Abstract
Pulsed electric field (PEF) is a novel method in liquid/semi-solid food processing, which has attracted attention due to its less destructive effects on nutritional compounds. This study aimed to optimize PEF treatment condition of kiwi-carrot juice using response surface approach to achieve higher pectin methylesterase (PME) inactivation with lower changes in ascorbic acid (AA) and phenolic compounds. For this purpose, samples were processed at PEF intensities of 22.22 to 55.56 kV/cm for 2400 to 4800 μs. The temperature of the samples increased in proportion to the increase in the intensity of PEF and the time of the process. Up to 43.03% PME inactivation was observed as a result of electroporation and increasing the temperature of fruit juice. The highest degradation rates recorded for AA and phenolic compounds were 32.19 and 12.08%, respectively. The juice cloud stability and color were totally affected by PME inactivation and degradation of AA and phenolic compounds. Juice treatment at 35.86 kV/cm for 2400 μs was recorded as the optimum process condition. This research proves that the PEF process in controlled conditions can be a suitable alternative/combination for conventional thermal methods to improve the nutritional quality and increase the shelf life of fruit juice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, various thermal and non-thermal processing methods have been introduced to guarantee the safety of the juice and prolong its shelf life [1]. One of the non-thermal techniques is the pulsed electric field (PEF), which uses a high-voltage pulsed field to inactivate microorganisms and has a minimal impact on the sensory quality and nutritional status of food. Electroporation and ohmic heating phenomena are the main PEF mechanisms affecting the food attributes. The process of electroporation creates holes in cell membranes that lead to the death of microorganisms or the removal of plant cytoplasm [2]. Fruit juices contain many ions that resist the passage of pulsed electric current. This resistance causes a rapid increase in the temperature of the fruit juice, which is known as the ohmic heating effect [3]. Vegetative microorganisms, thermal intolerant and sensitive to electroporation, are considered as the main safety concerns in the production of high acid juices (pH < 4.6) [4, 5]. Microbial studies are expensive and time-consuming. Hence, it is suggested to be replaced by analysis of enzymes having higher resistance than spoilage microorganisms [6]. Pectin methylesterase (PME) is among these enzymes with undesirable effects on the juice quality and shelf life. Considering its high resistance to the processing condition, it is known as the pasteurization index in high acid juices [7]. Studies have also shown that a higher activation energy is required to inactivate PME than to inactivate microorganisms by PEF treatment [8, 9]. In this regard, PME inactivation (about 80% and even more) was reported during PEF processing of different juices, including tomato juice (400 pulses, 24 kV/cm, < 15 °C) [7], Navelina orange juice (35 kV/cm, 1500 μs, < 37.5 °C) [9], Valencia orange juice (35 kV/cm, 184 ms, 61.9 °C) [10], orange-carrot juice (25kV/cm, 340 \(\mathrm{\mu s}\), 63 °C) [11] and red grapefruit juice (40 kV/cm, 10 \(\mathrm{\mu s}\), 50 °C) [12].
Carrot juice, as a popular and nutritious drink, is a good source of beta-carotene (vitamin A precursor) and lipophilic (carotenoids and xanthophyll) and hydrophilic (phenolic compounds) antioxidants [13]. It has been shown that the consumption of carrot juice reduces the possible risk of cardiovascular diseases and cancers [14]. The acidity of the carrot juice (pH ≈ 6) makes it vulnerable to spoilage by thermal resistance microorganisms. Therefore, high-temperature treatment condition is generally required to guarantee its shelf life. Thermal processing at high temperature reduces the nutritional value and visual quality of low-acid juices [6], while the high-acid juices need a moderate processing condition to provide an acceptable shelf life [8]. Hence, the pH of the low-acid juices could be adjusted by mixing them with a high-acid juice to avoid the necessity of juice processing under intensive processing conditions (such as high temperature). As well, mixing different juices can produce a juice with higher nutritional value and better organoleptic properties such as delicious taste (sweet–sour juice). Mixing the kiwi juice with carrot juice will be an effective way to obtain a high acidic product requiring mild heating or non-thermal processing such as PEF. Kiwifruit juice (pH \(\approx\) 3.5) brings a desirable aroma and sweet-acidic taste as well as high content of ascorbic acid, polyphenols, flavonoids, anthocyanins and other antioxidants [15, 16].
The desire for technologies that have less destructive effects on the nutritional value and appearance of juice has increased in recent years. One of these technologies, which is becoming important as an alternative to the conventional thermal process, is PEF treatment [9, 17, 18]. In this context, PEF has been claimed to inactivate microorganisms and enzymes, and preserve the quality attributes of different types of fruit juices [19]. The advantages of PEF technique compared to other thermal treatments include less energy consumption, continuous process, short process time and low process temperature.
In order to produce a juice with better quality and nutritional values, it is essential to control the processing condition. Response surface method (RSM) is an effective approach to describe and predict the effect of food treatment on its properties by developing different models. As well, RSM could be used to optimize and determine the best food processing condition. In this study, RSM was therefore performed to evaluate the influence of PEF processing conditions (PEF intensity and treatment time) on PME inactivation as well as nutritional values and color aspects of the kiwi-carrot juice. Finally, the optimized process condition was determined to produce a juice with the best quality.
Materials and methods
Juice preparation
To prepare the mixed juice, carrots (Nantes variety) and kiwi (Hayward variety) were purchased from the local market in Gorgan, Iran. After washing and peeling, the extraction procedure was carried out using a juicer (Panasonic, MJ-J176P, Japan). The obtained juice was then squeezed (mesh size 70). Based on the preliminary tests and required physicochemical properties, kiwi and carrot juice were mixed in a ratio of 70:30. Physicochemical properties of the fresh kiwi-carrot juice including pH, density, moisture content, total soluble solids and acidity (based on citric acid) were 3.80 ± 0.02, 1.01 ± 0.01 (kg/m3), 88.52 ± 0.03% (w.b), 10.10 ± 0.06°Brix and 0.55% ± 0.01, respectively. The juice was immediately subjected to PEF treatment.
Juice treatment
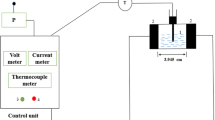
A continuous PEF system, equipped with a high voltage pulse converter, treatment chamber and syringe infusion pump, was used to treat the juice. The device was capable to generate an electric current up to 400 V by creating monopolar and bipolar square pulses with a frequency of 1 to 3000 Hz and a pulse width of 5 to 50 s. The energy stored in the capacitors is discharged into the treatment chamber by a pulse switch. The juice was processed using a square-wave bipolar pulse with a width of 1 ms with a frequency of 32 Hz. The treatment chamber comprises of two parallel stainless steel electrodes (10 mm in length and 0.5 mm in thickness) with a 0.45 mm distance. Electric field intensity is defined based on the ratio of the applied electric voltage to the gap space between the electrodes [20]. Therefore, the applied PEF intensities were 22.22, 38.89 and 55.56 kV/cm as the juice was processed at 1, 1.75 and 2.5 V, respectively. PEF treatment time was determined by adjusting the volumetric flow rate of the juice pumped through the two electrodes. PEF treatment was established for 2400, 3600 and 4800 μs by juice flow rates of 0.6, 0.9 and 1.2 mL/s, respectively. The T-type thermocouple was utilized to record the outlet juice temperature after PEF treatment. The treated juice was spontaneously cooled down in an ice-water bath (25 °C).
Analysis of physicochemical properties of kiwi–carrot juice
Pectin methylesterase activity
Pectin methylesterase activity (PME) was calculated based on the method proposed by Kimball [21]. First, 20 mL of the pectin saline solution (1 L solution containing 10 g of pectin and 15.3 g of sodium chloride salt) was added to 5 mL of kiwi–carrot juice. The pH of the solution was adjusted to 7.7 using NaOH (2 N and 0.05 N) at a constant temperature (30 °C). Then 0.1 mL of 0.05 N NaOH solution was added to the sample at once. The time required to produce acid by enzyme activity and decrease in the sample pH to 7 was recorded to define the enzyme activity unit (PEU) using Eq. 1:
where, “N” is normality (0.05 N). “V” and “V΄” are volume of the added NaOH (0.1 mL) and juice (5 mL), respectively. “t” represents the recorded time (min).
Ascorbic acid content
In this study, the iodine titration method was applied in measuring the AA content in kiwi–carrot juice [12]. To prepare the iodine solution, 5 g of potassium iodide and 0.268 g of potassium iodate were dissolved in a 500 mL flask with 200 mL of distilled water and 30 mL of 3 M sulfuric acid was then added to the resulting solution. The volume of solution with distilled water was finally increased up to 500 mL. In order to measure the amount of ascorbic acid content, 20 mL of kiwi–carrot juice was firstly mixed with 150 mL of distilled water. It was then titrated with the iodine solution until appearing a blue-blue color in the presence of 1% starch solution as an indicator. Taking 0.88 mg of AA equivalent to 1 mL of iodine solution, AA content (mg Ascorbic acid per 100 mg of the sample) was calculated using Eq. 2:
Total phenol content
Folin–Ciocalteu method was applied to measure the total phenol content of the juice [23]. According to this method, 20 μL of centrifuged juice (6000×g for 15 min) was thoroughly mixed with 1.16 mL of distilled water and then 100 μL of pure Folin reagent was added to it. After 5 min, 300 μL of 20% sodium carbonate solution was added to the prepared solution. The resulting solution was heated in a water bath (30 °C) for 30 min. Then, the absorption of the solution was read at 765 nm using a spectrophotometer (T-80, UV/VIS Double Beam Spectrophotometer). The amount of total phenol was expressed from the standard curve in terms of tannic acid concentration (mg/mL).
Cloud stability of the juice
According to the Versteeg, Rombouts, Spaansen and Pilnik [24] method, 5 mL of the kiwi-carrot juice was initially centrifuged (6000×g) for 10 min at room temperature (25 °C). To determine the cloud stability (turbidity), the adsorption of the supernatant was recorded using a spectrophotometer (T-80, UV/VIS Double Beam, PG Instrument, USA) at 660 nm. The absorbance of distilled water was considered blank.
Measurement of color parameters
The image processing method was used to evaluate the color aspects of the juice [25]. 10 mL of the sample was transferred to a laboratory plate (6 cm diameter and 1 cm height) and its image was taken by a scanner (Scanjet G2710, HP, USA). The scanner covered with a completely black and thick fabric was used to create a completely isolated environment to prevent unwanted effects of changing the ambient light. Images were saved in 600 dpi resolution in JPEG format. After transferring the images to the computer, L *, a * and b * values of the samples were extracted using ImageJ software version (1.47, Wayne Rasband, National Institutes of Health, USA). Total color difference (TCD), browning index (BI) and chroma were calculated according to Eqs. 3, 4, 5, 6:
Experimental design and optimization
In this study, the response surface method (RSM) as central composite design (CCD) was applied using Design-Expert Software (Version 10, 2017, Stat-Ease, Inc., Minneapolis, MN). RSM was used to predict the effect of processing conditions including processing time (A) and the PEF intensity (B) as independent variables. The range of each variable was selected after performing the initial pretreatments [26]. Different properties of kiwi-carrot juice (PME activity, AA content, cloud stability and color aspects) were introduced as the dependent variables. As shown in Table 1, this design consisted of four factorial points, four axial points and one central point. Three replications at each point of the design were used to assessment the error sum of squares. Different models were used to define each dependent parameter and the independent factors. These models were evaluated via analysis of variance (ANOVA). Optimization of the multiple responses was performed using numerical optimization performance of Design-Expert software. All the independent variables were within the studied ranges.
Results and discussion
Changes in the juice temperature during PEF treatment
PEF technology is one of the non-thermal processing techniques that is used to make food safe for consumption with short processing time and minimal heat generation. As seen in Fig. 1, PEF treatment caused the juice temperature to rise by up to 54 °C (from an initial temperature of 25 °C). As expected, samples exposed to higher PEF intensity for a longer period of time (p < 0.05) showed a greater increase in juice temperature (T). The ohmic heating phenomenon, which happens when an electrical current passes through the liquid between two electrodes, is closely related to the temperature rise [2, 20, 27]. The applied PEF intensity had more of an effect (twice as much as the processing time in Eq. 7) on the change in juice temperature (R2 = 0.986, adj-R2 = 0.977).
This result is in an accordance with previous studies. It is interesting that a rise in temperature about 21.9 °C (12.5 kV/cm, 62 Hz, 7.7 mL/s, 76.4 J/mL) and 36.5 °C (12.3 kV/cm, 94 Hz, 6.8 mL/s, 132.5 J/mL) in apple juice [28] and 12.38 °C to 43.13 °C (22.73 to 36.36 kV/cm, 3520 μs) in tomato juice processed with PEF have also been reported [29].
The changes in physicochemical properties of the juice during PEF processing
Pectin methylesterase inactivation
The PME activity of the fresh kiwi-carrot juice was 2.53 × 10–4 PEU. Statistical analysis revealed that the processing time, PEF intensity and their interaction had a considerable effect on PME inactivation (p < 0.05). PEF treatment at a higher intensity for a longer time caused a further decrease in the enzyme activity (Fig. 2). The highest PME inactivation (43.03%) was achieved at 55.55 kV/cm for 4800 μs, where the highest increase in the juice temperature was recorded (Fig. 1). Although it is believed that the denaturation of the protein in the structure of the enzyme occurs at high temperatures (above 40 °C), in this study, the inactivation of PME was observed at temperatures below 40 °C. This finding could be attributed to the effect of electroporation on the PME structure [6]. The results are consistent with previous studies. For example, PEF processing of the orange juice (35 kV/cm for 1500 μs) inactivated 80% of PME when the juice temperature was less than 37.5 °C [9], or Yeom, Zhang and Chism [10] reported that 40 °C (from 10 to 50 °C) increase in the orange juice temperature induced 54% more PME inactivation during PEF treatment (25 kV/cm for 250 ms). The extent of PME inactivation differs depending on the processing conditions and the type of juice [30, 31]. It has been reported that when the tomato juice was processed at 36.36 kV/cm for 3520 μs and at 24 kV/cm at 15 °C (400 pulses), PME inactivation was reported about 29.3% and 94% respectively [7]. Furthermore, in another study, the inactivation of PME enzyme in different plant sources was recorded as 87% inactivation of PME enzyme in orange and tomato juice, 83% in carrot juice and 45% in banana at 1.6 ms processing time and 19.1 kV/cm electric field strength [27].
Extending the PEF treatment time of orange-carrot juice from 280 to 330 μs resulted in only 2 °C (68 to 70 °C) with noticeable improvement (5%) in PME inactivation [19]. These results reflect the synergistic effect of electroporation and ohmic heating on the enzyme inactivation [32]. PME was completely inactivated during apple juice processing at 12.3 kV/cm (94 Hz and 6.8 mL/s), while lower PEF intensity (12.5 kV/cm, 62 Hz, 7.7 mL/s) caused 50% reduction in the activity of this enzyme [28].
In enzyme inactivation, the first thing that happens is the unfolding of the protein structure. These changes are of the covalent type, which leads to conformational changes in the protein structure. The second and third structures of enzymes are stabilized by hydrogen bonds, whose change by electric pulse fields may lead to the denaturation of the alpha helix structure of the enzymes and the disruption of the tetramer structure of the enzyme. Many studies indicate the destruction of alpha helical structure by PEF [33, 34]. There is also a direct relationship between loss of alpha-helical structure and enzyme inactivation [35].
During PEF processing, the quadratic model (Eq. 8) was chosen as the best model for predicting the PME inactivation (R2 = 0.993, adj-R2 = 0.982). This model showed a direct relationship between the PME inactivation and independent variables. Considering the presented coefficients, the change in the processing time had more impact on the PME inactivation than PEF intensity.
Effect of PEF treatment on ascorbic acid content
Ascorbic acid (AA) is a heat-sensitive nutrient that decreases significantly in different stages of processing or during the storage of fruit juices. Our results (Fig. 3) showed that the content of ascorbic acid decreased with increasing time and intensity of PEF (p < 0.05). AA content was almost preserved (99.89%) during PEF treatment at 22.22 kV/cm for 2400 μs, while a 32.19% decrease was observed during processing at the most intense condition. It was therefore clear that the non-thermal effect of PEF (electroporation phenomena) brings no considerable changes in the AA content [36]. In the same way, Odriozola-Serrano, Soliva-Fortuny and Martín-Belloso [37] reported only 13.5% reduction in the content of AA after tomato juice processing at 35 kV/cm for 1500 μs. In another study, AA retention in processed broccoli juice at 35 kV/cm for 500 μs (unipolar pulse) and 25 kV/cm for 1250 μs (bipolar pulse) were 90.1% and 67%, respectively [38].
There are other studies that have reported minimal effects on ascorbic acid for the PEF process. For example, PEF treatment did not show significant changes in the AA content of the different citrus juices (28 kV/cm, 100 μs) [39], tomato juice (40 kV/cm, 57 μs) [40] and sugarcane juice [18]. In general, it can be argued that due to the short time of PEF (microseconds), the increase in temperature during the process has minimal destructive effects on the structure of ascorbic acid.
From fitting the data with different statistical models, a linear model (Eq. 9) was suggested to describe the effect of process conditions on this vitamin content (R2 = 0.860, adj-R2 = 0.813). The coefficients showed that the effect of intensity was about two times higher than the processing time.
Effect of PEF treatment on total phenol content
Phenolic compounds are a group of secondary metabolites that are found in fruits and vegetables and have various health-giving properties such as antioxidant, anti-cancer and anti-inflammatory [41]. Total phenol content showed a different trend compared to ascorbic acid, so that it first slightly increased and then decreased (Fig. 4). The increase of phenolic compounds in the early stages of PEF treatment could be attributed to the release of the phenolic compounds within the juice as a result of the electroporation [30]. In overlay, the total phenol level in the kiwi-carrot juice was decreased between 2.01% to 12.08% during PEF treatment (p < 0.05). The degradation of phenolic compounds could be related to their thermal sensitivity. Conversely, Dziadek, Kopeć, Dróżdż, Kiełbasa, Ostafin, Bulski and Oziembłowski [42] reported that PEF treatment at 30 kV/cm (200–400 pulses) had no significant effect on the total phenol content of the apple juice. In general, the PEF processing as a novel non-thermal method significantly preserves phenolic compounds compared to the conventional thermal processing. Applying the PEF process led to an 18% reduction of phenolic compounds in longan juice, while the thermal process caused a 42% decrease of these compounds in longan juice [43].
From fitting of the data to various models (e.g., linear, quadratic, etc.), a quadratic model (Eq. 10) was chosen to predict the alteration in total phenol content of the juice (R2 = 0.996, adj-R2 = 0.0.988) in which the adverse effect of the PEF intensity and time were represented by negative coefficients.
Effect of PEF treatment on cloud stability
The PEF treatment improved the desirable cloudy appearance of the kiwi-carrot juice (Fig. 5). The statistical analysis revealed that both independent parameters had a significant effect on increasing the juice cloud value (p < 0.05). In general, depending on the juice conditions, cloud stability increased from 188.72 to 237.74% in different treatments. The change in cloud stability showed a similar trend with the PME inactivation, and increased proportionally with the increase in intensity and time of the process. It was shown that the PME activity decreases the turbidity of the juice by breaking the pectin structure [44]. Besides, observation of the cloud improvement in the treated juice with high PME activity referred to the effect of electroporation on more release of the cloud particles within the juice [3, 45]. Rayman et al. [45] reported that electroporation induced a 14.78% increase in the total pectin content in carrot juice after processing at 40 V for 60 s. The following equation shows the relationship between different process variables with cloud stability in coded form:
Equation (11) showed that cloud stability had a linear relationship with the intensity and time of the process (R2 = 0.919, adj-R2 = 0.891). From the coefficients of the variables, it is also clear that the independent variables had an almost equal and positive effect on cloud stability.
Effect of PEF treatment on the color properties
Color is the most important visual factor affecting the consumer acceptance. In other words, the color can indicate changes in the chemical contents of the product (such as ascorbic acid, antioxidants, pigments and etc.); hence, it is possible to control the nutritional value and quality of juice by monitoring it. Different color parameters are used frequently in describing the color of the juices such as L*, a* and b* values. L* ranges from 0 to 100 referring to ‘darkness’ to ‘lightness’, respectively. a* and b* values are represented as positive or negative values. Positive and negative values of a*-value shows ‘redness’ and ‘greenness’, respectively. Positive values of b*-value show ‘yellowness’ and its negative values reveal the ‘blueness’. These parameters are used in calculating other color properties including TCD, BI and chroma.
The L* index indicates the effect of processing conditions on the lightness of the juice color. The statistical analysis of the results showed that the use of different intensities of the PEF had a significant effect on L* (p < 0.05). In addition, as the process time increased, the lightness of the product decreased (p < 0.05). This trend was previously observed in the amount of lightness in orange juice and broccoli juice [46, 47]. The reason for the reducing lightness of the sample can be attributed to the destruction of compounds such as pigments, ascorbic acid and the change in the cloud of the sample. By applying high-intensity pulsed electric fields, the degradation rate of PME enzyme increases, and as a result, the amount of turbidity of preserved fruit juice and the lightness of the product decreases.
According to the increase in a* component, it can be concluded that the color of the juice changed to redness during longer processing (p < 0.05). Analysis of variance as well as comparison of the average changes in the a* showed that the intensity and time of the process have a significant effect on this component (p < 0.05). Changes in the b* index also showed that the yellowness increased in proportion to the increase in intensity and processing time (p < 0.05). The results are consistent with Zhou et al. [48].
Effect of PEF treatment on chroma index
Unlike the effect of PEF intensity, the processing time showed considerable impact on changes of chroma index (p < 0.05). As shown in Fig. 6, chroma index increased during the PEF treatment for a longer time. The linear model (Eq. 12) represented a positive correlation between the chroma and treatment time (R2 = 0.935, adj-R2 = 0.913). Chroma of the fresh orange juice (50.93) increased up to 53.70 after the application of PEF at 30 kV/cm for 100 μs [49]. However, Rivas, Rodrigo, Martínez, Barbosa-Cánovas and Rodrigo [19] reported that the chroma of mix orange-carrot juice were not affected by the PEF process (25 kV/cm for 280 μs). Wibowo et al. [28] also reported that no change in chroma index under different PEF conditions. The following equation shows the relationship between different process variables with chroma index in coded form:
Effect of PEF treatment on browning index (BI)
It was found that both independent variables and their interaction had a significant effect on BI of the kiwi-carrot juice (p < 0.05). The BI increased during processing under more intensive conditions (Fig. 7). Browning reactions, decreasing the juice acceptance and nutritional values, could occur through juice production stages such as juice extraction, processing, and storage. Several reactions are responsible in change of this color parameter. For example, Maillard’s reaction is responsible for browning development in low AA content juices. AA degradation and total phenol reduction are other factors involving in the BI development in juices like kiwi-carrot that are rich in antioxidants [50, 51].
During PEF treatment, an increase in the BI of mixed mandarin and Hallabong tangor juice was previously observed which was attributed to the reduction in the content of ascorbic acid and antioxidants [52]. Min et al. [53] and Arshad RN et all, [54] compared the effect of PEF and conventional thermal treatments on color aspects of juices. They found that the PEF treatment was more effective in the preservation of the ascorbic acid and preventing the BI development in the juices. Furthermore, Aguilo et al. [55] and [52] reported that the use of the high-intensity pulse process led to decrease in the lightness value in strawberry juice and increase in yellowness of apple juice which were attributed to the inactivation of peroxidase and polyphenol oxidase enzymes. In general, application of PEF had a negligible effect on BI of different citrus juices [39, 57].
Equation 13 was proposed to describe the effect of PEF treatment on BI of the kiwi-carrot juice (R2 = 0.996, adj-R2 = 0.990). It could be observed that the PEF intensity had a more important effect on this color attribute than the processing time.
Effect of PEF treatment on total color difference
Figure 8 shows the TCD alteration during PEF processing of the juice. The TCD of the juice increased between 2.15 and 9.27 in different treatments, depending on the PEF condition. However, the statistical analysis revealed that the PEF intensity and process time had no considerable effect on the TCD (p > 0.05). Nevertheless, TCD initially increased and then decreased at any PEF intensity. An increase in the juice temperature decreased the contents of AA and phenolic compounds that brought the rise in the a*. Besides, the more increase in the cloud stability caused by PME inactivation, lowered the juice lightness. Equation 14 is suggested to demonstrate the effect of process conditions on the TCD of the juice (R2 = 0.899, adj-R2 = 0.730).
During PEF treatment of orange juice at 25 kV/cm for 340 μs (72 °C) the TCD increased up to 7.32 [57]. Mixed mandarin and Hallabong tangor juice was primarily heated before PEF treatment [52]. They reported that TCD of the treated juices at 55 °C-19 kV/cm-102 μs and 70 °C-16 kV/cm-89 μs were 2.17 and 1.33, respectively. These results show that more intensive process conditions will result in higher TCD. In another study, PEF treatment of apple juice at 30 and 35 kV/cm for 75 μs [56] resulted in a TCD higher than 5 (with a yellowish color appearance) while lower TCD (up to 2) was observed at lower intensity even for longer time (24.8 kV/cm for 169 μs) [58]. Cserhalmi et al. [39] processed different citrus juices at 28 kV/cm for 100 μs and reported the TCD of the grapefruit, orange, lemon & tangerine juices were lightly increased to 0.45, 0.47, 0.59 & 2.44, respectively. The correlations of the TCD of the juice with independent variables were expressed as Eq. 14:
The optimization of the PEF process condition
The process condition was optimized to produce a juice with the best quality (Fig. 9). The highest levels of PME inactivation, AA retention, total phenol content and cloud stability as well as lowest changes in color aspects were the criteria of optimization. The software suggested optimum condition as juice processing at 35.86 kV/cm for 2400 μs (Fig. 9) which results in 8.10% PME inactivation, 97.82% AA retention, 90.30% total phenol content and 131.68% cloud stability as well as lowest 189.24% BI, 48.61% chroma and 3.794 TCD (desirability = 0.718). To validate the results, the experiments were carried out at the optimum PEF process condition and it was observed that there is no difference betweenthe predicted and actual results at the optimum process condition (p > 0.05).
Conclusion
In this study, a continuous PEF system was used in the processing of the kiwi-carrot juice. The results showed that processing at higher PEF intensity for a longer period increased the juice temperature causing changes in juice properties. During PEF treatment, different levels of PME inactivation (up to 43.03%) were observed as a result of electroporation and ohmic heating phenomena. It was also observed that this method was effective in preserving the AA and phenolic compounds. The maximum reduction in vitamin content was 19.32%, which was observed in the most intense PEF condition. Electroporation caused more release of cloud particles within the juice which improved the cloudiness of the juice. Besides, the cloud stability of kiwi-carrot juice increased proportionally with increasing process intensity and time, which could be related to PME inactivation. AA degradation and reduction in total phenol content enhanced the browning reactions and therefore BI. The changes in the color indices also showed that the value of a* (redness) and b* (yellowness) increased and L* (lightness) decreased. Different linear and quadratic models were also developed to evaluate and predict the effect of process conditions (PEF intensity and treatment time) on the juice properties. Finally, the optimum condition of 35.86 kV/cm–2400 μs was chosen with desirability of 0.718 for juice processing. This research showed that PEF treatment can be considered as alternative methods to thermal processing for the processing of fruit juices with better preservation of nutritional, sensory and marketing characteristics.
Data availability
Data will be made available on request.
References
E. Arjeh, M. Barzegar, M.A. Sahari, Effects of gamma irradiation on physicochemical properties, antioxidant and microbial activities of sour cherry juice. Radiat. Phys. Chem. 114, 18–24 (2015). https://doi.org/10.1016/j.radphyschem.2015.05.017
N. Kaur, A. Singh, Ohmic heating: concept and applications—a review. Crit. Rev. Food Sci. Nutr. 56(14), 2338–2351 (2016). https://doi.org/10.1080/10408398.2013.835303
A. Demirdöven, T. Baysal, J. Food Sci. Technol. 51(9), 1817–1826 (2014). https://doi.org/10.1007/s13197-012-0700-5
M. Turtoi, Inactivation of Saccharomyces cerevisiae using new non-thermal technologies. A review. Rom. Biotechnol. Lett. 19(1), 8901 (2014)
W.G. McGlynn, The importance of food ph in commercial canning operations. 2003: Oklahoma Cooperative Extension Service, Division of Agricultural Sciences and Natural Resources, Oklahoma State University.
S. Aghajanzadeh, A.M. Ziaiifar, Trends Food Sci. Technol. 71, 1–12 (2018). https://doi.org/10.1016/j.tifs.2017.10.013
A.C. Polydera, E. Galanou, N.G. Stoforos, P.S. Taoukis, J. Food Eng. 62(3), 291–298 (2004). https://doi.org/10.1016/S0260-8774(03)00242-5
S. Aghajanzadeh, A.M. Ziaiifar, Pasteurization of juices with non-thermal technologies, in Sustainable food processing and engineering challenges. ed. by C.M. Galanakis (Elsevier, Amsterdam, 2021), pp.25–73
F. Salehi, Int. J. Food Prop. 23(1), 1036–1050 (2020). https://doi.org/10.1080/10942912.2020.1775250
H. Yeom, Q. Zhang, G. Chism, J. Food Sci. 67(6), 2154–2159 (2002). https://doi.org/10.1111/j.1365-2621.2002.tb09519.x
D. Rodrigo, G. Barbosa-Cánovas, A. Martinez, M. Rodrigo, J. Food Prot. 66(12), 2336–2342 (2003). https://doi.org/10.4315/0362-028X-66.12.2336
J. Riener, F. Noci, D.A. Cronin, D.J. Morgan, J.G. Lyng, Euro. Food Res. Technol. 228(3), 373–379 (2009). https://doi.org/10.1007/s00217-008-0943-6
T.K. Koley, J. Nishad, C. Kaur, Y. Su, S. Sethi, S. Saha, S. Sen, B.P. Bhatt, J. Food Sci. Technol. (2020). https://doi.org/10.1007/s13197-020-04251-6
K.D. Sharma, S. Karki, N.S. Thakur, S. Attri, J. Food Sci. Technol. 49(1), 22–32 (2012). https://doi.org/10.1007/s13197-011-0310-7
Y. Peng, S.B. Cordiner, G.M. Sawyer, T.K. McGhie, R.V. Espley, A.C. Allan, R.D. Hurst, J. Funct. Foods 65, 103734 (2020). https://doi.org/10.1016/j.jff.2019.103734
H. Zhang, Q. Zhao, T. Lan, T. Geng, C. Gao, Q. Yuan, Q. Zhang, P. Xu, X. Sun, X. Liu, Foods 9(9), 1267 (2020). https://doi.org/10.3390/foods9091267
B. Ling, J. Tang, F. Kong, E. Mitcham, S. Wang, Food Bioprocess Technol. 8(2), 343–358 (2015). https://doi.org/10.1007/s11947-014-1398-3
K. Mukhtar, B.G. Nabi, R.N. Arshad, U. Roobab, B. Yaseen, M.M.A.N. Ranjha, R.M. Aadil, S.A. Ibrahim, Ultrasonics Sonochem. (2022). https://doi.org/10.1016/j.ultsonch.2022.106194
A. Rivas, D. Rodrigo, A. Martínez, G. Barbosa-Cánovas, M. Rodrigo, LWT-Food Sci. Technol. 39(10), 1163–1170 (2006). https://doi.org/10.1016/j.lwt.2005.07.002
E. Agcam, A. Akyildiz, G.A. Evrendilek, Food Chem. 165, 70–76 (2014). https://doi.org/10.1016/j.foodchem.2014.05.097
D.A. Kimball, Citrus processing: a complete guide. Springer Science & Business Media; 2012 Dec 6.
G. Kashyap, M. Gautam, J. Sci. Res. Phar. 1(2), 77–78 (2012). https://doi.org/10.9790/3008-1203035961
E. Arjeh, S.M. Khodaei, M. Barzegar, S. Pirsa, I. KarimiSani, S. Rahati, F. Mohammadi, Food Sci. Nutr. 10(12), 4238–4246 (2022). https://doi.org/10.1002/fsn3.3017
B. Tiwari, K. Muthukumarappan, C. Odonnell, P. Cullen, Innov. Food Sci. Emerg. Technol. 10(2), 166–171 (2009). https://doi.org/10.1016/j.ifset.2008.11.006
M. Saberi, A.M. Ziaiifar, M. Kashaninejad, M. Aalami, H.O. Mirzaei, K. Ghorbani, S. Aghajanzadeh, Prediction of the physicochemical properties of quince puree during thermal treatment using M5 decision tree. J. Food Bioprocess Eng. 2(2), 139–146 (2019)
E. Arjeh, M.K. Pirouzifard, Optimization of enzymatic pretreatment of raw sugar beet juice using response surface methodology. J. Food Sci. Tech. (Iran) 16(87), 343–354 (2019)
A. Espachs-Barroso, A. Van Loey, M. Hendrickx, O. Martín-Belloso, Innov. Food Sci. Emerg. Technol. 7(1–2), 40–48 (2006). https://doi.org/10.1016/j.ifset.2005.07.002
Wibowo, E.A. Essel, S. De Man, N. Bernaert, B. Van Droogenbroeck, T. Grauwet, A. Van Loey, M. Hendrickx, Innov. Food Sci. Emerg. Technol. 54, 64–77 (2019). https://doi.org/10.1016/j.ifset.2019.03.004
S. Aghajanzadeh, A.M. Ziaiifar, M. Kashaninejad, A. Rezaei Asl, The effect of infrared pre-heating and pulsed electric field on physicochemical properties of tomato juice. Iran. Food Sci. Technol. Res. J. 15(2), 297–307 (2019)
N. Chhikara, A. Panghal, D.N. Yadav, S. Mann, P. Bishnoi, Novel Technol. Food Sci. (2023). https://doi.org/10.1002/9781119776376.ch2
U. Roobab, A. Abida, J.S. Chacha, A. Athar, G.M. Madni, Molecules 27(13), 4031 (2022). https://doi.org/10.3390/molecules27134031
R. Buckow, P. Baumann, S. Schroeder, K. Knoerzer, J. Food Eng. 105(3), 545–556 (2011). https://doi.org/10.1016/j.jfoodeng.2011.03.019
Z. Alexandrakis, G. Katsaros, P. Stavros, P. Katapodis, G. Nounesis, P. Taoukis, Food Bioprocess Technol. 7, 853–867 (2014). https://doi.org/10.1007/s11947-013-1087-7
M. Šimunek, A.R. Jambrak, S. Dobrović, Z. Herceg, T. Vukušić, J. Food Sci. Technol. 51, 3577–3593 (2014). https://doi.org/10.1007/s13197-013-0958-2
M. de LourdesMeza-Jiménez, P.R. Pokhrel, LWT 109, 336–341 (2019). https://doi.org/10.1016/j.lwt.2019.04.037
N.D. Trung, N.N. Hoang, D.M. Hieu, Pulsed electric field for pasteurization of fresh sugarcane juice. ASEAN Eng. J. 8(1), 2586–9159 (2018). https://doi.org/10.11113/aej.v8.15496
I. Odriozola-Serrano, R. Soliva-Fortuny, O. Martín-Belloso, Innov. Food Sci. Emerg. Technol. 9(3), 272–279 (2008). https://doi.org/10.1016/j.ifset.2007.07.009
R. Sánchez-Vega, P. Elez-Martínez, O. Martín-Belloso, Innov. Food Sci. Emerg. Technol. 29, 70–77 (2015). https://doi.org/10.1016/j.ifset.2014.12.002
Z. Cserhalmi, A. Sass-Kiss, M. Tóth-Markus, Innov. Food Sci. Emerg. Technol. 7(1–2), 49–54 (2006). https://doi.org/10.1016/j.ifset.2005.07.001
S. Min, Q. Zhang, J. Food Sci. 68(5), 1600–1606 (2003). https://doi.org/10.1111/j.1365-2621.2003.tb12298.x
E. Arjeh, A. Masoumi, M. Barzegar, H.R. Akhavan, Strategies to reduce microbial contaminations and increase the shelf life of pistachio fruit: a review. Food Health 4, 24–37 (2021)
K. Dziadek, A. Kopeć, T. Dróżdż, P. Kiełbasa, M. Ostafin, K. Bulski, M. Oziembłowski, J. Food Sci. Technol. 56(3), 1184–1191 (2019). https://doi.org/10.1016/j.ifset.2014.12.002
Y.I. Zhang, B.E.I. Gao, M. Zhang, J. Shi, Y. Xu, J. Food Process. Preserv. 34(6), 1121–1138 (2010). https://doi.org/10.1111/j.1745-4549.2009.00441.x
S. Aghajanzadeh, A.M. Ziaiifar, M. Kashaninejad, Y. Maghsoudlou, E. Esmailzadeh, J. Food Eng. 185, 72–77 (2016). https://doi.org/10.1016/j.jfoodeng.2016.04.004
A. Rayman, T. Baysal, A. Demirdöven, Int. J. Food Sci. Technol. 46(4), 781–786 (2011). https://doi.org/10.1111/j.1365-2621.2011.02561.x
R.A.H. Timmermans, W.S.U. Roland, K. van Kekem, A.M. Matser, M.A.J.S. van Boekel, Foods 11(21), 3360 (2022). https://doi.org/10.3390/foods11213360
R. Sánchez-Vega, M.J. Rodríguez-Roque, P. Elez-Martínez, O. Martín-Belloso, J. Food Process. Preserv. 44(3), e14362 (2020). https://doi.org/10.1111/jfpp.14362
N. Zhu, Y. Zhu, N. Yu, Y. Wei, J. Zhang, Y. Hou, A. Sun, Food Chem. 274, 146–155 (2019). https://doi.org/10.1016/j.foodchem.2018.08.092
C. Cortés, M.J. Esteve, A. Frígola, Food Control 19(2), 151–158 (2008). https://doi.org/10.1016/j.foodcont.2007.03.001
C. Buvé, H.T.T. Pham, M. Hendrickx, T. Grauwet, A. Van Loey, Compr. Rev. Food Sci. Food Saf. 20(6), 5698–5721 (2021). https://doi.org/10.1111/1541-4337.12850
S. Aghajanzadeh, A.M. Ziaiifar, R. Verkerk, Food Rev. Int. (2022). https://doi.org/10.1080/87559129.2021.2012799
S.J. Lee, I.H. Bang, H.J. Choi, S.C. Min, Food Sci. Biotechnol. 27(3), 669–675 (2018). https://doi.org/10.1007/s10068-018-0311-7
S. Min, Z.T. Jin, S.K. Min, H. Yeom, Q.H. Zhang, J. Food Sci. 68(4), 1265–1271 (2003). https://doi.org/10.1111/j.1365-2621.2003.tb09637.x
R.N. Arshad, Z. Abdul-Malek, U. Roobab, M.A. Munir, A. Naderipour, M.I. Qureshi, A.E. Bekhit, Z.W. Liu, R.M. Aadil, Trends Food Sci. Technol. 1(111), 43–54 (2021). https://doi.org/10.1016/j.tifs.2021.02.041
I. Aguilo-Aguayo, G. Oms-Oliu, R. Soliva-Fortuny, LWT-Food Sci. Technol. 42(4), 813–818 (2009). https://doi.org/10.1016/j.lwt.2008.11.008
X. Bi, F. Liu, L. Rao, J. Li, B. Liu, X. Liao, J. Wu, Innov. Food Sci. Emerg. Technol. 17, 85–92 (2013). https://doi.org/10.1016/j.ifset.2012.10.008
C. Cortés, M.J. Esteve, D. Rodrigo, F. Torregrosa, A. Frígola, Food Chem. Toxicol. 44(11), 1932–1939 (2006). https://doi.org/10.1016/j.fct.2006.06.026
A. Sulaiman, M. Farid, F.V. Silva, Food Sci. Technol. Int. 23(3), 265–276 (2017). https://doi.org/10.1177/1082013216685484
Funding
This research was supported by Gorgan University.
Author information
Authors and Affiliations
Contributions
AMZ: Software, Formal analysis, Investigation, Writing—original draft. AD: Methodology, Visualization, Validation. ZM: Resources, Data curation, Visualization. SA: Methodology, Visualization, Validation. EA: Methodology, Visualization, Validation, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ziaiifar, A.M., Dezyani, A., Mokhtari, Z. et al. Response surface optimization of pulsed electric field processed kiwi–carrot juice: enzyme inactivation and evaluation of physicochemical and nutritional properties. Food Measure 18, 489–499 (2024). https://doi.org/10.1007/s11694-023-02164-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02164-4