Abstract
The yield and quality of sugar beet pulp pectin obtained by ultrasound-assisted extraction (UAE) were compared to those obtained by conventional heating. Extraction temperature (70–90) °C, extraction time (2–4) h and pH (1–1.5) were considered as the variables for the conventional extraction while ultrasound frequency (20–60) kHz, time (10–30) min and amplitude (60–100%) were considered as the variables for UAE. The optimal conditions for maximum yield of pectin for conventionally and ultrasonically extracted pectin were determined by the central composite design and the Box Behnken design, respectively. The optimum conditions of conventional extraction were the temperature of 90 °C, time of 4 h and pH of 1. The optimum conditions of UAE were ultrasound frequency of 20 kHz, time of 10 min, and ultrasound amplitude of 96%. Extraction using the optimized conditions of conventional heating and UAE achieved the best yield of 20.75% and 20.85%, respectively. The degree of methyl esterification, ferulic acid content and molecular weight of UAE pectin were higher than conventionally extracted pectin but the content of galacturonic acid in UAE pectin was lower than that of conventionally extracted pectin. The infrared spectra of both pectins revealed the occurrence of polysaccharide component. The pectin achieved by UAE had higher lightness value than conventionally extracted pectin, confirming its application in different products partially in bright food products. The UAE pectin possessed higher viscosity than conventionally extracted pectin. The addition of UAE pectin increased all of the DSC gelatinization parameters. Overall, UAE could be a promising scalable and economical approach to obtain pectin with unique characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugar beet pulp is a co-product of the sugar refining industry, the major part of which is now utilized for animal feed. Sugar beet pulp upon sugar extraction consists of almost 15–30% pectin (dry basis) (Adetunji et al. 2017). Pectin is basic polymer existing in the cell wall of plants (Qiu et al. 2009). They comprise two main structural domains, homogalacturonan, and rhamnogalacturonan-I, which are covalently linked to one another (Ishii and Matsunaga 2001). Homogalacturonan is a linear homopolymer of α-1,4-linked-D-galacturonic acid (GalA) which could be methyl esterified and acetylated to different degrees. Pectic substances are excellent gelling, thickening and emulsifying agents (Cserjési et al. 2011). Four groups of the chemical agents utilized for extraction of pectin. They are calcium-ion chelators, water and buffers, bases, and acids. The last ones are the strongest agents for pectin extraction due to their role to facilitate the insoluble pectin extraction which is firmly bound to the cell matrix of the plant (Sandarani 2017). Dilute alkaline solution was potentially efficient for extraction of intact rhamnogalacturonan-I with high neutral side chains while it might destroy the homogalacturonan backbone (Khodaei et al. 2016) and this can make large amount of alkaline waste. The chelating agents’ usage could be helpful for the pectin release from the plant cell wall by the egg-box model and therefore enhance the yield of pectin extraction (Caffall and Mohnen 2009). Nevertheless, chelating agents were observed to stay bounded with pectin even upon substantial dialysis (Renard and Thibault 1993). Water as an environmental friendly solvent has also gained remarkable attention, nevertheless, its low efficiency in yields is the main challenge of water solvent in extraction of pectin (Yeoh et al. 2008). Commercially, pectin is obtained by extracting the plant cell walls with mineral acids (Liu et al. 2010). This procedure is prolonged and causes pectin deterioration, so this process needs to be improved. Such procedures can cause environmental pollution by producing harmful waste substances. Hence, to obtain pectin with the desired characteristics, the methods available for extraction need to be corrected (Guo et al. 2012). Ultrasound-assisted extraction (UAE) is a valid eco-friendly process for the rapid and reproducible extraction of food samples. Totally the results of the published studies in the field of the UAE have been satisfactory. For instance, the extraction of sugar beet pulp pectin by the method of ultrasound-assisted subcritical water extraction has been investigated (Chen et al. 2015). The results showed this combined method led to the high yield of pectin-enriched materials (Chen et al. 2015).
The study about the UAE of polysaccharide from plant materials led to a shorter extraction time and lower energy needs (Yong-Guang et al. 2012). The study about the comparison between the application of conventional solvent (carried out in a HH-6 water bath), microwave apparatus and ultrasonic cleaner bath for obtaining of polysaccharides from mulberry leaves showed that the latter gave a remarkably high yield of polysaccharides (Ying et al. 2011). Dranca and Oroian (2019) studied the optimization of ultrasonic assisted treatment for the pectin extraction of apple pomace on yield, degree of esterification, and galacturonic acid content of the pectin. The highest yield of 9.183%, was achieved at 100% amplitude, pH of 1.8, solid–liquid rate of 1:10 g/mL, and time of 30 min. The results of FT-IR spectrum showed that the UAE pectin was similar to commercial apple pectin rather than to commercial citrus pectin in terms of chemical composition. The thermal behavior of the UAE pectin was affected by the orderly molecular arrangement and the narrower distribution of molecular weights, while the rheological characteristics of this pectin were affected by the galacturonic acid content and the morphological structure (Dranca and Oroian 2019). The enzymatic extraction of red beet pectin enhanced using applying ultrasonic treatment and agitation. The highest pectin yield can be reached, at an ultrasound frequency of 25 kHz combined with the rotational speed of 1000 rpm and, the pectin yield of ultrasonic-assisted and agitated enzymatic extraction (UAEE) at this condition reach nearly 80% for three hours of process duration (Velyamov et al. 2019). Shivamathi et al. (2019) studied the pectin extraction of custard apple peel and optimized the extraction process by RSM. The optimal conditions for the highest pectin yield (8.93%) were liquid–solid ratio of 23.52 mL g−1, ultra-sonication time of 18.04 min, temperature of 63.22 °C and pH solution of 2.3. They used commercially available fresh pectin for comparison purposes. The FTIR spectrum extracted pectin revealed the occurrence of polygalacturonic acid and polysaccharide components. The study of crystallinities of pectins displayed the narrow and sharp peaks of crystalline profiles of XRD for both pectins. The DSC analyses showed the closely related exothermic peaks of the extracted pectin and commercial pectin. According to the anti-nutritional property investigation, the extracted pectin was a toxic-free compound comprising around 20 mg/mL of antioxidant (Shivamathi et al. 2019). The utilization of ultrasound for extraction of pectin-containing plant materials (Adetunji et al. 2017) have been revealed; however, there is no comprehensive study of the comparison between ultrasound assisted and conventional heating extraction of pectin from sugar beet pulp. This work aimed to compare the yield and quality of pectin obtained by UAE and conventional heating.
Materials and methods
Samples preparation
The wet sugar beet pulps (SBP) supplied from the pulps being transported by the belt conveyor to the pulp press in the Khoy sugar factory (Khoy, East Azerbaijan Province, Iran). All used sugar beets were of Flores and Virginia varieties. Choosing wet SBP as a raw material instead of dry SBP, which have been used in previous researches, was because of the different physical treatments applied for SBP, especially drying which could have harmful effects on the cell wall, such as its collapse. Therefore, wet sugar beet pulps were dried at an oven of 40 °C for 24 h (until reaching the moisture content less than 3%), then they were crushed using a desktop hammer mill (Glen Creston Ltd, United Kingdom). Sugar beet pulp crushed to the extent that can pass through the 60 mesh sieve. Starch from corn (EC: 232 679 6) was purchased from Sigma Aldrich Chemical Co (St. Louis, MO) and used for the characterization of pasting and thermal properties.
Physico-chemical characteristics of sugar beet pulp (SBP)
Several physico-chemical characteristics of pectins were analyzed including moisture, ash, crude protein, crude fat with the Soxhlet extraction method and crude fiber contents according to AACC standard methods (AACC 2010).
Apparatus
A probe ultrasonic processor (Tapered tip: KE76, Code number: 530, BANDELIN SONOPULS, Germany) used for ultrasound treatment with the following parameters: HD2200, power 200 W, amplitude (10–100%), maximum admissible amplitude Setting (72%), frequency 2, 4, 6, 8 (cycle × 10%), maximum time of 99 min and 59 Sec, the length and diameter of horn tapered tip of 135 mm and 6 mm, respectively.
Conventional extraction of pectin
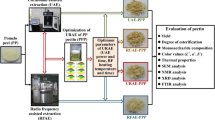
The extraction of pectin from the dried pulp (moisture content less than 3%) was carried out according to the method described by Lv et al. (2013) with a little modification. A three-variable-three-level central composite design (CCD) (Table S1) was used to determine the optimal conditions for maximum yield. Extraction temperature (70–90) (°C), extraction time (2–4) (h) and pH (1–1.5) were considered as the conventional extraction variables. The ratio of solid to liquid was chosen to be constant (1:20) for all treatments (Lv et al. 2013). Using a digital mechanical agitator (LCD Digital Overhead Stirrers and Magnetic Stirrer with speed of 50 to 2200 rpm, Shandong, China), these suspension samples were slowly stirred at 200 rpm. The suspensions pH was settled to the needed value by HCl (12 M). The suspensions temperature was adjusted with a temperature controlled water bath. Upon the reaction accomplishment, the obtaining slurries were cooled down, adjusted to pH 4.5 with 25% (w/w) NH3.H2O and filtered by means of a Buchner funnel under vacuum condition. The process was continued by the recovering of supernatant and measuring its volume. Precipitation and recovering of the pectin from the supernatant was carried out for 1 h at ambient temperature by two volumes of 100% ethanol and subsequent hand squeezing in a nylon cloth for the ethanol removal. Lastly, the sugar beet pulp pectin (SBPP) was twice washed with ethanol (100%), and squeezed in a nylon cloth for the remaining ethanol removal and dried at 50 ◦C for 5 h in a hot air oven.
Extraction of pectin using UAE
Similar to the conventional extraction method, the ratio of solid to liquid was chosen to be constant (1:20) for all treatments. UAE was carried out by the above-mentioned procedure for conventional extraction but the suspensions were subjected to an ultrasound probe instead of a water bath. A three-variable-three-level Box Behnken design (Table S2) was used to determine the optimal conditions for maximum yield. The UAE variables were ultrasound frequency (20–60) kHz, sonication time (10–30) min, and sonication amplitude (60–100%). The samples were slowly stirred with a magnetic stirrer at 200 rpm for 10 min. The pH of the suspension was adjusted to pH = 1 with 12 M HCl. The selection of this pH was conducted based on Jafarzadeh-Moghaddam et al. (2016) in which the optimized pH for maximum yield of sugar beet pulp pectin extracted by the conventional acid extraction method was pH = 1. Then Ultrasonic treatment was performed by pulsed ultrasonic probe processor according to the frequency (kHz), time (min), and sonication amplitude (%) listed in Table S2.
Purification
The extracted pectin was purified by the centrifugation process to remove insoluble materials (Yapo et al. 2007). Then, it was used for chemical, physical and rheological investigations.
Chemical analysis
Galacturonic acid (GalA) content of pectin was determined by high-performance anion exchange chromatography with a pulsed amperometric detector (HPAEC-PAD) according to the method described by Garleb et al. (1991). The constituents of pectin hydrolyzed to monomers as fully described by Garna et al. (2004). Hydrolysates (25 μl) were injected into the Dionex system (DX-500 Bio-LC, Dionex Corp) equipped with CarboPac PA-100 column (250 × 4 mm) in combination with a guard column (50 × 4 mm, Dionex Corp., Sunnyvale, CA); Elution was carried out with 0.1 M NaOH and 0.17 M NaOAc at a constant temperature of 30 °C, and at a flow rate of 1 ml/min. The column was washed post-injection with 0.5 M NaOH over 17 min followed by 20 min re-equilibration before the next injection. Pure galacturonic acid monohydrate (purity > 97%, Sigma-Aldrich) was used as an external standard. Individual neutral sugars were released from pectin molecules by acid hydrolysis with 1 M H2SO4 at 100 °C for 3 h and converted to alditol acetate according to Blakeney et al. (1983) method. Alditol acetate derivatives were separated and quantified by gas chromatography using a high-performance capillary column, BP1-methylsiloxane (30 mL, 0.32 mm ID, 0.25 lm film thickness, Scientific Glass Engineering, S.G.E. Pty. Ltd., Melbourne, Australia) mounted on a Hewlett–Packard HP-6890 series GC system. 2-Deoxy-d-glucose (purity > 98%, Sigma-Aldrich Chemical Co.,) was used as an internal standard. 2-Deoxy-d-glucose, L-rhamnose (Rha), L-arabinose (Ara), D-xylose (Xyl), D-galactose (Gal) and d-mannose (Man) were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO). Total neutral sugars (NS) were calculated as the sum of the individual neutral sugars. The ferulic acid content was determined by HPLC method after saponification and extraction of phenolic compounds. Gel permeation chromatography (GPC), combined with HPLC (Angilent1100, USA) was applied to determine molecular weight (MW). Six polysaccharides of MW of 12,200 to 830,000 g/mol were used for calibration of the column (Table S3).
FTIR characterization
The infrared spectra of pectin were recorded with a KBr (alkali halogenide)-pellet technique using a Tensor 27 FTIR spectrometer (Bruker Optik, Gmbh, Germany) at the frequency range of 4000–400 cm−1 and a wave number accuracy of 0.1 cm−1. All spectra were post-processed using OPUS software (Bruker Optik). 14 scans were measured for FTIR.
Color measurement
The color of pectins was evaluated by Hunterlab colorimeter (model Miniscan XE, USA), using the illuminant D65 (daylight). Three measurements were done. The NIST traceable white calibrated tile used for calibration (Misra and Yadav 2020).
Rheological measurements
Three different concentrations (0.5%, 0.75% and 1%, w/w) of each pectin solution were prepared in distilled water. A strain-controlled rheometer Anton Paar (DG 26.7) with a concentric cylindrical geometry, joint with a temperature controlled water bath was used for rheological examination. The steady shear analysis was made in shear rates ranged from 0.1 to 100 s−1 at 25 °C. Changes in viscosity by raising the temperature from 25 to 85 °C at a shear rate of 50 s−1 were also measured.
Pasting property measurements
Characterization of pasting characteristics of the mixture of normal corn starch and sugar beet pectin was performed with a Brabender micro viscoamylograph (version 4.1.0) according to the method described by Chen et al. (2015) with some modifications. After determination of starch moisture according to AACC 44-15A and correction of moisture content on the basis of 14% moisture content, the amount of starch was determined. Then samples were prepared by mixing the starch (which determined by above-mentioned method) and 100 mL distilled water or the pectin solution (1%, w/w). The mixture was stirred manually for 1 min. A 700 cmg cartridge was fitted with the rotation speed set at 75 rpm. The heating and cooling cycles were programmed in the following manner: the samples were heated at 7.5 °C/min from 30 °C to 95 °C, then held for 20 min at 95 °C, cooled to 50 °C at 7.5 °C/min, and then held for 20 min at 50 °C. The pasting temperature (Tp), peak viscosity (PV), hot paste viscosity (HPV), final viscosity, breakdown (BD) and setback (SB) values are recorded in Table 1.
Thermal analysis
Thermal measurements were done using differential scanning calorimetry (Netzsch DSC 200 F3, Germany) according to the method described by Chen et al. (2015). The pans which contained normal corn starch (5 mg) and distilled water (25 μl) or pectin solution (1%, w/w), were kept for 1 h at 25 °C, then were heated from 30 to 95 °C at 5 °C/min.
Statistical analysis
All experiments were done in triplicates. Optimization was carried out using Design-Expert software, version 10 (Stat-Ease, Inc, Minneapolis, US). The results of color attributes were assessed with the Independent-Samples T Test. The results of thermodynamic properties were assessed with Duncan multiple range tests (α = 0.05), using the SPSS 16.0 statistical software (SPSS Inc., Chicago, SPSS Inc.).
Results and discussion
Physico-chemical characteristics of sugar beet pulp (SBP)
The moisture content of pulp was 91%. The ash content was 0.3% w/w. The protein content was 6.1% (dry weight). The amount of lipid and crude fiber were 0.2% and 73.37% (dry weight), respectively.
Yield
In this study, since the frequency of the ultrasound probe processor was a multiple of 10, it was not possible to conduct experiments outside the specified range. Therefore, the Box Behnken design (BBD), which covers these ultrasound frequencies, was used instead of the central composite design (CCD). The conventionally extracted and UAE pectin yields obtained from all the experiments were listed in Table S1 and Table S2, respectively, according to response surface methodology (RSM) design. The normal probability at residuals of the yield for conventional extraction (Fig. S1a) and UAE (Fig. S1b) indicated no abnormality in the methodology adopted (R2 = 0.96). Multiple regression coefficients obtained by employing the least squares technique for the conventionally extracted and UAE pectin yield were summarized in Table S4. For both extraction procedure, the significance of each coefficient was determined by the F-value and P-value. For the conventionally extracted pectin yield, analysis of variance of the model showed that the generated model was significant (P = 0.0003) and the lack of fit was insignificant (P = 0.66). The R2, adj.R2, and CV values were 0.91, 0.84, and 6.76, respectively, indicating that the model can be used to navigate the design space. In the case of UAE pectin yield, analysis of variance of the model showed that the generated model was significant (P ≤ 0.05) and the lack of fit was insignificant (P = 0.7). The R2, adj.R2, and CV values were 0.91, 0.81, and 1.32, respectively, indicating that the obtained model can be used to navigate the design space. Optimization of conventional extraction condition indicated that time and temperature showed positive while the pH showed a negative linear impact on the pectin yield. Optimization of UAE condition indicated that frequency and amplitude showed positive while the time exhibited a negative linear impact on the yield. The optimum conditions of conventional extraction were estimated to be a temperature of 90 °C, time of 4 h and a pH of 1 (Fig. 1a). The optimum conditions of UAE were estimated to be ultrasound frequency of 20 kHz, time of 10 min and ultrasound amplitude of 96% (Fig. 1b). Extraction using the optimized conditions of conventional heating and UAE achieved the best yield of 20.75% and 20.85%, respectively. The yield of UAE pectin for the sonication time of 10 min gained 20.85%, which is not significantly different from the conventional heating yield (20.75%) after 4 h. The results displayed that by the application of ultrasound only for 10 min, the pectin extraction accelerated in comparison with the conventional extraction. UAE was over 24 times faster than conventional heating. It was due to the acoustic amplitude and the effect of mechanical vibration, leading to the change of velocity of pectin molecules with respect to time and the increment of the mass stream inside the pulp, enhancing the pectin yield (Vilkhu et al. 2008).
FTIR characterization
Figure 2a represents the transmittance spectrum of UAE pectin and Fig. 2b shows the transmittance spectrum of conventionally extracted pectin. FTIR spectra of UAE pectin exhibited good match with the spectrum of the conventionally extracted pectin due to their similar chemical compositions. The fingerprints region of the carbohydrates group revealed in the wavelength range of 950–1200 cm−1. The peak at around 1225 cm−1 is related to the C–C bond in the ring structure of pectin while the transmittance peaks between 990 and 1120 cm−1 are corresponded to the range for the spectral identification of GalA in pectin molecules (Dranca and Oroian 2019). Substantial changes take place in the spectral areas between 800 and 1200 cm−1 indicate the possible methylation or acetylation of pectin. Several specific peaks recorded in the spectra between 1750 and 2000 cm−1 indicate the possible esterification or desertification of pectins. According to Shivamathi et al. (2019), bands occurring between (1745 and 1760) cm−1 represents the carboxylic and ester group. Stretching bands at 1600–1630 and absorption peaks at 1730–1760 cm−1 correspond to C=O stretching vibrations of free carboxyl (COO–) and methyl-esterified (COO–R) groups, respectively (Wang et al. 2015). The spectrum shows several peaks correspond to the functional groups related to the native structure of pectin. The transmittance peak characteristic for polysaccharides that were found at around 2919 cm−1 in both the conventionally extracted pectin and UAE pectin spectra were due to C–H stretching of CH2 groups (Wang et al. 2016). Ample peaks around 3406–3600 cm−1 denote the OH group, which is found in the pectin structure. As shown in the figure, substantial changes take place in the spectral areas between 3000 and 3700 cm−1, which indicates possible water bounded by CH2 groups. The spectra of UAE and conventionally extracted sugar beet pectins revealed the occurrence of polysaccharide component.
Chemical analysis
The comparison between galacturonic acid (% w/w), the degree of methylation (DM) (% mol), degree of acetylation (DA) (% mol), neutral sugars (NS) (% w/w), ferulic acid (FA) (% w/w) and molecular weight (g/mol) of pectin obtained under the optimum condition by UAE and conventional heating illustrated in Table 2. The galacturonic acid content of the conventionally extracted pectin was higher than that of ultrasonically extracted one while the DM of ultrasonically extracted pectin was higher than that of conventionally extracted one. Both of the extracted pectins were high methoxy pectin. The degree of acetylation (DA) for conventionally and ultrasonically extracted pectin was 14.3% and 13.2% (mol), respectively. The amount of each neutral sugars (including Arabinose (Ara.) Rhamnose (Rha.), Galactose (Gal.), Glucose (Glu.), Xylose (Xyl.) and Mannose (Man.) in conventionally and UAE extracted pectin obtained under the optimal conditions showed in Table 2. The ferulic acid content (% w/w) of UAE pectin and conventionally extracted pectin was 0.9 and 0.8, respectively. This result was important because feruloyl groups are the main factors in the cross-linking of pectin molecules (Jung and Wicker 2012). The MW of UAE pectin was more than twice of the MW for conventionally extracted pectin. These values were in the range of 70,000 to 355,000 g/mol for pectin extracted by acid, which was reported by Levigne et al. (2002). The range of MW for pectin depends on its source and extraction procedure, so this value of MW probably was due to extraction condition. The lower MW of pectin gained in conventional extraction associated with heating time (temperature) could be ascribed to the degradation of pectin. It is ascribed to the fact that the pectin molecular weight declined with enhancing the temperature, which is as a result of pectin degradation. The higher MW of the pectin extracted by ultrasound may be related to the low temperature of UAE and also to the higher ferulic acid content which are the important factors in crosslinking of pectin molecules. The results of measuring the temperature at the center of becher when the seventeen experiments of UAE were done showed the temperature was in the range of 55 to 76 (°C); Considering the time of the UAE (10, 20, 30 min), ultrasound treatment cannot be effective in decomposing pectin. It was observed that the degree of methyl esterification, ferulic acid content and MW were higher for UAE pectin.
Color attributes
To evaluate how an extraction procedure influences the color of pectin, the mean values of experimental L, a, b parameters were measured. Fig. S2 shows two pectins obtained from sugar beet pulp by conventional heating (a) and UAE (b). A glance at the provided pictures reveals the bright color of UAE pectin as compared to conventionally extracted one. Table 3 confirms this significant difference between the color parameters of pectin extracted by the two above mentioned procedures. The lightness (L) of the UAE pectin was 64, while this value for conventionally extracted pectin was 46 thus the pectin obtained by UAE was lighter than conventionally extracted pectin. The conventionally extracted pectin exhibited significantly lower redness (a) and yellowness (b) values than those of UAE one, therefore, the redness and yellowness of the UAE pectin were also greater than that of conventionally extracted one. This result reflects the difference in pectin color extracted from the same starting raw material which was due to different extraction procedures. Color is an important element in food quality and consumer acceptance. It has been reported that in bright food products, beet pectin can affect the color of these products (Mesbahi et al. 2005). Considering this, the introduction a procedure to obtain pectin with suitable color would be greatly commendable. The color of UAE pectin was lighter than that of conventionally extracted one, therefore, because beet pectin extracted by ultrasound was brighter than conventionally extracted pectin, it would probably be a promising alternative to conventionally extracted one. Further works needed about the effect of beet pectin obtained by UAE, on the color of food products. As the results of FTIR showed the chemical composition of pectins was similar while the molecular structure of the UAE pectin was different from conventionally extracted one and this fact proved by the results obtained from color parameters. Color parameters of each pectin depend on the absorption of light by the pectin which in turn relies very strongly on electronic structure (energy levels) of pectin thus its molecular organization.
Rheological properties
The flow behavior of pectin solutions at concentration of 0.5%, 0.75% and 1% (w/w) was evaluated. As illustrated in Fig. 3a, all curves showed linear trends, therefore pectin solutions in the concentration range of 0.5–1% displayed nearly Newtonian behavior. Figure 3b shows that an increase in the concentrations caused an increase in viscosity for both extraction procedures. The range of apparent viscosity increased from 1.22 to 2 mPa s with the increased concentration of conventionally extracted pectin solutions, while this range increased from 2.6 to 5.2 mPa s for UAE pectin. When the flow behaviors at the same concentrations were compared, a pronounced difference in the apparent viscosity of pectin was observed between UAE and conventional heating. The pectin extracted by ultrasound exhibited higher viscosity than conventionally extracted pectin, in all concentrations. This observation could be ascribed to the higher MW of UAE pectin, which was an important element for the higher viscosity of its solutions compared to that of conventionally extracted. The higher viscosity of the UAE pectin sample extracted in this study in comparison to the conventionally extracted pectin was explained by the differences in the chemical structure of the two samples that were confirmed by FT-IR analysis. The examination of the temperature dependence of viscosity showed a nonlinear decrease in viscosity of both pectin solutions, when the temperature increased from 25 to 85 °C (Fig. 3c). This result is consistent with the findings by Min et al. (2011), which attributed to the lower molecular interactions at higher temperatures.
Pasting characteristics
Starch and hydrocolloids are usually utilized together in food systems to control moisture and water mobility as well as provide suitable texture, enhance overall quality or product stability, facilitate the process, or decline costs; therefore, understanding the interaction of starch and pectin is important (Shi and BeMiller 2002). Table 1 shows the pasting characteristics of the corn starch and starch pectin mixtures at a concentration of 1%. The pasting temperature (PT) is the temperature at which the rapid viscosity increase initiates, as observed with Brabender viscoamylograph. Both of the extracted pectins brought approximately a decrease in PT. The lower pasting temperature can be attributed to linking between amylose and added pectin molecules. Reduction of PT had been reported in more than 20 basic research papers which have named by Kim and BeMiller (2012). The lower value of peak viscosity (PV) related to the mixture of starch and conventionally extracted pectin. The use of UAE pectin increased the peak viscosity. The UAE pectin increased the viscosity at the beginning of cooling or hot paste viscosity (HPV) of the starch pectin mixture, while there was no significant difference between HPV values of the mixture containing conventionally extracted pectin and starch–water sample. UAE pectin increased the breakdown (BD) value of the starch while the conventionally extracted pectin decreased the BD value of the starch. Thus the mixture contained conventionally extracted pectin presented improved stability on the shearing process compared to starch. Both of the extracted pectins caused a reduction in final viscosity (FV). Abioye et al. (2011), reported that the lower FV could be considered as a signal of the viscous paste formation which can resist to shear stress during stirring. According to this result, the corn starch had a greater tendency to retrograde as compared to the starch with added pectin. Both of the extracted pectins reduced the setback value (SB) of starch-pectin mixtures as compared to the starch–water sample. The lower setback value approved that the starch pectin mixtures hardly retrograded over keeping in the refrigerator (Huang et al. 2012). All of the pasting parameters of starch decreased with the addition of conventionally extracted pectin, while the addition of UAE pectin caused a decrease in the PT, FV, and SB viscosity values of the starch.
Thermal properties
Table 1 shows onset temperature (TO), gelatinization peak temperature (TP) and completion temperature (TC) of starch with and without pectin. Both of the extracted pectins increased the TO of the starch. The TP of the starch was 71.3 °C while the TP of both starch pectin mixtures were 72 °C. There was no significant difference between the TP of mixtures containing conventionally or ultrasonically extracted pectin. The addition of UAE pectin increased the TC of the starch. The TC value of the mixture of starch with conventionally extracted pectin was close to that of starch. Both of the extracted pectins decreased the phase transition temperature range (TC–TO). The possible explanation for this trend was that the association of pectin and starch immobilized water molecules, reducing free water to starch ratio which subsequently result in declining the mass and heat transfer rates (Krüger et al. 2003). The enthalpy change (ΔH) increased with the addition of pectin. The exothermic peak stated that the sample degradation properties are correlated to its chemical composition profile. The closely related exothermic peaks of the UAE pectin at 71.9 °C and conventionally extracted pectin at 71 °C confirmed the same chemical constituent profile of both the samples. The enthalpy changes refer to the linkage between the pectin and starch, which reduces the motion of the starch chain, and as a consequence of this phenomenon more energy needs for melting. This observation fell in line with the conclusion of Chen et al. (2015). Therefore, the interaction between pectin solution and starch was stronger than that between water and starch. The addition of both conventionally and ultrasonically extracted pectin increased all of the DSC gelatinization parameters of starch except for TC of pectin obtained by heating. The pasting and thermal properties of the mixture of pectin and starch show that the functional properties of UAE pectin and conventionally extracted pectin were influenced by the extraction method.
Conclusion
This study compares the yield and quality of sugar beet pulp pectin extracted by UAE and conventional heating. The yield of UAE pectin for the sonication time of 10 min gained 20.85%, which was the same as the conventional heating yield (20.75%) after 4 h. UAE was over 24 times faster than conventional heating. The study showed that intermittent sonication results in better yields due to pulsation effects of ultrasound waves. The galacturonic acid content of UAE pectin was comparable to that of conventionally extracted pectin. It was observed that the yield, degree of methyl esterification, ferulic acid content and MW were higher for UAE pectin. The color of UAE pectin was lighter than that of conventionally extracted one and the redness and yellowness of UAE pectin were greater than that of conventionally extracted pectin. The UAE pectin solutions at concentrations of 0.5%, 0.75% and 1% (w/w) possessed higher viscosity than conventionally extracted pectin. Increasing temperature caused a non-linear decrease in viscosity of both pectin solutions. The conventionally extracted pectin decreased all of the pasting parameters of starch, but UAE pectin decreased PT, FV and SB viscosity values of the starch. The addition of both conventionally and ultrasonically extracted pectin increased all of the DSC gelatinization parameters of starch except for TC of pectin obtained by heating. All in all, the results offered a promising approach using UAE to reduce pectin extraction time on an industrial scale and provides pectin products that have their own special characteristics.
References
AACC (2010) Approved methods of analysis, 11th edn. AACC, St. Paul
Abioye VF, Ade-Omowaye BIO, Babarinde GO, Adesigbin MK (2011) Chemical, physico-chemical and sensory properties of soy-plantain flour. Afr J Food Sci 5:176–180
Adetunji LR, Adekunle A, Orsat V, Raghavan V (2017) Advances in the pectin production process using novel extraction techniques: a review. Food Hydrocoll 62:239–250
Blakeney AB, Harris PJ, Henry RJ, Stone BA (1983) A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr Res 113(2):291–299
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344(14):1879–1900
Chen H, Fu X, Luo Z (2015) Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem 168:302–310
Cserjési P, Bélafi-Bakó K, Csanádi Z et al (2011) Simultaneous recovery of pectin and colorants from solid agro-wastes formed in processing of colorful berries. Prog Agric Eng Sci 7(1):65–80
Dranca F, Oroian M (2019) Ultrasound-assisted extraction of pectin from Malus domestica ‘Fălticeni’ apple pomace. Processes 7(8):488
Garleb KA, Bourquin LD, Fahey-Jr GC (1991) Galacturonate in pectic substances from fruits and vegetables: comparison of anion exchange HPLC with pulsed amperometric detection to standard colorimetric procedure. J Food Sci 56(2):423–426
Garna H, Mabon N, Wathelet B, Paquot M (2004) New method for a two-step hydrolysis and chromatographic analysis of pectin neutral sugar chains. J Agric Food Chem 52(15):4652–4659
Guo X, Han D, Xi H et al (2012) Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: a comparison. Carbohydr Polym 88(2):441–448
Huang XQ, Tu ZC, Zhang XC, Wang H, Xiao H, Zhang QT, Liu YD (2012) Isolation and physicochemical properties of hardleaf oatchestnut (Castanopsis sclerophylla) starch. Appl Mech Mater 140:360–368
Ishii T, Matsunaga T (2001) Pectic polysaccharide rhamnogalacturonan II is covalently linked to homogalacturonan. Phytochemistry 57(6):969–974
Jafarzadeh-Moghaddam M, Peighambardoust SH, Hesari J, Azadmard-Damirchi S (2016) Optimization of ultrasonically assisted extraction of pectin from sugar beet pulp. Biol Forum Int J 8:356–362
Jung J, Wicker L (2012) Laccase mediated conjugation of sugar beet pectin and the effect on emulsion stability. Food Hydrocoll 28:168–173
Khodaei N, Karboune S, Orsat V (2016) Microwave-assisted alkaline extraction of galactan-rich rhamnogalacturonan I from potato cell wall by-product. Food Chem 190:495–505
Kim H-S, BeMiller JN (2012) Effects of hydrocolloids on the pasting and paste properties of commercial pea starch. Carbohydr Polym 88:1164–1171
Krüger A, Ferrero C, Zaritzky NE (2003) Modelling corn starch swelling in batch systems: effect of sucrose and hydrocolloids. J Food Eng 58:125–133
Levigne S, Ralet M-C, Thibault J-F (2002) Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr Polym 49(2):145–153
Liu L, Cao J, Huang J et al (2010) Extraction of pectins with different degrees of esterification from mulberry branch bark. Biores Technol 101(9):3268–3273
Lv C, Wang Y, Wang L et al (2013) Optimization of production yield and functional properties of pectin extracted from sugar beet pulp. Carbohydr Polym 95(1):233–240
Mesbahi G, Jamalian J, Farahnaky A (2005) A comparative study on functional properties of beet and citrus pectins in food systems. Food Hydrocoll 19:731–738
Min B, Lim J, Ko S et al (2011) Environmentally friendly preparation of pectins from agricultural byproducts and their structural/rheological characterization. Biores Technol 102(4):3855–3860
Misra NN, Yadav SK (2020) Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: kinetics, characterization and process economics. Food Hydrocoll 102:105592
Qiu N-X, Tian Y-X, Qiao S-T, Hong D (2009) Apple pectin behavior separated by ultrafiltration. Agric Sci China 8:1193–1202
Renard CM, Thibault JF (1993) Structure and properties of apple and sugar-beet pectins extracted by chelating agents. Carbohydr Res 244(1):99–114
Sandarani MDJC (2017) A review: different extraction techniques of Pectin. J Pharmacogn Nat Prod 3(3):1–5
Shi X, BeMiller JN (2002) Effects of food gums on viscosities of starch suspensions during pasting. Carbohydr Polym 50(1):7–18
Shivamathi CS, Moorthy IG, Kumar RV, Soosai MR, Maran JP, Kumar RS, Varalakshmi P (2019) Optimization of ultrasound assisted extraction of pectin from custard apple peel: potential and new source. Carbohydr Polym 225:115240
Velyamov SM, Jingilbaev SS, Akterian SG (2019) Ultrasound-assisted and agitated enzymatic extraction of pectin from red beet (Beta vulgaris L. var. conditiva) roots. Bulg J Agric Sci 25(1):196–202
Vilkhu K, Mawson R, Simons L, Bates D (2008) Applications and opportunities for ultrasound assisted extraction in the food industry—a review. Innov Food Sci Emerg Technol 9(2):161–169
Wang W, Ma X, Xu Y, Cao Y, Jiang Z, Ding T, Liu D (2015) Ultrasound-assisted heating extraction of pectin from grapefruit peel: optimization and comparison with the conventional method. Food Chem 178:106–114
Wang W, Ma X, Jiang P, Hu L, Zhi Z, Chen J, Liu D (2016) Characterization of pectin from grapefruit peel: a comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll 61:730–739
Yapo BM, Robert C, Etienne I et al (2007) Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem 100:1356–1364
Yeoh S, Shi JTAG, Langrish TAG (2008) Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination 218(1–3):229–237
Ying Z, Han X, Li J (2011) Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem 127:1273–1279
Yong-Guang BI, Ding-Long Y, Yu-min LI, Min-xia H (2012) Study on ultrasonic-assisted extraction of polysaccharide of Atractylis macroceohala koidz of experiment. Energy Procedia 17:1778–1785
Acknowledgements
Assistance provided by my research supervisors, Professor Javad Hesari and Professor Sodeif Azadmard-Damirchi was greatly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical statement
Our research did not include any human subjects and animal experiments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jafarzadeh-Moghaddam, M., Shaddel, R. & Peighambardoust, S.H. Sugar beet pectin extracted by ultrasound or conventional heating: a comparison. J Food Sci Technol 58, 2567–2578 (2021). https://doi.org/10.1007/s13197-020-04763-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04763-1