Abstract
Pectin methylesterases (PMEs) from two different orange sources, Valencia and Navel cv., were extracted and purified using cation exchange, heparin chromatography, and finally, gel filtration chromatography, yielding a single peak corresponding to a protein of molecular weight 34 and 35 kDa for Valencia and Navel PMEs, respectively. Effects of high pressure (HP) and thermal processing for various treatment durations on the activity of PMEs in Tris–HCl buffer solution (pH 7.5) were explored. Higher levels of pressure, temperature, and treatment duration resulted in an analogous reduction of the PME residual activity. HP-induced inactivation of both purified PMEs was described by a first-order kinetic model. Kinetic parameters were estimated and a multiparameter equation was developed to predict the PME inactivation rate constant at any combination of pressure and temperature conditions for both enzymes. The PME from Valencia orange peel appeared to be more heat- and pressure-sensitive compared to Navel PME. HP-induced conformational changes of the PME molecules were also investigated using circular dichroism (CD) spectroscopy. A direct comparison of the CD results for treated and untreated proteins reveals that pressure treatment has negligible effects upon far-UV CD spectra, while significant irreversible changes are depicted in near UV for both PMEs. It is, thus, evidenced that exposure to HP may lead to a structurally molten globulelike state, where the PME maintains a secondary structure of untreated protein molecules, while a tertiary structure is substantially affected bearing subsequent impact on substrate–enzyme binding interaction, leading to reduction of enzyme activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pectin methylesterase (PME, EC 3.1.1.11) can be found in cell walls of higher plants or it may be produced by microorganisms such as fungi. PME hydrolyses C6 methylesters of galacturonic acid residues in pectins, gradually decreasing their degree of esterification. The result of this reaction is a demethylated pectin that interacts with divalent cations, such as calcium, causing a precipitation of particles and a subsequent clarification of fruit juices or acts as substrate for other endo-acting enzymes such as polygalacturonases (PG) and pectate lyases (Van den Broeck et al. 2000a). Thus, overall quality of fruit and vegetable products (such as juice and pulp) is highly correlated to the effect of their endogenous PME. Control of PME activity is a prerequisite in food and beverages industry.
Conventional thermal treatment is widely used by the food and beverage industries for inactivation of PME of fruit products (Eagerman and Rouse 1976). However, processing at elevated temperatures also results in deterioration of organoleptic and nutritional characteristics and alteration of texture and color of treated products (Gervilla et al. 2000; Hendrickx and Knorr 2002). Alternative processing technologies such as high pressure (HP) processing, pulsed electric fields (PEF), ultrasounds, and HP carbon dioxide (HPCD) or combination of these technologies could substitute conventional pasteurization. These processes may inactivate microorganisms and enzymes (Castro et al. 1993; Katsaros et al. 2009a; b; Tiwari et al. 2009) while simultaneously better maintain sensory and nutritional properties and the overall quality of final products (Hendrickx et al.,1998; Barbosa-Cánovas 1999; Katsaros et al. 2010b).
In literature, there is a significant number of papers describing the combined effect of HP and temperature on PME activity from different fruits and vegetables, such as citrus-based foods, tomato-based foods, peach, strawberry, pepper, carrot, banana, and papaya. These studies had been carried out either directly in fruit juices and pieces or in extracted enzymes from plant tissues (Ly-Nguyen et al. 2003a; b; Balogh et al. 2004; Polydera et al. 2004; Guiavarc'h et al. 2005; Sila et al. 2007; Sampedro et al. 2008; Castro et al. 2008; Boulekou et al. 2010; Katsaros et al. 2010a). The results found in these papers show that when processed at same process conditions, degree of PME inactivation from different sources varies. This variation can only be partly attributed to intrinsic factors such as pH, ionic strength, composition of food, and purification level of enzyme. In general, more intense pressure and temperature process conditions enhance enzyme inactivation. In most cases, there is a synergistic effect of pressure and temperature (process combining pressure at a certain temperature results in faster inactivation when compared to enzyme inactivation by only thermal treatment at the same temperature). However, an antagonistic effect of pressure and temperature has also been observed. In these cases, the enzyme inactivation is slower when the enzyme is treated at a certain temperature combined with pressure, compared to the inactivation by only thermal processing. A synergistic effect of pressure and temperature may be observed for some enzymes when treated up to a certain pressure or/and temperature and an antagonistic effect may be observed for more intense process conditions and vice versa. Difference in remaining enzyme activity and resistance in process parameters can be attributed to the original enzyme structure and their structural changes when processed. Knowledge about the HP-induced conformational alterations in the protein structure of enzymes is of high importance, since it can contribute to elucidation of (1) how structural changes are correlated to residual enzyme activity, (2) different mechanisms that induce inactivation in thermal and HP processing, and (3) observed synergistic or antagonistic pressure and temperature effects on enzyme inactivation after combined processing.
In order to investigate the effect of process parameters on the structure of enzymes, purification procedure is essential. More specifically for citrus fruits, the purification and characterization of PMEs from different (citrus) varieties have been investigated from many researchers (Cameron et al. 2005; Laratta et al. 2008; Savary et al. 2010). The results of these studies showed that multiple forms of this enzyme are present in fruit tissues. Each form can be distinguished by its physical and biochemical characteristics such as molecular weight, isoelectric points, substrate affinity, and kinetic properties. Several studies have indicated existence of more than one form of PME found both in orange juice and orange peel (Rombouts et al. 1982). Versteeg, Rombouts, and Pilnik (1978) reported the existence of two enzymes (PE1 and PE2) in Navel orange peel, while Cameron, Baker, and Grohmann (1996) reported the existence of four forms of PMEs in Valencia orange.
Most of the studies on the inactivation by thermal and HP processing of crude or purified PME from various plant sources do not investigate structural changes associated with the processing. The pressure-induced changes in the rate of enzyme-catalyzed reactions may be attributed to changes in the structure of PME or/and changes in the reaction mechanism. It has been proposed that application of HP leads to disruption of native protein structures due to decrease in volume of the protein–solvent system upon unfolding, which arises from existence of voids in the folded state (Mozhaev et al. 1994). In order to understand better the PME structure/function relationships, the mechanism of HP-induced inactivation of PME is worth investigating.
Circular dichroism (CD) has been extensively used to give useful information about protein structure, extent and rate of structural changes, and ligand binding. CD is an effective technique for investigation of conformational changes in proteins that can occur as a result of changes in experimental parameters such as pH, pressure, temperature, and binding of ligands. CD analysis has been the subject of many studies and gives important additional information about the effect of temperature (Balan et al. 2006; Thanassoulas et al. 2011), pH (Ramos 2004; Ribeiro-Jr and Ramos 2004), charge (Ramos et al. 1999; Regis et al. 2005), and ligands (Balan et al. 2006; Ramos et al. 2007) in the secondary and tertiary structures of proteins and enzymes. Menéndez, Schwarzenbolz, and Henle (2006), working in the pressure range of 0.1–600 MPa with heat treatment at 40 °C, reported that HP processing at 600 MPa induced a loss of tertiary structure and a significant reduction in the alpha-helix content of microbial transglutaminase. Tedford, Smith, and Schaschke (1999) examined the structural changes to the milk protein β-lactoglobulin that were induced by the combined effects of pressure and temperature. Results indicated that the pressure effects were more significant in the tertiary structure than in the secondary structure of this protein. Similar studies are cited in the literature using other process technologies such as PEF (Zhong et al.,2007) and HPCD (Zhou et al.,2009), but in general, there is a lack of published information in the literature on the effect of HP treatment at ambient or elevated temperatures on the structure of PMEs. The main objective of our research was to comparatively study the effect of HP on the activity and structure of purified PMEs from two different sources of commercial significance, i.e., Valencia and Navel oranges.
Materials and Methods
Orange Peels Samples
Orange fruits (cv. Valencia and Navel) were directly obtained from the leading Greek citrus industry, a Greece-based producer-supplier of fruit juice concentrates and purees for the international fruit juice industry. Whole ripe oranges (of medium size, approximately 180 g each) were mechanically peeled (0.5 cm in depth) and the peels were grounded using a blender (Premier Chef KMC570, Kenwood, UK). No thermal treatment was applied. The pieces were immediately frozen in a forced circulation freezer (MDF-U442, Sanyo Electric Co., Ltd., Japan) and kept at −40 °C until use.
PME Activity Assay
PME activity was measured using a modification of the method of Rouse and Atkins (1955), as described in Boulekou et al. (2010). Juice samples of 2 ml adjusted to pH 7.5 with 0.2 N NaOH were added to 50 ml 1 % apple pectin (70–75 % degree of esterification, Fluka BioChemika, Buchs, Switzerland) solution containing 0.3 M NaCl that was also previously adjusted to pH 7.5 with 0.2 N NaOH at 30 °C. Consumption of NaOH (0.02 N) (Autotitrator TitraLab TIM 854, Radiometer Analytical, France) to keep the pH stable was recorded during a period of 20 min (sufficient time for an autotritator to estimate the NaOH consumption vs. time). The slope dV NaOH/dt was determined in the linear part of the titration curve. PME activity of the sample, which is calculated by Eq. 1 and expressed as microequivalents per minute and milliliter of sample, is directly proportional to the slope:
where A is the PME activity (units per milliliter), N NaOH is the normality of NaOH solution used for the titration (microequivalents per milliliter), and V is the volume of the sample (milliliters). Dilution of sample during pH adjustment was taken into consideration for the determination of PME activity of each sample.
Extraction and Purification of PME from Orange Peel (Valencia and Navel cv.)
The purified PME was produced from orange peel using the purification procedure as described by Cameron et al. (1996). PMEs were extracted after a number of steps such as centrifugation, ammonium sulfate fractionation (25–75 %), and ultrafiltration. The extracts were used for subsequent purification experiments. PMEs were purified by a combination of cation exchange, and heparin and gel filtration chromatography. The columns were attached to a fast protein liquid chromatography (FLPC) system (Bio-Rad, Hercules, CA, USA) and the eluate was spectrophotometrically measured at 280 nm. Chromatographic separation was performed at ambient temperature and the fractions received were stored at 4 °C immediately after collection. A 20-ml volume of enzyme extract was loaded in an UNO S (Bio-Rad, Hercules, CA, USA) column (2.5 × 10 cm) while continuously washed with 20 mM Tris–HCl buffer (pH 7.5) (TBS) until total removal of unbounded proteins. Bound molecules were eluted off using a gradient of a second buffer (TBS + 0.5 M NaCl) that steadily increases the ionic strength of the eluent solution. The procedure was repeated at least two times for more efficient purification. Online spectrophotometric analysis of the eluted fractions from major peak-containing PME activity (PE1 for Navel and Valencia peak 2) was pooled and concentrated at 4 °C with a Centricon microconcentrator (Amicon, 10-kDa cutoff membrane). Further separation of fractions was performed using chromatography on heparin Sepharose CL-6B. A 2.5 × 10-cm column was used with a 1.5 ml/min flow rate and elution was achieved using a second buffer (TBS + 0.5 M NaCl) that steadily increases the ionic strength of the eluent solution. Two milliliters of partially purified PME (received after cation exchange and heparin chromatography) was loaded into Bio-Gel P30 gel filtration column (Bio-Rad, Hercules, CA, USA). The column had previously been equilibrated in TBS buffer. The flow rate was 0.4 ml/min and fractions were collected every 11 min. Electrophoresis was performed after chromatography separation in order to check the purity of obtained PMEs. All fractions were analyzed for PME activity and protein concentration. The protein concentration was determined spectrophotometrically according to Bradford (Bradford 1976), while PME activity was measured as previously described. All the steps followed for enzyme extraction and purification are depicted in a flow diagram (Fig. 1).
Gel Electrophoresis
SDS-PAGE experiments were performed using a 4–20 % Mini-PROTEAN TGX™ precast polyacrylamide gel (Bio-Rad, Hercules, CA, USA) attached to a Mini-PROTEAN Tetra system (Bio-Rad, Hercules, CA, USA). Samples were boiled for 5 min at 100 °C in a buffer containing SDS (2.5 %) and β-mercaptoethanol (5 %). Gel staining was performed with Coomassie brilliant blue staining. An unstained MW marker (Fermentas, USA) composed of native proteins with precise molecular weights (beta-galactosidase (116 kDa), bovine serum albumin (66.2 kDa), ovalbumin (45.0 kDa), lactate dehydrogenase (35.0 kDa), REase Bsp98I (25.0 kDa), β-lactoglobulin (18.4 kDa), and lysozyme (14.4 kDa)) was used.
Kinetic Parameters of PME
The effect of substrate concentration was studied by determination of initial rates (V 0) of PME activity at different pectin concentrations. The K m values and maximum rates (V max) were determined using Lineweaver–Burk double reciprocal plot, in which the reciprocals of the initial rates of the PME activity were plotted against the reciprocals of the pectin concentrations used. Activity assays were conducted at 30 °C using apple pectin (as described in PME activity assay) solution ranging from 0.5 to 10 mg/ml containing 0.3 M NaCl.
Thermal Treatment
The effect of temperature treatment on the activity of PMEs was examined. Purified enzymes in Tris–HCl buffer solution (pH = 7.5) placed in thin-walled glass test tubes was immersed in water baths (WB/OB 7–45, MEMMERT GmbH + Co. KG, Schwabach, Germany) at temperatures in the range of 50–65 °C for various periods of time. During thermal treatment, temperature was constantly monitored and recorded every 2 s by a type T thermocouple placed in a representative glass test tube filled with the same enzyme solution (datalogger CR10X, Campbell Scientific, Leicestershire, UK). After heating, the samples were quickly cooled and assayed for their enzymatic activity at 25 °C. Storage before the assay at 0–5 °C (from 30 min to 48 h) did not alter the measured activities significantly.
High Hydrostatic Pressure Processing
HP inactivation experiments were conducted at various combinations of pressure (200–700 MPa) and temperature (30–55 °C) for appropriate processing times. Purified Valencia and Navel PMEs were diluted in 20 mM Tris–HCl buffer, pH 7.5, in a final concentration of 0.025 and 0.038 mg/ml, respectively. The diluted PMEs were placed into 2-ml polypropylene pouches and placed in preheated HP vessels for conduction of HP experiments. Processing time was determined at the planning of the experiments and was differentiated in each experiment depending on applied pressure and temperature (ranging from 5 to 40 min, according to the process conditions). A laboratory scale high hydrostatic pressure unit was used (Food Pressure Unit FPU 1.01, Resato International BV, Roden, the Netherlands) that consists of a pressure intensifier and a multivessel system (six vessels of 45 ml capacity each), with a maximum operating pressure and temperature of 1,000 MPa and 90 °C, respectively. The pressure-transmitting fluid used was polyglycol ISO viscosity class VG 15 (Resato International BV, Roden, the Netherlands). Process temperature in the vessels was achieved by liquid circulation in the outer jacket of the vessels controlled by a heating–cooling system. The desired value of pressure was set and after pressure build up (20 MPa/s), the pressure vessels were isolated. This point defined the zero time of the process. Pressure of each vessel was released (t release < 3 s) after a preset time interval (according to the experimental design) by opening the pressure valve. The temperature in the chambers was monitored during the process. In order to achieve the desired operating temperature during pressurization, the initial increase of adiabatic temperature during pressure build up was taken into account.
CD Spectroscopy
Conformational changes leading to differences in the secondary and tertiary structures of PMEs were determined with a JASCO J-715 spectropolarimeter (JASCO, Easton, MD, USA), equipped with a JASCO PTC 348 WI temperature controller. Wavelength scans in the far- (200 to 260 nm) and the near-UV (260 to 340 nm) regions were performed in Quartz Suprasil (Hellma, Germany) precision cells of 0.1 cm and 1-cm path length, respectively. Each CD spectrum was obtained by averaging five to eight successive accumulation of data with a wavelength step of 0.2 nm at a rate of 50 nm min−1, response time 2 s, and a bandwidth of 2 nm. In all cases 0.15–0.4 mg/ml protein concentration was used in 20 mM Tris–HCl, pH 7.5. Buffer spectra, obtained at identical conditions, have been subtracted from the raw data. The results in all experiments have been expressed as mean residue ellipticity in degree square centimeter per decimole. The CD spectra of all samples were measured immediately after thermal and HP treatments.
The far- and near-UV spectra were analyzed by curve-fitting software Origin 8.0 (OriginLab Corporation, USA) and for the calculation of secondary structural contents of the protein, the CDNN software (Institut für Biotechnologie, Martin-Luther Universität Halle-Wittenberg) was used.
Tryptophan Fluorescence Spectroscopy
Fluorescence emission spectra were obtained using a QuantaMaster UV VIS spectrofluorometer (Photon Technology International, Inc., Birmingham, UK). The experiments were carried out at 25 °C. In all cases, 0.34 mg/ml protein concentration was used in 20 mM Tris–HCl buffer (pH 7.5) loaded in a 4-ml quartz fluorometer cuvette. Any background signal (fluorescent or scattered light) from the buffer has been subtracted. The intrinsic protein fluorescence was measured by averaging three successive accumulations of data using the following parameters: excitation of 295 nm, excitation slit width of 0.85 nm, emission slit width of 1.00 nm, and 14 s integration time.
Kinetic Data Analysis
Values of enzyme activity (average of three different experiments) were plotted with time for various process conditions. The standard error between the three measurements is represented in the tables with ± standard deviation and in the figures with error bars (each point in a figure represents one average value of three measurements). The technique of model-based design of experiments was used for the experimental design in our study.
For statistical fit of the inactivation curves, least square regression analysis was conducted using SYSTAT 8.0 (SYSTAT 8.0 Statistics, 1998, SPSS Inc., Chicago, IL, USA) software. Dependence of inactivation rates on process parameters was described with appropriate mathematical equations, statistically treated by nonlinear regression.
PME inactivation rate constants (k, per minute) were estimated at all studied conditions to describe the effect of process time on their inactivation. The inactivation of PME was described by a first-order model for all pressure/temperature conditions studied (Eq. 2):
where A t is PME activity after processing for a time period t (microequivalents of H+ per minute per milliliter), A 0 is the initial PME activity (at time zero of processing, in microequivalents of H+ per minute per milliliter), t is the processing time (minutes), and k is the inactivation rate constant (per minute). The values of k were calculated from the negative slope of the regression of A t /A 0 vs. time. One-way ANOVA was applied to data and the differences between the means were tested by Duncan's test (P < 0.05). STATISTICA 7.0 (StatSoft Inc., USA) software was used for the statistical analysis.
The half-life time of the enzymes, defined as the time required by the enzyme to lose half of its initial activity, was also calculated. The calculation was based on Eq. 2, where A t /A 0 is equal to 0.5. The half-life time determination allows for easy and direct comparison of the inactivation of the two studied enzymes. The temperature dependence of the inactivation rate constant, k, as given by the Arrhenius equation (Eq. 3), was expressed in terms of activation energy, \( {E_{{{a_P}}}} \):
where T ref is the reference temperature, \( {k_{{{T_{\mathrm{ref}}}}}} \) is the inactivation rate (per minute) at T ref, and R is the universal gas constant (8.314 Jmol−1 K−1).
Pressure effect on the reaction rate constant, k, was expressed through the activation volume, \( {V_{{{a_T}}}} \) (milliliters per mole), and can be estimated by linear regression analysis using the Eyring equation:
where P ref is the reference pressure, \( {k_{{{P_{\mathrm{ref}}}}}} \) is the inactivation rate (per minute) at P ref, and R is the universal gas constant (8.314 Jmol−1 K−1). The effect of the applied pressure on the E a values was described by an exponential equation (Eq. 5), while the effect of process temperature on the V a values was described by a linear equation (Eq. 6) (Polydera et al. 2004):
Based on Eqs. 3 and 4 and taking into consideration the effect of pressure on E a values (Eq. 5) and the effect of temperature on V a values (Eq.6), the multiparameter equation (Polydera et al. 2004) was used to predict the inactivation rate constant at any combination of pressure and temperature conditions (Eq. 7). For each studied enzyme, the parameters of the equation were estimated using nonlinear regression routine (SYSTAT 8.0, Statistics 1998, SPCC Inc., Chicago, IL, USA):
Results and Discussion
Purification of Valencia and Navel Orange Peel PMEs
PMEs were purified from two different orange sources (Valencia and Navel cv.). The steps of purification included precipitation with ammonium sulfate, cation exchange, and heparin and gel filtration chromatography.
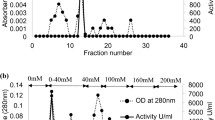
PMEs from both cultivars bounded strongly to a cation exchange column (UNOsphere S) media at pH equal to 7.5, whereas most of the other proteins were eluted. Upon increasing the NaCl gradient, four and two peaks of Valencia and Navel orange peel PMEs were eluted, respectively. The major peak of both PMEs (correlated to maximum enzyme activity) was eluted at 0.1 M NaCl (Fig. 2a, and d). These samples were rechromatographed on a cation exchange column at pH = 7.5 for further purification. Concentrated and pooled bounded PMEs containing fractions from cation exchange chromatography were next loaded onto the heparin Sepharose CL-6B column. Our decision to choose the heparin Sepharose for affinity chromatography was based on the structural similarities that exist between heparin and pectin. The PME activities that were eluted at 0.1 M NaCl from the heparin Sepharose for Navel and Valencia PMEs (Fig. 2b and e) were further purified by gel filtration using a Bio-Gel P30 column. After all these steps, only one single peak was finally received for both Valencia and Navel PMEs corresponding to a protein of molecular mass of 34 kDa and 35 kDa, respectively (Fig. 2c and f). As shown in Table 1, the final purification factors for the Valencia and Navel PME were estimated as 28.4 and 21.9, respectively. These values express the high level of purification obtained via the chromatography procedure. For Valencia oranges, the PME concentration obtained was 3,900 U/kg of peel, while for Navel oranges, the corresponding value was 2,250 U/kg of peel.
a and d Elution profile for the cation exchange chromatography of Valencia and Navel orange peel PMEs on UNOS column, respectively. UNO S was equilibrated in 20 mM Tris–HCl buffer solution (pH 7.5). b and e Elution profile for the chromatography of Valencia and Navel orange peel PMEs on heparin Sepharose CL-6B column, respectively. Heparin column was equilibrated in 20 mM Tris–HCl buffer solution (pH 7.5). c and f Elution profile for the gel filtration chromatography of Valencia and Navel orange peel PMEs on Bio-Gel P30 column, respectively. Bio-Gel P30 was equilibrated in 50 mM Tris–HCl buffer solution (pH 7.5)
Effect of Substrate Concentration
The K m (Michaelis constant) values of Navel and Valencia PMEs were determined after purification by cation exchange, and heparin and gel filtration chromatography. These values were calculated from Lineweaver–Burk double reciprocal (1/V vs. 1/[S]) plots. The K m of Valencia and Navel PMEs were calculated equal to 1.26 ± 0.09 and 1.39 ± 0.07 mg/ml, while the V max values were 4.24 ± 0.29 and 5.53 ± 0.27 mM/min, respectively.
Thermal Inactivation
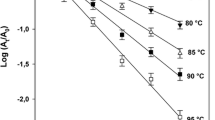
Thermal inactivation of both PME activity was investigated for temperatures from 50 to 65 °C. The enzyme inactivation was described by exponential curves, typical of first-order kinetics (Eq. 2). The values of k were calculated from nonlinear regression of Eq. 2. The exponential fit is illustrated in Fig. 3a. The square of the correlation coefficient of the fit, R 2, was above 0.982 for all thermal experiments. No residual activity was measurable after thermal processing for sufficiently long time at all studied temperatures.
a Thermal inactivation of purified PMEs (Valencia and Navel cv.) at temperatures 50 and 60 °C. Plots of A/A 0 vs. time; points are experimental measurements of the effect of 50 °C on the inactivation of Navel PME (unfilled square) and Valencia PME (unfilled diamond) and the effect of 60 °C on Navel PME (filled square) and Valencia PME (filled diamond), while the solid lines represent the nonlinear fit of Eq. 1. Insert The effect of temperature on the inactivation rate constant of both PMEs (T ref = 65 °C) (Navel PME (filled square) and Valencia PME (filled diamond)). b High pressure inactivation of purified PMEs (Valencia and Navel cv.) in 20 mM Tris–HCl buffer solution (pH = 7.5) at 600 MPa and 45 °C
For Valencia cv. Orange PME, the inactivation rate constants (k, per minute) obtained at each process temperature were estimated as 0.023, 0.057, 0.176, and 0.486 min−1 for processing at 50, 55, 60, and 65 °C, respectively. The corresponding values for Navel cv. Orange PME at the same process conditions were estimated as 0.011, 0.022, 0.110, and 0.351 min−1, respectively. For both Navel and Valencia inactivation rate constant values obtained, statistical analysis showed that each value is significantly different (p < 0.05) from the others. As evidenced by the obtained rates and by the plotting of the remaining enzyme activity vs. time for temperatures 50 and 60 °C (Fig. 3), the PME obtained from Valencia oranges appeared to inactivate faster when compared to Navel PME. The effect of temperature on the inactivation rate of both enzymes was described adequately by the Arrhenius equation (Eq. 3), as illustrated in Fig. 3a (insert). The temperature dependence of the thermal inactivation of PMEs expressed by the activation energy value were 186 ± 8 kJ/mol (R 2 = 0.985) for Valencia PME and 219 ± 33 kJ/mol (R 2 = 0.989) for Navel PME, respectively.
Inactivation of PMEs Subjected to HP Treatment
The effect of HP (200–700 MPa) combined with temperature (40–55 °C) on the inactivation of purified PMEs from Navel and Valencia orange peels in Tris–HCl buffer (pH 7.5) was studied and the stability of the two different PMEs was evaluated. The isothermal and HP inactivation of purified orange peel PMEs can be described by a first-order kinetic model (Eq. 2). The inactivation rate constants (k) and the half-life time (t 1/2) for purified PMEs were estimated at all combinations of pressures and temperatures studied.
As shown in Table 2, HP processing resulted in both enzymes' inactivation. Increased pressure or/and temperature and treatment duration led to faster inactivation (higher k values), indicating a synergistic effect of these process parameters on the inactivation. Statistically significant differences (p < 0.05) were observed between most of the k values obtained for the inactivation of Valencia and Navel PMEs (Table 2), having as variables both temperatures (differences between temperatures at each pressure) or/and pressures (differences between pressures at each temperature). In particular, k values of about 0.026 and 0.317 min−1 for inactivation of Navel PME and Valencia PME, respectively, were observed after processing at 600 MPa and 45 °C. The purified Navel PME appeared to be significantly more resistant compared to PME obtained from Valencia orange peel (Fig. 3b). The half-life times for Navel PME range from four- to 15-fold the corresponding values for Valencia PME (comparison at the same process conditions).
Comparing these data with those cited in the literature for pressure inactivation of orange PME in different matrices, it can be seen that the obtained k values of our research are significantly lower (Nienaber and Shellhammer 2001 ; Polydera et al. 2004). It's worth mentioning that other researchers have also observed several forms of orange (from Valencia cv. and Navel cv.) juice PMEs with different pressure stability (Van den Broeck et al. 2000b). Sampedro et al. (2008) found that PMEs were more pressure resistant when the purified enzyme was diluted in a buffer system (pH = 7.5) than in orange juices, showing that pH, matrix composition, and purification level play a significant role on the stability of PME. Our results are in accordance with the works of Sampedro et al. (2008) and Van den Broeck et al. (2000b) who kinetically studied the commercial orange peel PME inactivation in various model systems.
The effect of temperature on the inactivation rate constants of both studied purified PMEs was mathematically described by the Arrhenius equation (Eq. 3). For both enzymes, all the E a values were estimated (Table 3). Increase of pressure processing resulted in increased E a values, indicating higher temperature dependence of the enzyme inactivation rate constant at higher pressures.
The effect of pressure on the inactivation rate constants was described by the Eyring equation (Eq. 4). All the activation volume values, V a , were also estimated (Table 4) for each studied temperature. Negative activation volumes indicate that pressure increase favors PME inactivation at a given temperature. The effect of pressure on E a values and the effect of temperature on V a values can be expressed by Eqs. 5 and 6, respectively, or combined in Eq. 7, the parameters of which were estimated by nonlinear regression on all experimental data (Table 5). The multiparameter equation can be used to calculate the inactivation rate constant at any combination of pressure and temperature conditions. The experimentally obtained k values were very well correlated to the predicted values from the model (R-square of experimental vs. predicted values equal to 0.99 and 0.98 for Valencia and Navel PME, respectively).
Analysis of PME Structure by CD Spectroscopy
HP-induced as well as heat-induced structural changes upon the purified PME molecules from the two orange sources were investigated using CD spectroscopy. The CD spectra for the Valencia-extracted PME are presented in Fig. 4. Spectra in the far UV (wavelength region 200–260 nm) are presented in panel a and in the near UV in panel b. The native PME exhibits a specific minimum for β structures (Chang et al. 1978) at 217 nm. Analysis of the far-UV spectrum for the native molecule using CDNN software (Bohm et al. 1992) leads to 20.7 % alpha-helix content and 39.7 % β sheets and turns. These results are different from what has already been published for the crystallogarphically solved structure of PME from carrot (Johansson et al. 2002), where an 11 % alpha-helical content has been revealed (PDB code 1GQ8). The observed differences are likely due to the fact that CDNN fails to accurately characterize protein structures with a high content in β-structural elements, especially for analysis contained within the wavelength range from 200 to 260 nm (Böhm et al. 1992).
a Far-UV CD spectra for the untreated PME (Valencia cv.) in Tris–HCl (pH 7.5) at 30 °C (solid line); high pressure-treated PME at 30 °C and 200 MPa for 10 min (dashed line); at 30 °C and 400 MPa for 10 min (dotted line); and at 30 °C, 600 MPa for 10 min (dash-dotted line). b Near-UV CD spectra difference (∆θ vs. λ) between the untreated PME (Valencia cv.) in Tris–HCl (pH 7.5) at 30 °C and high pressure-treated PME at 30 °C and 200 MPa for 10 min (dashed line); at 30 °C and 400 MPa for 10 min (dotted line); and at 30 °C, 600 MPa for 10 min (dash-dotted line). c Far-UV CD spectra for the untreated PME (Navel cv.) in Tris–HCl (pH 7.5) at 40 °C (solid line); high pressure-treated PME, at 40 °C and 450 MPa for 10 min (dashed line); and at 40 °C and 700 MPa for 10 min (dotted line). d Near-UV CD spectra difference (∆θ vs. λ) between the untreated PME (Navel cv.) in Tris–HCl (pH 7.5) at 40 °C and high pressure-treated PME at 40 °C and 450 MPa for 10 min (dashed line) and at 40 °C, 700 MPa for 10 min (dotted line)
It has been shown that the application of pressure ranging from 400 to 800 MPa may result in the denaturation of monomeric proteins (Heremans 1982). In general, the pressure-induced conformational changes of an enzyme depend on its internal packing. The protein volume in solution contains voids and internal cavities, results of the imperfect packing of amino acid residues as well as contributions due to solvation of peptide bonds and amino acid side chains. For the Valencia PME, the HP-induced spectra in the far-UV region presented in Fig. 4a reveal negligible differences with respect to the native, independently of the pressure level used for the treatment, i.e., for pressures of 200, 400, and 600 MPa. This verifies that HP alone does not cause alterations upon the molecule's secondary structure. Instead, it supports the assumption that pressure bears a minimum effect upon the hydrogen bonds that are responsible for the secondary structure network maintenance (Mozhaev et al. 1994 , Messens et al. 1997).
On the other hand, the near-UV CD spectra of Valencia PME, associated to the molecule's tertiary structure (Woody 1995), reveal significantly altered patterns. These are illustrated in Fig. 4b, where the difference of ellipticity (∆θ) between the spectrum of the native enzyme and of the HP-processed molecules is plotted vs. the wavelength. The application of pressure leads to extensive, irreversible changes upon the enzyme's tertiary structure. The magnitude of these structural changes is greater for higher pressure values and is otherwise independent of the duration of the treatment for temperatures around 40 °C, well below the PME's thermal denaturation limits. Pressure is generally assumed to denature proteins by the destabilization of hydrophobic aggregates, thus allowing water molecules to be forced into the protein interior. This will, in turn, affect the molecule's tertiary structure (Hummer et al. 1998). Our results from the near-UV spectroscopy clearly demonstrate such trends and may well be correlated to the previously described significant PME activity reduction. Denatured protein molecules that retain the secondary structure of the native intact while loosing only the tertiary structure are generally described as intermediate, molten globule states (Mozhaev et al. 1996 , Vidugiris and Royer 1998; Foguel and Silva 1994).
The heat-induced denaturation of Valencia PME reveals a completely different picture from pressure. Results from CD spectroscopy are presented in Fig. 5a (far UV) and b (near UV). As it is clearly depicted, the far-UV spectrum of the PME at 70 °C, right above the molecule's denaturation temperature, is significantly altered from the native one, while all the recorded changes are irreversible. CDNN analysis reveals an intense, more than 50 %, reduction of the alpha-helical structural elements within nevertheless retains some residual alpha-helix content. Moreover, the near-UV spectra presented in Fig. 5b demonstrate an analogous dramatic loss of the PME tertiary structure when the temperature is raised to 70 °C. It is, thus, important to observe that while irreversible thermal unfolding leads to a final denatured state where both secondary and tertiary structures are severely affected, pressure-induced denaturation yields partially unfolded final states that retain most the secondary structure characteristics of the native while loosing tertiary structure depending on the applied pressure. This statement is in accordance with the results of the studies conducted by Ravindra and Winter (2003) and Mozhaev et al. (1996), which concluded that the secondary structure network is maintained after HP treatment.
a Far-UV CD spectra for the untreated PME (Valencia cv.) in Tris–HCl (pH 7.5) at 25 °C (solid line); thermal inactivation-treated PME at 55 °C for 5 min (dashed line), at 60 °C for 5 min (dotted line), at 65 °C for 5 min (dash-dotted line), and at 70 °C for 5 min (dash-dot-dotted line). b Near-UV CD spectra difference (∆θ vs. λ) between the untreated PME (Valencia cv.) in Tris–HCl (pH 7.5) at 25 °C and thermal inactivation-treated PME at 55 °C for 5 min (dashed line), at 60 °C for 5 min (dotted line), at 65 °C for 5 min (dash-dotted line), and at 70 °C for 5 min (dash-dot-dotted line)
This, of course, is reflected upon the residual activity obtained for the various processes: only 15 % for thermal treatment at 70 °C in atmospheric pressure vs. 88 % for treatment at 400 MPa and 30 °C and 80 % for treatment at 600 MPa, 30 °C. This is in agreement with theoretical assumptions that pressure denaturation only affects the incorporation of water molecules into the protein interior, while heat denaturation corresponds to the transfer of nonpolar groups to the solvent-accessible surface (Hummer et al. 1998).
Analogous CD spectroscopy results have been obtained for the Navel PME molecule. They are displayed in Fig. 4c and d. As in the case of Valencia PME, the native far-UV spectrum exhibits a minimum at 216 nm, while CDNN analysis yields a value of 23.6 % in helical content, which is an overestimation compared to the solved structure for the carrot PME (Johansson et al. 2002). The far-UV spectra for Navel PME treated at 450 and 700 MPa are statistically indistinguishable from the native state. On the other hand, the trend depicted in Fig. 4d clearly shows that the higher the pressure of the treatment, the higher the change in the tertiary structure with respect to the native. This result once again correlates with the residual activity obtained, 67 % for processing at 450 MPa and 33 % for processing at 700 MPa, 40 °C. As for the Valencia PME, the duration of the treatment did not affect the final pressure-denatured states.
Taking into consideration all the obtained results with regards the effect of pressure on the secondary and tertiary structures of both PMEs, it may be assumed that the pressure-induced degradation of PME structure is a result of the alteration of the tertiary structure with subsequent impact on the substrate–enzyme binding, leading to reduction of enzyme activity. Pressure-induced denatured states bear the characteristics of molten globule intermediate states. In order to further test this observation, we have performed fluorescent spectroscopy on the three HP-treated Valencia PME molecules (Fig. 6). A clear reduction in the intensity of the fluorescence is observed for the 400 and 600 MPa treated molecules, while no shift in the maximum wavelength is observed. This has previously been observed in molten globule intermediate states (Goto and Fink 1989), although literally in other cases, shifted to longer wavelength fluorescence emission spectra of pressure-induced molten globules have been recorded (Vidugiris and Royer 1998 , Foguel and Silva 1994).
Conclusion
Thermal and high-pressure inactivation of Valencia- and Navel-extracted PMEs were modeled by first-order kinetics. The inactivation rate constants of the treated enzyme were expressed as a function of temperature and pressure enabling a proper design of HP treatments. The effect of pressure on the activation volume, V a , was modeled by linear function and the effect of temperature on the activation energy, E a , by an exponential function, respectively. A synergistic effect of pressure and temperature under HP processing conditions was found. A developed mathematical model, which describes the PME inactivation rate as a function of pressure and temperature conditions, taking into account the dependence of both activation energy and activation volume on the applied pressure and temperature, respectively, allows better quantitative comparison of different treatments in the quest for an optimal process design. Overall, Navel orange peel PME appeared to be more pressure resistant than PME from Valencia orange peel.
HP-induced as well as heat-induced structural changes upon the purified PME molecules from the two orange sources have been investigated using CD spectroscopy. A characteristic for β structure (β sheets and turns) minimum at ~216 nm was observed for the studied PMEs.
Comparison of the CD spectra for the treated and the untreated enzymes reveals that exposure to HP may lead to a structurally molten globulelike state, where the PME maintains the secondary structure of the untreated protein molecules while the tertiary structure is significantly affected with subsequent impact on the substrate–enzyme binding, possibly leading to reduction of enzyme activity. Whether this may be attributed to the fact that pressure favors and strengthens the formation of hydrogen bonds that compose the secondary structure, but which has a major negative effect on hydrophobic interactions, which are responsible for maintaining the tertiary structure of a protein (Ravindra and Winter 2003), need to be further investigated.
References
Balan, A., Santa-Cruz, C. P., Moutran, A., Ferreira, R. C. C., Medrano, F. J., Pérez, C. A., et al. (2006). The molybdate-binding protein (ModA) of the phytopathogen Xanthomonas axonopodis pv citri. Protein Expression and Purification, 50(2), 215–222.
Balogh, T., Smout, C., Ly Nguyen, B., Van Loey, A., & Hendrickx, M. (2004). Thermal and high pressure inactivation kinetics of carrot pectinmethylesterase (PME): From model systems to real foods. Innovative Food Science & Emerging Technologies, 5(4), 429–436.
Barbosa-Cánovas, G. V. (1999). Preservation of foods with pulsed electric fields. London: Academic.
Böhm, G., Muhr, R., & Jaenicke, R. (1992). Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Engineering, 5(3), 191–195.
Boulekou, S., Katsaros, G., & Taoukis, P. (2010). Inactivation kinetics of peach pulp pectin methylesterase as a function of high hydrostatic pressure and temperature process conditions. Food and Bioprocess Technology, 3(5), 699–706.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72, 248–254.
Cameron, R. G., Baker, R. A., & Grohmann, K. (1996). Citrus tissue extracts affect juice cloud stability. Journal of Food Science, 62, 242–245.
Cameron, G. R., Savary, B. J., Hotchkiss, A. T., & Fishman, M. L. (2005). Isolation, characterization and pectin-modifying properties of a thermally tolerant pectin methylesterase from Citrus sinensis var. Valencia. Journal of Agricultural and Food Chemistry, 53(6), 2255–2260.
Castro, S., Saraiva, J., Lopes-da-Silva, J., Delgadillo, I., Van Loey, A., Smout, C., et al. (2008). Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chemistry, 107(4), 1436–1449.
Castro, A. J., Barbosa-Cánovas, G. V., & Swanson, B. G. (1993). Microbial inactivation of foods by pulsed electric fields. Journal of Food Processing and Preservation, 17(1), 47–73.
Chang, C. T., Wu, C.-S. C., & Yang, J. T. (1978). Circular dichroic analysis of protein conformation: Inclusion of the β-turns. Analytical Biochemistry, 91(1), 13–31.
Eagerman, B. A., & Rouse, A. H. (1976). Heat inactivation temperature–time relationships for pectin esterase inactivation in citrus juices. Journal of Food Science, 41(6), 1396–1397.
Foguel, D., & Silva, J. L. (1994). Cold denaturation of a repressor–operator complex: The role of entropy in protein-DNA recognition. Proceedings of the National Academy of Sciences, USA, 91(17), 8244–8247.
Gervilla, R., Ferragut, V., & Guamis, B. (2000). High pressure inactivation of microorganisms inoculated into ovine milk of different fat contents. Journal of Dairy Science, 83(4), 674–682.
Goto, Y., & Fink, A. L. (1989). Conformational states of beta-lactamase: Molten-globule states at acidic and alkaline pH with high salt. Biochemistry, 28(3), 945–952.
Guiavarc'h, Y., Segovia, O., Hendrickx, M., & Van Loey, A. (2005). Purification, characterization, thermal and high-pressure inactivation of a pectin methylesterase from white grapefruit (Citrus paradisi). Innovative Food Science and Emerging Technologies, 6(4), 363–371.
Hendrickx, M., Ludikhuyze, L., Van den Broeck, I., & Weemaes, C. (1998). Effects of high pressure on enzymes related to food quality.Trends of. Food Science and Technology, 9(5), 107–203.
Hendrickx M & Knorr D (2002) Ultra high pressure treatment of foods. Kluwer, New York, Aspen food engineering series.
Heremans, K. (1982). High pressure effects on proteins and other biomolecules. Annual Review of Biophysics and Bioengineering, 11, 1–21.
Hummer, G., Garde, S., Garcia, A. E., Paulaitis, M. E., & Pratt, L. R. (1998). The pressure dependence of hydrophobic interactions is consistent with the observed pressure denaturation of proteins. Proceedings of the National Academy of Sciences of the United States of America, 95(4), 1552–1555.
Johansson, K., El Ahmad, M., Friemann, R., Jornvall, H., Markovic, O., & Eklund, H. (2002). Crystal structure of plant pectin methylesterase. FEBS Letters, 514(2–3), 243–249.
Katsaros, G., Katapodis, P., & Taoukis, P. (2009a). High hydrostatic pressure inactivation kinetics of the plant proteases ficin and papain. Journal of Food Engineering, 91(1), 42–48.
Katsaros, G., Giannoglou, M., & Taoukis, P. (2009b). Kinetic study of the combined effect of high hydrostatic pressure and temperature on the activity of Lactobacillus delbrueckii ssp. bulgaricus aminopeptidases. Journal of Food Science, 74(5), 219–225.
Katsaros, G., Tsevdou, M., Panagiotou, T., & Taoukis, P. (2010a). Kinetic study of high pressure microbial and enzyme inactivation and selection of pasteurization conditions. International Journal of Food Science and Technology, 45(6), 1119–1129.
Katsaros, G., Tavantzis, G., & Taoukis, P. (2010b). Production of novel dairy products using actinidin and high pressure as enzyme activity regulator. Innovative Food Science and Emerging Technologies, 11(1), 47–51.
Laratta, B., Masi, L. D., Minasi, P., & Giovane, A. (2008). Pectin methylesterase in Citrus bergamia R. Purification, biochemical characterization and sequence of the exon related to the enzyme active site. Food Chemistry, 110(4), 829–837.
Ly-Nguyen, B., Van Loey, A. M., Smout, C., ErenOzcan, S., Fachin, D., Verlent, I., & Hendrickx, M. E. (2003a). Mild heat and high-pressure inactivation of carrot pectinmethylesterase: A kinetic study. Journal of Food Science, 68(4), 1377–1383.
Ly-Nguyen, B., Loey, A. M. V., Smout, C., Verlent, I., Duvetter, T., & Hendrickx, M. E. (2003b). Effect of mild-heat and high-pressure processing on banana pectin methylesterase: A kinetic study. Journal of Agricultural and Food Chemistry, 51(27), 7974–7979.
Menéndez, O. H., Schwarzenbolz, U. R., & Henle, T. (2006). Structural changes of microbial transglutaminase during thermal and high-pressure treatment. Journal of Agricultural and Food Chemistry, 54(5), 1716–1721.
Messens, W., Camp, J. V., & Huyghebaert, A. (1997). The use of high pressure to modify the functionality of food proteins. Trends in Food Science and Technology, 8(4), 107–112.
Mozhaev, V., Heremans, K., Frank, J., Masson, P., & Balny, C. (1994). Exploiting the effects of high hydrostatic pressure in biotechnological applications. Trends in Biotechnology, 12(12), 493–501.
Mozhaev, V., Heremans, K., Frank, J., Masson, P., & Balny, C. (1996). High pressure effects on protein structure and function. Proteins: Structure,Function and Bionformatics, 24(1), 81–91.
Nienaber, U., & Shellhammer, T. H. (2001). High-pressure processing of orange juice: Kinetics of pectinmethylesterase inactivation. Journal of Food Science, 66(2), 328–331.
Polydera, A., Galanou, E., Stoforos, N., & Taoukis, P. (2004). Inactivation kinetics of pectin methylesterase of Greek Navel orange juice as a function of high hydrostatic pressure and temperature process conditions. Journal of Food Engineering, 62(3), 291–298.
Ramos, C. H. I. (2004). A spectroscopic-based laboratory course for protein conformational studies. Biochemistry and Molecular Biology Education, 32(1), 31–34.
Ramos, C. H. I., Kay, M. S., & Baldwin, R. L. (1999). Putative interhelix ion pairs involved in the stability of myoglobin. Biochemistry, 38(30), 9783–9790.
Ramos, C. H. I., Weisbuch, S., & Jamin, M. (2007). Diffusive motions control the folding and unfolding kinetics of apomyoglobin pH 4 molten globule intermediate. Biochemistry, 46(14), 4379–4389.
Ravindra, R., & Winter, R. (2003). On the temperature-pressure free-energy landscape of proteins. Chem PhysChem, 4(4), 359–365.
Regis, W. C. B., Fattori, J., Santoro, M. M., Jamin, M., & Ramos, C. H. I. (2005). On the difference in stability between horse and sperm whale myoglobins. Archives of Biochemistry and Biophysics, 436(1), 168–177.
Ribeiro-Jr, E. A., & Ramos, C. H. I. (2004). Origin of the anomalous circular dichroism spectra of many apomyoglobin mutants. Analytical Biochemistry, 329(2), 300–306.
Rombouts, F. M., Versteeg, C., Karman, A. H., & Pilnik, W. (1982). Pectinesterases in component parts of citrus fruits related to problems of cloud loss and gelation in citrus products. In P. Dupuy (Ed.), Use of enzymes in food technology (pp. 483–487). Paris, France: Technique et documentation Lavoisier.
Rouse, A. H., & Atkins, C. D. (1955). Pectinesterase and pectin in commercial orange juice as determined by methods used at the Citrus Experiment Station. Bulletin of the University of Florida Agricultural Experiment Station, 570, 1–19.
Sampedro, F., Rodrigo, D., & Hendrickx, M. (2008). Inactivation kinetics of pectin methyl esterase under combined thermal–high pressure treatment in an orange juice–milk beverage. Journal of Food Engineering, 86(1), 133–139.
Savary, B. J., Vasu, P., Nuñez, A., & Cameron, R. G. (2010). Identification of thermolabile pectin methylesterases from sweet orange fruit by peptide mass fingerprinting. Journal of Agricultural and Food Chemistry, 58, 12462–12468.
Sila, D., Smout, C., Satara, Y., Truong, V., Van Loey, A., & Hendrickx, M. (2007). Combined thermal and high pressure effect on carrot pectinmethylesterase stability and catalytic activity. Journal of Food Engineering, 78(3), 755–764.
Tedford, L. A., Smith, D., & Schaschke, C. J. (1999). High pressure processing effects on the molecular structure of ovalbumin, lysozyme and β-lactoglobulin. Food Research International, 32(2), 101–106.
Thanassoulas, A., Nomikos, M., Theodoridou, M., Stavros, P., Mastellos, D., & Nounesis, G. (2011). Thermal and chemical denaturation of the BRCT functional module of human 53BP1. International Journal of Biological Macromolecules, 49(3), 297–304.
Tiwari, B. K., Muthukumarappan, K., O'Donnell, C. P., & Cullen, P. J. (2009). Inactivation kinetics of pectin methylesterase and cloud retention in sonicated orange juice. Innovative Food Science and Emerging Technologies, 10(2), 166–171.
Van den Broeck, I., Ludikhuyze, L. R., Van Loey, A. M., & Hendrickx, M. (2000a). Effect of temperature and/or pressure on tomato pectinesterase activity. Journal of Agricultural and Food Chemistry, 48(2), 551–558.
Van den Broeck, I., Ludikhuyze, L. R., Van Loey, A. M., & Hendrickx, M. E. (2000b). Inactivation of orange pectinesterase by combined high-pressure and -temperature treatments: A kinetic study. Journal of Agricultural and Food Chemistry, 48(5), 1960–1970.
Versteeg, C., Rombouts, F. M., & Pilnik, W. (1978). Purification and some characteristics of two pectinesterase isoenzymes from orange. Lebensmittel-Wissenschaft und Technologie, 11, 267–274.
Vidugiris, G. J. A., & Royer, C. A. (1998). Determination of the volume changes for the pressure-induced transitions of apomyoglobin between the native, molten globule and unfolded states. Biophysical Journal, 75(1), 463–470.
Woody, R. W. (1995). Circular dichroism. Methods in Enzymology, 246, 34–71.
Zhong, K., Wu, J., Wang, Z., Chen, F., Liao, X., & Hu, X. (2007). Inactivation kinetics and secondary structural change of PEF treated POD and PPO. Food Chemistry, 100(1), 115–123.
Zhou, L., Wu, J., Hu, X., Zhi, X., & Liao, X. (2009). Alterations in the activity and structure of pectin methylesterase treated by high pressure carbon dioxide. Journal of Agricultural and Food Chemistry, 57(5), 1890–1895.
Acknowledgments
The authors would like to thank Professor P. Christakopoulos and Lecturer E. Topakas for offering their expert advice and experience on enzyme purification assessment. This research has been cofinanced by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alexandrakis, Z., Katsaros, G., Stavros, P. et al. Comparative Structural Changes and Inactivation Kinetics of Pectin Methylesterases from Different Orange Cultivars Processed by High Pressure. Food Bioprocess Technol 7, 853–867 (2014). https://doi.org/10.1007/s11947-013-1087-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1087-7