Abstract
Background
A short-term randomized controlled trial shows that the one anastomosis gastric bypass (OAGB) is a safe and effective alternative to the Roux-en-Y gastric bypass (RYGB).
Objective
The aim of this study is to evaluate the OAGB at our University Hospital between 2006 and 2013.
Patients
One thousand patients have undergone an OAGB. Data were collected on all consecutive patients. The mean follow-up period was 31 months (SD, 26.3; range, 12–82.9), and complete follow-up was available in 126 of 175 patients (72 %) at 5 years after surgery.
Results
Mortality rate was 0.2 %. Overall morbidity was 5.5 %; 34 required reoperations: i.e., 6 leaks, 5 obstructions, 5 incisional hernias, 7 biliary refluxes, 2 perforated ulcers, 2 bleeds, 2 abscesses, and 1 anastomotic stricture. Four patients were reoperated for weight regain. Overall rate of marginal ulcers was 2 % (n = 20), all in heavy smokers. Conversion from an OAGB to a RYGB was required in nine cases (0.9 %): seven for intractable biliary reflux, two for a marginal ulcer. At 5 years, percent excess body mass index loss was 71.6 ± 27 %. One hundred patients with type-2 diabetes, with a mean preoperative HbA1C of 7.7 ± 1.9 %, were followed for >2 years; the total resolution rate was 85.7 %.
Conclusion
This study confirms that the OAGB is an effective procedure for morbid obesity with comparable outcomes to RYGB; in addition, it seems to be safer with lower morbidity. Its technical simplicity represents a real advantage and makes it an option that should be considered by all bariatric surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morbid obesity has become a public health problem in most countries. It leads to a high incidence of complications that decrease life expectancy, even in young adults. Medical treatment of obesity is known to bring disappointing results; however, bariatric surgery has been proved to be efficient for the treatment of morbid obesity, and for decreasing even mortality and complication rates [1].
Our surgical team has a 20-year experience in laparoscopic bariatric surgery. In 1995, we began performing restrictive procedures (vertical banded gastroplasty, VBG; then laparoscopic adjustable gastric banding, LAGB). In 2002, we began laparoscopic Roux-en-Y gastric bypass (RYGB), and then began laparoscopic mini-gastric bypasses or one anastomosis gastric bypasses (OAGB) in 2006. With time, our techniques have evolved. We stopped performing vertical banded gastroplasty because of its lack of long-term efficiency [2], but found predictive factors for the success of LAGB in a nationwide study, and followed these indications since 2007 [3]. We used to perform the RYGB using the Lönroth technique, which consists of an omega loop raised up to the gastric pouch, in which both anastomoses are created and then separated by a section converting the omega into a Roux-en Y gastric bypass [4].
In 2005, a prospective randomized trial compared OAGB to RYGB and showed better outcomes for OAGBs at 2 years [5]. Even though this was a small trial, we considered that because the report was from a reputable journal and had shown that a shorter procedure gave the same results as RYGB, and with less complication, everybody should use it. However, in spite of its proven efficiency, OAGB has raised criticism and controversy, especially regarding its potential risk of bile reflux [6]. In addition, more recently, some long-term series on OAGB have shown promising results in terms of security and efficiency [7–11].
After having reported the complications on our first 1000 LAGB procedures [12], we then published the outcomes of our 100 first OAGB procedures [13]. These prospective data enabled us now to report on our first 1000 OAGB procedures, which have been conducted over a 7-year period.
Materials and Methods
This study was conducted in the Department of Digestive Surgery, at the George Pompidou European Hospital (Paris, France), which is a University Public Hospital reference center for bariatric surgery. Between October 2006 and December 2013, 1000 obese patients underwent OAGB for morbid obesity; demographic and clinic data were prospectively collected from the preoperative evaluations. Pre- and post-surgical management was conducted according to our 20 years of experience in bariatric surgery. The inclusion criteria were essentially the recommendations of the National Institutes of Health Consensus Development Panel, made in 1991. All patients were evaluated for surgical treatment by a multidisciplinary and integrated medical team. Our multidisciplinary team offers adjustable bands according to predictive factors for a successful LAGB [3]: i.e., patients are aged <40 years, with a preoperative body mass index (BMI) of <50 kg/m2, they agree to accept and follow dietary rules, and agree to be surveyed for the long term. An OAGB was performed in most other cases. Endoscopic and histological diagnosis for Barrett’s esophagus and severe gastroesophageal reflux disease (GERD) were considered contraindication for OAGB and candidates for RYGB. Written informed consent was obtained from all patients before surgery. The patients’ follow-up visits were scheduled for 1, 3, 6, and 12 months after surgery, and then annually. Data sources included office and hospital charts, follow-up notes, interviews with patients, physicians’ reports, and telephone contacts. Data were entered into a computer database that was maintained prospectively.
Weight loss was determined as a change in mean BMI and mean percent excess body mass index loss (%EBMIL: initial BMI − current BMI / (initial BMI – 25) * 100), with a BMI of 25 denoting the upper limit of normal, according to the actual adopted standard measure [14]. Weight-loss failure was defined by a %EBMIL of <25 %. We assessed complications according to if they occurred before (early) or after 3 months (late). Severe and major complications were defined according to the Clavien–Dindo score [15]: a score of IIIa for an intervention without general anesthesia and a score of IIIb indicated that further interventional procedures were needed under general anesthesia. Resolution of comorbidities was defined by normalization of clinical and biological parameters, and not needing treatment. The primary endpoints were mortality and morbidity rates. Secondary outcomes were weight loss and resolution of comorbidities. The study was approved by the hospital’s ethics committee.
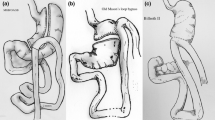
The OAGB Procedure
All surgical procedures were carried out by six different surgeons using the same standardized technique. Details of the surgical procedure have been published previously [13], and are similar to those described by Rutledge [16]. A long and narrow gastric tube was created by applying one horizontal 45-mm roticulator Endo-GIA® stapler (Covidien, Cincinnati, OH, USA) at the angle of lesser curvature, just above the left branch of the crow’s foot, and then four to five vertical 60-mm roticulator Endo-GIA® staple cartridges were placed upwards to the angle of His, and calibrated along a 32-Fr bougie, similar to the vertical part of a sleeve gastrectomy. No reinforcement was done on the staple line. Sectioning of the greater omentum into a bivalve was performed. The jejunum was mounted antecolically at 200 cm from the ligament of Treitz, and an end-to-side anastomosis was performed with the gastric tube, using a posterior 45-mm Endo-GIA® stapler and an anterior running suture. The Petersen’s defect was never closed. A nasogastric tube was introduced into the efferent loop, and a closed vacuum drain was placed behind the anastomosis, both, until the second postoperative day. The anastomosis was finally checked using an intraoperative methylene-blue test.
Statistical Analyses
When normally distributed, results were reported as mean values, standard deviation (SD), and range when necessary. When the distribution was not normal, results were expressed as median values with interquartile range (IQR). Categorical data were compared between independent groups by the chi-squared or Fisher’s exact test, as appropriate. McNemar’s test was used to compare paired groups. For continuous data, the independent-sample Student’s t test was used, after using Levene’s test to assess the equality of variance. All statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL). A p value of ≤0.05 was considered statistically significant.
Results
Between May 2002 and October 2007, 348 patients underwent a RYGB; after this period, an OAGB was offered for 1000 patients between 2006 and 2013, except for those who had a Barrett’s esophagus (n = 11), who still had a RYGB. During the same lapse of time, patients who reached the predictive factors for success [3] were offered a LAGB (n = 1140). No patient declined to have the procedures. In the overall series, the median age at surgery was 41.8 years (IQR, 17.5), and 71.2 % were female subjects. Median weight at surgery was 127 kg (IQR, 31), and median BMI at surgery was 45.7 kg/m2 (IQR, 9). One hundred seventy-seven cases were revision surgeries after restrictive procedures (VBG n: 11; LAGB n: 125; sleeve gastrectomy n: 41), and 82.3 % were primary cases. The clinical characteristics of the patients are detailed in Table 1.
Patients were followed up at 1 (n = 666/803 [83 %]), 3 (n = 264/448 [59 %]), and 5 years (n = 126/175 [72 %]), and the mean follow-up period was 31 months (SD, 26.3; range, 12–82.9).
Mortality and Morbidity Rates
All complications are summarized in Table 2. Overall morbidity rate was 5.5 % (n = 55); 35 patients (3.5 %) presented with an early complication and 20 patients (2 %) presented with a late complication.
There were two deaths: one 65-year-old woman on day 45 post-surgery, from a massive pulmonary embolism (BMI = 62), and one 63-year-old man on day 35 post-surgery, from a myocardial infarction.
Reoperations
Within the 7 years of the study, further surgery was necessary in 34 subjects, 30 for major complications (16 early complications and 14 late complications) and 4 for gastric pouch dilatations, which lead to weight regain.
Early Reoperations
There were six cases of leaks: three located on the gastrojejunal anastomosis; one colic leak (after removal of the transverse colon from a huge umbilical hernia) and two in the gastric remnant, one following obstruction of the biliary limb secondary to a stenotic marginal ulcer. Five patients were reoperated on before the ninth postoperative day for incarceration of the small bowel into a trocar wound.
Intra-abdominal infection occurred in two patients: one patient underwent a mini-laparotomy for a perianastomotic abscess without evidence of leakage: simple evacuation of the abscess with drainage was done, and postoperative antibiotics were provided. The other patient presented on day 1 post-surgery with peritonitis caused by traumatic injury of the afferent loop, at 2 cm proximal to the anastomosis; the afferent segment was resected and the biliary limb was re-sutured to the alimentary limb at 20 cm below the gastrojejunal anastomosis.
One patient presented with a hemoperitoneum that required laparoscopic splenectomy on day 1. Another patient required an endoscopy on day 0 and an injection of adrenalin for anastomotic bleeding. One patient underwent endoscopic balloon dilation of an anastomotic stricture 2 months after surgery.
Late Reoperations
There were two late ulcer perforations with peritonitis (at 9 and 35 months after OAGB). The patients had an emergency laparotomy with lavage of the peritoneal cavity. A suture was not safe at that time because of scar tissue and peritonitis: thus, catheterization of the perforation was done using T-tubes and drainage. The postoperative follow-ups were uneventful. The T-tubes were removed after 3–4 months. Proton-pump-inhibitor therapy was continued over the long term. Seven patients had an intractable biliary reflux. They were reoperated on after a mean of 23 months: they had a mean BMI of 25.7 kg/m2. These patients were then cured after conversion using a RYGB: the gastroesophageal reflux (GERD) then disappeared. Another five patients required laparotomy for the treatment of intestinal obstruction caused by visceral adhesions.
Weight Loss
Changes in terms of %EBMIL are shown in Table 3. At 5 years, mean BMI was 31.5 ± 6.2 kg/m2 (range, 19–51) and the mean %EBMIL was 71.6 ± 27 % (range, 8.75–124). The nadir of weight loss occurred at 24 months, with a mean BMI of 30.1 ± 5.5 kg/m2. At 4–5 years, the %EBMIL did not significantly differ from that after 1 year. Comparison between the %EBMIL between primary and revisional OAGB showed a significant difference at 1 year, but not later on. Patients aged <40 years had significantly better results, but only after 5 years. Patients with an initial BMI of <50 kg/m2 had a significantly better %EBMIL at 1 year only, but not later on.
Weight-Loss Failure
Five percent (n = 49) of patients had a ≤25 % EBMIL and had to be considered as weight-loss failures. In this population, the mean preoperative BMI was 42.5 ± 6.4 kg/m2 and the %EBMIL was 13.8 ± 12.6 % at 5 years; 40 % of these patients had a prior LAGB, but the differences between the primary and revisional surgery were not statistically significant.
Dilatation of the gastric pouch occurred in four patients, 24 months post-OAGB. The dilatation was assessed by an upper gastrointestinal series. Revision surgery was done by pouch trimming using a calibration tube for all patients, at ~4 years post-OAGB, when patients had a mean BMI of 39.3 kg/m2 and a mean %EBMIL of 40.3 %. There was no per- or postoperative morbidity. At 5 years, these patients’ mean BMI was 35.9 kg/m2 and %EBMIL was 55.7 %. Three of these four patients had a prior LAGB before OAGB.
Malnutrition
Malnutrition occurred in two patients who had excessive weight loss (%EBMIL >100 % and albuminemia <30 g/L). Their mean BMI at 5 years was 19 kg/m2 and %EBMIL was 124 and 122 %. They are still being treated in a specialized medical unit with parenteral alimentation and psychiatric support, and a reversal of the OAGB is planned.
Comorbidities
Remission rate from type-2 diabetes (defined by the American Diabetes Association as glycated hemoglobin of <6.5 % without any medication) was 85.7 %, after a mean follow-up period of 26 months, without any recurrence of diabetes. At 2 years, the resolution rate was 80.6 % for dyslipidemia, 52.1 % for high blood pressure, 50 % for sleep apnea, and 36.5 % had less joint pain.
Discussion
RYGB is considered a gold-standard treatment [17], but still remains one of the most challenging laparoscopic procedures with a long learning period [18] and, sometimes, an increased morbidity rate [19].
Technical Aspects
The technical difficulty of RYGB is mainly related to the gastrojejunal anastomosis, but also to enteroenterostomy. To avoid tension on the mesentery, some surgeons recommend performing a retro-colic Roux-en-Y limb [20], which actually increases the challenge. Others prefer to perform an ante-colic Roux-en-Y limb, but advise to section the great omentum into a bivalve to reduce tension. The enteroenterostomy can also cause complications, such as leaks [21] and kinking or internal hernias on the mesenteric defect [22]. Performing an OAGB has two advantages: lower ante-colic gastrojejunal anastomosis, which is much easier to perform than the higher gastrojejunal anastomosis used in RYGB, and it includes one less anastomosis.
Complications
In 2001, Rutledge [16] published the first results from 1274 OAGB procedures: He concluded, it was a simpler technique than RYGB, the operative time was shorter, there were fewer complications, and OAGB had the same efficacy as RYGB in term of weight loss. One recent prospective randomized trial [5] has demonstrated that OAGB is a simpler and safer (1.8 vs. 3.2 % severe complications) alternative to RYGB. Another report from the same author, that was not randomized, reported a similar efficacy at 5 years in term of excess weight loss (72.9 vs. 60.1 %) [9].
Our series is not comparative, but confirms the good early and long-term results of OAGBs with a low rate of major complications. There were two deaths: both patients were aged >60 years. Even though they occurred at more than 1-month post-surgery, both patients had comorbidities that may not have been adequately considered when deciding upon surgery.
The 0.6 % rate of leakage is low, and is similar to those reported in other series [7–11]: this may be because a good blood supply was preserved in the thinner gastric tube and jejunal loop without mesenteric interruption. The leak rate with RYGB is low in some series: i.e., from 0.19 % [23] to 1.2 % [24], but can also reach 3.1 % in other series [17].
One of the classic concerns with a gastrojejunal bypass is the occurrence of marginal ulcers at the edge of the gastrojejunal anastomosis, which are caused by the small gastric pouch continuing to secrete acid. Two ulcers were revealed by late peritonitis. Both patients were heavy smokers; they had lost all their excess weight, and had stopped taking proton-pump inhibitors. Overall, the number of marginal ulcers (2 %) was low and was equivalent to that found after RYGB [19]. The low rate of ulcers was probably because we routinely tested for Helicobacter pylori preoperatively, the small size of our gastric pouches, and the routine prescription of proton-pump inhibitors. If the gastric pouch is larger in OAGB and thus, is likely to secrete more acid, it may play a role in the incidence of ulcers; thus, it must be long and narrow, and the bile can then also buffer the ulcerogenic effect of acid within the anastomosis [25]. Smoking was a risk factor for these ulcers throughout this series, as it was also for RYGB. We think that patients who continue to smoke after an OAGB need to keep taking proton-pump inhibitors.
Even if we did not observe any cases of internal hernia after OAGB, others have so, although none of these incidences have been published yet. There is no mesenteric defect after OAGB, which represents an important difference compared to RYGB, where the peritoneum has to be closed and where the incidence of an internal hernia can reach 9 % [17,22], even several years after a RYGB [26].
Biliary Reflux
Critics of the OAGB procedure compare it to the first bypasses performed by Mason in 1969 [27], which consisted of horizontal section of the proximal part of the stomach and then raising the bowel loop to form an omega shape. The proximity of the anastomosis to the esophagus caused incapacitating biliary reflux, which led the author to abandon the omega procedure for the Roux-en-Y procedure. Seven cases of biliary reflux necessitated us to convert to a RYGB at ~2 years post-OAGB in patients who had lost all their excess weight. The conversion had a spectacular effect on biliary reflux, and patients also maintained their weight loss. This conversion required the resection of the anastomosis to restore the digestive tract by a linear side-to-side enteroenterostomy, and then performing a regular RYGB by cutting the gastric pouch higher, with 1.5-m-long alimentary limb.
However, GERD is rarely a problem in OAGB because the anastomosis is placed low in the stomach. Although it is normal to find bile around the anastomosis in the medium- and long-term, it is rarely seen as far up as the esophagus. OAGB actually creates an anatomy where reflux is intuitively promoted, as opposed to the standard RYGB. This is one of the major criticisms raised against the OAGB; unfortunately, our data were insufficient to determine if this criticism is unfounded. We can only mention that among the postoperative gastroscopic biopsies conducted, we found foveolar hyperplasia, a sign of biliary reflux, in only 13/76 (17.1 %) cases at 2 years and in 2/43(4.6 %) cases at 4 years, with no dysplasia or metaplasia [28]. We have no experience of the anti-reflux technique, as described by others [29]. The gastric pouch must be long and narrow. Stapling must be vertical, perpendicular to the incision in the pouch, and above the posterior surface of the stomach so that the afferent loop comes from the back, and is higher than the efferent loop. If this biliary reflux ever becomes intractable, conversion to a RYGB can then be easy and effective.
Risk of Cancer
A common criticism leveled at OAGB is the potential risk of esophageal and gastric cancer. The concern is to know whether increased bile acid in the stomach can lead to chronic gastritis, which has potential carcinogenic effects. There are actually two different questions.
-
1.
Does biliary reflux toward the esophagus carry a risk of esophageal adenocarcinoma?
-
2.
Does the presence of bile in the stomach increase the risk of stomach cancer in the long term?
Concerning the risk of esophageal adenocarcinoma, the main risk factor is GERD, but also alcohol and smoking. GERD can actually cause the presence of Barrett’s esophagus which is associated with increased risk of developing an invasive cancer. Columnar epithelial dysplasia as seen in Barrett’s esophagus is a premalignant lesion [30]. It has even been confirmed that acid reflux increases this risk, but not biliary reflux [31]. However, until now, no case of esophageal adenocarcinoma has been described in the literature after OAGB, although some studies have shown that LAGB [32] and sleeve gastrectomy [33] can increase acid reflux and cause Barrett’s esophagus and esophageal adenocarcinoma. Among the 33 esophagogastric cancers reported after bariatric procedures [34], 15 were after restrictive bariatric procedures, i.e., LAGB, vertical banded gastroplasty, and sleeve gastrectomy. Criticisms regarding the increased risk of cancer can thus be applied to both LAGB and sleeve gastrectomy. Criticism also comes from experimental studies [35] on mice, which do not have the same mucosal anatomy close to the esophagogastric junction. In the rodent stomach, the esophagogastric junction consists of squamous cells, is aglandular, and, thus, is more at risk of developing adenocarcinoma than in humans [36].
OAGB has been wrongly compared to a Billroth II gastrectomy for ulcers. The risk of cancer was not increased in humans in more than 500 patients with Billroth II procedure over 30 years [37]. According to another study, the risk of cancer was slightly increased in the 20–30 years after a Billroth II procedure, but these patients also had an ulcer [38], and we know that gastric ulcers double the risk of cancer. We should also remember that the effects of H. pylori, which were not known at that time, were not taken into consideration, and are now known as a primary carcinogenic factor [39]. OAGB differs from Billroth II gastrectomy as the gastric tube is long and narrow, and the biliopancreatic fluids are diluted at 2 m from the ligament of Treitz.
Although four cancers have been reported after a loop bypass [34], three were detected in the excluded stomach and, thus, were unrelated to the OAGB. One was found in the gastric pouch 26 years after surgery (in 1980), which was probably not caused by the OAGB (first described in 2001). Fourteen cases of cancer have been diagnosed after a RYGB, including five located in the excluded stomach. Lastly, but not least, Rutledge began OAGB surgery in 1996, almost 20 years ago. Until now, as far as we know, no case of cancer has been reported in the gastric pouch [40]. If there had been a single cancer caused by OAGB, this would have been widely published.
To conclude, biliary reflux and the risk of cancer are theoretical and have not been confirmed in over 20 years. However, cancer may take 20 to 30 years to develop [41]. Thus, because we perform surgery on relatively young patients, they should be informed of this (theoretical) risk and, thus, given the option to have a standard RYGB if they prefer. However, patients should similarly be informed when they choose a sleeve gastrectomy or a LAGB.
Weight Loss and Comorbidities
In terms of weight loss, we report a %EBMIL of >70 % at 5 years, which is globally similar to that reported in the literature [7–10]. High-risk patients (revision surgery and super-obese patients) had less %EBMIL at 1 year, but not later on. Younger patients (aged <40 years) had significantly better %EBMIL results after 5 years. Our results confirm that OAGB has better weight-loss results than those reported for RYGB [9]. These outcomes can be explained by a longer bypass limb used in OAGB compared to RYGB. In OAGB, the intestinal loop is bypassed at 200 cm from the Treitz ligament, whereas in RYGB, the biliopancreatic limb varies from 50 to 130 cm. Particularly, in super-obese patients, studies have shown that the length of the biliopancreatic limb can modify weight loss [42,43]. With regard to long-term efficiency, the only study available on OAGB at 10 years reported an excess weight loss of 72 ± 19.3 % [9]. Our study had a mid-term follow-up of 31 months, but showed some results for up to 5 years: these are similar to those in a recent report [10], and confirm the trend of significant and sustained weight reduction, as has been emphasized in a recent meta-analysis [44].
Malnutrition
Two patients had excessive weight loss with hypoalbuminemia, which will probably require reversion of the bypass. Because of its higher malabsorptive component, OAGB has been reported to give a lower hemoglobin level by 1–5 years compared to RYGB. However, the albumin level remained similar before and after surgery in both types of bypass [9]. We do not have complete data on postoperative albumin levels after OAGB, but any problems we encountered always involved noncompliant patients.
Our study has several limitations. The biological data are not complete and the dropout rate of patients during follow-up was significant. In spite of our efforts, one-third of our patients were lost to follow-up at 5 years, which could have changed the overall results. We did not record glycated hemoglobin, albumin, or hemoglobin for all patients, and were not able to focus on biological nutritional deficiencies.
Conclusions
In our more than 7-years experience, OAGB appears to be effective, safe, simple, and easy to reverse, with manageable and acceptable complications. Until now, we have not been able to find any formal argument against the use of OAGB. A longer follow-up period, with more subjects and randomized trials, is needed to confirm when OAGB can be the best option.
References
Sjostrom L, Narbro K, Sjostrom D, et al. Effect of bariatric surgery on mortality in Swedish obese subjects. NEJM. 2007;357:741–52.
Balsiger BM, Poggio JL, Kelly KA, et al. Ten and more years after vertical banded gastroplasty as primary operation for morbid obesity. J Gastrointest Surg. 2000;4:598–605.
Chevallier JM, Païta M, Rodde-Dunet MH, et al. Predictive factors of outcome after gastric banding: a nationwide survey on the role of center activity and patient’s behavior. Ann Surg. 2007;246:1034–9.
Lönroth H, Dalenbäck J, Hagling E, et al. Laparoscopic gastric bypass. Surg Endosc. 1998;10:636–8.
Lee WJ, Yu PJ, Wang W, et al. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity. Ann Surg. 2005;242:20–8.
Fisher BL, Buchwald H, Clark W, et al. Mini-gastric bypass controversy. Obes Surg. 2001;11:773–7.
Carbajo M, Garcia-Caballero M, Toledano M, et al. One-anastomosis gastric bypass by laparoscopy: results of the first 209 patients. Obes Surg. 2005;15(3):398–404.
Noun R, Skaff J, Riachi E, et al. One thousand consecutive mini-gastric bypass: short- and long-term outcome. Obes Surg. 2012;22(5):697–703.
Lee WJ, Ser KH, Lee YC, et al. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg. 2012;22(12):1827–34.
Musella M, Susa A, Greco F, et al. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg Endosc. 2014;28(1):156–63.
Kular KS, Manchanda N, Rutledge R. A 6-year experience with 1,054 mini-gastric bypasses- first study from Indian subcontinent. Obes Surg. 2014;24:1430–5.
Chevallier JM, Zinzindohoué F, Douard R, et al. Complications after laparoscopic adjustable gastric banding for morbid obesity: experience with 1000 patients over 7 years. Obes Surg. 2004;14:407–14.
Chakhtoura G, Zinzindohoué F, Ghanem Y, et al. Chevallier JM primary results of laparoscopic mini-gastric bypass in a French obesity-surgery specialized university hospital. Obes Surg. 2008;18:1130–3.
Deitel M, Gawdat K, Melissas J. Reporting weight loss 2007. Obes Surg. 2007;17:565–8.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg. 2001;11:276–80.
Schauer P, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic gastric bypass for morbid obesity. Ann Surg. 2000;232:515–29.
Schauer P, Ikramuddin S, Hamad G, et al. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc. 2003;17(2):212–5.
Su-Hsin C, Stoll C, Jihyun S, et al. The effectiveness and risks of bariatric surgery, an updates systematic review and meta-analysis. JAMA Surg. 2014;149(3):275–87.
Hwang RF, Swartz DE, Felix EL. Causes of small bowel obstruction after laparoscopic gastric bypass. Surg Endosc. 2004;18(11):1631–5.
Jacobsen HJ, Nergard BJ, Leifsson BG, et al. Management of suspected anastomotic leak after bariatric laparoscopic Roux-en-Y gastric bypass. Br J Surg. 2014;101:417–23.
Higa K, Ho T, Boone K. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg. 2003;13:350–4.
Dillemans B, Sakran N, Van Cauwenberge S, et al. Standardization of the fully stapled laparoscopic Roux-en-Y gastric bypass for obesity reduces immediate postoperative morbidity and mortality: a single center study of 2606 patients. Obes Surg. 2009;19:1355–64.
Suter M, Calmes JM, Paroz A, et al. Results of Roux-en-Y gastric bypass in morbidly obese vs superobese patients. Arch Surg. 2009;144:312–8.
Rieu PN, Jansen JB, Biemond I, et al. Short-term results of gastrectomy with Roux-en-Y or Billroth II anastomosis for peptic ulcer. A prospective comparative study. Hepato-Gastroenterology. 1992;39(1):22–6.
Paroz A, Calmes JM, Giusti V, et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity: a continuous challenge in bariatric surgery. Obes Surg. 2006;16:1482–7.
Mason EE, Ito C. Gastric bypass in obesity. Surg Clin N Am. 1967;47:1345–135.
Chevallier JM, Trelles N, Arienzo R, et al. Endoscopic findings after laparoscopic omega loop gastric bypass. Obes Surg. 2011;21:956.
Garcia-Caballero M, Carbajo M. One anastomosis gastric bypass, safe and efficient surgical procedure for treating morbid obesity. Nutr Hosp. 2004;19(6):372–5.
Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143(3):199–211.
Erichsen R, Robertson D, Farkas DK, et al. Erosive reflux disease increases risk for esophageal adenocarcinoma, compared with non erosive reflux. Clin Gastroenterol Hepatol. 2012;10(5):475–80.
Korswagen LA, Schrama JG, Bruins Slot W, Hunfeld MA. Adenocarcinoma of the lower esophagus after placement of a gastric band. Obes Surg. 2009;19(3):389–392. 21.
Howard DD. Gastroesophageal reflux after sleeve gastrectomy in morbidly obese patients. Surg Obes Relat Dis. 2011;7(6):709–13.
Scozzari G, Trapani R, Toppino M, et al. Esophagogastric cancer after bariatric surgery: systematic review of the literature. Surg Obes Relat Dis. 2013;9:133–42.
Nishijima K, Miwa K, Miyashita T, et al. Impact of the biliary diversion procedure on carcinogenesis in Barrett’s esophagus surgically induced by duodenooesophageal reflux in rats. Ann Surg. 2004;240:57–67.
Frantz JD, Bretton G, Cartwright ME, et al. Proliferative lesions of the non-glandular and glandular stomach of rats . In: Guides for Toxicologic Pathology STP/ARF/AFIP, Washington,DC,1991.
Bassily R, Smallwood RA, Crotty B. Risk of gastric cancer is not increased after partial gastrectomy. J Gastroenterol Hepatol. 2000;15:762–5.
Csendes A, Burgos AM, Smok G, et al. Latest results (12–21 years) of a prospective randomized study comparing Billroth II and Roux-en-Y anastomosis after a partial gastrectomy plus vagotomy in patients with duodenal ulcers. Ann Surg. 2009;249:189–94.
Ito M, Takata S, Tatsugami M, et al. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol. 2009;44:365–71.
Chun-Chi Wu, Wei-Jei Lee, Long-Han Ser, et al. Gastric cancer after mini-gastric bypass surgery: A case report and literature review. Asian J Endosc Surg. 2013;303–306.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354–62.
Brolin RE, Kenler HA, Gorman JH, et al. Long-limb gastric bypass in the superobese: a prospective randomized study. Ann Surg. 1992;215:387–95.
Kalfarentzos F, Skroubis G, Karamanakos S, et al. Biliopancreatic diversion with Roux-en-Y gastric bypass and long limbs: advances in surgical treatment for superobesity. Obes Surg. 2011;21:1849–58.
Padwal R, Klarenbach S, Wiebe N, et al. Bariatric surgery: a systematic review and network metaanalysis of randomized trials. Obes Rev. 2011;12:602–21.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
All the authors declare that they have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chevallier, J.M., Arman, G.A., Guenzi, M. et al. One Thousand Single Anastomosis (Omega Loop) Gastric Bypasses to Treat Morbid Obesity in a 7-Year Period: Outcomes Show Few Complications and Good Efficacy. OBES SURG 25, 951–958 (2015). https://doi.org/10.1007/s11695-014-1552-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1552-z