Abstract
Background
Marginal ulcerations (MU) are a common and concerning complication following Roux-en-Y gastric bypass (RYGB) surgery. The aim of the present study was to examine the progression of MU and identify risk factors for the need for surgical intervention in patients with MU following RYGB.
Methods
A New York state longitudinal administrative database was queried to identify patients who underwent RYGB between 2005 and 2010 and who were followed for at least 4 years for the development of MU using ICD-9 and CPT codes. Patients with perforation as their first presentation of MU were excluded. Multivariable Cox proportional hazard model was built to identify risk factors for surgical intervention. Hazard ratios (HR) with 95% confidence intervals (CI) were reported.
Results
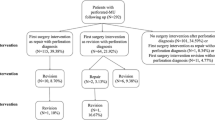
We identified 35,075 patients who underwent RYGB. Mean age was 42.47 ± 10.90 years and most were female (81.08%). There were 2201 (6.28%) patients with MU, of which 204 (9.27% of MU; 0.58% of RYGB overall) required surgery. The estimated cumulative incidence of having surgical intervention 1, 2, 5, and 8 years after MU diagnosis was 6% (95% CI 5–7%), 8% (95% CI 7–9%), 13% (95% CI 11–14%), and 17% (95% CI 13–20%), respectively. At time of MU diagnosis, younger age (HR 0.93 every 5 years, 95% CI 0.87–0.99), white race (HR 1.60, 95% CI 1.15–2.23), and weight loss (HR 2.82, 95% CI 1.62–4.88) were independent risk factors for subsequent surgical intervention for MU. Estimated cumulative incidence of MU recurrence was 15% (95% CI 9–22%) and 24% (95 CI% 15–32%) at 6 and 12 months after surgical intervention.
Conclusions
The need for surgical intervention for MU after RYGB is uncommon. Young age, white race, and marked weight loss are risk factors for surgical intervention. Such patients may benefit from early intensive medical therapy at the time of MU diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In response to rising obesity epidemic in the United States, bariatric surgery has emerged as the most effective means of achieving sustained weight loss and offers substantial amelioration of its related comorbidities [1, 2]. Of the estimated 216,000 bariatric procedures performed in 2016 in the United States, 40,392 (18.7%) were Roux-en-Y gastric bypasses (RYGB) [3]. Marginal ulcerations (MU), which are ulcers that develop at the gastrojejunal anastomosis, are a common and concerning complication following RYGB surgery. Symptoms of MU may include heartburn, abdominal pain, nausea, and diarrhea, but up to 61% of patients are asymptomatic [4]. The reported incidence of marginal ulcers varies between 0.6 and 16% [5, 6], likely due to differences in how MU is defined and diagnosed as well as the methods used to screen for them [7].
The etiology of MU is likely multifactorial. Identified risk factors for the development of marginal ulcers include nonsteroidal anti-inflammatory drug use, corticosteroid use, nicotine use, foreign body reactions to staples or suture material, and Helicobacter pylori infection [5, 8]. The clinical impact and optimal treatment of MU remain unclear. Some patients with MU undergo surgical intervention due to persistence of symptoms despite medical therapy or in the setting of complications such as intestinal perforation, which has a reported incidence of 0.83% [9]. Given the wide variation in disease severity, ranging from asymptomatic diagnosis to recurrent MU requiring multiple reinterventions, the clinical impact of this complication remains poorly understood. The aim of the present study was to examine the progression of MU and identify risk factors for subsequent surgical intervention in patients with non-perforated MU following RYGB, so these risks may be mitigated through changes in future practices.
Materials and methods
This study was approved by the Institutional Review Board (IRB) at the Stony Brook University Medical Center and the New York State Department of Health Data Governance Committee. We searched the Statewide Planning and Research Cooperative System (SPARCS) longitudinal database for all patients who underwent RYBG between 2005 and 2010 in the state of New York and who were followed for at least 4 years for the development of MU using ICD-9 and CPT codes. Patients with perforation as their first presentation of MU were excluded due to the fact that these patients almost universally require immediate surgical intervention.
For all inpatient records and outpatient records before 2008, the primary procedure code column contains the ICD-9 code either 44.31 or 44.39 (open RYGB), or 44.38 (laparoscopic RYGB) and primary diagnosis code of either 278.00, 278.01, or 278.02. For outpatient records after 2008, either of the 7 CPT code column contains 43,644 or 43,645 and primary diagnosis codes of either 278.00, 278.01, or 278.02. For all patients who had multiple RYGB records found during the study period, only their first records were used as initial RYGB. In addition, if a patient’s earliest RYGB record had a diagnosis code of v4586, this patient was viewed as having a revision procedure as the first record and hence was excluded in the analysis. Any patients records with age < 18, having in-hospital death, having unknown insurance type, or without exact unique patient identification were excluded.
With the use of a specific identifier, patients were followed across the state for subsequent diagnosis of MU and surgical intervention. Surgical intervention included repair (procedure codes: 44.41, 44.69, 44.6, 44.61, 44.62, 44.63, 44.4, 44.40, 44.41, 44.49, 44.74, 44.73, 44.79) or revision (procedure code: 44.5, 44.31, 44.38, 44.39, 43.89, 44.96, 43.7, 43.81, 43.9, 43.91, 43.99, 46.93). Twenty-seven patients who had revision and repair at the same time were treated as revision.
Multivariable Cox proportional hazard (PH) model was built to identify risk factors for surgical intervention. Hazard ratios (HR) with 95% confidence intervals (CI) were reported as indicated. Univariate Cox PH models were utilized to examine the marginal association between the risk of having surgical intervention after first-time non-perforated MU diagnosis and patients’ characteristics, comorbidities at the time of having MU diagnosis, and complications at the time of their original RYGB procedure. Any factors with p value < 0.1 based on univariate Cox PH models were further considered in the multivariable Cox PH model. Comorbidities and complications that afflicted < 10 patients were not considered as possible predictors. Statistical significance level was set at 0.05. Descriptive analysis and Cox PH models were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC); cumulative incidence results were performed using R packages “cmprsk” based on R 3.3.1.
Results
Between 2005 and 2010, we identified 35,075 patients who underwent initial RYGB in the state of New York, with follow-up to 2014. Mean patient age was 42.47 ± 10.90 years, and most were female (81.08%). There were 2201 (6.28%) patients diagnosed with MU and 204 (9.27% of MU; 0.58% of entire RYGB cohort) required a surgical intervention 248 days (interquartile range 51–824 days) after MU diagnosis. The demographic characteristics of the population are listed in Table 1. The estimated cumulative incidence of having surgical intervention at 1, 2, 5, and 8 years after MU diagnosis was 6% (95% CI 5–7%), 8% (95% CI 7–9%), 13% (95% CI 11–14%), and 17% (95% CI 13–20%), respectively (Fig. 1).
At the time of MU diagnosis, young age (HR 0.93 for every 5 year increase in age, 95% CI 0.87–0.99), white race (HR 1.60, 95% CI 1.15–2.23), and profound weight loss (HR 2.82, 95% CI 1.62–4.88) were independent risk factors for subsequent surgical intervention for MU after adjusting for hypertension, diabetes, chronic blood loss anemia, and tobacco use, while patients with chronic blood loss anemia (HR 0.22, 95% CI 0.05–0.88) were less likely to have surgical intervention for MU after adjusting for other confounding factors (Table 2). Estimated cumulative incidence of MU recurrence following surgical intervention was 15% (95% CI 9–22%) and 24% (95% CI 15–32%) at 6 and 12 months after surgical intervention (Table 3). The estimated incidence of MU recurrence following repair was 17% (95% CI 1.3–33%) and 23% (95% CI 4.4–41%) at 6 and 12 months after surgical intervention. The estimated incidence of MU recurrence following revision was 15% (95% CI 7.4–22%) and 24% (95% CI 14–33%) at 6 and 12 months after surgical intervention.
Discussion
Our study shows that the estimated cumulative incidence of having surgical intervention 8 years after non-perforated MU diagnosis was 17%. This falls within the wide range of 9–31% seen in the previous literature [10,11,12]. However, the true burden of refractory MU is revealed by the considerable recurrence rate following surgical intervention. El-Hayek et al. found that 33% of patients had recurrence of MU after surgical intervention [11]. Our study showed similar MU recurrence rate of 15% and 24% at 6 and 12 months after surgical intervention. Importantly, MU recurrence is very common after surgical intervention, and patients may possibly benefit from prolonged or even lifelong medical prophylaxis.
MU following RYGB, while often asymptomatic, has the potential to incur significant morbidity including intractable abdominal pain, fistula formation, persistent bleeding, and perforation. While the majority of patients with non-emergent complications of MU can be managed medically with proton pump inhibitors, sucralfate, and avoidance of causative factors, some patients will go on to undergo surgical intervention. Studies suggest that the most common indications for surgical intervention for MU include perforation, refractory disease, presence of gastrogastric fistula, and active bleeding [10,11,12,13,14,15,16]. A retrospective review of 2535 patients who underwent RYGB identified MU in 59 patients (2.3%), and of these, surgical intervention was required in 26 patients (44.1%) [12]. Of the 26 operative cases, 12 (20.3%) were performed for perforation, seven (13.5%) for chronic and refractory ulcers, five (8.5%) for associated gastrogastric fistula, and two (3.4%) for active bleeding [12].
While the need for surgical intervention for MU after RYGB is uncommon, with an incidence of < 1% in our study, once MU is diagnosed, the rate of surgical intervention is considerable. Risk factors for subsequent surgical intervention for MU have not been previously delineated. Based on our data, patients of younger age, white race, and those with marked weight loss are at higher risk for surgical intervention, and may potentially benefit from early intensive medical therapy at the time of initial MU diagnosis.
The limitations of the study include the retrospective nature of the design, the fact that there were no endoscopic diagnoses, and the fact that the medical therapy could not be controlled for as it was an unknown entity in this study. Our study is also limited by the absence of information about operative time and technique for both primary RYGB as well as for subsequent surgical interventions when indicated. Importantly, the SPARCS database is a hospital-based data source. Patients who are diagnosed and managed solely in the outpatient setting would be missed using this design. It is therefore likely that the recurrence rate following surgical intervention and the true incidence of MU at baseline are higher than what we report herein.
References
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F (2015) Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 386:964–973
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F (2012) Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366:1577–1585
Surgery American Society for Metabolic and Bariatric Surgery (2016) Estimate of bariatric surgery numbers, 2011–2016
Dallal RM, Bailey LA (2006) Ulcer disease after gastric bypass surgery. Surg Obes Relat Dis 2:455–459
Sapala JA, Wood MH, Sapala MA, Flake TM Jr (1998) Marginal ulcer after gastric bypass: a prospective 3-year study of 173 patients. Obes Surg 8:505–516
MacLean LD, Rhode BM, Nohr C, Katz S, McLean AP (1997) Stomal ulcer after gastric bypass. J Am Coll Surg 185:1–7
Coblijn UK, Lagarde SM, de Castro SM, Kuiken SD, van Wagensveld BA (2015) Symptomatic marginal ulcer disease after Roux-en-Y gastric bypass: incidence, risk factors and management. Obes Surg 25:805–811
Bendewald FP, Choi JN, Blythe LS, Selzer DJ, Ditslear JH, Mattar SG (2011) Comparison of hand-sewn, linear-stapled, and circular-stapled gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass. Obes Surg 21:1671–1675
Altieri MS, Pryor A, Yang J, Yin D, Docimo S, Bates A, Talamini M, Spaniolas K (2018) The natural history of perforated marginal ulcers after gastric bypass surgery. Surg Endosc 32:1215–1222
Patel RA, Brolin RE, Gandhi A (2009) Revisional operations for marginal ulcer after Roux-en-Y gastric bypass. Surg Obes Relat Dis 5:317–322
El-Hayek K, Timratana P, Shimizu H, Chand B (2012) Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned? Surg Endosc 26:2789–2796
Moon RC, Teixeira AF, Goldbach M, Jawad MA (2014) Management and treatment outcomes of marginal ulcers after Roux-en-Y gastric bypass at a single high volume bariatric center. Surg Obes Relat Dis 10:229–234
Racu C, Dutson EP, Mehran A (2010) Laparoscopic gastrojejunostomy revision: a novel approach to intractable marginal ulcer management. Surg Obes Relat Dis 6:557–558
Lo Menzo E, Stevens N, Kligman M (2011) Laparoscopic revision of gastrojejunostomy and vagotomy for intractable marginal ulcer after revised gastric bypass. Surg Obes Relat Dis 7:656–658
Wendling MR, Linn JG, Keplinger KM, Mikami DJ, Perry KA, Melvin WS, Needleman BJ (2013) Omental patch repair effectively treats perforated marginal ulcer following Roux-en-Y gastric bypass. Surg Endosc 27:384–389
Chang PC, Huang CK, Tai CM, Huang IY, Hsin MC, Hung CM (2017) Revision using totally hand-sewn gastrojejunostomy and truncal vagotomy for refractory marginal ulcer after laparoscopic Roux-en-y gastric bypass: a case series. Surg Obes Relat Dis 13:588–593
Acknowledgements
We acknowledge the biostatistical consultation and support provided by the Biostatistical Consulting Core at School of Medicine, Stony Brook University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Aurora Pryor receives honoraria for speaking for Ethicon, Medtronic, Stryker, and Gore, and is a consultant for Medicines Company, Merck, Intuitive, BAROnova, Obalon Therapeutics. Dr. Pryor also has ownership interest in Transenterix. Dr. Konstantinos Spaniolas is on the advisory board for Mallincktodt and received a research grant from Merck. Donglei Yin, Drs. Owen Pyke, Jie Yang, Tyler Cohn, Salvatore Docimo, Andrew Bates, and Mark Talamini have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Pyke, O., Yang, J., Cohn, T. et al. Marginal ulcer continues to be a major source of morbidity over time following gastric bypass. Surg Endosc 33, 3451–3456 (2019). https://doi.org/10.1007/s00464-018-06618-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-06618-5