Abstract
In this study, optimization of ohmic heating (OH) process parameters (temperature and voltage gradient) to inactivate polyphenoloxidase (PPO) of not-from-concentrate (NFC) apple juice was conducted. Response surface methodology was used for optimization of OH parameters, where the voltage gradient and temperature on the PPO activity in the NFC apple juice was evaluated. Then the optimized condition was used to produce the NFC apple juice and the quality parameters were evaluated and compared to NFC apple juice prepared by conventional heating (CH). The studied parameters were: PPO activity, total phenolic, total carotenoids, ascorbic acid, cloud value, color as well as physical properties (i.e., TSS, acidity, electric conductivity and viscosity). The reduction of PPO activities was 97 and 91% for OH (at 40 V/cm and 80 °C) and CH (at 90 °C and 60 s), respectively. The reduction of the ascorbic acid was 66.8% for OH significantly lower than the 80% for CH treated samples. The total extracted phenolic content was increased by 5.4 and 2.5% with OH and CH treatments, respectively. The decrease in the concentration of total carotenoids for OH (13.17%) was significantly lower than for CH (34.23%). The color values (L*, a*, b* and ΔE) were only significantly increased in the OH treatment. OH is a potential mild thermal treatment in the production of apple juice with improved functional properties instead of conventional methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple juice is one of the preferred juices for consumption due to its health benefits (i.e. containing large amounts of antioxidant compounds, fibers, carbohydrates, minerals and other health benefits) (Włodarska et al. 2016). Not-from-concentrate (NFC) juice is defined as a juice that has not undergone concentration or dilution during processing with only removal of the insoluble pulp, skin, and seeds and subjected to pasteurization for reducing both microbial load and enzyme activity. Then the juice is deaerated by N2 gas and refrigerated at 0–2 °C under strictly controlled conditions to be stored more than one year (Clark 2009). Polyphenoloxidase (PPO) is one of the important enzymes responsible for browning and color changes in most of the fruit juices. PPO in the presence of oxygen can oxidize the phenolic compounds, catalyzing the aerobic regioselective oxidation of monophenols to o-diphenols followed by dehydrogenation to brown o-quinones. Thermal pasteurization of apple juice depends on the thermal destruction of PPO, which has a higher thermal stability than many vegetative microorganisms (Gong et al. 2015).
Conventional heating (CH) is a widely used method to inhibit microbial and enzymatic activities (causing deterioration and spoilage of juices) for prolonging the shelf life of juices. However, thermal treatment (using more than 80 °C) can cause deterioration in the fruit juice characteristics (i.e., color alterations, flavor damages, vitamins and other nutritional losses) (Mena et al. 2013). Therefore, the food industry is looking for alternative technologies either thermal or non-thermal that able to inactivate the undesired microbial and enzymatic activities with minimum effect on the quality characteristics of fruit juices (Chemat and Khan 2011). Ohmic heating (OH) is a thermal treatment of food by passing alternating electric current through food components (Knirsch et al. 2010). The food serves as an electrical resistor to heat in a very short time. The OH device consists of two electrodes in the direct contact with the food where alternating current is directly passed through the food. Heat transfer behavior of conventional heating occurs from a heated surface to the product interior by means of convection and conduction, while in OH it occurs volumetrically in nature (inside-outside heat transfer pattern) and has the potential to reduce over-processing (Lima 2007). OH is similar to high temperature short time (HTST) treatments and function as a conventional heating process of juices with additional benefits of rapid and uniform heating. OH is a rapid and uniform heating technique which leads to inactivation of the microbial load and enzymes activity in a shorter time with a smaller loss of ascorbic acid and strange smell compared to conventional heating (Machado et al. 2010).
OH has shown promising results in the inactivation polyphenoloxidase, peroxidase and pectinmethylesterase inpineapple, papaya and orange juices (Ramaswamy et al. 2005; Demirdöven and Baysal 2014). Enzymatic activity has a negative impact on juice quality and it should be controlled in every step of food processing (Demirdöven and Baysal 2009). Makroo et al., (2016) studied the effect of OH (at 24 V/cm—90 °C) and hot-water (at 90 °C) for 15, 30, 45 and 60 s on fresh watermelon juice. They found that PPO activity was reduced to 36.15 and 8.87% in 60 s by hot-water and OH, respectively. Also, Icier et al. (2008) conducted the inactivation curves of PPO on grape juice at different OH holding time at 30 V/cm. They found that PPO activity was decreased with increasing time and temperature (in the range of 60 to 90 °C). However, there is no reported data available on the optimization of ohmic heating parameters for PPO inactivation in the elstar apple juice. Therefore, the objective of this study is to investigate the effect of ohmic heating on the inactivation of PPO in the apple juice as well as to optimize the temperature and voltage gradient conditions using response surface methodology (RSM). The NFC apple juice is then produced with optimum OH condition and the quality parameters are evaluated and compared with the conventional heating.
Materials and methods
Chemicals
Polyvinyl poly pyrrolidone (PVPP), catechol, 2,6-dichlorophenol-indophenol (DCPIP), sodium bicarbonate, l-ascorbic acid, butylated hydroxytoluene (BHT), hexane, acetone, methanol, oxalic acid, folin–ciocalteau reagent, gallic acid, sodium carbonate and NaOH from (Sigma-Aldrich Chemical Co., Denmark) were used.
Materials
Fresh apple fruits (Malus Domestica, cv. Elstar) were purchased from a local supermarket in Copenhagen, Denmark. The unblemished fruits were selected, washed, dried by tissue paper and cut into four pieces. The stems, seeds, and overripe portions were discarded. The juice was extracted (Extractor, PHILIPS, HR 1865-700W, China) and filtered through sterilized double-layered muslin cloth. The residual of apple juice was divided into three parts i.e. control, conventional heating (CH) and ohmic Heating (OH) to determine the quality properties and PPO inactivation after each treatment. All samples were quickly cooled to 4 °C (ice bath) and stored at − 18 °C to stop all reactions until further analysis.
Processing methods
Conventional heating
Apple juice (80 ml) was heated at 90 °C for 60 s in a clean 250 ml glass bottle using a shaker water bath (Julabo, SW22, Germany). The temperature was measured during the experiments by a thermocouple (Pico, TC-08, UK) within the center vertical axis of the apple juice bottles without agitation.
Ohmic heating
An ohmic heater (BCH ltd., Lancashire, UK) with an ohmic unit consisting of a holding cell made of W500 grade polyethylene-polypropylene with variable size adjustment and mountings for temperature loggers (K-type) was used. Experimental set up of the ohmic heating is shown in Fig. 1. A maximal supply at 230 voltage using alternating current (60 Hz, sinusoidal) was installed with the ohmic heater, a titanium electrode with high corrosion resistance in chloride environments (Pedersen et al. 2016). A distance between the electrodes and a width of the chamber were set to 3.945 and 9.5 cm, respectively. After the system was sealed, 80 ml of apple juices were ohmically heated up to different temperatures (60, 70 and 80 °C) at 60 Hz frequency using different voltages (30, 35 and 40 V/cm). Voltage, current and temperature data were recorded per second during the heating. The temperature of each sample was assumed uniform in the cell, where the maximum difference among the measured temperatures at different treatments was ± 1 °C. The experiments were performed in triplicate. After each treatment, the samples were cooled quickly to 4 °C in an ice bath.
Experimental design
The effects of voltage gradient and temperature (independent variables) on PPO activity (response) of the NFC apple juice were investigated using response surface methodology (RSM). A 3-level factorial design (32) was used: voltage gradient (30, 35 and 40 V/cm) and temperature (60, 70 and 80 °C).
The 32 factorial design was set up for two factors, with three coded levels (− 1, 0, and + 1), as illustrated in Table 1. The significant terms in the model were found by analysis of variance (ANOVA) for the response (PPO activity) and validation of the equation was investigated by model ANOVA statistics. The regression coefficients were used to make statistical calculations to generate response surface plot from the model (trial version of Design Expert Version 10.0.6 software). The generalized second-order polynomial model was used in the response surface analysis, which is described by (Eq. 1):
where Y is the response variable, χ1 (voltage gradient) and χ2 (temperature) are the independent variables. Regression coefficients are: ao is for intercept, a1 and a2 are for the linear term, a11 and a22 are for the quadratic term and a12 is for cross product (interaction) term. The experimental data were fitted to a second-order polynomial model (Eq. 1) to obtain the regression coefficients.
The adequacy of the model was checked using the R2, adjusted-R2, predicted-R2 (should be above 0.90) and prediction error sum of squares (PRESS), where a large predicted R2 and a low PRESS show a good model fitting (Myers and Montgomery 1995). Moreover, the effects of factors were compared at a particular point in the design space using the perturbation plot. Response surface and contour plots were then generated.
A desirability function was used for the optimization of OH parameters (voltage gradient and temperature) for PPO inactivation. For each response (y), a desirability function d(y) ranging from 0 to 1 and completely dependent on closeness to the lower and upper limits. The desirability value ranges are from 0 (representing a completely undesirable value of y) to 1 (completely desirable or ideal response value). Depending on whether a particular response is to be maximized, minimized or assigned to a target value, numerous desirability functions can be used (Derringer and Suich 1980).
In this study, the main objective of optimization is minimizing the PPO activity (response, y), therefore the desirability function is described by Eq. 2:
where L and U are the lower and upper limit values of the response y, respectively. Minimization of the polynomials by desirability function method was carried out using a trial version of design expert version 10.0.6 software.
Physical analysis
Total soluble solids (TSS) was determined by placing two drops of prepared apple juice in a portable refractometer (Model no. p 300003, UK) and the TSS was read directly from the refractometer.
Electric conductivity (EC) of apple juice samples was measured using a conductivity meter (WTW82362 Weinheim, LF323 Instrument, Germany) at 22 ± 1 °C.
The color of apple juice was measured using a colorimeter (Model CR-200; Konica Minolta, Japan) and the parameters of color L* (lightness), a* (redness), b* (yellowness) and ΔE (total color differences) were evaluated. ΔE was calculated using Eq. (3):
where subscript “o” refers to the color reading of control sample used as the reference and a high ΔE value indicates a large change in the color from the reference sample.
The viscosity of apple juice samples (50 ml) was measured at room temperature using a model DV-II viscometer (Brookfield Engineering Laboratories, Inc, Stoughton, MA, USA), spindle 5 with speed 100 rpm.
Cloud value was measured as a supernatant absorbance at 650 nm using a Unicam UV–VIS (UV2) spectrophotometer with a reference of distilled water (Versteeg et al. 1980).
Chemical analysis
Titratable acidity, apple juice (10 ml) were mixed with 40 ml distilled water and titrated against standardized 0.1 N NaOH to the phenolphthalein end point (pH 8.2 ± 0.1). The acidity was expressed as g malic acid/100 ml juice (Redd et al. 1986).
Ascorbic acid was determined using a 2,6-dichlorophenol indophenol (DCPIP) visual titration method (Helrich 1990). Five ml of an apple juice was immediately added to 5 ml of 1% oxalic acid to halt any degradation of ascorbic acid and then titrated with standardized dye solution (DCPIP). An auto-titrator (Dos Bio-5, 665 Dosimat, Metrohm, Swiss) was used to deliver the dye to the sample until a pink endpoint (color should persist for ≥ 15 s). The results obtained were expressed as mg of ascorbic acid per 100 ml.
Total phenolic content was measured by Folin–Ciocalteu method with the following modifications (Abdullakasim et al. 2007). Five ml of apple juice was mixed with 5 ml of 80% methanol in a 15 ml centrifuge tubes (Sarstedt) and then the tubes were centrifuged at 4000 rpm for 20 min at 4 °C (Sigma 4-16KS, Germany). For analysis, 100 μl appropriately diluted sample or standard solution at various concentrations was mixed with 100 μl Folin–Ciocalteu reagent and 3000 μl deionized water and vortexed. After 10 min incubation at room temperature (rt), 100 μl of 20% sodium carbonate solution was added with immediate mixing and incubated at rt for 2 h in the dark. The absorbance at 765 nm using a Microplate reader (Biotek Synergy 2 Microplate reader, U.S.A) was measured. Gallic acid was used as standard and total phenolic contents of the samples were expressed as mg of gallic acid per 100 ml.
Total carotenoids content was measured according to Lee and Castle (2001) with some modifications. Five ml of apple juice and 10 ml of hexane/methanol/acetone, 50/25/25, v/v with 0.1% BHT were mixed and centrifuged for 10 min 4000 rpm at 4 °C. The absorbance of the supernatant phase was measured at 450 nm. The total carotenoid content was calculated as µg β-carotene per g using an extinction coefficient of 2505 in hexane (Ritter and Purcell 1981).
PPO activity was determined by the method of Trejo-Gonzalezl and Soto-Valdez (1991) with the following modification. The enzyme was extracted using 5 ml apple juice that mixed with 5 ml of 0.2 M sodium phosphate buffer (pH 6.8) containing 2% (w/v) polyvinyl poly pyrrolidone (PVPP) and then centrifuged (Sigma 3MK, Labrzentrifugen, GmbH, Germany) at 10,000 g, 4 °C for 30 min. The supernatant was collected for the enzyme assay. The standard reaction mixture contained 2 ml of 0.05 M catechol in 0.05 M sodium phosphate buffer (pH 6.8), and 1 ml of extract, were incubated for 3 min at 25 °C. The increase in the absorbance at 420 nm (Microplate reader) was measured and compared with a control in which the enzyme extract was substituted by water. PPO activity (1unit) was defined as the increase in the absorbance by 0.001/min.
Statistical analysis
The measured results in Sects. 2.4 and 2.5 were statistically analyzed by analysis of variance (ANOVA) using the software SPSS 13 (SPSS Inc., Chicago IL, USA) with the Duncan test to evaluate differences between the treatments at levels of significance (p ≤ 0.05). Each experiment was repeated at least three times; means and standard deviations were calculated.
Results and discussion
Effect of process parameters on the PPO activity
Based on inactivation of PPO and changes in the color and ascorbic acid, ohmic heating parameters—voltage range of 30–40 V/cm and temperature range of 60–80 °C were selected (Table 1) for RSM to evaluate the effect of OH parameters on PPO activity and optimum process parameters. This was done due to the observation that a voltage gradient > 40 V/cm leads to adverse color changes in the apple juice (observed during a pretest experiment, data not given) and a similar result was reported for orange juice by Demirdöven and Baysal (2014). On the other hand, temperatures > 80 °C at 40 V/cm causes a juice bubbles leading to the loss of the juice during heating and also further deterioration of the color and other quality characteristics (Icier et al. 2008; Demirdöven and Baysal 2014). The holding time for all OH treatments was set to 60 s. This was to avoid long treatment time that leads to further deterioration of the quality characteristics especially the color and ascorbic acid (Demirdöven and Baysal 2014).
Table 1 presents the measured PPO activity during the OH heating. Optimization of ohmic heating parameters was carried out by applying second order polynomial equation (Eq. 1) and the regression coefficients for independent variables were obtained by multiple regression analysis.
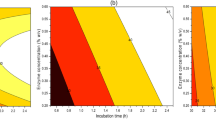
Table 2 shows the obtained results—the effect of voltage gradient and temperature on the PPO activity in the apple juice at 95% confidence interval using ANOVA analysis. The model shows a good fit with the measured PPO activity: a high significant and having less variation around the mean (R2 = 0.989, i.e., 98.9% of the response variability could be explained by the fitted model). The adj-R2 value (0.984) for the model did not differ dramatically compared to R2—stating a high degree of correlation between the experimental and predicted values. The lack-of-fit was not significant (p > 0.05). Based on these results, the model was satisfactory for predicting the PPO activity in the experimental ranges.
The negative linear effect of voltage gradient (χ1) and temperature (χ2) were found to be significant for the response variable (PPO activity) and the quadratic effect of temperature (χ 22 ) on PPO activity was also found to be significant (p < 0.05). However, the effect of interaction (χ1χ2) and the quadratic of voltage gradient (χ 21 ) were insignificant (p > 0.05).
The non-significant variables were removed and the fitted second order polynomial equation showed as (Eq. 4):
where χ1: voltage gradient (V/cm) and χ2: temperature (°C), are the coded values.
Second order polynomial models obtained in this study were used for a response (PPO activity) to determine the specified optimum conditions. As illustrated in Fig. 2a, the PPO activity decreases with increasing voltage gradient and temperature. The model equation, perturbation, and 3D response surface plots show the significant influence of factor B (temperature) on PPO activity at low temperature, while was less significance at high temperature. On the other hand, the effect of factor A (voltage gradient) is lower than B on PPO activity (Fig. 2b). Perturbation plot (Fig. 2b) revealed a sequence of the relative influence of the operating parameters on the target response as follows: temperature > voltage gradient. The optimum condition for OH of NFC apple juice were obtained at the minimum PPO activity by applying desirability function. Ohmic heating (OH) at 40 V/cm and 80 °C was selected as an optimum condition for inactivation of PPO in apple juice, which gave the best result for the PPO inactivation (0.39 U/ml/min). The obtained optimum OH condition was used to produce the juice and then compared to the CH in Sect. 3.2.
Physical and chemical characteristics of juice
Table 3 presents the effects of CH and OH on PPO activity and color values (L*, a*, b* & ΔE). The PPO activity decreased significantly (p ≤ 0.05) in both CH and OH treatments. The reduction of PPO activities was 97.13 and 91.35% for OH and CH, respectively.
The decrease in the PPO was due to the effect of heating in both OH and CH in addition to the presence of voltage gradient, which could influence biochemical reactions by changing molecular spacing and increasing interchain reactions regarding the OH treatment (Castro et al. 2004). At the same time, Leizerson and Shimoni (2005); Demirdöven and Baysal (2014) found that for orange juice the reduction in the PME activity during OH was higher than by CH. Chutintrasri and Noomhorm (2006) found the inhibition of PPO activity using conventional thermal treatment activity in a pineapple was increased rapidly above 75 °C because of the denaturation (proteins of the PPO), which they found that the PPO inhibition was 93 and 98.8% for 5 min at 85 and 90 °C, respectively.
A significant increase in the color values (L*, a*, b* & ΔE) for the OH, while no significant changes for the CH were observed (Table 3). The ΔE value was 1.77 for OH and 0.72 for CH, with the larger ΔE for the OH treated sample indicating a larger color change (from the reference sample, fresh) compared to the CH. The changes in the color values (L*, a*, b* & ΔE) during thermal treatments might be due to the migration of moisture due to other chemical changes (Leizerson and Shimoni 2005).
Table 4 presents the effects of ohmic and conventional heating on total phenolics, total carotenoids, ascorbic acid, cloud value, total soluble solids (TSS), titrable acidity (TA), electric conductivity (EC) and viscosity of apple juice. Compared to control (30.56 mg/100 ml), the total phenolic content increased slightly (but not significantly) for OH (32.22 mg/100 ml) and CH (31.33 mg/100 ml) treated samples. The increase in the total phenolic content could be attributed to the increased extractability of total phenolic components due to the changes in the tissue matrix induced by heating (Mcinerney et al. 2007), or a disruption of complexes between polyphenols and proteins (Girgin and El 2015). During OH, the alternating current has a synergistic effect on the release of total phenolic contents that could be exposed to Folin–Ciocalteu reagent used in the determination of phenolic content (Roy et al. 2009).
The ascorbic acid content in the OH treated sample (Table 4) is significantly higher than the CH treated sample. This shows that OH treatment is a better processing method to retain the heat-sensitive ascorbic acid than CH. This is due to OH is faster than CH: i.e., at 40 V/cm it takes 52 s to reach 80 °C for OH and 490 s to reach 90 °C for CH, see Fig. 3. Moreover, the higher ascorbic acid content in the OH compared to the CH, could be due to increased cell permeability that leads to easier release of cell components to the liquid part of the juice. (Demirdöven and Baysal 2014). The content of vitamin C in 71 Danish apple cultivars ranged from less than 1 to 27 mg/100 ml juice, with an average of 6.4 mg/100 ml (Varming et al. 2013). For elstar apple juice, the ascorbic acid content was similarly reported as 6.4 mg/100 g (Planchon et al. 2004); 7.4 mg/100 g (Podsedek et al. 2000) and 4.8 mg/100 g (Varming et al. 2013). Varming et al. (2013) observed also that after 5 min at rt after pressing apple juice of different cultivars the ascorbic acid content was degraded by 39–91%. Our significant (p ≤ 0.05) reduction of ascorbic acid agree with Demirdöven and Baysal (2014) who observed that the decrease in ascorbic acid for OH was lower than for CH.
A significant (p ≤ 0.05) decrease in the total carotenoids content due to heat treatment for OH (73.21 μg/100 g) and CH (55.45 μg/100 g) compared to control sample (84.32 μg/100 g) was recorded. Similar significant reductions have previously been reported for conventional thermal treatment of orange juice (Lee and Coates (2003); Gama and Sylos (2007)). Cloud value of the OH (0.361 A) and of the CH (0.242 A) treated samples were significantly increased compared to the control sample (0.076 A), indicating more cloud stability and enzyme inhibition.
From Table 4 there is no significant differences between OH and CH treated samples in the TSS, and TA (as a Malic acid %) content. Also, Abid et al. (2014) and Demirdöven and Baysal (2014) observed no significant differences of TSS for their orange samples treated by OH and CH. The electrical conductivity (EC) of OH (0.188 S/cm) and CH (0.177 S/cm) samples were significantly increased (p > 0.05) compared to the control (0.173 S/cm). This increase may be attributed to the increase in the ionic mobility as a result of structural changes in the apple tissue, e.g. cell wall protopectin breakdown, the expulsion of non-conductive gas bubbles, tissue softening, and a lowering in aqueous phase viscosity (Darvishi et al. 2012).
Viscosity for both OH (2.2 cP) and CH (2.2 cP) treatments were decreased compared to the control (2.4 cP). This reduction may be due to the effect of heat treatment, which cause degradation of pectins by acid hydrolysis (Diaz et al. 2007).
Figure 3 shows the relationship between temperature and time during OH (at 30, 35 and 40 V/cm) heating of apple juice and CH. OH treatment is much faster than the conventional heating (at 90 °C). Moreover, the increase in the voltage gradient during OH treatment shortens the heating time to the desired temperature (e.g., the time needed to reach 80 °C: 52 s (at 40 V/cm) < 69 s (at 35 V/cm) < 79 s (at 30 V/cm). This reduces the total time (heating + holding time), which resulted in better quality of the final product (e.g., reduce the loss of ascorbic acid, total carotenoids and color).
Conclusion
In this study, ohmic heating condition (i.e., temperature, 60–80 °C and voltage gradient, 30–40 V/cm) for PPO inactivation in apple juice were determined. An improvement in apple juice quality was achieved by using OH process condition at 80 °C and 40 V/cm due to the higher inactivation of PPO activity in comparison to CH. The total extracted phenolic content was increased by 5.4% with OH and 2.5% with CH compared to fresh apple juice. The loss of ascorbic acid and carotenoids contents of OH treated sample was less than the CH treated sample. The color values (L*, a*, b* and ΔE) was improved using OH compared to CH. These results indicated that OH could be used as a mild thermal treatment for inactivation of PPO as well as improving the quality characteristics of NFC apple juice.
Abbreviations
- OH:
-
Ohmic heating
- PPO:
-
Polyphenoloxidase
- CH:
-
Conventional heating
- RSM:
-
Response surface methodology design
- NFC:
-
Not-from-concentrate
References
Abdullakasim P, Songchitsomboon S, Techagumpuch M et al (2007) Antioxidant capacity, total phenolics and sugar content of selected Thai health beverages. Int J Food Sci Nutr 58:77–85. https://doi.org/10.1080/09637480601140946
Abid M, Jabbar S, Wu T et al (2014) Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason Sonochem 21:93–97. https://doi.org/10.1016/j.ultsonch.2013.06.002
Castro I, Macedo B, Teixeira JA, Vicente AA (2004) The effect of electric field on important food processing enzymes comparison of inactivation kinetics under conventional and ohmic heating. J Food Sci 69(9):696–701. https://doi.org/10.1016/j.jfoodeng.2007.08.002
Chemat F, Khan MK (2011) Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem 18:813–835. https://doi.org/10.1016/j.foodres.2010.07.005
Chutintrasri and Noomhorm (2006) Thermal inactivation of polyphenoloxidase in pineapple puree. LWT Food Sci Technol 39:492–495
Clark JP (2009) Case studies in food engineering. Chapter 6: fruit and vegetable juice processing. Case studies in food engineering. Food engineering series, p 224
Darvishi H, Hosainpour A, Nargesi F (2012) Ohmic heating behaviour and electrical conductivity of tomato paste. J Nutr Food Sci 2:9. https://doi.org/10.4172/2155-9600.1000167
Demirdöven A, Baysal T (2009) Ohmic heating applications on fruit and vegetable products. In: International conference on bio and food electrotechnologies, 22–23 October 2009, Compiègne, France, pp 294–300
Demirdöven A, Baysal T (2014) Optimization of ohmic heating applications for pectin methylesterase inactivation in orange juice. J Food Sci Technol 51(9):1817–1826. https://doi.org/10.1007/s13197-012-0700-5
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12:214–219
Diaz JV, Anthon GE, Barrett DM (2007) Effect of pH, temperature and degree of methyl esterification on the non-enzymatic degradation of citrus pectin and pectate during prolonged heating. J Agric Food Chem 55:5131–5136
Gama JJT, Sylos CM (2007) Effect of thermal pasteurization and concentration on carotenoid composition of Brazilian Valencia orange juice. Food Chem 100:1686–1690. https://doi.org/10.1016/j.foodchem.2005.01.062
Girgin N, El SN (2015) Effects of cooking on in vitro sinigrin bioaccessibility, total phenols, antioxidant and antimutagenic activity of cauliflower (Brassica Oleraceae L. var. Botrytis). J Food Compos Anal 37:119–127. https://doi.org/10.1016/j.jfca.2014.04.013
Gong Z, Li D, Liu C, Cheng A, Wang W (2015) Partial purification and characterization of polyphenol oxidase and peroxidase from chestnut kernel. LWT Food Sci Technol 60:1095–1099. https://doi.org/10.1016/j.lwt.2014.10.012
Helrich K (1990) Official methods of analysis of the association of official analytical chemists, vol 2, 15th edn. The Association of Official Analytical Chemists, Arlington
Icier F, Yıldız H, Baysal T (2008) Polyphenoloxidase deactivation kinetics during ohmic heating of grape juice. J Food Eng 85(3):410–417. https://doi.org/10.1016/j.jfoodeng.2007.08.002
Knirsch KC, Alves dos Santos CA, de Oliveira Martins, Soares Vicenteb A et al (2010) Ohmic heating e a review. Trends Food Sci Technol 21:436–441. https://doi.org/10.1016/j.tifs.2010.06.003
Lee HS, Castle WS (2001) Seasonal change of carotenoid pigments and color in Hamlin, Earlygold, and Budd Blood orange juices. J Agric Food Chem 49:877–882. https://doi.org/10.1021/jf000654r
Lee HS, Coates GA (2003) Effect of thermal pasteurization on Valencia orange juice color and pigments. Food Sci Technol 36:153–156. https://doi.org/10.1016/S0023-6438(02)00087-7
Leizerson S, Shimoni E (2005) Stability and sensory shelf life of orange juice pasteurized by continuous ohmic heating. J Agric Food Chem 53:4012–4018. https://doi.org/10.1021/jf047857q
Lima M (2007) Ohmic heating: Quality improvements. Encyclopedia of Agricultural, Food, and Biological Engineering 1:1–3. https://doi.org/10.1111/j.1745-4549.1999.tb00395.x
Machado LF, Pereira RN, Martins RC, Teixeira JA, Vicente AA (2010) Moderate electric fields can inactivate Escherichia coli at room temperature. J Food Eng 4:520–527. https://doi.org/10.1016/j.jfoodeng.2009.08.035
Makroo HA, Saxena J, Rastogi NK, Srivastava B (2016) Ohmic heating assisted polyphenol oxidase inactivation of watermelon juice: effects of the treatment on pH, lycopene, total phenolic content, and color of the juice. J Food Process Preserv. https://doi.org/10.1111/jfpp.13271
Mcinerney JK, Seccafien CA, Stewart CM, Bird AR (2007) Effects of high-pressure processing on antioxidant activity, and total carotenoid content and availability, in vegetables. Innov Food Sci Emerg Technol 8:543–548. https://doi.org/10.1016/j.ifset.2007.04.005
Mena P, Vegara S, Martí N et al (2013) Changes on Indigenous Microbiota, Colour, Bioactive Compounds and Antioxidant Activity of Pasteurised Pomegranate Juice. Food Chem 141(3):2122–2129. https://doi.org/10.1016/j.foodchem.2013.04.118
Myers RH, Montgomery DC (1995) Response surface methodology, process and product optimization using designed experiments, 2nd edn. Wiley, New York
Pedersen SJ, Feyissa AH, Brøkner Kavli ST, Frosch S (2016) An investigation on the application of ohmic heating of cold water shrimp and brine mixtures. J Food Eng 179:28–35. https://doi.org/10.1016/j.jfoodeng.2016.01.022
Planchon V, Lateur M, Dupont P, Lognay G (2004) Ascorbic acid level of Belgian apple genetic resources. Sci Hortic 100:51–61. https://doi.org/10.1016/j.scienta.2003.08.003
Podsedek A, Wilska-Jeszka J, Anders B, Markowski J (2000) Compositional characterisation of some apple varieties. Eur Food Res Technol 210:268–272
Ramaswamy R, Balasubramanıam VM, Sastry SK (2005) Ohmic heating of foods-fact sheet for food processors. Ohio State University (OSU). http://ohioline.osu.edu/fsefact/0004.html. Accessed 22 Apr 2009
Redd JB, Hendrix CM, Hendrix DL (1986) Quality control manual for citrus processing plants, book 1. Intercity, Safety Harbor, FL
Ritter E, Purcell AE (1981) Carotenoid analytical methods. In: Bavernfeind JC (ed) Carotenoids as colorants and vitamin A precursors. Academic Press, New York, pp 815–883
Roy MK, Juneja LR, Isobe S, Tsushida T (2009) Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chem 114:263–269. https://doi.org/10.1016/j.foodchem.2008.09.050
Trejo-Gonzalezl A, Soto-Valdez H (1991) Partial characterization of polyphenoloxidase extracted from ‘Anna’ apple. J Am Soc Hortic Sci 4:672–675
Varming C, Petersen MA, Toldam-Andersen TB (2013) Ascorbic acid contents in Danish apple cultivars and commercial apple Juices. LWT Food Sci Technol 54:597–599. https://doi.org/10.1016/j.lwt.2013.06.024
Versteeg C, Rombouts FM, Spaansen CH, Pilnik W (1980) Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. J Food Sci 45:969–971. https://doi.org/10.1111/j.1365-2621.1980.tb07489.x
Włodarska K, Pawlak-Lemańska K, Górecki T, Sikorska E (2016) Perception of apple juice: a comparison of physicochemical measurements, descriptive analysis, and consumer responses. J Food Qual 39(4):351–361. https://doi.org/10.1111/jfq.12208
Acknowledgement
Tarek G. Abedelmaksoud would like to thank The Danish Agency for Higher Education for a research grant for his stay as a guest Ph.D. student for one year at Food Production Engineering Research Group, Technical University of Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Rights and permissions
About this article
Cite this article
Abedelmaksoud, T.G., Mohsen, S.M., Duedahl-Olesen, L. et al. Optimization of ohmic heating parameters for polyphenoloxidase inactivation in not-from-concentrate elstar apple juice using RSM. J Food Sci Technol 55, 2420–2428 (2018). https://doi.org/10.1007/s13197-018-3159-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3159-1