Abstract
Purpose
One-anastomosis gastric bypass (OAGB) is an established bariatric procedure performed worldwide. We developed a modification of OAGB leaving a bridge at the cranial 2 cm of the fundus as a gastro-gastric fistula to allow for endoscopic access to the bypassed stomach. We present the preliminary results of 44 patients who underwent this technique in our hospital.
Methods
We analyzed, retrospectively, data collected prospectively on 44 patients who underwent our bridged one-anastomosis gastric bypass (BOAGB) procedure between September, 2018 and November, 2020.
Results
The mean age of the patients was 45.2 ± 9.3 years (range 20–66 years). The mean preoperative body mass index (BMI), weight, and HbA1c values were 41.5 ± 6.4 kg/m2 (range 35–59), 116 ± 22.7 kg, and 8.2 ± 2.1%, respectively. After a median follow-up period of 18 months (11–26 months), the mean postoperative BMI was 28.4 ± 3.2 kg/m2 (range 21–38), the mean total weight loss was 35.8 ± 13.5 kg (range 20–80 kg), and the mean percentage of excess weight loss (%EWL) and the percentage of total weight loss (%TWL) were 79.8 ± 16.1% (range 47–109) and 30.6 ± 6.9% (range 19–48), respectively. The mean postoperative HbA1c level was 6.3 ± 0.9%. There were two early complications (stenosis and bleeding) and one late complication (marginal ulcer).
Conclusion
Patients who underwent BOAGB lost weight similarly to those who underwent OABG as reported in the literature, without an apparent increase in complications related to the technique. Randomized studies with longer term follow-up are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One-anastomosis gastric bypass (OAGB) is now an established bariatric procedure across the world [1]. It is a modification of the gastric bypass procedure proposed by Mason in 1967 [2]. One of the drawbacks of all gastric bypasses for obesity, including the more popular Roux-en-Y gastric bypass (RYGB), is blocked endoscopic access to the bypassed stomach and duodenum after the procedure.
Lack of usual endoscopic access may delay the diagnosis of gastric cancer in the bypassed stomach [3, 4] and also make endoscopic access to the biliary tree difficult [5, 6]. Considering the growing problem of choledocholithiasis, after gastric bypass [7], this is an issue that needs to be addressed. It also has implications for the diagnosis and management of peptic ulcer disease and its complications after gastric bypass [8].
We modified the original OAGB procedure to leave a communication between the gastric pouch and the fundus to allow for endoscopic access to the bypassed stomach and proximal small intestine. To our knowledge, this has not been described in the published scientific literature on any of the gastric bypasses. Therefore, it is unclear if this modification is safe and effective and we need to establish if it has any effect on ulceration rates or weight loss outcomes. We describe our technique and the preliminary results of bridged one-anastomosis gastric bypass (BOAGB) in our initial 44 patients who underwent this procedure.
Materials and methods
This study was conducted according to the principles of the Helsinki Declaration and was approved by the Research Ethics Committee of the Istinye University, School of Medicine (28.12.2018/44). All participants were informed about the modified technique, verbally and by reading brochures. Signed informed consent was given by all participants.
All patients were evaluated for their suitability for bariatric surgery by a multidisciplinary team of a surgeon, an endocrinologist, a psychologist, a dietitian, and an anesthesiologist. Inclusion criteria were a body mass index (BMI) > 40 kg/m2 or a BMI > 35 kg/m2 with at least one co-morbidity. The exclusion criteria were a history of esophagitis, Barrett’s esophagus, severe gastroesophageal reflux disease resistant to proton pump inhibitors (PPI), hiatal hernia, and previous bariatric surgery.
Prospectively collected data were evaluated retrospectively. Data included demographics, pre- and postoperative BMI, excess weight loss (EWL), total weight loss (TWL) mean pre- and postoperative glycated hemoglobin (HbA1c) level, pre- and postoperative co-morbidities, pre- and postoperative medications, length of hospital stay, number of used cartridges, postoperative complications. Data analysis was performed with SPSS (version 20.0; IBM, Armonk, USA).
Surgical technique
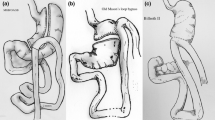
Figure 1 shows a schematic illustration of BOAGB, which was performed using four or five trocars. The procedure started with the creation of an opening in the hepatogastric ligament at the level of incisura angularis. Then, a 45 mm purple linear stapler (Covidien Endo GIA, Tri-Staple© Technology, Mansfield) was placed parallel and 3–4 cm away from the pylorus, at the level of incisura angularis. An orogastric tube (39F-bougie) was introduced, and the following fires were placed according to the orogastric tube, cranially, the same as for OAGB. Before the last firing, a passage, approximately 2 cm-long, was left in the uppermost stomach. This passage could be measured by either passing a 39F-bougie toward the fundus and firing the last cartridge against the bougie, or by measuring the diameter of the remaining stomach bridge with the jaws of a laparoscopic instrument (Fig. 2).
After completing the gastric transaction, we measured a small bowel segment 150–250 cm from the Treitz’s ligament, depending on the patient's BMI, and performed GJ anastomosis. GJ was performed at 150 cm for those with a BMI 35–39 kg/m2, at 200 cm for those with a BMI 40–44 kg/m2, and at 250 cm for those with a BMI 45 kg/m2 or above. The staple line was observed for bleeding, and any bleeding points were clipped or suture ligated. The stapler entrance site was closed with a single-layer hand-sewn technique. A methylene blue test was performed to evaluate any narrowing or staple-line leaks. A drain was placed.
Post-operative management and follow-up
On postoperative day (POD) 1, an upper gastrointestinal (GI) hydrosoluble contrast swallow (Gastrografin®-Bracco Diagnostics, Canada) study was done to check patency and that there were no leaks. Under fluoroscopy, the radio-opaque density travelled easily through the GJ anastomosis to the jejunal loops, but not to the remnant stomach. On X-ray, the Gastrografin® passage was seen as an inverted Y-shape (Fig. 3). Coronal and axial computed tomography images (CT) showed the Gastrografin® passing through the small-bowel from the stomach pouch, but not into the remnant fundus (Figs. 4, 5) The urine catheter was removed, and the patient mobilized actively. A clear fluid diet was started with sips of ∼30 ml. The drain was removed and the patient was discharged on POD 2 or 3 with the discharge recommendations of the dietician. The patients were checked 3, 6, and 12 months postoperatively.
In accordance with our routine clinical practice for all bariatric procedures, the patients were prescribed proton pump inhibitors (PPIs) and sucralfate daily for 1 month, high-protein compounds and calcium for 3 months, and a daily mineral-vitamin supplement for 1 year. Specific mineral or nutritional supplements were given as needed for deficiencies identified during follow-up.
Results
Between September, 2018 and January, 2020, 44 patients underwent BOAGB surgery in our hospital. We analyzed data collected until November, 2020, retrospectively. Table 1 summarizes the patients’ demographic and weight loss data, and their postoperative results. The mean age at the time of surgery was 45.2 ± 9.3 years (range 20–66 years) and 21 of the 44 patients (47.8%) were women. The mean preoperative BMI was 41.5 ± 6.4 kg/m2 (range 35–59) and the mean preoperative HbA1c level was 8.2 ± 2.1%.
Eleven patients were lost to follow-up, but data collected over 11–26 months were available for 33 patients, achieving a 75% follow-up rate. The median follow-up in this study was 18 (11–26) months. The mean postoperative BMI was 28.4 ± 3.2 kg/m2 (range 21–38). The mean total weight loss was 35.8 ± 13.5 kg (range 20–80) and the mean percentage of excess weight loss (%EWL) and percentage total weight loss (%TWL) were 79.8 ± 16.1% (range 47–109) and 30.6 ± 6.9% (range 19–48), respectively. The mean postoperative HbA1c level was 6.3 ± 0.9%.
Table 2 summarizes the co-morbidities of the patients and Table 3 lists the medications, especially of those with type II diabetes mellitus (T2DM) patients who use insulin, oral anti-diabetics (OADs), or both. Only 3 of the 16 T2DM patients who complied with 18 months of follow-up had an HbA1c value of 7% and above. However, the HbA1c levels of these three patients dropped from 10.2 to 8%, from 10.8 to 7.3%, and from 8.5 to 7.6%, respectively. One of these patients still requires insulin, but the other two continue to take OADs only. Table 4 shows the mean C-peptide levels. The C-peptide levels of the three patients with T2DM were less than 2 nmol/L and two of three stopped using the drug for diabetes postoperatively. One was lost to follow up.
There were two early complications (within 30 days of surgery) and no mortality. The two early complications were stenosis of the GJ anastomosis and stapler line bleeding/hematoma. Early endoscopic intervention was initiated within 24 h of the operation for two patients. Endoscopic balloon dilation was done for the stenosis and continuity of the gastrointestinal passage was achieved by endoscopic morcellation of the hematoma for the stapler line bleeding. The remnant stomach and GJ anastomosis were washed out and checked for bleeding. There was one late complication (> 30 days) in a heavy-smoker who continued to smoke after the operation. She was readmitted to the emergency department 2 months after surgery with stomach spasms and severe epigastric pain. A marginal ulcer was identified on the jejunal site of the GJ anastomosis, which was treated successfully with PPIs—sucralfate and the patient was discharged 2 days later.
Discussion
We reported our preliminary results of performing BOAGB in a series of 44 patients. The mean follow-up period was 18 months (11–26 months) and the follow-up rate was 75%, similar to the follow-up reported by Larjani et al. and Taha et al. In the cohort trial published by Larjani et al. [9] in 2016, the overall attendance rates to follow-up appointments after bariatric surgery at 3, 6, 12, and 24 months were 78.1%, 68.0%, 75.0%, and 41.0%, respectively. In the study published by Taha et al. [10] in 2017, 1124 (73.9%) of 1520 patients had been followed up for 12 months. In the original study published by Rutledge in 2001, the follow-up rate was 89% by the end of the 43 months [11].
OAGB was described initially by Rutledge in 1997 and reported in 2001 [11]. As a trauma surgeon, Rutledge encountered a patient with a gunshot wound and decided that duodenal exclusion with a Billroth II anastomosis was the appropriate reconstruction in this case. This was the inspiration for performing OAGB [12]. After its benefits in terms of weight loss and diabetes remission were identified, it became a method used in metabolic and bariatric surgery [13, 14]. Like Rutledge’s experience, our modification originated from two difficult revisional surgeries for dense and thick fibrotic tissue at the upper part of the fundus in our first two patients, following laparoscopic adjustable gastric banding in one and laparoscopic Nissen fundoplication in the other. Since we did not find stapler firing reliable in that area, it prompted us to leave that opening as a gastro-gastric fistula. We concluded that this fistula is an advantage in that it can be used for endoscopic interventions. Having seen the same effect as OAGB after several months, we proceeded to publish the promising results of our five cases in 2019 [15].
OAGB has multiple advantages over RYGB, with higher 1-year and 2-year EWL, a higher T2DM remission rate, and a shorter operation time [13, 16]. The YOMEGA trial showed that OAGB is not inferior to RYGB for achieving weight loss and metabolic improvement at 2 years [17]. In that study, 60% of patients with T2DM had complete remission after OAGB vs. 38% after RYGB. The partial remission rates were 10 and 6%, respectively. OAGB is effective not only for morbidly obese patients with a BMI > 35 kg/m2, but also for T2DM patients with a lower BMI ≤ 35 kg/m2 [13]. Osman Abouzeid et al. [18], evaluated 17 patients with T2DM and a BMI 25–30 kg/m2, who underwent OAGB-MGB surgery and claimed that OAGB is an efficient metabolic procedure and could be integrated into the treatment algorithm of T2DM for patients with BMI 25–30 kg/m2.
In the current study, the mean total weight loss was 35.8 ± 13.5 kg (range 20–80 kg), the mean %EWL was 79.8 ± 16.1% (range 47–109) and the %TWL was 30.6 ± 6.9% (range 19–48). Among the 16 T2DM patients who completed an 18-month follow-up, the overall diabetic remission rate was 82.5% with decreased HbA1c mean from 8.2 ± 2.1 to 6.3 ± 0.9% in that period. Only 3 of the 16 T2DM patients had an HbA1c value of 7% or above. Nevertheless, their HbA1c levels dropped from 10.2 to 8%, from 10.8 to 7.3%, from 8.5 to 7.6%, respectively. One patient continues to use insulin, but the other two take OADs only. Because of the small number of participants, we could not conclude definitively that BOAGB is as effective as OAGB for diabetic remission; however, the data of our study, although limited, shows the promise of our modified technique.
The early (within 30 days) and late (> 30 days) complication rates after OAGB range from 1.96 to 10.0% and 0.5 to 6.1%, respectively, and the mortality rate ranges from 0 to 0.5%. Recent systematic reviews report an incidence of marginal ulcer after OAGB between 0.6 and 4% [1, 10, 19]. In our preliminary results, the early complication rate was 4.5% and the late complication rate was 2.3%. The early complications were stenosis of GJ anastomosis and stapler line bleeding/hematoma, and the late complication was marginal ulcer. The early and late mortality rates and conversion rates were 0%. These results are complementary to the results of the largest published studies.
BOAGB has several advantages over OAGB. First, BOAGB does not involve touching or destroying the Angle of His. Second the risk of bleeding from the short gastric vessel during dissection of the fundus for the last stapler firing is much lower than in OAGB. Third, the risk of leakage from the upper side of the pouch is theoretically lower, since there is an opening between the newly formed pouch and the gastric remnant. Finally, the most important advantage of BOAGB is that it allows us to perform endoscopic intervention through the GGF postoperatively [15]. Although GGF may seem like a critical disadvantage of the BOAGB technique, it is in fact the purpose of this technique. Because GGF stimulates weight regain and causes marginal ulceration, hemorrhage, perforation, or stricture, it is accepted as a complication after RYGB, with an estimated incidence ranging from 1 to 6% [20, 21]. Since the volume of the stomach pouch is 25 ml or less after RYGB, most of the food can pass easily into the remnant stomach via the GGF, which may result in weight regain. Thus, GGF can be called a complication after RYGB. Conversely, in OAGB there is a bigger stomach pouch with a capacity of 150 ml, so less food can pass through the GGF into the remnant stomach. Furthermore, OAGB is more malabsorptive and less restrictive than RYGB [22]. In our opinion, the main question to be answered is: “Is GGF a complication after OAGB?” We could not find any data related to GGF following OAGB in the bibliographic databases Medline, Embase, PubMed, or Google Scholar. There is only one case report of GGF causing severe bile reflux after laparoscopic conversion of sleeve gastrectomy to OAGB [23]. The incidence of marginal ulcer after OAGB ranges from 0.6–4% [1, 10, 19]. In the current study, it was 2.3%, consistent with that after OAGB and this occurred in only one patient, who was a heavy smoker. Thus, there is not sufficient evidence to claim that the GGF after OAGB predisposes to this complication.
We adjusted the 2 cm diameter opening, because the outer diameter of the distal part of the duodenoscope is 13.7 mm [24]. If ERCP is indicated, it can be performed via the 2 cm diameter GGF in BOAGB. We adjust the opening by two methods. First, the 39F-bougie is passed toward the fundus and the last cartridge is fired against the bougie. Second, the diameter of the bridge can be measured with the jaws of a laparoscopic instrument. It is easy to measure a 2 cm diameter with the jaws of a laparoscopic instrument on the anterior surface of the stomach, but difficult to adjust an appropriately sized fistula on the posterior surface of the stomach. The standardization and better calibration of the ‘bridge’ remains an important challenge to be resolved. The aim of this study was to find a solution for the remnant stomach without losing the metabolic and bariatric effects of the original OAGB procedure. We may lose either the endoscopic access or the metabolic and bariatric effect of BOAGB, should we do not standardize GGF size properly.
Little is known about remnant gastric cancer after OAGB-RYGB and the true incidence is unknown. In a 2019 literature review, gastric remnant cancer was identified in 17 patients who had undergone RYGB [25]. The inability to perform endoscopic follow-up because of the closed remnant stomach after the bypassed procedure may delay the diagnosis of gastric cancer [3, 4]. This also has a negative effect on the diagnosis and management of peptic ulcer disease and its complications in these patients [8]. Postoperative upper gastrointestinal (GI) bleeding is a rare but dangerous complication, with an incidence of 0.6%—4%, which should be managed by an endoscopic intervention [26]. After other bypassed techniques, it is impossible for an endoscope to be passed into the remnant stomach to investigate upper GI bleeding. For emergency intervention, laparoscopic endoscopy is mandatory, under general anesthesia. [15, 21, 25, 26]. It is difficult to determine the percentage of OAGB patients who will need endoscopic surveillance; however, our modified technique allows us to reach both the remnant and gastric pouch via GGF by endoscopy. In the current study, endoscopy was performed for 10 (22.7%) patients (Fig. 6). Another study reported the incidence of symptomatic gallstones after RYGB and OAGB to be 14.5% and 7.5%, respectively [29]. Evaluation of the biliary tree by ERCP may also be very difficult [5, 6] in patients who have had gastric bypass and it is unknown whether this can be done easily via GGF. Performing ERCP after RYGB and OAGB, remains challenging. There are some options such as a balloon-assisted, laparoscopy-assisted approach with gastrostomy creation to access the gastric remnant, and endoscopic ultrasound-directed transgastric ERCP. However, these procedures are time-consuming, more invasive, and require resources, and the success rates are low, ranging from 55–63% [30,31,32]. We found only one case report of ERCP done via GGF after RYGB [33]. In the current study, there were no indications to perform ERCP.
The number of patients and follow-up periods in clinical studies conducted to develop new or modified techniques are ethically controversial. De Paula et al. [34] published their new technique “Laparoscopic sleeve gastrectomy with ileal interposition” with a study of 19 patients and a mean follow-up of 11.6 months, Mercan et al. [35] published their new technique “Endoscopic retroperitoneal adrenalectomy” with a study of eight patients, and Sánchez-Pernaute et al. [36] published their modified technique “Single Anastomosis Duodeno–Ileal Bypass with Sleeve Gastrectomy (SADI-S)” with a study of 50 patients and 1–3-year follow-up. Similarly, there were 44 patients in our study with a median follow-up of 18 months.
The limitations of this study were the short follow-up period and the small sample size. Moreover, we did not have the opportunity to test the bridge opening for an ERCP post-operatively in any of the patients. Although the results of 18-month follow-up after BOAGB are promising, the long-term results of the gastric bridge after 3–5 years are not yet clear. Scarring in the long term may lead to stenosis and inhibit endoscopic intervention or surveillance. Conversely, stretching of the ‘bridge’ may result in loss of the bariatric and metabolic effects of the operation [15].
In conclusion, added to the advantages of OAGB, BOAGB eliminates the most important disadvantage of losing endoscopic access to the remnant stomach. Considering this advantage, we believe that BOAGB might be one of the most promising physiological and anatomical of all the modified techniques described so far. If the long term weight loss and metabolic effects of BOAGB are equivalent to those of OAGB, it might be adapted more widely to patients of all ages. The current results are encouraging; however, further randomized controlled trials with longer follow-up are needed.
References
Parmar CD, Mahawar KK. One anastomosis (Mini) gastric bypass is now an established bariatric procedure: a systematic review of 12,807 patients. Obes Surg. 2018;28(9):2956–67.
Mason EE, Ito C. Gastric bypass in obesity. Surg Clin N Am. 1967;47(6):1345–51.
Tinoco A, Gottardi LF, Boechat ED. Gastric cancer in the excluded stomach 10 years after gastric bypass. Case Rep Surg. 2015;2015:468293.
Ali S, Chaar A, Frandah W, Altoos R, Sattar Z, Hasan M. Exploring the excluded stomach: a case series of novel endoscopic techniques to diagnose gastric cancer in the excluded stomach after Roux-en-Y gastric bypass surgery. Cureus. 2018;10(6):2825.
Wisneski AD, Carter J, Nakakura EK, Posselt A, Rogers SJ, Cello JP, et al. Ampullary stenosis and choledocholithiasis post Roux-En-Y gastric bypass: challenges of biliary access and intervention. HPB (Oxford). 2020;22(10):1496–503.
Snauwaert C, Laukens P, Dillemans B, Himpens J, Looze DD, Deprez PH, et al. Laparoscopy-assisted transgastric endoscopic retrograde cholangiopancreatography in bariatric Roux-en-Y gastric bypass patients. Endosc Int Open. 2015;3(5):458–63.
Overby DW, Richardson W, Fanelli R. Choledocholithiasis after gastric bypass: a growing problem. Surg Obes Relat Dis. 2014;10(4):652–3.
Patrascu S, Ponz CB, Ananin SF, Soler EMT. A delayed acute complication of bariatric surgery: gastric remnant haemorrhagic ulcer after Roux-en-Y gastric. J Minim Access Surg. 2018;14(1):68–70.
Larjani S, Spivak I, Guo MH, Aliarzadeh B, Wang W, Robinson S, et al. Preoperative predictors of adherence to multidisciplinary follow-up care postbariatric surgery. Surg Obes Relat Dis. 2016;12(2):350–6.
Taha O, Abdelaal M, Abozeid M, Askalny A, Alaa M. Outcomes of omega loop gastric bypass, 6-years experience of 1520 cases. Obes Surg. 2017;27(8):1952–60.
Rutledge R. The mini-gastric bypass experience with the first 1,274 cases. Obes Surg. 2001;11:276–80.
Deitel M, Hargroder D, Peraglie C. Mini-gastric bypass for bariatric surgery increasing worldwide. Austin J Surg. 2016;3(3):1092.
Parmar CD, Zakeri R, Mahawar K. A systematic review of one anastomosis/mini gastric bypass as a metabolic operation for patients with body mass index ≤ 35 Kg/m2. Obes Surg. 2020;30(2):725–35.
Abdel-Rahim MM, Magdy MM, Mohamad AA. Comparative study between the effect of sleeve gastrectomy and mini-gastric bypass on type 2 diabetes mellitus. Diabetes Metab Syndr. 2018;12:949–54.
Sumer A, Atasoy D, Barbaros U, Savas OA, Eren E, Yurdaisik I, et al. Bridged mini gastric bypass a novel metabolic and bariatric operation. Bariatr Surg Pract Patient Care. 2019;14(2):62–7.
Wang FG, Yan WM, Yan M, Song MM. Outcomes of mini vs Roux-en-Y gastric bypass: a meta-analysis and T systematic review. Int J Surg. 2018;56:7–14.
Robert M, Espalieu P, Pelascini E, Caiazzo R, Sterkers A, Khamphommala L, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomized, open-label, non- inferiority trial. Lancet. 2019;393(10178):1299–309.
Osman Abouzeid TA, Ain Shoka AA, Abdelsameeatia KS. From diabetes remedy to diabetes remission; could single-anastomosis gastric bypass be a safe bridge to reach target in non-obese patients? Asian J Surg. 2019;42(1):307–13.
Carbajo MA, Luque-de-Leon E, Jimenez JM, Solorzano JOD, Miranda MP, Alija MJC, et al. Laparoscopic one-anastomosis gastric bypass: technique, results, and long-term follow-up in 1200 patients. Obes Surg. 2016;27:1153–67.
D’Hondt M, Vansteenkiste F, Van Rooy F, Devriendt D. Gastrogastric fistula after gastric bypass-is surgery always needed? Obes Surg. 2006;16:1548–51.
Rabl C, Peeva S, Prado K, James AW, Rogers SJ, Posselt A, et al. Early and late abdominal bleeding after Roux-en-Y gastric bypass: sources and tailored therapeutic strategies. Obes Surg. 2011;21:413–20.
Solouki A, Kermansaravi M, Davarpanah Jazi AH, Kabir A, Farsani TM, Pazouki A. One-anastomosis gastric bypass as an alternative procedure of choice in morbidly obese patients. J Res Med Sci. 2018;23:84.
Haddad A, Bashir A, Nimeri A. Gastrogastric fistula: an unusual cause for severe bile reflux following conversion of sleeve gastrectomy to one anastomosis gastric bypass. Obes Surg. 2018;28:2151–3.
Olympus.www.olympus-europa.com/medical/ Accessed 9 Mar 2019
Tornese S, Aiolfi A, Bonitta G, et al. Remnant gastric cancer after Roux-en-Y gastric bypass: narrative review of the literature. Obes Surg. 2019;29(8):2609–13.
Dick A, Byrne TK, Baker M, et al. Gastrointestinal bleeding after gastric bypass surgery: nuisance or catastrophe? Surg Obes Relat Dis. 2010;6(6):643–7.
Issa H, Al-Saif O, Al-Momen S, Bseiso B, Al-Salem A. Bleeding duodenal ulcer after Roux-en-Y gastric bypass surgery: the value of laparoscopic gastroduodenoscopy. Ann Saudi Med. 2010;30:67–9.
Tran TT, Pauli E, Lyn-Sue JR, Haluck R, Rogers AM. Revisional weight loss surgery after failed laparoscopic gastric banding: an institutional experience. Surg Endosc. 2013;27:4087–93.
Sneineh MA, Harel L, Elnasasra A, et al. Increased incidence of symptomatic cholelithiasis after bariatric Roux-En-Y gastric bypass and previous bariatric surgery: a single center experience. Obes Surg. 2020;30(3):846–50.
Choi EK, Chiorean MV, Coté GA, Hajj IIE, Ballard D, Fogel EL, et al. ERCP via gastrostomy vs double balloon enteroscopy in patients with prior bariatric Roux-en-Y gastric bypass surgery. Surg Endosc. 2013;27(8):2894–9.
Schreiner MA, Chang L, Gluck M, Irani S, Gan SI, Brandabur JJ, et al. Laparoscopy-assisted ve sus balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75(4):748–56.
Haber GB. Double balloon endoscopy for pancreatic and biliary access in altered anatomy (with videos). Gastrointest Endosc. 2007;66(3):47–50.
Simons-Linares CR, Chahal P. ERCP through gastrogastric fistula in a patient with Roux-en-Y gastric bypass anatomy. Obes Surg. 2019;29(4):1370–1.
De Paula AL, Macedo ALV, Prudente AS, Queiroz L, Schraibman V, Pinus J. Laparoscopic sleeve gastrectomy with ileal interposition (“neuroendocrine brake”)—pilot study of a new operation. Surg Obes Relat Dis. 2006;2:464–7.
Mercan S, Seven R, Ozarmagan S, Tezelman S. Endoscopic retroperitoneal adrenalectomy. Surgery. 1995;118(6):1071–6.
Sánchez-Pernaute A, Herrera MA, Pérez-Aguirre ME, Talavera P, Cabrerizo L, Matía P, et al. Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). One to three-year follow-up. Obes Surg. 2010;20:1720–6.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Informed consent
Informed consent was obtained from all participants in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sumer, A., Mahawar, K., Aktokmakyan, T.V. et al. Bridged one-anastomosis gastric bypass: technique and preliminary results. Surg Today 51, 1371–1378 (2021). https://doi.org/10.1007/s00595-021-02264-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02264-y