Abstract
Pectin, a complex polysaccharide present in the middle lamella of plants, may appear in different structures depending on its origin and extraction techniques. Citrus and apple fruits are among the main sources of pectin extraction, but there is ongoing research on alternative fruit sources and fruit waste from processing industries. In the food industry, pectin is widely used as an additive in jams, jellies, low-calorie foods, and stabilisers for acidified milk products. To meet the increasing demand and reduce waste, it is best to explore other non-conventional sources or modify existing sources to obtain pectin with the desired quality attributes. This review aims to cover various extraction methods, including the potential of green solvents such as ionic liquids and deep eutectic solvents, to extract pectin. Finally, this review discusses the role of pectin in the development of innovative pectin-based food products.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2016, almost 34 thousand metric tons of pectin was used globally in various industries, such as the food, pharmaceutical, cosmetic, and dietary industries. According to Yuldasheva et al. [1], pectin consumption worldwide is expected to increase by 3.7% by the end of 2026, reaching 48,735 metric tons. Pectin is a versatile ingredient that has gained significant attention in the food industry owing to its unique properties. The Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) committee considers that pectin is safe to be consumed without any limits that are accepted across nations, underscoring its critical role in food manufacturing [2]. Pectin can be extracted from various plant sources including fruits, vegetables, and agricultural waste by-products [3]. However, the food processing industry is confronted with the challenge of obtaining pure pectin from native plants as an ingredient in food products. This is due to the variability in pectin content and composition among different plant species, including plant genotype, ripening stage, and extraction method. In addition, the extraction process requires precise selection of raw materials, optimisation of extraction conditions, and purification steps [4, 5]. The properties of pectin, including molecular weight, degree of esterification, and gelation behaviour, vary depending on the source, extraction method, and conditions used. This property is important for determining their suitability for different food applications. For example, pectin with a high molecular weight and low degree of esterification (< 50%) is suitable for gelling applications, whereas pectin with a low molecular weight and high degree of esterification (> 50%) is more suitable for emulsifying applications [6, 7].
Previous studies have focused on the emulsion properties of pectin, comparisons between various pectin extraction methods, and the impact of different extraction parameters on pectin yield and characteristics [8,9,10,11]. Recently, issues have arisen concerning the limited understanding of the origins of pectin, inadequate extraction parameters, and lack of standardised methods for evaluating and characterising pectin attributes. Therefore, this review aims to address this gap by providing a comprehensive overview of pectin, including its sources, extraction methods, properties, and potential applications in food products. The scope of the study encompasses various plant sources and extraction techniques, with a particular focus on the conventional method towards a more environmentally friendly approach. This study contributes to the existing body of knowledge on pectin and serves as a comprehensive resource for researchers, industry professionals, and other stakeholders with an interest in pectin, particularly within the food industry.
Overview of pectin
Pectin is a high molecular weight carbohydrate naturally found in all plant cell walls. Pectin is indeed a complex group, as illustrated in Fig. 1, composed of alpha-1,4-linked D-galacturonic acid (GaIA), which is partly methyl esterified, and its stereochemistry encompasses several neutral sugars such as galactose, rhamnose, and arabinose [12]. Variations in the number of GaIA zones in pectin structure may significantly affect the physicochemical characteristics of pectin, depending on the botanical origin and maturity of the source material. For example, the monosaccharide composition of pectin varies among oranges, lemons, lime, and grapefruit, as reported by Kaya et al. [13]. They found that lime had the highest GaIA content (29.74%) among the others, whereas orange, lemon, and grapefruit only had GaIA contents of 24.08%, 24.33% and 25.91%, respectively. These variation in monosaccharide composition between the four citrus sources indicated the minor heterogeneity in pectin structure. The variation in molecular weight of pectin from orange and grapefruit was clearly observed by Sayah et al. [14], with a molecular weight ranging from 1.544 × 105 to 2.472 × 105 g/mol for orange and a molecular weight ranging from 1.639 × 105 to 2.405 × 105 g/mol for grapefruit. Notably, the obtained molecular weight of pectin from grapefruit was slightly decreased in comparison with pectin from orange. This is because the high GaIA content within grapefruit pectin structure contributes to a lower degree of methylation (DM), thereby reducing its average molecular weight.
Molecular structure of pectin adapted from Patil et al. [15]
Pectin can be classified as poor methoxy (PMP) or high methoxy (HMP). PMP requires the presence of calcium ions and acids to form a gel through calcium cross-linking, whereas HMP forms a gel through a combination of sugar and acid content [16]. Both PMP and HMP have unique properties that make them suitable for various applications in the food industry. The choice between PMP and HMP depends on the specific requirements of the food product and the desired pectin functionality. For instance, PMP is preferable as a food additive to improve the texture and stability of products and for the development of biocomposite films for food packaging applications [17, 18]. HMP is a highly suitable gelling agent, stabiliser, emulsifier, and thickener for marmalades, sauces, milk, and low-fat products [19,20,21]. Thus, the integrated utilisation of plant waste is crucial for minimising chemical residues.
The structure and sources of pectin
The structure of a plant cell wall, as shown in Fig. 2, can be divided into four layers: plant cell, secondary cell walls (S3, S2, and S1), primary cell wall, and middle lamella. The primary cell wall is the outermost layer of the cell wall and is composed of cellulose, hemicellulose, and pectin. Secondary cell walls are found in some plant cells and are composed of cellulose, lignin, and other compounds. The middle lamella is a thin layer that separates adjacent plant cells, primarily pectins. According to Hassan et al. [22], pectin is responsible for cohesion between adjacent plant cells and plays a critical role in plant growth and development by forming a gel-like substance that firmly holds adjacent cells. Overall, the structure of plant cells is complex, with multiple layers of cell walls containing different compounds.
Commercial and non-commercial sources
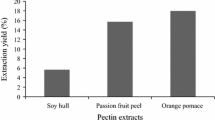
Commercial sources of pectin, such as citrus fruits and apple pomace, are characterised by their ability to produce pectin with high galacturonic acid content (GaIA) (> 65%) and high degree of esterification (DE) (> 50%) [25, 26]. Citrus is a genus of flowering trees and shrubs belonging to the Rutaceae family. The plants in this genus produce citrus fruits, which are juicy, have a rough outer skin, and come in shades of yellow, green, and orange, which can be sour or sweet. Oranges, lemons, lime, and grapefruit are examples of citrus fruits, as their peels are the main sources of commercialised pectins. The pectin yields from orange peel, lemon peel, lime peel, and grapefruit peel were reported to be within the range of 12.93–29.05%, 16.45–16.60%, 5.20–23.59%, 22.09–30%, respectively [6, 27,28,29]. This indicates that even among the fruits consists of same genus, the pectin yields were different, probably due to the different recovery techniques, parameters and solvent used. Apple pomace refers to the solid residue that remains after milling and pressing apples to produce cider, juice, and purees. It is a byproduct of apple processing and is composed of a mixture of peel, core, seed, calyx, stem, and pulp. This waste was a natural source of pectic substances. The maximum pectin yield for apple pomace has been reported to be in the range of 15–20% [30]. Thus, pectin from apple pomace is widely extracted as an important commercial source of food products.
Many other non-commercial sources of pectin are less explored or not usually harnessed because of less knowledge of their characteristics regarding galacturonic acid content and degree of esterification, which may be higher or lower. Examples of non-commercial sources are guava, papaya, mango, and jackfruit waste. Guava waste, known for its high methoxylated pectin content, is a good source of antioxidants. Although guava is reported to rich in pectin, however, the amount of pectin extracted reported in the literature were not exceed 2.4% [31, 32]. The low pectin extracted from guava is expected to be due to its genetic makeup, ripening process, postharvest treatments, and storage conditions. For example, the ripening process of guava requires the action of pectinase, whereas postharvest treatments involve the use of hot water. The use of pectinase contributes to the hydrolysis of pectin, whereas hot water inhibits pectin solubility within guava, resulting in a relatively low pectin content [33, 34]. Papaya waste, including peels and seeds, can also be used to obtain pectin via various extraction methods. The pectin source in papaya waste is greater in the peel, ranging from 2.8% to 16%, than in its seeds, with a maximum of 8.65% [35]. Mango peel possesses a pectin content of approximately 20–30%, which can vary depending on factors such as maturity stage, extraction time, and the method employed [36, 37]. The influence of maturity stage on pectin yield was demonstrated in a study conducted by Azad [38], which showed that pectin yield from mango peel was 4.6% for premature, 9% for mature, and 18.5% for overripe fruit. Hence, it is crucial to select samples from a store or farm based on their maturity grade, as different fruits may possess varying degrees of maturity.
Sunflowers and sugar beets are potential sources of pectin, which can be obtained from their heads, stalks, and pulp. The pectin yield from these sources can vary significantly, ranging from 15 to 25% for sunflowers and 15–30% for sugar beets [39, 40]. These differences were attributed to variations in the extraction methods and the ability of the solvent to release pectin from the cell wall. Jackfruit is a tropical fruit belonging to the Artocarpus genus. Jackfruit is widely acknowledged for its distinctive sweet flavour and aroma. However, its potential as a source of pectin has been relatively unexplored despite the fact that the peel constitutes a significant proportion of the fruit's overall mass. Thus, given the size and abundance of this fruit, it represents a promising resource for pectin extraction. According to Nidhina et al. [41], different parts of the jackfruit, such as seeds, kernels, and peels, were reported to have a range of 21–25%, 23–28%, 25–30% of pectin, respectively. Their study showed that the accumulation of pectin was higher in the peel than in other parts. In contrast, Xu et al. [42] and Wignyanto et al. [43] found that pectin content in jackfruit peels ranged from 4.7% under wet conditions to 22.5% under dry conditions. These studies indicate that drying reduces the moisture content within the peel, leading to a higher yield of pectin. Thus, the sample was dried before pectin was extracted from various sources, as listed in Table 1.
Pectin extraction techniques
Extraction is an essential method to obtain a specific compound from a source or origin. This process is commonly used in the food industry to retrieve pectin from natural plants and fruit wastes. As depicted in Fig. 3, the pectin extraction process comprises four major stages: selection of raw plant materials, sample preparation, choice of extraction methods, and post-extraction. The stages were nearly identical, and the differences were the types of solvents, technologies, and conditions used. Conventionally, pectin extraction from raw plant materials involves mixing, stirring, and heating with acids at high temperatures. Although this approach is effective to some extent, it has several limitations, including corrosive acid hazards, environmental risks, and potential for pectin degradation [45]. To address these challenges, modern pectin extraction methods utilising enzymes, microwaves, ultrasonication, subcritical water, and green solvents have been developed to enhance extraction yields and efficiency, while preserving the functional properties of pectin and reducing solvent consumption [46,47,48,49,50]. The differences between traditional extraction methods and modern techniques are necessary to help researchers select the most suitable method according to their specific requirements.
Hot acid extraction
Hot acid extraction (HAE) is the most common and conventional method used for obtaining pectin. As shown in Fig. 4, acids are used as extraction solvents because of their ability to degrade and solubilise various plant components. Plant material, such as fruit peel or agro-industrial waste, is washed and dried to remove impurities and contaminants. The sample was heated in acidified water for a specific temperature and duration. Common acids used as extraction solvents include sulfuric, phosphoric, and hydrochloric acids. However, these acids show some limitation because of their highly corrosive nature, potential environmental hazards, and thermal degradation via beta elimination and debranching, which lead to low-quality of pectin [35, 51]. The hydrogens from the acid react with the ionised carboxyl groups of pectin molecules, causing the pectin chains to become soluble and form gel-like substances [52]. After extraction, filtration of the hot acid extract was necessary to remove any solid particles or pulp. The addition of a precipitating agent, such as ethanol, to the cooled filtrate is important because it leads to the precipitation of pectin from the solution. Therefore, separation processes such as filtration or centrifugation are vital to obtain the final product.
The percentage of pectin yield extracted using strong acids varies depending on the acid type, concentration, and extraction conditions. In a study by Jong et al. [53], on durian rinds, mineral acids such as hydrochloric, sulfuric, and nitric acids successfully recovered pectin ranging from 0.93 to 9.95%. According to them, the stronger the acidity of the acid, the better the recovery yield. This is because strong acids have a better ability to break down plant cell walls and release pectin. Specifically, strong acids can donate more protons to pectin molecules, leading to the hydrolysis of pectin bonds. This process breaks down pectin molecules into smaller fragments, making them soluble in the extraction solution and increasing the yield. However, the extraction of pectin from grape pomace using sulfuric, nitric, and citric acids showed pectin yields of 5.80, 5.84, and 6.01%, respectively [30]. Studies conducted by Jong et al. [53] and Spinei and Oroian [30] showed that, even when using the same acids (e.g. nitric acid: 9.95% versus 5.84%), the recovered pectin yields were completely different which might be due to the different sources used as the extraction medium. Therefore, selecting the appropriate plant and fruit sources is crucial for food researchers to obtain pectin with higher recovery yields.
Recently, many organic acids such as citric and acetic acids have been used for pectin extraction. Compared with mineral acids, organic acids are much safer and have been shown to achieve higher or equal yields of pectin [54]. For example, approximately 8.38% pectin was extracted from watermelon rind using citric acid at an extraction temperature of 80 °C for an extraction time of 3 h and a solid-to-liquid ratio of 1:25 g/mL, compared to only 6.52% pectin extracted when hydrochloric acid was used [55]. In addition, these organic acids have been reported to have chelating properties, mild extraction, and less pectin degradation owing to their less corrosive nature. However, these acids are more expensive than common mineral acids, although their yields are nearly the same [56]. Overall, the extraction of pectin using acid is simple and requires minimal equipment. Both minerals and organic acids have good potential for pectin extraction; however, they have some drawbacks that need to be considered. Mineral acids are better for extracting pectin, but their highly corrosive nature limits their use in large-scale industrial applications, whereas organic acids are more expensive than mineral acids.
Enzyme-assisted extraction
Enzyme-assisted extraction (EAE) is a green approach for improving extraction yield. This method is utilised to release compounds that are bound on the interior side and are not accessible via conventional solvent extraction. EAE is based on the inherent ability of enzymes to catalyse reactions with exceptional specificity, hydrolyse cell wall components, and disrupt the structural integrity of plant cell walls. EAE can also be used to extract pectin from various plants using pectinases, enzymes derived from fungi, or other sources [56]. Before treating the plant material with pectinases, the samples must be pre-treated to remove impurities and enhance the accessibility of pectin for extraction. This may involve washing, grinding, and drying of the sample [57, 58]. As illustrated in Fig. 5, an enzyme (pectinase) was mixed with the selected plant materials to extract pectin. Therefore, the extracted pectin is purified to remove unwanted components which may involve filtration, centrifugation, or other separation techniques. After the desired extraction time, pectinases are typically inactivated to inhibit their activity. This is typically achieved by heating a mixture to a high temperature for a short period [59]. In EAE, three main processes occur: depolymerisation of pectin, cleavage of glycosidic bonds, and a decrease in water-holding capacity after the disruption of pectin structure by pectinase. Pectinases break down pectin polymers via hydrolysis, trans-elimination, and de-esterification [60]. These enzymes target the ester bond that holds pectin molecules together, leading to degradation of the pectin structure. By breaking these bonds, pectinases facilitate the breakdown of pectin polymers into monomeric sugars, such as galacturonic acid. This results in a decrease in the water-holding capacity of pectin, which helps to obtain a higher yield during the extraction process [52, 56].
Several studies have reported on the use of enzymes for pectin extraction. For example, Jiang et al. [61] showed that pectin from hawthorn pomace has a low molecular weight and high degree of esterification, with a pectin yield of 21.57%. The EAE of pectin from passion fruit peels using a combination of pectinases and cellulases achieved 24.61% pectin by optimising enzyme dosage, pH, and extraction temperature [62]. In other studies, pectin yields of 18.95% and 17.86% obtained when enzymes celluclast and viscoferm were used, respectively [63]. These two studies showed that the selection of enzymes and their combinations affected the percentage yield of pectin. Surprisingly, a combination of two or more enzymes leads to an increased yield compared with the use of one type of enzyme alone [64]. In addition, commercial pectinase enzymes extract pectin from orange peels with a higher pectin yield (11.6%) than conventional hot acids (7.9%) [65]. Another study by Hennessey-Ramos et al. [66] showed a similar finding, in which the EAE of pectin from orange peels was found to be more effective with 19.2% than the traditional hot acid method with 14.8% pectin obtained. These studies suggest that EAE eliminates the major disadvantages of conventional acid extraction of pectin, such as the high temperature and low pH of the extracting solution. Overall, the EAE method for pectin offers several advantages, including selectivity, efficiency, and green procedure with low energy consumption and safety [53, 58]. However, this method is time consuming, specific, sensitive, and expensive [35, 53, 67]. Therefore, further research is required to reduce these drawbacks.
Microwave-assisted extraction
Microwave-assisted extraction (MAE) has been applied in various fields including analytical chemistry, natural product isolation, and pharmaceuticals. MAE uses microwave energy to facilitate extraction of natural products from various matrices, particularly plant materials. This is a new, green, and efficient approach to extraction technologies. As shown in Fig. 6, the MAE of pectin involves subjecting a selected sample in contact with a selected solvent under microwave radiation, typically in the frequency range of 0.915–2.45 GHz via heating and continuous dipole rotation [68]. Theoretically, when microwave power is applied, the sample absorbs energy, resulting in enhanced solvent heating. This heat helps disrupt the cell walls and facilitates the diffusion of pectin from the sample matrix. The enhancement in the extraction process was attributed to an increase in the kinetic energy of the molecules, which subsequently accelerated the mass transfer of pectin [69, 70]. This increased solubility of pectin led to a better recovery yield owing to improved process efficiency.
Schematic diagram of MAE adapted from Martins et al. [71]
Research conducted by Karbuz and Tugrul [72] showed that pectin can be extracted from lemons and mandarins using MAE with pectin yields of 9.71% and 7.60%, respectively. The extraction was conducted using nitric acid as the solvent at 360 W microwave power in a short amount of time, which was only 1 min. However, pectin extraction could not be performed for more than 3 min because the resulting mixture was very thick and could not be centrifuged. This proves that longer exposure to microwave irradiation and a very high microwave power caused the pectin chain molecules to deteriorate, thereby affecting the pectin recovery rate and efficiency. Nevertheless, the efficiency of MAE was confirmed by Kute et al. [73], who compared the effectiveness of the two methods for orange peel powder with extraction yields of 15.76% and 8.78% for MAE and hot acid extraction, respectively. The MAE resulted in a higher penetrative power, which implies faster heating rates than conventionally using a hotplate stirrer. This high rate increases thermal efficiency, resulting in a very high yield of pectin.
Still, the quality of pectin obtained using MAE was comparable to that obtained using conventional acid extraction. This was clearly observed by Rodsamran and Sothornvit [74], who compared the degree of esterification (DE) and galacturonic acid (GaIA) content of lime peels extracted using hydrochloric acid with and without microwaves. They reported that the DE value increased from 72.81% to 77.04%, and the GaIA content decreased from 95.53% to 79.29% when microwave irradiation was employed to recover pectin. The high DE was probably due to the successful demethylation and fragmentation of the polygalacturonic chains, whereas the low GaIA indicated that the extracted pectin had a low purity. In contrast, Tran et al. [75] reported that the DE and GaIA contents of pectin extracted from jackfruit rags using the MAE method were 61.53% and 64.61%, respectively. These studies proved that pectin extracted from jackfruit rags and lime peels has different attributes based on their DE and GaIA contents. In conclusion, the MAE of pectin has several advantages such as reduced extraction time, increased yield, lower energy consumption, and reduced solvent consumption [76, 77]. However, the specialised equipment required for MAE may not be readily available in all laboratories, and pectin has a high potential for degradation at high temperatures and pressures during microwave heating. As a result, further research is needed to determine the scalability of MAE and evaluate its suitability for the extraction of pectin and other temperature-sensitive compounds. These limitations should be considered when selecting an appropriate extraction method.
Ultrasonic assisted extraction
Ultrasonic-assisted extraction (UAE) is a nonthermal extraction technique used to recover pectin from diverse plant sources. This method employs ultrasonic baths or probes conducted at low frequencies (> 20 kHz) and high power of generator (80–200 W), as shown in Fig. 7. The extraction method is based on the cavitation process, which creates bubbles by disrupting and collapsing the cell walls to free target molecules and allowing solvent diffusion. This is because ultrasound waves generate rapid movement of solvents, resulting in a higher mass transfer speed and accelerated extraction. This reduces the extraction time and lowers the required process temperature, which reduces the chances of undesired pectin modifications and preserves pectin quality in terms of a narrow molecular weight distribution range, orderly arrangement, and a high degree of esterification [72].
The percentage yield of pectin extracted using the UAE method varied depending on ultrasound power, pH, and extraction time. For instance, pectin from sour orange peel was extracted via UAE at a microwave power of 150 W, pH 1.5, and an extraction time of 10 min, with a maximum yield of 28.07% [79]. In contrast, a low pectin yield of 6.2% was obtained from chayote under the optimised conditions of a liquid-to-solid ratio of 50 mL/g, extraction temperature of 70 °C, and an extraction time of 40 min [80]. According to Abidin et al. [81], the highest pectin yield obtained from watermelon rind using UAE in sulfuric acid is approximately 10.60%. However, 9.87% of pectin was recovered using only sulfuric acid. This trend is comparable to that reported in a study on sugar beet pectin, with yields of 20.85% obtained using the UAE method and 20.75% obtained using conventional acid-heating methods [39]. The two studies showed that there was not much difference in pectin yields between the two methods. However, the effect of ultrasound was clearly observed in a study on mango peel residue, with yields of 50% and 31% with and without ultrasonic treatment, respectively [82]. This significant difference in pectin yield highlights that ultrasound effectively enhanced the extraction process, as the cell wall of the mango peel residue was successfully disrupted which later facilitated the release of more pectin.
UAE provides several benefits such as increased yield, better extractability, and enhanced pectin quality. This statement is supported by a study conducted by Siddiqui et al. [83], who successfully extracted pectin from sweet lime peel with 36.4% yield, equivalent weight of 740.3 mg and methoxyl content of 7.1%. The reported methoxyl content which falls within the standard range of 2.5–7.2%, proved that UAE enhanced the number of methyl alcohols within the pectin structure, which means that the extracted pectin has a high ability to form gels. In addition, ultrasonic treatment can significantly affect the extraction yield, galacturonic acid content, and degree of esterification of the extracted pectin. Dranca and Oroian [84] used UAE to extract pectin from apple pomace and obtained a recovery yield of 9.18%, a galacturonic acid content of 98.127 g, and a degree of esterification of 83.20% at optimum conditions of 100% amplitude, a pH of 1.8, and a solid-to-liquid ratio of 1:10 g/mL within an extraction time of 30 min. This study proved that the extracted pectin has a good attribute owing to a high degree of esterification, and the use of ultrasound treatment was quite efficient.
Despite the highly advantageous value of UAE, there are some limitations that need to be considered when implementing this method to recover pectin from fruit peels. The UAE instrument is expensive and sometimes causes undesirable changes in pectin molecules owing to intense mechanical and cavitation effects. As Yeh and Lai [85] noted, the use of UAE led to a significant decrease in the intrinsic viscosity (25.72 dL/g) and molecular weight (6.69 × 105 g/mol) of pectin compared to the citrate buffer-extracted pectin, which had a molecular weight of 19.03 × 105 g/mol and intrinsic viscosity of 108.64 dL/g. This suggests that strong cavitation effects may promote the release of proteins, which could modify the charge density, conformation, and physicochemical properties of extracted pectin. Therefore, UAE shows great potential as a method for recovering pectin from plants and fruit peel. However, this process is complex and expensive, and requires expertise. Thus, the optimisation of the extraction parameters is crucial for each application to achieve the desired results, as failure to do so can lead to lower yields, lower quality, and reduced functionality.
Subcritical water extraction
Subcritical water extraction (SWE) is a green extraction technology that uses water at temperatures and pressures below its critical point to extract less polar compounds from various sources such as fruits, vegetables, and herbs [86]. Subcritical water is maintained in a liquid state under high pressure at temperatures between 100 and 374 °C [86]. This technique is relatively new and is considered a promising alternative to conventional acid-extraction methods. The process involves three important steps: diffusion of solutes through particles, distribution, and convection of solutes through the layer of stagnant fluid outside the particles. The key elements of SWE are temperature and pressure. The temperature of the SWE process can be adjusted to target specific compounds, which a higher temperature used for extracting less polar substances and a lower temperature used for extracting more polar compounds [86]. The pressure of the SWE system is regulated using a pressure regulator, typically constructed from stainless steel, and must be set above the vapour pressure at a given temperature to maintain the water in the liquid state [87].
Figure 8 shows a schematic of the SWE method which has been used in several studies. For example, fresh sunflower heads had a maximum yield of 6.57 ± 0.6% under optimal conditions of extraction pressure of 8 bar, at a temperature of 120 °C, extraction time of 20 min, and a liquid–solid ratio of 7 mL/g [88]. In contrast, pectin extracted from jackfruit peel waste using SWE at an extraction temperature of 138 °C for an extraction time of 9.15 min and a liquid–solid ratio of 7.03 mL/g had a yield of 149.6 g/kg (w/w) [89]. These studies showed that a variety of pectin sources exhibit different pectin yields, qualities, and extraction conditions. Additionally, SWE of pectin from sugar beet pulp was effective for up to an extraction time of 20 min, as the pectin yield decreased beyond this time, indicating that the extraction time achieved its optimum value [39]. The decline in pectin yield after 20 min proved that pectin began to degrade and lose its nature, resulting in a lower yield value. In comparison of SWE to conventional acid extraction, SWE is obviously more efficient with a highly significant yield in a reasonably short extraction time, owing to the high reactivity of the ions generated from the dissociation of water under subcritical conditions. This aligns with the findings of Muñoz-Almagro et al. [90], who obtained 10.9% and 8.3% using SWE and conventional acid extraction, respectively. They mentioned that the high difference in pectin yield was related to the high dielectric and solubility properties of subcritical water, as it significantly increased the diffusion, vapour pressure, and mass transfer rate, leading to a reduction in the energy for division in solute-to-matrix interactions which later increased the extraction efficiency. However, the quality of pectin extracted using SWE is generally low compared to that extracted using other methods. For example, pectin from sugar beet pulp extracted through SWE has a significantly lower molecular weight (23.51 kDa) than the molecular weight of pectin (102.27 kDa) extracted through conventional acid extraction [91]. The decrease in molecular weight was probably attributed to the hydrolysis and decomposition of pectin under subcritical temperatures and pressures.
Schematic diagram of SWE adapted from Xiao et al. [92]
Overall, the SWE method for pectin extraction is advantageous because of the nature of the water. Water is non-toxic, inexpensive, readily available, and easily disposed of, making it a cost-effective and environmentally friendly solvent for extraction [80, 93, 94]. SWE has been shown to have a high extraction rate, is suitable for heat-sensitive materials such as pectin, and can behave like an organic solvent [86, 89]. However, there are major drawbacks that need to be considered, such as pectin degradation at high temperatures, energy-intensive processes, and inconsistent pectin quality owing to inconsistent extraction conditions [80, 87]. Therefore, the extraction parameters should be carefully optimised to obtain the maximum pectin yield without compromising pectin quality.
Extraction of pectin using green solvents
Ionic Liquid extraction
In recent years, there has been increasing interest in the use of more ecologically friendly solvents for biomass extraction. ILs have been investigated as alternatives to traditional organic solvents or acids owing to their unique properties. ILs can be used to extract various compounds from various matrices, including pectin, natural products, metals, and organic pollutants. ILs are entirely composed of ions and typically consist of organic cations and inorganic anions. Some common ILs are based on imidazolium, pyridinium, and phosphonium cations [95]. Figure 9 shows examples of ILs include 1-ethyl-3-methyl-imidazolium acetate, and 1-butyl-3-methylimidazolium chloride. ILs have been studied as potential green solvents because of their negligible vapour pressure, high thermal stability, and recyclability [96]. However, their toxicity, non-biodegradability, and high cost have limited their application as green solvents.
In pectin extraction, IL disrupt the ionic bonds that link pectin polymers to the cell wall [96, 97]. The IL breaks the bonds, releases the pectin from the sample matrix which then solubilise in the ILs solution throughout the extraction process. Thus, separation is performed by filtration and centrifugation to obtain pure pectin. According to Xiao et al. [98], ILs offer a higher extraction efficiency and selectivity than conventional extraction methods. In addition, IL can reduce pectin degradation and denaturation because they are thermally stable. Hence, extraction with IL can be performed at significantly lower temperatures to increase efficiency. Despite the many advantages of ILs, there are some drawbacks that need to be considered. For example, ILs have the potential to interact with pectin molecules, subsequently altering their properties and affecting their processing and yield [99]. ILs have a high affinity for polar and charged molecules such as pectin, which leads to a possible interaction between them, resulting in the formation of IL-pectin complexes. These interactions reduce pectin solubility and hinder its extraction from plant materials [100, 101]. This is because different IL have different pectin dissolution abilities owing to their polarities. Therefore, choosing ILs with the correct concentrations and compositions is crucial for obtaining pectin with a high recovery yield.
A few studies have reported the extraction of pectin using ILs. For instance, Wang et al. [102] extracted pectin from ponkan mandarin peels using a few of developed ILs that consists of cholinium cation with different amino acid-derived anions which were alanine, serine, cysteine, proline, aspartic acid, valine, leucine, and phenylalanine, respectively. Among the ILs, choline with leucine showed the best ability as a solvent extractor for pectin, with a maximum recovery yield of 13.42% under optimum conditions of 54% IL solution, 100 min extraction time, and a solid-to-liquid ratio of 1:17 g/mL. However, the recovery of pectin using ILs can be coupled with MAE or UAE to enhance the extraction efficiency, as reported by Liu et al. [103]. They used 3-methyl-1-(4-sulfonylbutyl) imidazolium hydrogen sulphate as a solvent extractor for pectin from pomelo peels, with combined ultrasonic and microwave-assisted extraction (UMAE) and obtained a maximum yield of 328.64 ± 4.19 mg/g at the optimal conditions of 10 mM IL concentration, 15 min extraction time, 360 W microwave power, and a solid-to-liquid ratio of 1:27 g/mL. The pectin yield was significantly higher than that recovered via conventional heating (194.24 ± 5.71 mg/g) and UAE (269.31 ± 5.71 mg/g), both of which used aqueous hydrochloric acid. The high pectin content was attributed to the double acidic sites within the IL, which were capable of forming multiple pectin extractions. Therefore, it can be suggested that acidic IL is expected to increase pectin yield compared to non-acidic IL.
Deep eutectic solvents extraction
Deep eutectic solvents (DESs) are emerging as green and sustainable solvents for efficient pectin extraction. DES was first introduced by Abbott et al. [104], and its use as a solvent extractor for obtaining pectin was first reported by Liew et al. [105]. DES can be prepared by mixing hydrogen bond acceptors (HBA) with hydrogen bond donors (HBD). HBA commonly originates from quaternary ammonium salts, whereas HBD commonly originates from a group of polyamides (–CONH2), polycarboxylic acids (–COOH), and polyalcohols (–OH). DES exhibit properties similar to those of IL, including a wide liquid range, low melting point, and non-reactivity [106, 107]. Even so, DES offer a great advantage over IL because they are non-toxic, cheaper, and easier to prepare. Figure 10 shows examples of HBA and HBD that can be used to prepare DES for extraction. Different structures of HBA and HBD can contribute to a variety of combinations, producing a wide range of DES polarities. For this reason, DES are said to be able to extract polar and non-polar compounds. Therefore, numerous studies have been undertaken to examine the efficacy of DES in the extraction of pectin.
DES performs better than conventional hot acids as a solvent extractor for pectin recovery. Shafie et al. [108] compared the ability of ChCl-citric acid monohydrate (CAM) based DES with CAM in extracting pectin from Averrhoa bilimbi. They found that the pectin yield (14.14%) extracted using ChCl-CAM was higher than the pectin yield (9.34%) extracted using CAM only. From the reported results, it was noted that the enhancement in pectin yield extracted using DES was approximately 5% higher than CAM, demonstrating the efficiency of DES for pectin recovery. The reason for the more effective extraction with DES is attributed to its ability to induce a highly acidic condition that facilitates extensive hydrolysis, breaking the bond linkages between pectin and the cell wall constituents of plants, thus releasing the pectin molecules. Therefore, isolation and purification processes such as filtration or centrifugation can be utilised to obtain pure pectin, as it is more accessible to extraction. However, Shafie et al. [108] noted that the quality of pectin extracted using CAM had better water holding capacity, emulsifying, and antioxidant activity than pectin extracted using ChCl-CAM. They suggested that this could be due to the more linear, less branched structure of DES-extracted pectin compared to CAM-extracted pectin. Thus, the choice of extraction medium, either DES or CAM, significantly influences not only the yield but also the structural and functional properties of the extracted pectin, necessitating further research on the new formulation of DES to optimise both yield and quality.
The combination of DES components is an essential factor affecting the extraction efficiency. For example, pectin extracted from Brazilian berry waste using citric acid–glucose DES coupled with subcritical water extraction (SWE) improved pectin yield by enhancing pectin solubility in water [109]. This is because the addition of DES to SWE can modify the polarity of the solvent and the hydrogen-bonding properties, which can increase its ability to dissolve pectin [90]. This modification leads to higher extraction efficiency and improved pectin yield compared to conventional SWE. Previously, Zhang et al. [110] used ChCl-glycerol to extract pectin from shitake mushrooms and compared the extraction pectin yield based on hot water extraction (HWE), SWE, and SWE-assisted DES at the optimal conditions of 147.23 °C extraction temperature, 1:40 g/mL as the solid-to-liquid ratio, a water content of 39.76% and an extraction time of 15.58 min. According to them, the yield obtained using SWE-assisted DES (6.26 ± 0.08%) was higher than SWE (5.25 ± 0.05%) and HWE (5.35 ± 0.11%). Therefore, the utilisation of DES in conjunction with diverse methodologies represents a highly promising strategy for attaining a substantial yield of pectin during the recovery process.
In addition, natural DES, such as ChCl-glycerol, ChCl-lactic acid, and potassium carbonate-glycerol, were studied by Chen et al. [111] to extract pectin from apple pomace. Natural DES was used as a pretreatment prior to hot water extraction of pectin, assisting in loosening cell wall interactions for pectic polysaccharide extraction. This approach improved the subsequent water extraction yield up to five times (yield: 1.5% for hot water, 8.0% for ChCl-glycerol, 7.8% for ChCl-lactic acid, and 9% for potassium carbonate-glycerol). Meanwhile, pectin extracted from Averrhoa bilimbi using ChCl-citric acid at a molar ratio of 1:1 was found to be 14.44% under optimum conditions of an extraction temperature of 80 °C, an extraction time of 2.5 h and a percentage of DES of 3.74% (w/v) [112]. It is worth noting that the DES-extracted pectin has qualities such as oil holding capacity (2.40 g/g), foaming capacity (133.33%), and water-holding capacity (3.70 g/g), making it suitable for use as a food additive or a natural biopolymer.
Yield and quality of pectin are strongly affected by the mole ratio of DES. As studied by Fauzan et al. [113], changing the mole ratio of potassium carbonate-lactic acid (1:4, 1:7, and 1:20) contributes to different yields, moisture content, galacturonic acid content, and methoxyl content of pectin extracted from Kepok banana peels. They reported that potassium carbonate-lactic acid showed the highest abilities to extract pectin with a yield of 5.81% compared to other ratios. This is because at a mole ratio of 1:7 and 1:20, the pectin yield decreased to 0.15% and 0.00%, respectively, showing that at the right combination, DES can extract more pectin from the same number of peels. As a result, Fauzan et al. [113] extracted pectin at optimised conditions of a mole ratio of 1:4 for potassium carbonate-lactic acid and an extraction temperature of 90 °C, resulting in pectin with a moisture content of 99.8%, a galacturonic acid content of 75.56%, and a methoxyl content of 2.79%. Therefore, adjusting the mole ratio of DES is one of the key factors in maximising the quality and quantity of pectin.
In another study, nine types of ChCl-based DES were tested as extractors to recover pectin from dragon fruit peels using microwave ultrasonic-assisted extraction (MUAE) technique. Among these trials, only ChCl-glucose-water yielded 19.39% pectin, which is higher than that of other tested DES [114]. This pectin recovery was obtained under optimum conditions with a solid-to-liquid ratio of 35.25 mg/L, a DES-to-water ratio of 3.37 mL/mL, an extraction time of 14.26 min, a microwave power of 240 W, and a sonication time of 46.07 min. In addition, sugar-based acidic DES were compared with amino-based acidic DES to extract pectin from pomelo peel. Among the DES, only lactic acid–glucose-water yielded the highest pectin yield (23.01%) [105]. More studies have focused on using acid-based DES, as they have been shown to have a better extraction pectin yield. Recently, ChCl-malonic acid was found to be one of the best solvents for extracting pectin from pomelo peel compared to ChCl-glucose. This ChCl-malonic co-solvent yielded 94% pectin and 52% degree of esterification after optimisation at an extraction temperature of 80 °C with a sonication time of 60 min at pH < 3.0 [115]. Overall, it was noted that acid-based DES is a better choice for pectin extraction, implying that it breaks down the sample matrix more effectively. Table 2 summarises the reviewed studies regarding the use of DES in various pectin extraction techniques with their optimised conditions.
Application of pectin in food technologies
Gelling and thickening agent
Pectin is a versatile and unique fibre with wide applications in the food industry. Pectin is used as a gelling agent in canned jams and jellies. The formation of pectin gel is complex and is mainly governed by the degree of esterification (DE), which leads to the production of high-methoxyl (HM) and low-methoxyl (LM) pectin, as illustrated in Fig. 11 [116]. The benefits of HM and LM pectin differ in terms of their gelling and thickening capabilities. HM pectin, with a DE of over 50%, is capable of forming a sturdy gel and exhibits good thermal stability, making it suitable for a wide range of temperatures [117]. In contrast, LM pectin with a DE of less than 50% can form gels with weaker gel strength and a larger porous matrix, resulting in poor water-holding capacity. However, LM pectin possesses enhanced solubility and amphiphilicity, making it more suitable for food products that require solubility and emulsifying properties [118,119,120]. Thus, it is evident that HM and LM pectins offer distinct properties and can be utilised based on the desired gelling and thickening characteristics.
Types of pectin a HM b LM adapted from Belkheiri et al. [56]
In HM pectin, hydrogen bonds and hydrophobic interactions bind the individual pectin chains together at a soluble solid sugar content above 60% and a pH value between 2.8 and 3.6. These bonds are formed when water is bound to sugar and forces pectin strands to stick together in a three-dimensional molecular net that creates a macromolecular gel [5]. However, if the gel formation is too strong, syneresis or a granular texture may occur, whereas weak gelling leads to excessively soft gels. Syneresis in pectin gels refers to the expulsion of liquid from a gel structure. This phenomenon is a crucial parameter for determining pectin quality, with lower syneresis indicating better stability and gel strength. This agrees with Free-Manjarrez et al. [121], who reported that pectin gel strength increased from 55 to 96 gf when the percentage of syneresis was reduced from 4.20 to 2.71%. In contrast, LM pectin forms a gel in the presence of calcium ions and at a pH between 2.8 and 3.6. Gel formation of LM pectin was completely different from that of HM pectin. In LM, the gel results from an ionic connection between the carboxyl groups of the two pectin molecular chains through a calcium bridge and the joint action of hydrogen bonding [122]. Han et al. [123] used a mathematical model to demonstrate the influence of pH, calcium ions, sucrose, and pectin concentrations on the gelation of LM pectin. They revealed that the pectin gel was stable at a pH range of 3.5 to 5.0, and an increase in the concentration of calcium ions resulted in a strengthened gel owing to the formation of calcium bridges at the dissociated carboxyl groups of pectin. Furthermore, an increase in pectin concentration provided more hydrophobic interactions and crosslinking junctions between pectin chains, whereas an increase in sucrose content resulted in more hydroxyl groups stabilising the junction zones and promoting hydrogen bonds to immobilise free water.
Pectin can also be harnessed as a thickener in juices, bakery, confectionary and dairy products. The hydrophilic regions of pectin allow it to absorb and retain water, thereby increasing juice viscosity [124]. Pectin also binds to juice components, such as sugars and proteins, which allows the formation of a network that can trap water and thicken the juice [125]. Furthermore, pectin can work synergistically with other thickeners such as gelatin, locust bean gum, and guar gum to enhance juice stability [126]. This allows greater control over the texture of the juice and prevents the separation of solids and liquids from the juice. This reduces syneresis, which is the release of liquid from the gel, and helps maintain the desired consistency. Adjusting the concentration of pectin is of utmost importance, because it significantly affects its thickening ability. For example, utilising concentrated pectin results in a viscous and stable juice, making it less likely to separate or become watery over time. According to Zhu et al. [127], there is a direct correlation between pectin concentration and viscosity as well as shear-thinning resistance. They reported that increasing the pectin concentration from 1 to 4% of sea buckhorn high methoxyl pectin solution resulted in an increase in its viscosity from 0.07 to 0.27 Pa at the shear rate of 1 s−1. This phenomenon occurred because a high number of pectin molecules led to an increase in intermolecular forces, resulting in more viscoelastic chain structures. Therefore, understanding the relationship between pectin concentration and thickening properties is crucial to optimise its use in food applications.
Emulsifier and stabiliser
In low-calorie foods, pectin acts as an emulsifier by reducing the interfacial tension between oil and water, allowing it to easily mix and form a stable emulsion [116]. Emulsions are mixtures of two immiscible liquids, such as oil and water, which are held together by an emulsifier. The availability of hydrophilic and hydrophobic regions in pectin molecules allows them to interact with water and oil, and act as emulsifiers. Additionally, pectin stabilises emulsions by forming a protective layer around oil droplets, preventing them from coalescing and separating from the aqueous phase [128]. Figure 12a shows that the pectin layer prevents the aggregation of oil droplets, whereas Fig. 12b illustrates the formation of a separate oil phase due to the absence of pectin as an emulsifier. Thus, pectin can act as a replacement for sugars or fat in food products because it mimics functionalities such as texture, mouthfeel, and sweetness, without adding significant calories. In ice cream, pectin is used as a stabiliser to maintain the structure and firmness, prevent syneresis, hinder melting, and improve frozen stability [129]. Additionally, the development of a functional ice cream with unique nutritional value, such as natural antioxidants, pigments, vitamins, low fat, and free from synthetic additives, is also possible, highlighting the versatility of pectin in conjunction with other ingredients [130, 131].
Emulsification process with a pectin and b without pectin adapted from Jiang et al. [132]
Structural differences in pectin significantly affect its emulsifying and stabilising ability in making a good quality product which is related to the degree of esterification. Schmidt et al. [133] reported different monosaccharide composition contribute to different values of DE. From the analysis, they observed that apple had the highest content of arabinose with a value of 158.03 ± 2.79 mg/g, whereas carrot, onion and tomato has the highest content of galactose which values of 66.95 ± 5.94 mg/g, 291.11 ± 8.57 mg/g, and 47.97 ± 5.03 mg/g, respectively, which the DE within apple, carrot, onion, and tomato samples, were reported to be 78.41 ± 0.83%, 63.02 ± 2.26%, 56.41 ± 0.21% and 54.29 ± 0.32%, respectively. These values were greater than 50%, indicating that the pectin samples had high potential for better emulsifying ability. This result was supported by Neckebroeck et al. [134], who performed a dynamic interfacial tension experiment and monitored the oil droplet size for 14 days. All samples were able to lower the interfacial tension and the oil droplet size remained constant, implying that neither coalescence nor flocculation occurred in these emulsions.
The pH and polarity of pectin play significant roles in the emulsifying and stabilising properties of pectin-based food products. Research conducted by Zhao et al. [135] demonstrated the effect of emulsifying capacity of citrus pectin based on acid treatment at various pH levels (1–7). They found that molecular size of citrus pectin aggregate and reduce to smaller size at pH 2–7, which improved its interfacial capacity and emulsifying ability. Hence, the distribution of pectin works well at the interface. The emulsions were found to be unstable at pH 1, owing to the electrostatic attraction caused by the partially positively charged citrus pectin. Crosslinking modification has been found to significantly affect the molecular weight and viscosity of pectin, which plays a critical role in enhancing its functionalities. According to Lin et al. [136], the addition of genipin as a crosslinker to pectin has been reported to increase the molecular weight of sugar beet pectin from 582.6 ± 23.1 to 1441.6 ± 32.7 kg/mol and viscosity from 14.12 ± 0.18 to 23.2 ± 0.18 mPa.s, resulting in an increase in interfacial tension from 12.30 ± 0.38 to 14.14 ± 0.14 mN/m. Therefore, it is essential to select an appropriate pH range and add crosslinkers to maintain pectin functionality as an emulsifier in food products to achieve good quality food products.
Dietary fibre
Fruits and vegetables contain pectin, which serves not only to maintain their structure but also to contribute to dietary fibre intake. Examples of fruits rich in pectin include apples, oranges, and carrots. Furthermore, certain whole foods, such as fruit by-products and yellow passionfruit peel flour, naturally contain high levels of pectin. These can also function as sources of dietary fibre and have a positive impact on blood glucose levels [137,138,139]. Given that pectin is found in the cell walls of plants, consuming whole plant-based foods like legumes and certain grains can provide dietary fibre from pectin. This, in turn, aids digestion and helps regulate blood sugar levels in the human body. As a fibre, pectin increases the viscosity of digestive contents by forming a gel-like substance. This makes it more difficult for the body to quickly break down and absorb sugars, resulting in a slower release of glucose into the bloodstream [140]. Consequently, consuming foods high in pectin can lead to increased feelings of fullness, better hunger control, reduced food intake, decreased risk of spikes that can trigger cravings, and increased satiety.
Pectin offers various health benefits that extend beyond its utilisation in food processing. It possesses the ability to reduce cholesterol levels and support gut health. Being a dietary fibre, pectin remains undigested in the small intestine and undergoes fermentation in the colon through the action of bacteria. This process of fermentation generates short-chain fatty acids (SCFAs) that exert positive effects on the gut microbiota, fostering a well-balanced microbial ecosystem and the proliferation of beneficial gut bacteria, notably bifidobacterial [141]. These bacteria play a critical role in preserving gastrointestinal health and functionality. By influencing the gut microbiota, pectin has the potential to enhance the functionality of the intestinal barrier, thereby mitigating the risk of gastrointestinal disorders and diabetes, while simultaneously improving digestive health. Moreover, pectin holds implications for allergies and body composition. It interacts with immune cells in the gut, reinforces the mucus layer, and fortifies the intestinal barrier, consequently restraining excessive immune reactions triggered by allergens [142]. Through the reduction of allergic sensitivities, pectin effectively alleviates inflammation within the body, subsequently curbing fat accumulation and contributing to an overall reduction in body composition. As a result, pectin is highly esteemed in the development of functional and health-oriented food products, such as yogurt, fruit gummies, and fibre supplements.
The natural fibre pectin has the potential to inhibit cancer development by transforming into a form that obstructs cancer-promoting proteins, particularly in the colon and breast. Emran et al. [143] demonstrate that a specific type of pectin known as modified citrus pectin (MCP) can impede the proliferation of cancer cells and possibly mitigate their harmful effects. This occurs because MCP enhances the functionality of natural killer (NK) cells and T helper cells, thereby bolstering the body's immune system and enabling more effective elimination of cancer cells. Consequently, MCP can effectively deter the formation of tumours within the human body. As a result, food manufacturers can consider incorporating MCP into health bars and dietary supplements, thereby targeting consumers seeking food products with supplementary health benefits. Moreover, MCP could be recommended for inclusion in bakery products and snacks, facilitating its consumption as part of a regular diet. This offers individuals a convenient means to potentially lower their risk of cancer through dietary choices.
Fat replacer
Phosphates are used in meat products to improve texture, flavour, and water retention. However, excessive phosphate consumption can have negative effects on human health. According to Sharefiabadi et al. [2], a high intake of phosphates is associated with various health risks, such as heart disease and weakened bones. Therefore, it is important to limit phosphate consumption for good health. As a result, there is a growing trend in food formulation to reduce the use of phosphates or find natural alternatives that can replicate their effects without the associated health risks. This emphasizes the significance of discovering safer alternatives for the well-being of consumers. One potential substitute for phosphates is pectin. Pectin can increase the pH value and enhance the water retention capacity of meat products, making them safer and healthier to consume [2, 144]. By retaining more moisture during cooking, meat products become juicier, more tender, and more cost-effective to produce. Pectin can also serve as a fat replacer in meat products, reducing calorie content without compromising texture or taste, which fat typically provides. This was demonstrated in a study by Mendez-Zamora et al. [145], where a combination of 15% pectin and 15% inulin effectively replaced animal fat in sausages, thus presenting pectin as a potential ingredient for creating healthier meat options without sacrificing quality. Similarly, mango peel pectin has been found to preserve the physical characteristics of meat products, including vibrant colour, thereby enhancing their visual appeal and desirability to consumers [138]. Consequently, the development of low-calorie meat products with favourable qualities becomes feasible, potentially contributing to weight management and reducing the risks associated with high-fat diets, such as obesity and cardiovascular diseases.
Food packaging and preservation
Pectin, a natural biopolymer, is gaining increasing recognition for its potential applications in food packaging and preservation due to its biodegradable nature, non-toxic properties, and exceptional film-forming ability. Films based on pectin can be created using various techniques such as casting, spraying, and extrusion. Casting and spraying are part of the wet process, which requires careful control of microbial contamination and the use of non-toxic solvents to ensure the safety and quality of the film. In the casting method, a liquid form of pectin is spread onto a surface and allowed to dry, resulting in the formation of a film. On the other hand, spraying involves the application of a pectin solution onto a surface or directly onto food products, requiring specialized equipment capable of finely atomizing the pectin mixture to ensure the formation of an even and continuous film. Additionally, extrusion is a dry process that involves pushing the pectin mixture through a "die" tool to shape and form films. Among these three methods, casting is expected to be the most commonly used, simple, and cost-effective method for film preparation, particularly on a laboratory scale. In contrast, spraying and extrusion are less accessible to beginners due to their complexity and higher costs [146].
According to Pereira et al. [147], the combination of pectin, water, and glycerol produces a solution that can be dried to create thin, flexible films suitable for food packaging. In this context, pectin plays a crucial role as the primary component of the film, providing structure and flexibility by forming a gel-like network when mixed with water. The presence of water as a solvent allows for the dissolution of pectin, ensuring the even distribution of all constituents and facilitating a consistent film formation process. Additionally, glycerol acts as a plasticiser, enhancing the film's flexibility and reducing its brittleness by minimizing the intermolecular forces within the pectin structure. However, Pereira et al. [147] also included Salicornia ramosissima as an additional additive to improve various properties of the pectin-based films. These properties include thickness (0.25 ± 0.01 mm; control 0.18 ± 0.01 mm), colour parameters a* (4.96 ± 0.03; control 3.29 ± 0.16) and b* (28.62 ± 0.51; control 12.74 ± 0.75), water vapor permeability (1.62 × 10–9 ± 1.09 × 10–10 g/m.s.Pa; control 1.24 × 10–9 ± 6.58 × 10–11 g/m.s.Pa), water solubility (50.50 ± 5.00%; control 11.56 ± 5.56%), and biodegradation capacity (90% after 21 days in soil). As a result, the developed films demonstrate improved abilities to protect food by establishing a stronger barrier, reducing translucency, and effectively managing moisture without compromising structural integrity. These characteristics contribute to the preservation of food freshness, while the films readily decompose in the environment, making them an environmentally friendly choice.
Pectin-based films have the potential to extend the freshness of food by incorporating specific ingredients such as antimicrobial and antioxidant agents. Antimicrobials like tetracycline, zinc oxide, and copper sulphide have the ability to create an inhospitable environment for bacteria and fungi [148,149,150]. They achieve this by breaking down the cell walls of these microorganisms or inhibiting their growth, thereby preventing food spoilage. On the other hand, antioxidants such as malic acid, acerola, and de-esterified oligogalacturonides safeguard food from oxidation, a process that leads to degradation as a result of oxygen exposure [151,152,153]. These antioxidants neutralise free radicals and prevent them from reacting with the food, thereby preserving its quality and freshness. Bhatia et al. [154] conducted a study comparing pectin-based films with and without the addition of an antioxidant agent. They observed that the inclusion of 0.2% Melissa officinalis (MOEO) in 1.5% pectin-based films increased the DPPH and ABTS radical scavenging activity from 18.56% to 59.03% and from 64.31% to 73.75%, respectively. This increase was attributed to the high phenolic content in Melissa officinalis (MOEO), which is known for its radical-scavenging properties and enhances the antioxidant capabilities of the films. Meanwhile, Ngo et al. [155] incorporated nanochitosan as an antimicrobial agent in pectin-based films to inhibit the growth of harmful microorganisms such as E. coli, S. cerevisiae, A. niger, and C. gloeosporioides. They found that the effectiveness of nanochitosan in suppressing microbial growth improved with higher concentrations. This can be attributed to nanochitosan's ability to disrupt the outer layer of microbes or interfere with their essential functions, thereby ensuring food safety. Therefore, the inclusion of these two agents in pectin-based films is vital for the development of an active food packaging system. This innovative approach goes beyond simple barrier properties, actively working to prolong the shelf life of food.
Challenges and future directions of pectin extraction
Briefly, pectin can be extracted from various fruit wastes, including peel, pulp, and pomace. Nevertheless, new sources of fruit waste may yield higher or lower pectin levels, necessitating ongoing research to identify suitable alternatives. Each source of fruit waste may also differ in pectin quality and yield. Pectin extraction methods involve the use of conventional acids, enzymes, microwaves, ultrasonics, and subcritical water. Each method has its own advantages and disadvantages, which differ in terms of efficiency, yield, and quality of the extracted pectin. It is anticipated that in the future, researchers will delve further into the development of methods for pectin extraction that are safe, non-toxic, environmentally friendly, and efficient with a high yield. This may involve the use of solvents and techniques, such as IL and DES, which are known for their sustainable properties. However, several challenges must be addressed, including the optimal integration of cations and anions in ILs, as well as the appropriate combination of hydrogen bond acceptors and donors in DES at specific molar ratios. This is because each combination exhibits unique properties, particularly polarities that may or may not be suitable for pectin extraction. It is imperative to note that the safety and quality of pectin utilised in food products are subject to strict regulations outlined by esteemed organisations such as the Food and Agriculture Organization (FAO). Industrial pectin must adhere to these guidelines and contain a minimum of 65% polygalacturonic acid to attain the E440 level, which is mandatory for authorising its use as a food additive [143, 156, 157]. Thorough investigation of the physicochemical properties of green solvents before their use as pectin extractors is of utmost importance for food researchers. Therefore, the quality of the extracted pectin can be evaluated to ensure that it meets the standards set by FAO.
References
O. Yuldasheva, G. Sassi, and N. Goto, Assessment of domestic material consumption for sustainable consumption and production in Uzbekistan, (2018).
E. Sharefiabadi, M. Serdaroğlu, Pectin: properties and utilization in meat products. Food Health 7(1), 64–74 (2021). https://doi.org/10.3153/fh21008
M.H. Mahmoud, F.M. Abu-Salem, D.E.S.H. Azab, A comparative study of pectin green extraction methods from apple waste: characterization and functional properties. Int. J. Food Sci. (2022). https://doi.org/10.1155/2022/2865921
F.M. Kpodo et al., Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 72, 323–330 (2017). https://doi.org/10.1016/j.foodhyd.2017.06.014
C. Mellinas, M. Ramos, A. Jiménez, M.C. Garrigós, Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials (2020). https://doi.org/10.3390/ma13030673
V. Chandel, D. Biswas, S. Roy, D. Vaidya, A. Verma, A. Gupta, Current advancements in pectin: extraction, properties and multifunctional applications. Foods (2022). https://doi.org/10.3390/foods11172683
M. Celus et al., Structurally modified pectin for targeted lipid antioxidant capacity in linseed/sunflower oil-in-water emulsions. Food Chem. 241, 86–96 (2018). https://doi.org/10.1016/j.foodchem.2017.08.056
S. Huang et al., Pectin stabilized fish gelatin emulsions: physical stability, rheological, and interaction properties. Front. Nutr. (2022). https://doi.org/10.3389/fnut.2022.961875
A.D. Kurniawati, C. Hidayat, Formation of coconut oil by–product protein concentrate-pectin through electrostatic interaction to improve emulsifying properties. Agrihealth J. Agri-Food Nutr. Public Health. (2023). https://doi.org/10.20961/agrihealth.v4i1.70577
Y. Liang et al., Extraction of pectin from passion fruit peel: composition, structural characterization and emulsion stability. Foods (2022). https://doi.org/10.3390/foods11243995
H. Pourramezan, F. Khodaiyan, S.S. Hosseini, Extraction optimization and characterization of pectin from sesame (Sesamum Indicum L.) capsule as a new neglected by-product. J. Sci. Food Agric. (2022). https://doi.org/10.1002/jsfa.12014
X. Wang, Q. Chen, X. Lü, Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 38, 129–137 (2014). https://doi.org/10.1016/j.foodhyd.2013.12.003
M. Kaya, A. Sousa, M.-J. Crepeau, S.O. Sørensen, M.-C. Ralet, Characterization of citrus pectin samples extracted under different conditions: influence of acid type and pH of extraction. Ann. Bot. (2014). https://doi.org/10.1093/aob/mcu150
M.Y. Sayah et al., Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS ONE (2016). https://doi.org/10.1371/journal.pone.0161751
B. Patil, D. Patel, J. Patil, R. L. Nibe, Extraction of pectin from citrus peels—a review. Int. J. Adv. Res. Sci. Commun. Technol. (2022).
S.Q. Liew, W.H. Teoh, R. Yusoff, G.C. Ngoh, Comparisons of process intensifying methods in the extraction of pectin from pomelo peel. Chem. Eng. Proc. Process Intensification 143, 107586 (2019). https://doi.org/10.1016/j.cep.2019.107586
S.T. Fan, F. Fang, A. Lei, J. Zheng, F. Zhang, Effects of salts on structural, physicochemical and rheological properties of low-methoxyl pectin/sodium caseinate complex. Foods (2021). https://doi.org/10.3390/foods10092009
A. Nešić, S. Meseldzija, G. Cabrera-Barjas, A. Onjia, Novel biocomposite films based on high methoxyl pectin reinforced with zeolite Y for food packaging applications. Foods (2022). https://doi.org/10.3390/foods11030360
A. Zoghi, S. Vedadi, Z.H. Esfahani, H.A. Gavlighi, A review on pectin extraction methods using lignocellulosic wastes. Biomass Conversion Biorefinery. (2021). https://doi.org/10.1007/s13399-021-02062-z
N. Muñoz-Almagro, F. Rico-Rodriguez, P.J. Wilde, A. Montilla, M. Villamiel, Structural and technological characterization of pectin extracted with sodium citrate and nitric acid from sunflower heads. Electrophoresis 39(15), 1984–1992 (2018). https://doi.org/10.1002/elps.201800130
M.T.B. Pacheco, M. Villamiel, R. Moreno, F.J. Moreno, Structural and rheological properties of pectins extracted from industrial sugar beet by-products. Molecules (2019). https://doi.org/10.3390/molecules24030392
M.K. Hassan, J.A. McInroy, J.L. Jones, D. Shantharaj, M.R. Liles, J.W. Kloepper, Pectin-rich amendment enhances soybean growth promotion and nodulation mediated by bacillus velezensis strains. Plants (2019). https://doi.org/10.3390/plants8050120
M.C.N. Picot-Allain, B. Ramasawmy, M.N. Emmambux, Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: a review. Food Rev. Intl. 38(3), 282–312 (2022). https://doi.org/10.1080/87559129.2020.1733008
S.Y. Chan, W.S. Choo, D.J. Young, X.J. Loh, Pectin as a rheology modifier: origin, structure, commercial production and rheology. Carbohydr. Polym. 161, 118–139 (2017). https://doi.org/10.1016/j.carbpol.2016.12.033
J.L. Vukušić et al., Reshaping apple juice production into a zero discharge biorefinery process. Waste Biomass Valorization (2020). https://doi.org/10.1007/s12649-020-01245-5
S. Chamyuang, S. Duangphet, A. Owatworakit, U. Intatha, J. Nacha, P. Kerdthong, Preparation of pectin films from coffee cherry and its antibacterial activity. Trends Sci. (2021). https://doi.org/10.48048/tis.2021.34
O.A. Fakayode, K.E. Abobi, Optimization of oil and pectin extraction from orange (Citrus sinensis) peels: a response surface approach. J. Anal. Sci. Technol. (2018). https://doi.org/10.1186/s40543-018-0151-3
A. Göksu, G. Duran, S. Çilingir, M. Çevik, S. Sabancı, Performance evaluation of pectin extraction from grapefruit peel powder by ohmic heating. J. Food Process. Preserv. (2022). https://doi.org/10.1111/jfpp.16813
S. Sabancı, M. Çevik, A. Göksu, Investigation of time effect on pectin production from citrus wastes with ohmic heating assisted extraction process. J. Food Process Eng (2021). https://doi.org/10.1111/jfpe.13689
M. Spinei, M. Oroian, The influence of extraction conditions on the yield and physico-chemical parameters of pectin from grape pomace. Polymers (Basel) (2022). https://doi.org/10.3390/polym14071378
Poonam, K.V. Singh, S.S. Rathour, I.S. Dawar, A. Uikey, A. Kushwah, Physico-chemical evaluation of different cultivars of guava under gird region of Madhya Pradesh, India. Int. J. Plant Soil Sci. (2023). https://doi.org/10.9734/ijpss/2023/v35i12745
S.H. Spiller et al., Modifications in the methods to extract pectin from Cv. ‘Pedro Sato’ guavas during ripening. Braz. J. Food Technol. (2018). https://doi.org/10.1590/1981-6723.03217
D. Dolkar et al., Biochemical changes in guava (Psidium guajava) fruits during different stages of ripening. Indian J. Agric. Sci. (2017). https://doi.org/10.56093/ijas.v87i2.67659
S. Supapvanich, P. Boonyaritthongchai, C. Techavuthiporn, R. Tepsorn, P. Youryon, Physicochemical quality maintenance and bioactive compounds enhancement of Thai guava fruit Cv. ‘Kim Ju’ by using combinative hot water and methyl Jasmonate immersion. Emirates J. Food Agric. (2019). https://doi.org/10.9755/ejfa.2019.v31.i5.1958
S. Madhuvanthi, K. Selvapriya, R.A. Nirmala, A. Agalya, N. Jeya, Extraction and characterization of pectin derived from underutilized papaya seeds as a value-added product. J. Appl. Nat. Sci. 14(1), 127–132 (2022). https://doi.org/10.31018/jans.v14i1.3269
Y. Ma, J. Luo, Y. Xu, Co-preparation of pectin and cellulose from apple pomace by a sequential process. J. Food Sci. Technol. 56(9), 4091–4100 (2019). https://doi.org/10.1007/s13197-019-03877-5
J. Luo, Y. Xu, Y. Fan, Upgrading pectin production from apple pomace by acetic acid extraction. Appl. Biochem. Biotechnol. 187(4), 1300–1311 (2019). https://doi.org/10.1007/s12010-018-2893-1
Md.A.K. Azad, Isolation and characterization of pectin extracted from lemon pomace during ripening. J. Food Nutr. Sci. (2014). https://doi.org/10.11648/j.jfns.20140202.12
M. Jafarzadeh-Moghaddam, R. Shaddel, S.H. Peighambardoust, Sugar beet pectin extracted by ultrasound or conventional heating: a comparison. J. Food Sci. Technol. 58(7), 2567–2578 (2021). https://doi.org/10.1007/s13197-020-04763-1
X. Ma, J. Yu, J. Jing, Q. Zhao, L. Ren, Z. Hu, Optimization of sunflower head pectin extraction by ammonium oxalate and the effect of drying conditions on properties. Sci. Rep. (2021). https://doi.org/10.1038/s41598-021-89886-x
K. Nidhina et al., Physicochemical and functional properties of pectin extracted from the edible portions of jackfruit at different stages of maturity. J. Sci. Food Agric. (2023). https://doi.org/10.1002/jsfa.12391
S. Xu et al., Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT. (2018). https://doi.org/10.1016/j.lwt.2018.01.007
W. Wignyanto, N. Rahmah, A.D. Margani, The best solvent and extraction time in pectin production made from waste of jackfruit (bark and straw). Agroindustrial J. (2017). https://doi.org/10.22146/aij.v3i1.25030
M. Spinei, M. Oroian, The potential of grape pomace varieties as a dietary source of pectic substances. Foods. (2021). https://doi.org/10.3390/foods10040867
M. Guzel, N. Tektas Tasan, Ö. Akpinar, Alternative pectin production methods and sources. AGROFOR (2020). https://doi.org/10.7251/agreng2001038g
L. Benassi, I. Alessandri, I. Vassalini, Assessing green methods for pectin extraction from waste orange peels. Molecules (2021). https://doi.org/10.3390/molecules26061766
K. Rafińska et al., Enzyme-assisted extraction of plant material—new functional aspects of the process on an example of Medicago sativa L. Ind. Crops Prod. (2022). https://doi.org/10.1016/j.indcrop.2022.115424
Y. Wandee, D. Uttapap, P. Mischnick, Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocoll. 87, 237–244 (2019). https://doi.org/10.1016/j.foodhyd.2018.08.017
G. Mao et al., Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: targeting rhamnogalacturonan I. Trends Food Sci. Technol. 94, 65–78 (2019). https://doi.org/10.1016/j.tifs.2019.11.001
M. Sarah, I.M. Hasibuan, E. Misran, S. Maulina, Optimization of microwave-assisted pectin extraction from cocoa pod husk. Molecules (2022). https://doi.org/10.3390/molecules27196544
K.A. Ninga, Z.S.C. Desobgo, S. De, E.J. Nso, Pectinase hydrolysis of guava pulp: effect on the physicochemical characteristics of its juice. Heliyon (2021). https://doi.org/10.1016/j.heliyon.2021.e08141
R. Juneja, M. Kaur, Overview on pectin and its pharmaceutical uses. Int. J. Innov. Res. Eng. Manag. (2022). https://doi.org/10.55524/ijirem.2022.9.1.72
S.H. Jong, N. Abdullah, N. Muhammad, Effect of acid type and concentration on the yield, purity, and esterification degree of pectin extracted from durian rinds. Results Eng. (2023). https://doi.org/10.1016/j.rineng.2023.100974
A.A. Sundarraj, R. Thottiam Vasudevan, G. Sriramulu, Optimized extraction and characterization of pectin from jackfruit (Artocarpus integer) wastes using response surface methodology. Int. J. Biol. Macromol. 106, 698–703 (2018). https://doi.org/10.1016/j.ijbiomac.2017.08.065
K.Y. Lee, W.S. Choo, Extraction optimization and physicochemical properties of pectin from watermelon (Citrullus lanatus) rind: comparison of hydrochloric and citric acid extraction. J. Nutraceuticals Food Sci. (2020). https://doi.org/10.36648/nutraceuticals.5.1.1
A. Belkheiri et al., Extraction, characterization, and applications of pectins from plant by-products. Appl. Sci. 11(14), 6596 (2021). https://doi.org/10.3390/app11146596
F.C. Cruz-Casillas, T. García-Cayuela, V. Rodriguez-Martinez, Application of conventional and non-conventional extraction methods to obtain functional ingredients from jackfruit (Artocarpus heterophyllus lam.) tissues and by-products. Appl. Sci. (Switzerland) (2021). https://doi.org/10.3390/app11167303
X. Duan, Y. Zhu, C. Shu, J. Gao, F. Liu, S. Pan, Extraction of pectin from satsuma mandarin peel: a comparison of high hydrostatic pressure and conventional extractions in different acids. Molecules (2022). https://doi.org/10.3390/molecules27123747
F. Dranca, M. Oroian, Optimization of pectin enzymatic extraction from malus domestica ‘fălticeni’ apple pomace with celluclast 1.5L. Molecules (2019). https://doi.org/10.3390/molecules24112158
J. Singh Jadaun, Pectinase: a useful tool in fruit processing industries. Nutr. Food Sci. Int. J. (2018). https://doi.org/10.19080/nfsij.2018.05.555673
Y. Jiang, J. Du, L. Zhang, W. Li, Properties of pectin extracted from fermented and steeped hawthorn wine pomace: a comparison. Carbohydr. Polym. 197, 174–182 (2018). https://doi.org/10.1016/j.carbpol.2018.06.001
J. Vasco-Correa, A.D. Zapata, Enzymatic extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) at laboratory and bench scale. LWT 80, 280–285 (2017). https://doi.org/10.1016/j.lwt.2017.02.024
K.A.T.C. Israel, J.F.R. Amian, Z.J.S. Garibay, V.E.B. Leyeza, A.J.T. Sarte, A comparative study on characteristics of pectins from various fruit peel wastes extracted using acid and microbial enzymes. J. Microbiol. Biotechnol. Food Sci. 9(2), 216–221 (2019). https://doi.org/10.15414/jmbfs.2019.9.2.216-221
W.S. Abou-Elseoud, E.A. Hassan, M.L. Hassan, Extraction of pectin from sugar beet pulp by enzymatic and ultrasound-assisted treatments. Carbohydr. Polym. Technol. Appl. (2021). https://doi.org/10.1016/j.carpta.2021.100042
H. Shinde, A. Rathod, P. Karande, A. Thokal, and A. professor, “Extraction of pectin from orange peels: a review,” JETIR, 2022. Available: www.jetir.orgg512
L. Hennessey-Ramos, W. Murillo-Arango, J. Vasco-Correa, I.C.P. Astudillo, Enzymatic extraction and characterization of pectin from cocoa pod husks (Theobroma cacao L.) using celluclast® 1.5 L. Molecules (2021). https://doi.org/10.3390/molecules26051473
C.G. Nadar, A. Arora, Y. Shastri, Sustainability challenges and opportunities in pectin extraction from fruit waste. ACS Eng. Au 2(2), 61–74 (2022). https://doi.org/10.1021/acsengineeringau.1c00025
M. Sarah, F. Hanum, M. Rizky, and M. F. Hisham, “Microwave-assisted extraction of pectin from cocoa peel,” in IOP Conference Series: Earth and Environmental Science, Institute of Physics Publishing, (2018). https://doi.org/10.1088/1755-1315/122/1/012079
C.M.P. Freitas, R.C.S. Sousa, M.M.S. Dias, J.S.R. Coimbra, Extraction of pectin from passion fruit peel. Food Eng. Rev. 12(4), 460–472 (2020). https://doi.org/10.1007/s12393-020-09254-9
G.J. Swamy, K. Muthukumarappan, Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 220, 108–114 (2017). https://doi.org/10.1016/j.foodchem.2016.09.197
R. Martins, A. Barbosa, B. Advinha, H. Sales, R. Pontes, J. Nunes, Green extraction techniques of bioactive compounds: a state-of-the-art review. Processes (2023). https://doi.org/10.3390/pr11082255
P. Karbuz, N. Tugrul, Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 58(2), 641–650 (2021). https://doi.org/10.1007/s13197-020-04578-0
A.B. Kute, D. Mohapatra, N. Kotwaliwale, S.K. Giri, B.P. Sawant, Characterization of pectin extracted from orange peel powder using microwave-assisted and acid extraction methods. Agric. Res. 9(2), 241–248 (2020). https://doi.org/10.1007/s40003-019-00419-5
P. Rodsamran, R. Sothornvit, Microwave heating extraction of pectin from lime peel: characterization and properties compared with the conventional heating method. Food Chem. 278, 364–372 (2019). https://doi.org/10.1016/j.foodchem.2018.11.067
N.T.K. Tran, V.B. Nguyen, T. Van Tran, T.T.T. Nguyen, Microwave-assisted extraction of pectin from jackfruit rags: optimization, physicochemical properties and antibacterial activities. Food Chem. 418, 135807 (2023). https://doi.org/10.1016/j.foodchem.2023.135807
N. Tongkham, B. Juntasalay, P. Lasunon, N. Sengkhamparn, Dragon fruit peel pectin: microwave-assisted extraction and fuzzy assessment. Agric. Nat. Resour. 51(4), 262–267 (2017). https://doi.org/10.1016/j.anres.2017.04.004
A. M. Sari, D. Ishartani, and P. S. Dewanty, “Effects of microwave power and irradiation time on pectin extraction from watermelon rinds (Citrullus lanatus) with acetic acid using microwave assisted extraction method,” in IOP Conference Series: Earth and Environmental Science, Institute of Physics Publishing, (2018). https://doi.org/10.1088/1755-1315/102/1/012085
N.A.A.R. Zahari, G.H. Chong, L.C. Abdullah, B.L. Chua, Ultrasonic-assisted extraction (UAE) process on thymol concentration from Plectranthus amboinicus leaves: kinetic modeling and optimization. Processes (2020). https://doi.org/10.3390/pr8030322
C.H. Feng, Optimizing procedures of ultrasound-assisted extraction of waste orange peels by response surface methodology. Molecules (2022). https://doi.org/10.3390/molecules27072268
N.A. Patience, D. Schieppati, D.C. Boffito, Continuous and pulsed ultrasound pectin extraction from navel orange peels. Ultrason. Sonochem. (2021). https://doi.org/10.1016/j.ultsonch.2021.105480
S. A. Sarah Zainal Abidin, N. Shuhada Ahmad Badarudin, and S. R. Ab Mutalib, “Ultrasound-Assisted Extraction Increases Pectin Yield from Watermelon (Citrullus Lanatus) Rind,” in 2020 11th IEEE Control and System Graduate Research Colloquium, ICSGRC 2020—Proceedings, Institute of Electrical and Electronics Engineers Inc., (2020), pp. 291–294. https://doi.org/10.1109/ICSGRC49013.2020.9232531
K. Kumar, S. Srivastav, V.S. Sharanagat, Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason. Sonochem. (2021). https://doi.org/10.1016/j.ultsonch.2020.105325
A. Siddiqui, K. Chand, N.C. Shahi, Effect of process parameters on extraction of pectin from sweet lime peels. J. Inst. Eng. India Ser. A 102(2), 469–478 (2021). https://doi.org/10.1007/s40030-021-00514-3
F. Dranca, M. Oroian, Ultrasound-assisted extraction of pectin from Malus domestica ‘Fălticeni’ apple pomace. Processes (2019). https://doi.org/10.3390/PR7080488
Y.C. Yeh, L.S. Lai, Effect of extraction procedures with ultrasound and cellulolytic enzymes on the structural and functional properties of Citrus grandis Osbeck seed mucilage. Molecules (2022). https://doi.org/10.3390/molecules27030612
Y. Cheng, F. Xue, S. Yu, S. Du, Y. Yang, Subcritical water extraction of natural products. Molecules (2021). https://doi.org/10.3390/molecules26134004
K. Radovanović et al., Subcritical water extraction as an effective technique for the isolation of phenolic compounds of achillea species. Processes (2023). https://doi.org/10.3390/pr11010086
X. Ma, J. Jing, J. Wang, J. Xu, Z. Hu, Extraction of low methoxyl pectin from fresh sunflower heads by subcritical water extraction. ACS Omega 5(25), 15095–15104 (2020). https://doi.org/10.1021/acsomega.0c00928
W. Li, Z.-G. Fan, Y. Wu, Z. Jiang, R. Shi, Eco-friendly extraction and physicochemical properties of pectin from jackfruit peel waste with subcritical water. J. Sci. Food Agric. (2019). https://doi.org/10.1002/jsfa.9729
N. Muñoz-Almagro, L. Valadez-Carmona, J.A. Mendiola, E. Ibáñez, M. Villamiel, Structural characterisation of pectin obtained from cacao pod husk. Comparison of conventional and subcritical water extraction. Carbohydr. Polym. 217, 69–78 (2019). https://doi.org/10.1016/j.carbpol.2019.04.040
S.H. Peighambardoust, M. Jafarzadeh-Moghaddam, M. Pateiro, J.M. Lorenzo, R. Domínguez, Physicochemical, thermal and rheological properties of pectin extracted from sugar beet pulp using subcritical water extraction process. Molecules (2021). https://doi.org/10.3390/molecules26051413
S. Xiao et al., Subcritical water extraction of ursolic acid from Hedyotis diffusa. Appl. Sci. (Switzerland) (2017). https://doi.org/10.3390/app7020187
E. Mlyuka, M. Mbifile, S. Zhang, Z. Zheng, and J. Chen, “Strategic Applications and the Challenges of Subcritical Water Extraction Technology in Food Industries,” 2018. [Online]. Available: http://epg.science.cmu.ac.th/ejournal/ReviewArticle
J. Zhang, C. Wen, H. Zhang, Y. Duan, H. Ma, Recent advances in the extraction of bioactive compounds with subcritical water: a review. Trends Food Sci. Technol. 95, 183–195 (2020). https://doi.org/10.1016/j.tifs.2019.11.018
S. Gao, J. Jin, M. Abro, R. Song, M. He, X. Chen, How to select ionic liquids as extracting agents systematically: a special case study for extractive denitrification processes. RSC Adv. 11(2), 700–710 (2020). https://doi.org/10.1039/d0ra09316e
S.P.M. Ventura, F.A.E. Silva, M.V. Quental, D. Mondal, M.G. Freire, J.A.P. Coutinho, Ionic-liquid-mediated extraction and separation processes for bioactive compounds: past, present, and future trends. Chem. Rev. 117(10), 6984–7052 (2017). https://doi.org/10.1021/acs.chemrev.6b00550
E.S. Morais, A.C. Lopes, M.G. Freire, C.R. Freire, J.P. Coutinho, A.D. Silvestre, Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules (2020). https://doi.org/10.3390/molecules25163652
J. Xiao, G. Chen, N. Li, Ionic liquid solutions as a green tool for the extraction and isolation of natural products. Molecules (2018). https://doi.org/10.3390/molecules23071765
Y. Zhang, Y. Cao, H. Wang, Multi-interactions in ionic liquids for natural product extraction. Molecules (2021). https://doi.org/10.3390/MOLECULES26010098
K. Sarabandi, S.H. Peighambardoust, A.S. Mahoonak, S.P. Samaei, Effect of different carriers on microstructure and physical characteristics of spray dried apple juice concentrate. J. Food Sci. Technol. (2018). https://doi.org/10.1007/s13197-018-3235-6
X. Peng, G. Yang, Y. Shi, Y. Zhou, M. Zhang, S. Li, Box–Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. (2020). https://doi.org/10.1038/s41598-020-60339-1
R. Wang, Y. Chang, Z. Tan, F. Li, Applications of choline amino acid ionic liquid in extraction and separation of flavonoids and pectin from ponkan peels. Separation Sci. Technol. (Philadelphia) 51(7), 1093–1102 (2016). https://doi.org/10.1080/01496395.2016.1143006
Z. Liu, L. Qiao, F. Yang, H. Gu, L. Yang, Brönsted acidic ionic liquid based ultrasound-microwave synergistic extraction of pectin from pomelo peels. Int. J. Biol. Macromol. 94, 309–318 (2017). https://doi.org/10.1016/j.ijbiomac.2016.10.028
A.P. Abbott, D. Boothby, G. Capper, D.L. Davies, R.K. Rasheed, Deep Eutectic Solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J. Am. Chem. Soc. 126(29), 9142–9147 (2004). https://doi.org/10.1021/ja048266j
S.Q. Liew, G.C. Ngoh, R. Yusoff, W.H. Teoh, Acid and Deep Eutectic Solvent (DES) extraction of pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Biocatal. Agric. Biotechnol. 13, 1–11 (2018). https://doi.org/10.1016/j.bcab.2017.11.001
P. Janicka et al., Trends in the new generation of green solvents in extraction processes. Curr. Opin. Green Sustain. Chem. (2022). https://doi.org/10.1016/j.cogsc.2022.100670
S. Armenta, F.A. Esteve-Turrillas, S. Garrigues, M. de la Guardia, Alternative green solvents in sample preparation. Green Anal. Chem. 1, 100007 (2022). https://doi.org/10.1016/j.greeac.2022.100007
M.H. Shafie, C.Y. Gan, A comparison of properties between the citric acid monohydrate and deep eutectic solvent extracted Averrhoa bilimbi pectins. J. Food Meas. Charact. 14(5), 2889–2897 (2020). https://doi.org/10.1007/s11694-020-00533-x
L. Benvenutti, A.A.F. Zielinski, S.R.S. Ferreira, Subcritical water extraction (SWE) modified by deep eutectic solvent (DES) for pectin recovery from a Brazilian berry by-product. J. Supercrit. Fluids 189, 105729 (2022). https://doi.org/10.1016/j.supflu.2022.105729
J. Zhang et al., Subcritical water enhanced with deep eutectic solvent for extracting polysaccharides from lentinus edodes and their antioxidant activities. Molecules (2022). https://doi.org/10.3390/molecules27113612
M. Chen, X. Falourd, M. Lahaye, Sequential natural deep eutectic solvent pretreatments of apple pomace: a novel way to promote water extraction of pectin and to tailor its main structural domains. Carbohydr. Polym. 266, 118113 (2021). https://doi.org/10.1016/j.carbpol.2021.118113
M.H. Shafie, R. Yusof, C.-Y. Gan, Deep eutectic solvents (DES) mediated extraction of pectin from Averrhoa bilimbi: optimization and characterization studies. Carbohydr. Polym. 216, 303–311 (2019). https://doi.org/10.1016/j.carbpol.2019.04.007
A. Fauzan, G. Prihandini, A. Yudha, R. Sihaloho, Optimization of pectin extraction from kepok banana peel (Musa Acuminata Balbisiana Colla) using natural deep eutectic solvent (NADES). Fluida 16(1), 69–74 (2023)
N.N.T. Tien, N.L. Le, T.T. Khoi, A. Richel, Characterisation of dragon fruit peel pectin extracted with natural deep eutectic solvent and sequential microwave-ultrasound-assisted approach. Int. J. Food Sci. Technol. 57(6), 3735–3749 (2022). https://doi.org/10.1111/ijfs.15699
A. Elgharbawy et al., Natural deep eutectic solvent-assisted pectin extraction from pomelo peel using sonoreactor: experimental optimization approach. Processes 7(7), 416 (2019). https://doi.org/10.3390/pr7070416
D. Gawkowska, J. Cybulska, A. Zdunek, Structure-related gelling of pectins and linking with other natural compounds: a review. Polymers (2018). https://doi.org/10.3390/polym10070762
H. Li, J. Rao, B. Chen, Tyramine modification of high and low methoxyl pectin: physicochemical properties, antioxidant activity, and gelation behavior. Food Hydrocoll. 144, 108949 (2023). https://doi.org/10.1016/j.foodhyd.2023.108949
S.H. Jong, N. Abdullah, N. Muhammad, Optimization of low-methoxyl pectin extraction from durian rinds and its physicochemical characterization. Carbohydrate Polymer Technol. Appl. 5, 100263 (2023). https://doi.org/10.1016/j.carpta.2022.100263
W. Liang, J. Liao, J.-R. Qi, W. Jiang, X. Yang, Physicochemical characteristics and functional properties of high methoxyl pectin with different degree of esterification. Food Chem. 375, 131806 (2022). https://doi.org/10.1016/j.foodchem.2021.131806
M. Zioga, V. Evageliou, Formation and physicochemical properties of insoluble complexes resulted from high methoxyl pectin—protein interactions. Food Hydrocoll. 142, 108806 (2023). https://doi.org/10.1016/j.foodhyd.2023.108806
S. Free-Manjarrez, L. Mojica, H. Espinosa-Andrews, N. Morales-Hernández, Sensory and biological potential of encapsulated common bean protein hydrolysates incorporated in a Greek-style yogurt matrix. Polymers (Basel) (2022). https://doi.org/10.3390/polym14050854
T.M.T. Vo, T. Kobayashi, P. Potiyaraj, Viscoelastic analysis of pectin hydrogels regenerated from citrus pomelo waste by gelling effects of calcium ion crosslinking at different pHs. Gels. (2022). https://doi.org/10.3390/gels8120814
W. Han et al., Mathematical model of Ca2+ concentration, pH, pectin concentration and soluble solids (sucrose) on the gelation of low methoxyl pectin. Food Hydrocoll. 66, 37–48 (2017). https://doi.org/10.1016/j.foodhyd.2016.12.011
T. Vanitha, M. Khan, Role of pectin in food processing and food packaging, in Pectins. ed. by M. Masuelli (IntechOpen, Rijeka, 2019). https://doi.org/10.5772/intechopen.83677
C. Lara-Espinoza, E. Carvajal-Millán, R. Balandrán-Quintana, Y. López-Franco, A. Rascón-Chu, Pectin and pectin-based composite materials: beyond food texture. Molecules (2018). https://doi.org/10.3390/molecules23040942
H. Yang, C.C. Tsai, J.S. Jiang, C.C. Hua, Rheological and textural properties of apple pectin-based composite formula with Xanthan gum modification for preparation of thickened matrices with dysphagia-friendly potential. Polymers (Basel) (2021). https://doi.org/10.3390/polym13060873
Y. Zhu, K. Liu, M. Yuen, T. Yuen, H. Yuen, Q. Peng, Extraction and characterization of a pectin from sea buckthorn peel. Front. Nutr. (2022). https://doi.org/10.3389/fnut.2022.969465
X. Shuai et al., The effects of pectin structure on emulsifying, rheological, and in vitro digestion properties of emulsion. Foods (2022). https://doi.org/10.3390/foods11213444
C. Bilbao-Sainz, A.J.G. Sinrod, B. Chiou, T. McHugh, Functionality of strawberry powder on frozen dairy desserts. J. Texture Stud. 50(6), 556–563 (2019). https://doi.org/10.1111/jtxs.12464
N. Zhexenbay et al., Development technology of functional soft ice cream using beet pectin concentrate and probiotic. Eastern-Eur. J. Enterprise Technol. (2022). https://doi.org/10.15587/1729-4061.2022.265966
M.B. Güler-Akın, F. Avkan, M.S. Akın, A Novel functional reduced fat ice cream produced with pea protein isolate instead of milk powder. J. Food Process. Preserv. (2021). https://doi.org/10.1111/jfpp.15901
W. Jiang, J.-R. Qi, Y. Huang, Y. Zhang, X.-Q. Yang, Emulsifying properties of high methoxyl pectins in binary systems of water-ethanol. Carbohydr. Polym. 229, 115420 (2020). https://doi.org/10.1016/j.carbpol.2019.115420
U.S. Schmidt, L. Schütz, H.P. Schuchmann, Interfacial and emulsifying properties of citrus pectin: interaction of pH, ionic strength and degree of esterification. Food Hydrocoll. 62, 288–298 (2017). https://doi.org/10.1016/j.foodhyd.2016.08.016
B. Neckebroeck et al., Structural and emulsion stabilizing properties of pectin rich extracts obtained from different botanical sources. Food Res. Int. (2021). https://doi.org/10.1016/j.foodres.2020.110087
S. Zhao et al., Citrus oil emulsions stabilized by citrus pectin: the influence mechanism of citrus variety and acid treatment. J. Agric. Food Chem. (2018). https://doi.org/10.1021/acs.jafc.8b04711
J. Lin, H. Meng, X. Guo, S. Yu, Enhancing the emulsification and photostability properties of pectin from different sources using genipin crosslinking technique. Foods (2022). https://doi.org/10.3390/foods11162392
F. García-Carrizo, C. Picó, A.M. Rodríguez, A. Palou, High-esterified pectin reverses metabolic malprogramming, improving sensitivity to adipostatic/adipokine hormones. J. Agric. Food Chem. 67(13), 3633–3642 (2019). https://doi.org/10.1021/acs.jafc.9b00296
I.C. Pappa, J.G. Bloukas, I.S. Arvanitoyannis, Optimization of salt, olive oil and pectin level for low-fat frankfurters produced by replacing pork backfat with olive oil. Meat Sci. 56(1), 81–88 (2000). https://doi.org/10.1016/S0309-1740(00)00024-3
N.M. Paderin, Y.N. Saveliev, S.V. Popov, The effect of pectin of tansy, Tanacetum vulgare L., on anxiety and overeating food rich in fats and sugars in mice in modelling binge eating. Vopr. Pitan. 89(6), 14–22 (2020). https://doi.org/10.24411/0042-8833-2020-10074
Y. Bai, R.G. Gilbert, Mechanistic understanding of the effects of pectin on in vivo starch digestion: a review. Nutrients (2022). https://doi.org/10.3390/nu14235107
J.L.S. Donadio, S.B.R. do Prado, M.M. Rogero, J.P. Fabi, Effects of pectins on colorectal cancer: targeting hallmarks as a support for future clinical trials. Food Funct. 13(22), 11438–11454 (2022). https://doi.org/10.1039/D2FO01995G
P. Jiang, Y. Ren, Y. Zhang, Effects of pectin on intestinal microbiota and human health. Theor. Nat. Sci. 4(1), 321–330 (2023). https://doi.org/10.54254/2753-8818/4/20220581
T. Bin Emran et al., Pectin: a bioactive food polysaccharide with cancer preventive potential. Molecules (2022). https://doi.org/10.3390/molecules27217405
M. Han, H.C. Bertram, Designing healthier comminuted meat products: effect of dietary fibers on water distribution and texture of a fat-reduced meat model system. Meat Sci. 133, 159–165 (2017). https://doi.org/10.1016/j.meatsci.2017.07.001
G. Méndez-Zamora et al., Fat reduction in the formulation of frankfurter sausages using inulin and pectin. Food Sci. Technol. (Brazil) 35(1), 25–31 (2015). https://doi.org/10.1590/1678-457X.6417
J. Huang, Z. Hu, L. Hu, G. Li, Q. Yao, Y. Hu, Pectin-based active packaging: a critical review on preparation, physical properties and novel application in food preservation. Trends Food Sci. Technol. 118, 167–178 (2021). https://doi.org/10.1016/j.tifs.2021.09.026
D.G.M. Pereira, J.M. Vieira, A.A. Vicente, R.M.S. Cruz, Development and characterization of pectin films with salicornia ramosissima: biodegradation in soil and seawater. Polymers (Basel) (2021). https://doi.org/10.3390/polym13162632
N.M. Al-Enazi, K. Alsamhary, F. Ameen, Evaluation of citrus pectin capped copper sulfide nanoparticles against Candidiasis causing Candida biofilms. Environ. Res. (2023). https://doi.org/10.1016/j.envres.2023.115599
P.R. Sougandhi et al., Multifunctional bio-nanocomposite films of carboxymethyl cellulose and pectin with incorporated zinc oxide nanoparticles. Lett. Appl. NanoBioSci. (2023). https://doi.org/10.33263/LIANBS123.085
Y.V. Chekunkov et al., Citrus pectin based complexes for the tetracycline delivery. Food Hydrocoll. Health (2022). https://doi.org/10.1016/j.fhfh.2022.100100
K.S. Eça, M.T.C. Machado, M.D. Hubinger, F.C. Menegalli, Development of active films from pectin and fruit extracts: light protection, antioxidant capacity, and compounds stability. J. Food Sci. 80(11), C2389–C2396 (2015). https://doi.org/10.1111/1750-3841.13074
B.E. Teleky et al., Development of pectin and poly(vinyl alcohol)-based active packaging enriched with itaconic acid and apple pomace-derived antioxidants. Antioxidants (2022). https://doi.org/10.3390/antiox11091729
X. Qi et al., Structural characterization and anti-oxidation activity evaluation of pectin from Lonicera japonica Thunb. Front. Nutr. (2022). https://doi.org/10.3389/fnut.2022.998462
S. Bhatia et al., Physical, chemical, barrier, and antioxidant properties of pectin/collagen hydrogel-based films enriched with melissa officinalis. Gels (2023). https://doi.org/10.3390/gels9070511
T.M.P. Ngo, T.H. Nguyen, T.M.Q. Dang, T.X. Tran, P. Rachtanapun, Characteristics and antimicrobial properties of active edible films based on pectin and nanochitosan. Int. J. Mol. Sci. (2020). https://doi.org/10.3390/ijms21062224
J.A. Pérez, K. Gómez, L. Vega, Optimization and preliminary physicochemical characterization of pectin extraction from watermelon rind (Citrullus Lanatus) with citric acid. Int. J. Food Sci. (2022). https://doi.org/10.1155/2022/3068829
A. Mortensen et al., Re-evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives. EFSA J. (2017). https://doi.org/10.2903/j.efsa.2017.4866
Acknowledgements
The authors acknowledge facilities support from the Universiti Teknologi MARA and scholarship from MyBrainSc 2023 provided by the Ministry of High Education (MOHE) of Malaysia.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Mohammad Amin Wan Chik: Investigation, Data curation, Writing-Original draft preparation. Rizana Yusof: Supervision, Writing-Reviewing and Editing. Muhammad Hakimin Shafie: Supervision, Writing-Reviewing,Validation. Roziana Mohamed Hanaphi: Supervision, Writing-Reviewing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan Chik, M.A., Yusof, R., Shafie, M.H. et al. The versatility of pectin: a comprehensive review unveiling its recovery techniques and applications in innovative food products. Food Measure 18, 6101–6123 (2024). https://doi.org/10.1007/s11694-024-02632-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02632-5