0% in 2019 [1]. This appears to be a global trend and bariatric surgery has emerged as an effective treatment [2]. Sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB) are the two commonest bariatric procedures worldwide [2, 3]. One-anastomosis gastric bypass (OAGB), introduced by Rutledge in 1997, has recently gained popularity and is currently the third commonest bariatric procedure worldwide [4, 5]. It is suggested to have a technically less demanding learning curve whilst offering comparable benefits to RYGB [5, 6].
Bariatric procedures are currently advocated as the recommended treatment for severe obesity, as they achieve better outcomes when compared to conservative treatment [6, 7]. Despite this, patients have identified risks of surgery and complications as barriers to opting for surgical treatment [8]. It is, therefore, important to understand them better.
Marginal ulceration (MU) at the anastomosis between the gastric pouch and the small intestine is a recognised complication of gastric bypass procedures. Though some studies have suggested that the risk of MU is higher with OAGB compared to RYGB [4, 9,10,11], there is no robust data on the MU after OAGB in scientific literature. In particular, there is currently no published systematic review focussing on MU following OAGB. The purpose of this systematic review was to understand the incidence, risk factors, and management of MU following OAGB.
Methods
This systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. A systematic review protocol was prospectively registered on PROSPERO (CRD42022362594). The primary outcome of this study was to identify the incidence rate of MU following OAGB. The secondary outcomes included understanding its risk factors, investigation, and management of MU.
Literature Search Strategy
Databases including MEDLINE, Scopus, and Cochrane Library were used to systematically identify all studies on OAGB where authors had reported on MU. The search was conducted from inception until the 21st of September 2022. The following Medical Subject Headings were used: “Mason’s loop”, “mini-gastric bypass”, “mini-gastric bypass”, “single anastomosis gastric bypass”, “single-anastomosis gastric bypass”, “single anastomosis (mini-) gastric bypass”, “one anastomosis (mini-) gastric bypass”, “one anastomosis gastric bypass”, “one-anastomosis gastric bypass”, “omega gastric bypass”, “omega-loop bypass”, “omega loop bypass” AND “Peptic ulcer disease”, “marginal ulceration”, “marginal ulcer”, “anastomotic ulcer”, “ischemic ulcer”, “ulcers” (Appendix).
Inclusion and Exclusion Criteria
We included patients of all ages who underwent an OAGB for obesity. This review includes randomised controlled trials, non-randomised controlled trials, and observational studies. Only English-language articles were included. We excluded case reports, ongoing trials, and studies on revisional OAGB surgeries.
Study Selection
Titles and/or abstracts of studies obtained from our search were screened independently by two reviewers (S.L. and S.S.). Ninety-four studies were obtained in full text and independently reviewed for eligibility by both reviewers (S.L. and S.S.). Any discrepancies were resolved by discussion with a third reviewer (K.M.).
Data Extraction
A total of thirty-two articles were included in our systematic review. Two authors (S.L. and S.S.) reviewed all eligible studies independently and extracted data using a standardised Microsoft Excel worksheet. The following data were included: author; year of publication; inclusion period; study design; the number of participants; participants’ characteristics; mean age; mean body mass index; associated risk factors; surgical techniques; postoperative complications; investigation and treatment of MU. Any discrepancies were then discussed among the two reviewers (S.L. and S.S.) and if required the third reviewer (K.M.).
Quality of Assessment
Two reviewers (S.L. and S.S.) independently assessed the risk of bias in the included studies using the Jadad scoring system for randomised control trials [13] and the Newcastle-Ottawa Scale (NOS) for non-randomised controlled trials [14].
Statistics
All statistical analyses were conducted in R version 4.0.3 [15], using the meta package [16]. Funnel plots were visually inspected to assess publication bias. The pooled incidence of MU was calculated using the generic inverse variance method after log transformation. Where cohorts from multiple studies overlapped in time and institution, only the largest cohort was included in the meta-analysis. Pooled estimates used the DerSimonian-Laird estimator for between-study variance and are reported with associated 95% confidence intervals (CIs) [17]. A random effects model was chosen owing to the expected heterogeneity between studies.
Results
Study Characteristics
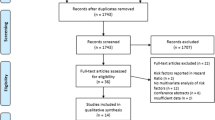
A total of thirty-two articles were included in our systematic review. Figure 1 outlines the PRISMA flowchart. Our initial search resulted in 264 articles. After removing duplicates, thorough screening, and full-text assessment, 32 articles were included in our study. These articles comprised nine prospective cohort studies, 19 retrospective cohort studies, and three randomised controlled trials. A total of 8868 patients met the inclusion criteria and were included for data analysis. A summary of study characteristics is outlined in Table 1.
Patient Demographics and Comorbidities of the Baseline Population
The mean age and preoperative body mass index (BMI) were 40.9 ± 4.5 years and 47.6 ± 5.6 kg/m2, respectively. Among the patient cohort, approximately 72% were females (6388/8868); 20.6% (1825/8868) had preoperative gastroesophageal reflux disease; 46.3% (4105/8868) were treated for preoperative hypertension; and 30.3% (2690/8868) were diagnosed with type 2 diabetes mellitus. Basic patient demographics and comorbidities are summarised in Table 2.
Surgical Technique
OAGB was performed laparoscopically in all studies. The mean operative time was 90.6 ± 33.5 minutes. Various biliopancreatic limb (BPL) lengths were used, mostly measuring 200 cm (n = 8) [11, 18,19,20,21,22,23,24]. Some studies adjusted the BPL length according to BMI (n = 3) [25,26,27]. Four studies did not describe the BPL length used [28,29,30,31].
The size of the bougie used to create the gastric pouch was reported to be inconsistent. Most studies reported using 36 Fr (n = 10) [18, 23, 32,33,34,35,36,37,38,39] followed by 38 Fr (n = 5) [11, 20, 27, 29, 40], 34 Fr (n = 2) [41, 42], 30 Fr (n = 2) [26, 43], 39 Fr (n = 1) [25], 28 Fr (n = 1) [5], 28–36 Fr (n = 1) [21], 36–42 Fr (n = 1) [44], and 34 Fr or 36 Fr (n = 1) [45]. Eight studies did not specify the size of bougie used [19, 22, 24, 28, 30, 31, 46, 47].
Six studies described performing single-layer running sutures for stapler site closure [18, 25, 27, 36, 42, 45]. The length of the gastric pouch was only mentioned in a few studies (Table 3). Saarinen et al. described a fixed length of 15 cm for the gastric pouch [40], whereas others had varying lengths of gastric pouches: 16–22 cm [43], 12–18 cm (n = 1) [33], and 16–18 cm (n = 1) [44].
Postoperative Care
The average length of stay was 3.6 ± 1.0 days. Only 14.1% (1252/8868) of patients were documented to have been prescribed proton-pump inhibitors (PPI) after surgery with various duration reported: 1 month (n = 44) [25], 3 months (n = 295) [29, 34], 6 months (n = 605) [30, 31], 12 months (n = 190) [39], and unspecified (n = 118) [19, 24]. A small minority of patients, 5.2% (463/8868), reported developing reflux symptoms after surgery.
Incidence of MU
The overall incidence of MU was 2.59% (95% CI 1.89–3.52) (Fig. 2). The incidence of MU was 2.89% (95% CI 1.96–4.23) in units across Europe compared to 1.70% (95% CI 0.67–4.25) in Asia (Fig. 3). The incidence of MU was 2.40% (95% CI 0.74–7.57) in units across the Middle East and North America (Fig. 3).
Clinical Presentation, Investigation, and Treatment for MU
Out of the 228 patients with MU, only 82 had their diagnosis timeline recorded. Of those, 65% were diagnosed within the first 12 months of surgery. Approximately 23.7% (54/228) were diagnosed with MU on endoscopy for the following symptoms: dyspepsia (n = 8) [19, 20], symptomatic reflux (n = 38) [41, 45], unspecified gastrointestinal symptoms (n = 2) [31], gastrointestinal bleed (n = 2) [39], and symptomatic anaemia (n = 2) [42]. In contrast, Saarinen et al. performed a routine endoscopy for patients 6 months after surgery, at which 10% (4/40) of patients were found to have MU [40]. Pizza et al. and Baksi et al. have also mentioned performing routine endoscopies for asymptomatic patients, which resulted in diagnosing three and four MU, respectively [11, 38]. Sumer et al. reported one patient returning to the emergency department 2 months after surgery with severe epigastric pain [25]. Fifteen studies did not specify how MU was diagnosed.
The treatment method was only available for 68.0% (n = 156/228) of the patients. Of these, 89.7% were treated conservatively with PPIs and 10.3% required surgery. Rutledge et al. reported three patients proceeding with revisional surgery due to failed medical treatment of MU [5]. Mustafa et al. described one patient requiring conversion to RYGB to treat perforated MU and another requiring revisional surgery due to stricture at GJ anastomosis secondary to chronic MU [47]. Shivakumar et al. described performing an omental patch repair in one patient with MU diagnosed 11 months after surgery [36]. Endoscopic haemostasis had to be carried out in one patient with bleeding MU [42]. Of patients, 31.6% (72/228) did not have specified treatment mentioned in the studies.
Risk of Bias
The average NOS score was 5.56. There was inadequate comparability of cohorts with a lack of adjustment for confounding. All three RCTs did not specify the total number and reason for withdrawals. Two RCTs were deemed low risk of bias [36, 39] (≥3/5), whilst one RCT did not report the method of randomisation nor blinding of participants in the published article [40]. The full assessment of the risk of bias for each study can be found in Table 4.
Discussion
To our knowledge, this is the first systematic review and meta-analysis on MU after OAGB. We have found an overall incidence rate of MU at 2.59% after OAGB. The median presentation time of MU following OAGB is within 12 months. PPI prophylaxis prescription was described to have been given to 14.1% of patients, with variable total duration reported. Of patients with MU, 89.7% were managed conservatively with PPIs. However, the course of treatment remains inconclusive.
PPI Prophylaxis
The duration of PPI prophylaxis after OAGB also remains inconclusive. A recent survey suggested variable duration of postoperative PPI prescription among surgeons: 20.2%, 43.6%, and 18.6% reported 1, 3, and 6 months respectively [48]. Ying et al. suggested 1 year of PPI prophylaxis due to the low incidence of MU reported following RYGB [49]. Our review has shown that the majority of MU (63%) presented within the first 12 months following OAGB. In light of these findings, PPI for MU prophylaxis should be recommended for at least a year following OAGB, if not longer. It is the authors’ current practice to recommend PPI prophylaxis for 5 years after both types of gastric bypass procedures after obesity [50].
Diagnosis
In 2020, the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) committee published guidelines on the timing of routine endoscopy after OAGB. Routine endoscopy has been suggested for patients at year 1 and years 2 to 3 following OAGB to minimise the risk of undiagnosed upper gastrointestinal or Barrett’s oesophagus malignancies [51].
Management of MU
The review of included studies revealed significant variations in the management of MU. Jammu et al. suggested intravenous antibiotics (cefotaxime, tinidazole) and PPI for 5 days, followed by oral PPI for 6 months [20]. Baksi et al. reported a similar treatment consisting of oral PPI and sucralfate for 2 months [11]. On the contrary, Piazza et al. treated three patients with MU with 4–5 days of intravenous PPI. Regardless, most patients were successfully managed conservatively and only a small proportion of patients, 10.3%, needed revisional surgeries [5, 32,33,34, 36, 38, 42, 45, 47].
Risk Factors
We did not observe any consistent association between the BPL length and the incidence of MU. Bertrand et al. conducted a retrospective cohort study and reported a significantly lower rate of MU in patients who had undergone OAGB with BPL length of 150 cm compared to 200 cm (OR = 0.4) [52]. Liagre et al. reported a MU rate of 1.22% (3/245) in OAGB with BPL length of 150 cm [34]. With a similar BPL length of 150 cm, Mustafa et al. concluded that 5% (10/198) of MU occurred in patients following OAGB [47]. In contrast, ElAbd et al. did not report any MU in a retrospective cohort study consisting of 40 patients undergoing OAGB with BPL length of 150cm [37]. Schmitz et al. reported a MU rate of 7.3% (11/150) with a BPL length of 250 cm [43]. On the other hand, Markopoulos et al. reported no MU in 36 patients undergoing OAGB with a BPL length of 250 cm [46].
There is currently limited data on the causative factors of MU following OAGB. Chevallier et al. reported a 10% (2/20) rate of MU following OAGB, and both participants were smokers [53]. Similarly, Pizza et al. concluded a 1.2% (3/241) rate of MU following OAGB in which all three participants were heavy smokers and consumed alcohol at least three times a week. All three patients needed conversion to RYGB [38]. Sumer et al. reported a 2.3% (1/44) rate of MU, occurring in one patient who was a heavy smoker [25]. Winstanley et al. reported a 4.5% rate of MU following OAGB in patients with hiatal hernia [30].
A long, narrow gastric pouch has been recommended to counteract acid build-up at the anastomosis site, which could lead to an increased risk of MU [53]. Wilson et al. conducted a retrospective study of 1001 participants and subsequently reported that both smoking (aOR = 30.6) and the use of non-steroidal anti-inflammatory drugs (NSAIDs) (aOR = 11.5) contributed to the formation of MU following RYGB [54]. Beran et al. recently published a systematic review and meta-analysis which suggested the following risk factors of MU in patients who have had RYGB: helicobacter pylori (HP) infection (OR 4.97 [2.24–10.99]), smoking (OR 2.50 [1.76–3.54]), and diabetes mellitus (OR 1.80 [1.15–2.80]) [55]. Bhayani et al. concluded that preoperative hypertension is a significant predictive factor of MU in patients undergoing RYGB [56].
Limitations and Strengths
Our study has several limitations. Firstly, we have found that most studies did not routinely report risk factors that may contribute to MU. There were only three studies with reported data on smoking. Therefore, we could not establish any causative links between potential risk factors and MU. Instead, we reported results from studies included separately and compared them accordingly. As a result, there has been a lack of multivariable analyses resulting in difficulties when comparing results in most studies and, thus, confounding bias. Secondly, our review consisted mainly of retrospective studies. As a result, there were gaps in data reporting, including unspecified timings of endoscopy performed when diagnosing MU. Thus, it was difficult to determine the exact presentation time of marginal ulcers in each study. In addition, endoscopic findings and morbidity and mortality rates were not routinely reported. Moreover, there was no standardised description of the routine practice of gastroenterologists when encountering MU in the current literature. Hence, there seemed to be a lack of clarification on whether MUs managed by gastroenterologists were routinely reported to the surgical team resulting in a potential risk of underreporting of incidence of MU.
Our study is the first to have succinctly summarised current literature on MU, and we hope our findings will guide clinicians in decision-making when treating this condition.
Conclusion
Our study concluded an estimated overall incidence of MU at 2.59% (95% CI 1.89–3.52); the risk of underreporting remains. The majority of MU can be treated conservatively with PPI. Future studies on risk factors, clinical presentation, investigation, and treatment of MU are recommended.
References
Baker C. Obesity statistics. commonslibrary.parliament.uk, [online] 1(3336). 2023. Available at: https://commonslibrary.parliament.uk/research-briefings/sn03336/. Accessed 31 July 2023.
Small P, Md R, Kamal Mahawar F, Frcsed M, Walton P, Frcp M, Kinsman R. The United Kingdom National Bariatric Surgery Registry Third Registry Report 2020 on behalf of the NBSR Data Committee. 2020. Available at: https://e-dendrite.com/Publishing/Reports/Bariatric/NBSR2020.pdf. Accessed 31 July 2023.
Schauer P, Ikramuddin S. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–29.
Angrisani L, Santonicola A, Lovino P, Ramos A, Shikora S, Kow L. Bariatric surgery survey 2018: similarities and disparities among the 5 IFSO chapters. Obes Surg. 2018;2021(31):1937–48.
Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg. 2001;11:276–80.
Peterko AC, Mazul Sunko B, Mirosevie G, et al. Combined sleeve gastrectomy and mini gastric bypass in a new bariatric procedure of mini gastric bypass and proximal sleeve gastrectomy. Acta Clin Croat. 2013;52:316–20.
Franco JV, Ruiz PA, Palermo M, et al. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21:1458–68.
Wharton S, Serodio KJ, Kuk JL, Sivapalan N, Craik A, Arts MA. Interest, views and perceived barriers to bariatric surgery in patients with morbid obesity. Clin Obes. 2016;6(2):154–60.
Martinino A, Bhandari M, Abouelazayem M, Abdellatif A, Koshy RM, Mahawar K. Perforated marginal ulcer after gastric bypass for obesity: a systematic review. Surg Obes Related Dis. 2022;18(9):1168–75.
Magouliotis DE, Tasiopoulou VS, Tzovaras G. One anastomosis gastric bypass versus Roux-en-Y gastric bypass for morbid obesity: an updated meta-analysis. Obes Surg. 2019;29:2721–30.
Baksi A, Kamtam DNH, Aggarwal S, Ahuja V, Kashyap L, Shende DR. Should surveillance endoscopy be routine after one anastomosis gastric bypass to detect marginal ulcers: initial outcomes in a tertiary referral centre. Obes Surg. 2020;30:4974–80.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Jadad AR et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12.
GA Wells, B Shea, D O’Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 31 July 2023.
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. <https://www.R-project.org/>. Accessed 31 July 2023.
Schwarzer G. General Package for Meta-Analysis [R package meta version 4.18-0]. 2021.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trial. 1986;7(177e):188.
Miller KA, Radauer M, Buchwald JN, McGlennon TW, Ardelt-Gattinger E. 5-year results of banded one-anastomosis gastric bypass: a pilot study in super-obese patients. Obes Surg. 2020;30(11):4307–14. https://doi.org/10.1007/s11695-020-04824-6.
Kular KS, Manchanda N, Rutledge R. A 6-year experience with 1,054 mini-gastric bypasses-first study from Indian subcontinent. Obes Surg. 2014;24(9):1430–5. https://doi.org/10.1007/s11695-014-1220-3.
Jammu GS, Sharma R. A 7-year clinical audit of 1107 cases comparing sleeve gastrectomy, Roux-En-Y gastric bypass, and mini-gastric bypass, to determine an effective and safe bariatric and metabolic procedure. Obes Surg. 2016;26(5):926–32. https://doi.org/10.1007/s11695-015-1869-2.
Campanelli M, Bianciardi E, Benavoli D, Bagaglini G, Lisi G, Gentileschi P. Laparoscopic banded one anastomosis gastric bypass: a single-center series. J Obes. 2022;29(2022):4942052. https://doi.org/10.1155/2022/4942052.
Lee WJ, Yu PJ, Wang W, Chen TC, Wei PL, Huang MT. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005 Jul;242(1):20–8. https://doi.org/10.1097/01.sla.0000167762.46568.98.
Kansou G, Lechaux D, Delarue J, Badic B, Le Gall M, Guillerm S, Bail JP, Thereaux J. Laparoscopic sleeve gastrectomy versus laparoscopic mini gastric bypass: one year outcomes. Int J Surg. 2016;33(Pt A):18–22. https://doi.org/10.1016/j.ijsu.2016.07.051.
Noun R, Riachi E, Zeidan S, Abboud B, Chalhoub V, Yazigi A. Mini-gastric bypass by mini-laparotomy: a cost-effective alternative in the laparoscopic era. Obes Surg. 2007;17(11):1482–6. https://doi.org/10.1007/s11695-008-9426-x.
Sumer A, Mahawar K, Aktokmakyan TV, Savas OA, Peksen C, Barbaros U, Mercan S. Bridged one-anastomosis gastric bypass: technique and preliminary results. Surg Today. 2021;51(8):1371–8. https://doi.org/10.1007/s00595-021-02264-y.
Rheinwalt KP, Plamper A, Rückbeil MV, Kroh A, Neumann UP, Ulmer TF. One anastomosis gastric bypass-mini-gastric bypass (OAGB-MGB) versus Roux-en-Y gastric bypass (RYGB)-a mid-term cohort study with 612 patients. Obes Surg. 2020;30(4):1230–40. https://doi.org/10.1007/s11695-019-04250-3.
Charalampos T, Maria N, Vrakopoulou VGZ, Tania T, Raptis D, George Z, Emmanouil L, Konstantinos A. Tailored one anastomosis gastric bypass: 3-year outcomes of 94 patients. Obes Surg. 2019;29(2):542–51. https://doi.org/10.1007/s11695-018-3572-6.
Elkerkary MA, Adly OA, Elhadary MKE, et al. Comparison between the effect of laparoscopic sleeve gastrectomy and laparoscopic mini-gastric bypass on type 2 diabetes mellitus in obese patients: a prospective study. World J Lap Surg. 2021;14(2):131–5.
Olmi S, Oldani A, Cesana G, Ciccarese F, Uccelli M, De Carli SM, Villa R, David G, Giorgi R, Zanoni AAG. Laparoscopic one anastomosis gastric bypass versus laparoscopic one anastomosis gastric bypass with Braun anastomosis: what’s better? J Laparoendosc Adv Surg Tech A. 2019;29(11):1469–74. https://doi.org/10.1089/lap.2019.0218.
Winstanley J, Ahmed S, Courtney M, Sam M, Mahawar K. One anastomosis gastric bypass in patients with gastrooesophageal reflux disease and/or hiatus hernia. Obes Surg. 2021;31(4):1449–54. https://doi.org/10.1007/s11695-020-05149-0.
Mari A, Khoury T, Daud G, Lubany A, Safadi M, Sbeit W, Pellicano R, Mahamid M. The yield, effectiveness and safety of gastroscopy in management of early postbariatric upper gastrointestinal pain. Minerva Chir. 2020;75(3):164–8. https://doi.org/10.23736/S0026-4733.20.08282-6.
Tasdighi E, Mousapour P, Khalaj A, Sadeghian Y, Mahdavi M, Valizadeh M, Barzin M. Comparison of mid-term effectiveness and safety of one-anastomosis gastric bypass and sleeve gastrectomy in patients with super obesity (BMI ≥ 50 kg/m2). Surg Today. 2022;52(5):854–62. https://doi.org/10.1007/s00595-021-02387-2.
Bhandari M, Nautiyal HK, Kosta S, Mathur W, Fobi M. Comparison of one anastomosis gastric bypass (OAGB) and Roux-en-Y gastric bypass (RYGB) for treatment of obesity: a five-year study. Surg Obes Related Dis. 2019; https://doi.org/10.1016/j.soard.2019.05.025.
Liagre A, Martini F, Kassir R, Juglard G, Hamid C, Boudrie H, Van Haverbeke O, Antolino L, Debs T, Petrucciani N. Is one anastomosis gastric bypass with a biliopancreatic limb of 150 cm effective in the treatment of people with severe obesity with BMI > 50? Obes Surg. 2021;31(9):3966–74. https://doi.org/10.1007/s11695-021-05499-3.
Piazza L, Ferrara F, Leanza S, Coco D, Sarvà S, Bellia A, Di Stefano C, Basile F, Biondi A. Laparoscopic mini-gastric bypass: short-term single-institute experience. Updates Surg. 2011;63(4):239–42. https://doi.org/10.1007/s13304-011-0119-y.
Shivakumar S, Tantia O, Goyal G, Chaudhuri T, Khanna S, Ahuja A, Poddar A, Majumdar K. LSG vs MGB-OAGB-3 year follow-up data: a randomised control trial. Obes Surg. 2018;28(9):2820–8. https://doi.org/10.1007/s11695-018-3255-3.
ElAbd R, AlMutairi R, Alhaj A, AlKhayat H, Jamal MH. One-anastomosis gastric bypass as a primary bariatric surgery: initial experience and short-term outcomes. Bariatric Surg Prac Patient Care. 2021:220–5. https://doi.org/10.1089/bari.2020.0063.
Pizza F, D'Antonio D, Lucido FS, Tolone S, Dell'Isola C, Gambardella C. Postoperative Clinical-endoscopic follow-up for GERD and gastritis after one anastomosis gastric bypass for morbid obesity: how, when, and why. Obes Surg. 2020;30(11):4391–400. https://doi.org/10.1007/s11695-020-04805-9.
Pizza F, D'Antonio D, Lucido FS, Tolone S, Del Genio G, Dell'Isola C, Docimo L, Gambardella C. The Role of ursodeoxycholic acid (UDCA) in cholelithiasis management after one anastomosis gastric bypass (OAGB) for morbid obesity: results of a monocentric randomized controlled triaL. Obes Surg. 2020;30(11):4315–24. https://doi.org/10.1007/s11695-020-04801-z.
Saarinen T, Pietiläinen KH, Loimaala A, Ihalainen T, Sammalkorpi H, Penttilä A, Juuti A. Bile reflux is a common finding in the gastric pouch after one anastomosis gastric bypass. Obes Surg. 2020;30(3):875–81. https://doi.org/10.1007/s11695-019-04353-x.
Slagter N, Hopman J, Altenburg AG, de Heide LJM, Jutte EH, Kaijser MA, Damen SL, van Beek AP, Emous M. Applying an anti-reflux suture in the one anastomosis gastric bypass to prevent biliary reflux: a long-term observational study. Obes Surg. 2021;31(5):2144–52. https://doi.org/10.1007/s11695-021-05238-8.
Meydan C, Raziel A, Sakran N, Gottfried V, Goitein D. Single anastomosis gastric bypass-comparative short-term outcome study of conversional and primary procedures. Obes Surg. 2017;27(2):432–8. https://doi.org/10.1007/s11695-016-2336-4.
Schmitz SM, Alizai PH, Kroh A, Schipper S, Brozat JF, Plamper A, Neumann UP, Rheinwalt K, Ulmer TF. Clinical outcomes after one anastomosis gastric bypass versus sleeve gastrectomy in super-super-obese patients. Surg Endosc. 2022;36(6):4401–7. https://doi.org/10.1007/s00464-021-08790-7.
Szymański M, Marek I, Wilczyński M, Janczy A, Bigda J, Kaska Ł, Proczko-Stepaniak M. Evaluation of esophageal pathology in a group of patients 2 years after one-anastomosis gastric bypass (OAGB) - Cohort study. Obes Res Clin Pract. 2022;16(1):82–6. https://doi.org/10.1016/j.orcp.2021.12.001.
Musella M, Susa A, Greco F, De Luca M, Manno E, Di Stefano C, Milone M, Bonfanti R, Segato G, Antonino A, Piazza L. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg Endosc. 2014;28(1):156–63. https://doi.org/10.1007/s00464-013-3141-y.
Markopoulos G, Skroubis G, Kalfarentzos F, Kehagias I. Comparison of one anastomosis gastric bypass versus standard Roux-en-Y gastric bypass versus a variant of biliopancreatic diversion, in a case-matched, non-superobese population: 6 years of follow-up. Prz Gastroenterol. 2022;17(2):152–61. https://doi.org/10.5114/pg.2021.108453.
Mustafa A, Rizkallah NNH, Samuel N, Balupuri S. Laparoscopic Roux-En-Y gastric bypass versus one anastomosis (loop) gastric bypass for obesity: a prospective comparative study of weight loss and complications. Ann Med Surg (Lond). 2020 18;55:143-147. 10.1016/j.amsu.2020.04.040. Erratum in: Ann Med Surg (Lond). 2020 Nov 18;60:701.
Giannopoulos S, Athanasiadis DI, Clapp B, Lyo V, Ghanem O, Puzziferri N, Stefanidis D. American Society for Metabolic and Bariatric Surgery Research Committee. Proton pump inhibitor prophylaxis after Roux-en-Y gastric bypass: a national survey of surgeon practices. Surg Obes Relat Dis. 2023;19(4):303–8. https://doi.org/10.1016/j.soard.2022.10.002.
Ying VW, Kim SH, Khan KJ, et al. Prophylactic PPI help reduce marginal ulcers after gastric bypass surgery: a systematic review and meta-analysis of cohort studies. Surg Endosc. 2015;29(5):1018–23. https://doi.org/10.1007/s00464-014-3794-1.
Mahawar KK, Parmar C, Graham Y. Procedure and patient selection in bariatric and metabolic surgery. Minerva Chir. 2019;74(5):407–13. https://doi.org/10.23736/S0026-4733.19.08121-5.
Brown WA, Johari Halim Shah Y, Balalis G, Bashir A, Ramos A, Kow L, Herrera M, Shikora S, Campos GM, Himpens J, Higa K. IFSO position statement on the role of esophago-gastro-duodenal endoscopy prior to and after bariatric and metabolic surgery procedures. Obes Surg. 2020;30(8):3135–53. https://doi.org/10.1007/s11695-020-04720-z.
Bertrand T, Rives-Lange C, Jannot AS, et al. 150-cm versus 200-cm biliopancreatic limb one-anastomosis gastric bypass: propensity score-matched analysis. Obes Surg. 2022;32(9):2839–45. https://doi.org/10.1007/s11695-022-06203-9.
Chevallier JM, Arman GA, Guenzi M, et al. One thousand single anastomosis (omega loop) gastric bypasses to treat morbid obesity in a 7-year period: outcomes show few complications and good efficacy. Obes Surg. 2015;25(6):951–8. https://doi.org/10.1007/s11695-014-1552-z.
Wilson JA, Romagnuolo J, Byrne TK, Morgan K, Wilson FA. Predictors of endoscopic findings after Roux-en-Y gastric bypass. Am J Gastroenterol. 2006;101(10):2194–9. https://doi.org/10.1111/j.1572-0241.2006.00770.x.
Beran A, Shaear M, Al-Mudares S, et al. Predictors of marginal ulcer after gastric bypass: a systematic review and meta-analysis [published online ahead of print, 2023 Feb 16]. J Gastrointest Surg. 2023; https://doi.org/10.1007/s11605-023-05619-7.
Bhayani NH, Oyetunji TA, Chang DC, Cornwell 3rd EE, Ortega G, Fullum TM. Predictors of marginal ulcers after laparoscopic Roux-en-Y gastric bypass. J Surg Res. 2012;177(2):224–7. https://doi.org/10.1016/j.jss.2012.06.003.
Acknowledgements
The authors thank Hugh Hanchard for helping with the initial literature search.
Author information
Authors and Affiliations
Contributions
Study conceptualisation: K.M., S.L., S.S.
Data analysis: S.L., C.V.
Writing the manuscript and approving the finalised manuscript: S.L., S.S., C.V., K.M.
Corresponding author
Ethics declarations
Ethical Approval
Formal consent is not required for this type of study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• The overall estimated incidence of MU after OAGB was 2.59%.
• Approximately 65% of patients presented within the first 12 months after surgery.
• The majority of patients (89.7%) are treated conservatively with PPIs.
Appendix Search strategy
Appendix Search strategy
Ovid MEDLINE(R) ALL <1946 to August 10, 2022>
1 | exp *Peptic Ulcer/ | 61,705 |
2 | (marginal ulcer or marginal ulceration or marginal ulcers).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 490 |
3 | stomal ulceration.mp. | 39 |
4 | (stomal ulcers or stomal ulcer).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 156 |
5 | 2 or 3 or 4 | 671 |
6 | (gastric adj bypass).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 16,341 |
7 | (bariatric adj bypass).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 26 |
8 | exp *Gastric Bypass/ | 9793 |
9 | 6 or 7 or 8 | 16,354 |
10 | 5 and 9 | 308 |
11 | 10 | 308 |
12 | limit 11 to (English language and humans and yr=“2015–Current”) | 128 |
13 | proton pump inhibitor.mp. or exp *Proton Pump Inhibitors/ | 17,160 |
14 | proton pump inhibitors.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 17,792 |
15 | 13 or 14 | 24,634 |
16 | 9 and 15 | 149 |
17 | prophylaxis.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 12,0005 |
18 | prophylactic.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 87,767 |
19 | 17 or 18 | 19,1174 |
20 | 16 and 19 | 17 |
21 | 10 | 308 |
22 | limit 21 to (human and English language and exclude medline journals and yr=“2019–Current”) | 65 |
23 | 20 not 10 | 4 |
24 | 23 | 4 |
25 | limit 24 to (English language and exclude medline journals) | 4 |
26 | incidence.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 97,3337 |
27 | 9 and 26 | 1487 |
28 | (ulcer or ulcers or ulceration).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 21,0204 |
29 | 27 and 28 | 137 |
30 | 29 not 10 | 45 |
31 | 30 | 45 |
32 | limit 31 to (English language and humans and yr=“2019–Current”) | 6 |
33 | 9 and 15 and 19 and 28 | 15 |
34 | 33 not 30 | 15 |
35 | 33 not 20 | 0 |
Scopus
“mason’s loop” OR “mini-gastric bypass” OR “mini-gastric bypass” OR “single anastomosis gastric bypass” OR “single-anastomosis gastric bypass” OR “single anastomosis (mini-) gastric bypass” OR “one anastomosis (mini-) gastric bypass” OR “one anastomosis gastric bypass” OR “one-anastomosis gastric bypass” OR “omega gastric bypass” OR “omega-loop bypass” OR “omega loop bypass” AND “Peptic ulcer disease” OR “marginal ulceration” OR “anastomotic ulcer” OR “ischemic ulcer” OR “ulcers” OR “ulcera*”
Cochrane
mason’s loop OR mini-gastric bypass OR mini-gastric bypass OR single anastomosis gastric bypass OR single-anastomosis gastric bypass OR single anastomosis (mini-) gastric bypass OR one anastomosis (mini-) gastric bypass OR one anastomosis gastric bypass OR one-anastomosis gastric bypass OR omega gastric bypass OR omega-loop bypass OR omega loop bypass in Title Abstract Keyword AND Peptic ulcer disease OR marginal ulceration OR anastomotic ulcer OR ischemic ulcer OR ulcers OR ulcera* in Title Abstract Keyword
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S., Supparamaniam, S., Varghese, C. et al. Marginal Ulcers Following One-Anastomosis Gastric Bypass: a Systematic Review and Meta-analysis. OBES SURG 33, 2884–2897 (2023). https://doi.org/10.1007/s11695-023-06762-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06762-5