Abstract
The effect of high-intensity pulsed electric fields (HIPEF) processes on Listeria innocua inhibition, physicochemical parameters and activity of oxidative enzymes of mango juice was evaluated to set the optimal HIPEF treatment time. Quality parameters, microbial population and bioactive compounds of HIPEF-treated (35 kV/cm, 1800 μs) and thermally treated (TT) (90 °C, 60 s) mango juices were studied and compared with those non-treated during 75 days of storage at 4 °C. HIPEF treatment for 800 μs ensured 5 log reductions of L. innocua. Polyphenoloxidase (PPO), lipoxygenase (LOX) and peroxidase (POD) residual activities were significantly reduced to 70, 53 and 44%, respectively, at treatment times of 1800 μs. Similar sensory properties compared with fresh mango juice were attained from product treated at 1800 μs. Moreover, fresh mango juice colour (L* = 38.79, h° = 106.57) was preserved after HIPEF treatment throughout storage. Moulds and yeasts and psychrophilic bacteria counts in HIPEF-treated (1800 μs) mango juice remained below 6 log cycles CFU/mL up to 2 months of refrigerated storage. The content of total phenolic compounds in those HIPEF-treated increased from 333 to 683 μg of GAE/mL from day 0 to the end of storage. Hence, the application of HIPEF may be a feasible treatment in order to ensure microbiological stability, high bioactive compound content and fresh-like characteristics of mango juice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mango (Mangifera indica L.), one of the most harvested tropical fruits, is widely used to produce juices due to its well-appreciated sensorial attributes (FAO 2003, 2012; Nanjundaswamy 1998). Furthermore, this fruit is a rich source of bioactive compounds such as phenolics and carotenoids; hence, mango consumption could have health benefits in preventing degenerative diseases (Rawson et al. 2011; Schieber et al. 2000).
Mango juice can undergo quality-degrading reactions triggered by microbial growth population and quality-degrading enzymes, among others. Therefore, preservation treatments are required to ensure its safety and quality stability. On one hand, thermal treatment is commonly used in the juice industry because of its well-known effectiveness in the inactivation of microorganisms and quality-degrading enzymes (Mercadante and Rodriguez-Amaya 1998; Soliva-Fortuny et al. 2009). However, undesired chemical, physical and sensorial changes as well as reduction of bioactive compound content have been observed in thermally treated juices (Sánchez-Moreno et al. 2005; Wibowo et al. 2015). On the other hand, non-thermal treatments allow not only to obtain microbiologically stable fruit juices but also a better preservation of sensorial and nutritional characteristics than conventional treatments (Chen et al. 2013). Hence, high-intensity pulsed electric fields (HIPEF) technology has been considered a feasible non-thermal technique for the preservation of liquid foods. The electric field strength and treatment time are reported as the main parameters of HIPEF treatment to induce an electric potential across cell membrane conducting the cell damage (Morales-de la Peña et al. 2010).
Several studies have proved the efficiency of HIPEF on the inactivation of microorganisms such as Listeria innocua, which is one of the main foodborne microorganisms in fruit juices (Huang et al. 2012; Mosqueda-Melgar et al. 2007; Timmermans et al. 2014). Nevertheless, published data evidenced that the degree of microbial inactivation is strongly dependent on the HIPEF conditions (Jiménez-Sánchez et al. 2017). With regard to enzyme activity, peroxidase (POD), polyphenoloxidase (PPO) and lipoxygenase (LOX) catalyse some reactions affecting sensory and nutritional properties in fruit juices. HIPEF treatments from 20 to 35 kV/cm have halved enzymatic activity in tomato and orange juices (Aguiló-Aguayo et al. 2010; Vervoort et al. 2011). Moreover, HIPEF seems to maintain quality characteristics including colour, soluble solids and viscosity as well as retain bioactive compounds of fruit juices (Buckow et al. 2013; Odriozola-Serrano et al. 2008).
Despite of the noteworthy literature using HIPEF treatment for fruit juice quality preservation, no studies comparing the effects of HIPEF and thermal treatment on quality changes of mango juice have been found. Therefore, the objectives of the present work were firstly to select the HIPEF treatment time capable to inactivate L. innocua and to reduce enzymatic activity in mango juice while preserving its fresh-like sensorial attributes, and secondly, to compare the effect of HIPEF and thermal treatments on microbial stability, activity of oxidative enzymes, total carotenoids and phenolics content, antioxidant capacity and physicochemical properties in mango juice throughout 75 days of refrigerated storage.
Materials and Methods
Mango Juice
Mangoes (M. indica L.) cv. tommy atkins were purchased from a local wholesale market (Lleida, Spain). Each fruit was washed, dried and peeled, and the seed was discarded. The pulp was squeezed and then centrifuged at 5400×g during 5 min at 4 °C (AVANTI™ J-25 Beckman; Instruments Inc.; Fullerton, CA) and vacuum filtered to obtain mango juice (MJ). MJ electric conductivity (1.54 ± 0.02 mS/cm), soluble solids (12.77 ± 1.11 °Brix) and pH (3.67 ± 0.14) were measured.
HIPEF Treatments
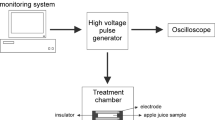
HIPEF treatments were performed using a continuous flow bench scale system (OSU-4F, Ohio State University, Columbus, OH) that generates squared wave pulses. The flow rate was 60 mL/min controlled by a speed pump (model 752210-25, Cole Palmer Instrument Company, Vermon Hills, IL). The treatment chamber device consisted of eight co-linear chambers disposed in series and each pair of chambers had a thermocouple to control temperature. The outlet treatment temperature of juice was kept below 40 °C using a cooling coil, which was connected before and after each pair of chambers and submerged in an ice-water shaking bath. Based on previous literature, constant electric field strength (35 kV/cm), pulse frequency (200 Hz) and width (4 μs) were kept to apply pulses in bipolar quadratic mode, while different treatment times were assayed (50, 100, 200, 400, 800, 1200, 1600, 1800 and 2000 μs). According to the results of microbial and enzymatic inactivation of HIPEF-treated mango juice, the treatment conditions were set for subsequent study of preservation of mango juice along the storage.
Thermal Treatment
MJ was heat-treated at 90 °C for 60 s. The juice was pumped with a peristaltic pump (model D-21V, Dinko, Barcelona, Spain) and passed through a tubular stainless steel heat exchange coil system (University of Lleida, Lleida, Spain). Immediately after heating, the tubular stainless steel was immersed in an ice-water bath at 4 °C and thereafter MJ was packaged (Odriozola-Serrano et al. 2009).
Packaging and Storage
Treated MJ was bottled directly from the treatment systems in sterilised 100 mL polypropylene bottles and leaving the minimum headspace volume. Non-treated MJ was bottled thereafter the juice preparation. Once filled, the containers were tightly closed and stored in darkness under refrigeration (4 ± 1 °C) until analysis. Non-treated and treated MJ were analysed twice a week the first 3 weeks and once a week until day 75.
L. innocua Culture, Inoculation and Enumeration
L. innocua IPL 1.17 (Institute Pasteur de Lille; Lille, France) was cultured in tryptone soy broth (TSB) with 0.6% yeasts extract (Bioakar Diagnostic; Beauvais, France) and incubated at 35 °C with continuous agitation at 200 rpm for 15 h to obtain cells in stationary growth phase. The final concentration reached in the culture was 108–109 colony-forming units (CFU)/mL. MJ was inoculated with L. innocua to have an initial concentration of 107–108 CFU/mL and then HIPEF treated. Treated and non-treated MJ was serially diluted in saline peptone water (Bioakar Diagnostic; Beauvais, France) for L. innocua enumeration; the cells were spread on Palcam agar plates (Bioakar Diagnostic; Beauvais, France) and incubated at 35 °C for 24–48 h as stated by ISO 11290-2 method (1998). Colonies were counted, and the results were expressed as log10 CFU per millilitre.
Microbial Evaluation During Storage
Enumeration of psychrophilic microorganisms in MJ on plate count agar (PCA; Biokar Diagnostic; Beauvais, France) was carried out after the incubation at 5 ± 1 °C for 10 days (ISO 17410, 2001 Method). Mould and yeast counts were determined with the ISO 7954, 1987 Method using chloramphenicol glucose agar (CGA; Biokar Diagnostic; Beauvais, France) and incubated 2–4 days at 25 ± 1 °C. Colonies were counted, and the results were expressed as log10 CFU per millilitre. Counts below the detection limit (1.0 log CFU/mL) were considered no detectable colonies. The criterion for completing the storage study was established as the time at which a microbial population of 106 CFU/mL (Salvia-Trujillo et al. 2011).
Physicochemical Analysis
Electric conductivity (Testo 240 conductivity-meter; Testo GmBh & Co; Lenzkirch, Germany), pH (Crison 2001 pH-meter; Crison Instruments S.A; Barcelona, Spain), soluble solid content (Atago RX-1000 refractometer; Atago Company Ltd.; Japan), viscosity using a spindle SP61 at 100 rpm and 5 °C (Brookfield, Stoughton, MA) and colour (Minolta CR-400; Konica Minolta Sensing, Inc., Osaka, Japan) of MJ were measured. Colour equipment was set up for illuminate D65 and 10° observer angle and calibrated using a standard white reflector plate. MJ (10 mL) were placed in petri dishes (3.5 cm × 3.5 cm), and colour was measured using the CIE L*, a* and b* scales. Additionally, hue angle (h°) was calculated as the arctan of the b* and a* quotient (measure of red = 0 or 36°, yellow = 90°, green = 180°) (Hunter 1987).
Enzyme Activity Evaluation
Peroxidase
POD activity was determined using the method described by Elez-Martínez (2006) with some modifications. The enzyme extract for POD activity measurement was obtained by the homogenisation of 10 mL of MJ and 20 mL of sodium phosphate buffer 0.2 M at pH 6.5. The homogenate was centrifuged at 24,000×g for 15 min at 4 °C (AVANTI™ J-25, Beckman Instruments Inc.; Fullerton, CA, USA). The supernatant was filtered throughout a Whatman paper (no. 1), and the resulting liquid constituted the enzymatic extract. POD activity was assayed spectrophotometrically (CECIL CE 2021 spectrophotometer Cecil Instruments Ltd., Cambridge, UK) in a 1-cm path cuvette by adding at 0.1 mL of enzymatic extract 2.7 mL of sodium phosphate buffer (0.05 M, pH 6.5), 0.1 mL phenylenediamine (1%) and 0.1 mL hydrogen peroxide (1.5%). The oxidation of p-phenylenediamine was determined at 470 nm measuring the absorbance every 10 s during 3 min. The absorbance values were referred to a sample blank containing all reagents except hydrogen peroxide, which was substituted by distilled water. POD activity was obtained from the slope of the linear portion of the curve. One unit of POD activity was defined as the change of absorbance per minute and millilitre of enzymatic extract at 22 °C.
Polyphenoloxidase
PPO activity was determined by the method of Vásquez-Caicedo et al. (2007) with some modifications. For the extraction of the enzyme, 5 g of MJ were mixed with 0.5 g polyvinylpolypyrrolidone (PVPP) and 4.5 g McIlvaine buffer solution (pH 6.5) consisting of 35% of 0.1 M citric acid and 75% 0.2 M disodium phosphate. The mixture was homogenised and centrifuged at 23,000×g for 15 min at 4 °C (Centrifuge AVANTI™ J-25, Beckman Instruments Inc.; Fullerton, CA). The supernatant was filtered with Whatman paper (no. 1) to obtain the enzyme extract. PPO activity was measured using a spectrophotometer (CECIL CE 2021; Cecil Instruments Ltd., Cambridge, UK) at 400 nm by adding 100 μL enzyme extract and 3 mL of 0.5 M cathecol solution and obtaining the absorbance every 10 s during 3 min. A blank of cathecol without extract was used. The PPO activity was obtained from the slope of the linear portion of the curve; 1 unit of PPO activity was defined as a change of 1 unit of absorbance per minute and millilitre of enzyme extract at 22 °C.
Lipoxigenase
LOX activity was determined by the method described by Anthon and Barrett (2003) with modifications. The enzyme extract was obtained by mixing 20 mL of MJ with 5 mL of a solution containing 0.5 M phosphate buffer (pH 6.5) and 0.5% Triton X-100 and centrifugation for 10 min at 10,000×g at 4 °C (Centrifuge AVANTI™ J-25, Beckman Instruments Inc.; Fullerton, CA). The pellet was discarded, and the supernatant was filtered with Whatman paper (no. 1). The LOX activity of the enzyme was measured by mixing 2 mL phosphate buffer at 0.1 M (pH 6.5), 40 μL linoleic acid and adding 100 μL enzymatic extract. The reaction was measured with a spectrophotometer (CECIL CE 2021; Cecil Instruments Ltd., Cambridge, UK) at 234 nm each at 10 s during 3 min. The activity was calculated from the slope of the linear portion of the curve. A blank was prepared with 2 mL phosphate buffer at 0.1 M mixed with 1 mL linoleic. One unit of LOX activity was defined as a change of 1 unit of absorbance per minute and per millilitre of enzyme extract at 22 °C.
Enzymatic activity was expressed as percentage of residual activity (RA %) which was calculated by the quotient between the enzyme activity of treated (AEt) and the non-treated (AE O ) MJ.
Bioactive Compounds and Antioxidant Activity Determination
Total Carotenoids
The determination of total carotenoids was performed according to Robles-Sánchez et al. (2009). MJ (5 mL) were added to 20 mL of tetrahydrofuran (THF) and homogenised with an Ultra-Turrax T 25 basic (IKA® WERKE, Germany). An aliquot was filtered throughout a no. 1 Whatman paper. Total carotenoids were measured spectrophotometrically (CECIL CE 2021 spectrophotometer; Cecil Instruments Ltd., Cambridge, UK) at 470 nm, quantified using β-carotene as an external standard and expressed as microgrammes of β-carotene equivalent per MJ per millilitre.
Total Phenolic
The content of total phenolic compounds (TP) was determined according to the Folin-Ciocalteu colorimetric method described by Singleton et al. (1998) with slight modifications. MJ (0.5 mL) was mixed and homogenised with saturated sodium carbonate solution (10 mL) and Folin-Ciocalteu reagent (10 mL). After 1 h in dark storage, absorbance was measured at 765 nm (CECIL CE 2021 spectrophotometer; Cecil Instruments Ltd., Cambridge, UK). TP content was calculated on the basis of a standard curve of gallic acid and expressed as microgrammes of gallic acid equivalent (GAE) per MJ per millilitre.
Antioxidant Capacity
Antioxidant capacity was determined by a radical-scavenging activity (RSA) assay evaluated as bleaching of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. MJ (10 mL) was centrifuged at 3500×g for 20 min at 4 °C in a Centrifuge AVANTI™ J-25 (Beckman Instruments Inc.; Fullerton, CA, USA). The reaction mixture constituting 10 μL of supernatant, 3.9 mL of methanolic DPPH (0.0025 g L−1) and 90 μL of distilled water was carried out. The samples were shaken vigorously and kept in the dark for 30 min. The absorption of the samples was measured with a spectrophotometer (CECIL CE 2021 Cecil Instruments Ltd., Cambridge, UK) at 515 nm against a blank of methanol without DPPH (Odriozola-Serrano et al. 2008). The results were expressed as percentage of DPPH inhibition as shown in Eq. 1 where A o is de absorbance of DPPH reagent and A s is the absorbance of the MJ sample reaction with DPPH.
Sensory Evaluation
A total of 30 non-trained panellists participated in the sensory test of treated and non-treated MJ on the day of processing. A hedonic scale from 0 (dislike) to 10 (extremely like) was used to rate the colour, flavour and overall acceptance. MJ (30 mL) processed by HIPEF (35 kV, 1800 μs, 200 Hz, 4 μs), heat (90 °C, 60 s) and non-treated (NT) were served at 16 ± 1 °C in transparent cup coded with three digits randomly numbered. Moreover, a glass containing potable water and a piece of non-salted cracker were provided to panellists to eliminate the residual taste between samples (Mosqueda-Melgar et al. 2012).
Statistical Analysis
All the treatments were assayed in duplicate, and two replicate analyses were carried out for each sample to obtain the mean values and standard deviations (SD) for each analysed parameter. The analysis of variance (ANOVA) and least significant differences (LSD) was performed in order to find statistical differences (p ≤ 0.05). All statistical analyses were conducted with Statgraphics plus Centurion XV software version 15.1.02 (StatPoint Technologies, Inc.).
Results and Discussion
Effect of HIPEF Treatment on Mango Juice
L. innocua Inactivation
A maximal reduction of L. innocua survival of 5.7 log units was achieved after applying HIPEF (35 kV/cm, 200 Hz, 4 μs) for 800 μs to MJ (Fig. 1). The longer is the HIPEF treatment time up to 800 μs, the higher the decrease of microbial population. As described in Fig. 1, no significant differences in the L. innocua inactivation levels were appreciated at HIPEF treatments from 800 to 2000 μs. According to microbiological criteria proposed by FDA (2004) for fruit juices, 5 log reductions of target microorganisms should be accomplished for obtaining safe product. Similarly, Mosqueda-Melgar et al. (2007) achieved 5 log reductions of L. innocua population in melon juice treated by HIPEF (35 kV/cm, bipolar square wave, 4 μs pulses and 200 Hz) as treatment times increased up to 1250 μs. Previous research explained the effect of increasing HIPEF treatment time on microbial inactivation by the formation of membrane pores triggering to membrane destabilisation and cell rupture (Vega-Mercado et al. 1997). Although the efficacy of HIPEF (20 kV/cm, 90 Hz and 130 L/h) against L. innocua was also proved in orange juice (Timmermans et al. 2014), less studies have been found to reduce more than 5 log units at 800 μs. The low pH (4.1) and conductivity (1.71 mS/cm) of MJ could cause L. innocua cells more sensible to damage. Indeed, Amiali et al. (2006) reported that lowering ionic concentration cause an increase of the treatment chamber resistance, which could enhance the microbial inactivation levels. Wouters et al. (1999) observed better reduction of L. innocua in solutions with low pH (4.0) and conductivity (2.7 mS/cm) than in alkaline solutions.
Enzyme Activity
HIPEF treatment applied for 1800 μs reduced at 70, 53 and 44% for PPO, LOX and POD activities in the MJ, respectively (Fig. 2). Differently, at treatment times below 1800 μs, when no significant reduction of enzymatic activity was observed, various deleterious reactions affecting loss of nutritive value and yellow colour might occur in MJ.
A reduction of the RA as increasing HIPEF treatment time has also been reported by Aguiló-Aguayo et al. (2008) and Aguiló-Aguayo et al. (2010), who reached 10 and 30% of RA for PPO and LOX, respectively, in strawberry treated by HIPEF (35 kV/cm, 1000 μs, 200 Hz and 4 μs). HIPEF treatment, known to be conducted through cell electroporation, might benefit the contact between enzyme and substrate released from the cell; hence, no complete inactivation was achieved in MJ (Huang et al. 2012). The effect of HIPEF at 1800 μs might cause an irreversible conformational change of the globular protein chain of enzymes in MJ. An enzyme denaturation might be a feasible reason for enzymatic activity reduction (Luo et al. 2010).
The studied oxidative enzymes followed similar pattern of inactivation. Nevertheless, differences on the RA between LOX and the other oxidative enzymes in MJ at the longest treatment time were observed (Fig. 2). This could indicate a different level of HIPEF effect on the enzymatic structure. PPO and POD structures contain a prosthetic group, thereby, the influence of electric fields on changing the structure of copper-containing enzyme has been reported scarcely since it can be considered tightly bound organic molecules (Sharma et al. 2013). Otherwise, conformational changes in LOX structure, with no prosthetic group, could occur easily. Moreover, other authors have reported that charges separation of tertiary structure occurred in LOX native conformation leading an almost complete inactivation of LOX, when long treatments and high voltage are used in enzymatic solution, but not in PPO (Luo et al. 2010). In agreement with the available scientific literature, electrochemical effect of HIPEF may affect the local electrostatic fields in proteins and disrupt electrostatic interactions of peptide chains leading to conformational changes in enzymes (Buckow et al. 2013). Therefore, HIPEF treatment had greatest degree of activity reduction on LOX compared with PPO and POD in HIPEF-treated MJ at 2000 μs.
Physicochemical Parameters
HIPEF treatment had no significant effect (p ≥ 0.05) on pH and conductivity of MJ when different treatment times were applied. Average values in pH and conductivity of treated MJ were 4.1 ± 0.1 and 1.71 ± 0.01 mS/cm, respectively. In a similar way, Zhang et al. (2010) and Aguilar-Rosas et al. (2007) reported that both HIPEF-processed longan and apple juice did not show pH differences with the non-treated products. Other reports indicated no change of conductivity after HIPEF treatment (Mosqueda-Melgar et al. 2012; Vega-Mercado et al. 1997). Although no effect of HIPEF treatment time on TSS content or viscosity of MJ was observed, differences between HIPEF-treated and non-treated MJ were detected. Non-treated MJ (10.8 ± 0.7 °Brix and 4.0 ± 0.3 mPa s) presented lower average values of TSS and viscosity compared with HIPEF-treated (35 kV/cm, 200 Hz, 4 and 2000 μs) MJ (12.9 °Brix ± 0.6 and 5.4 mPa s ± 1.1). Cserhalmi et al. (2006) and Falade et al. (2004) reported an increase in TSS and viscosity of citrus juices treated by HIPEF (28 kV/cm, 100 μs, 2 μs-bipolar pulses at 100 Hz), which were attributed to the breakdown cell effect releasing soluble solids from the cell. Moreover, changes in HIPEF-treated MJ compared with the non-treated might be also attributed to a decline of the pectinolitic enzyme activity, which could enable to maintain pectin content in MJ and hence higher TSS and viscosity (Espachs-Barroso et al. 2006).
Figure 3 shows a non-significant changes of L* value of MJ from 50 to 2000 μs. Similarly, the h° value was maintained in the range of 74.5 to 73.9 in HIPEF-treated MJ as treatment time increased (Fig. 3). Thus, HIPEF treatment preserved characteristic colour of MJ. The significant reduction of enzymatic activity in HIPEF-treated MJ might prevent quality-degrading oxidative reactions (Pathare et al. 2012). The present results are aligned with previous studies, where colour of HIPEF-treated orange (Cortés et al. 2008) and carrot juice (Quitão-Teixeira et al. 2007) were preserved as in fresh juices. Carrot, orange and mango juice have similar yellow colour tonality, which could be mainly attributed to carotenoid compounds. Thus, yellow colour might be preserved whether great content of natural pigments such as carotenoids is maintained.
Sensory Evaluation of Mango Juice
Figure 4 shows the influence of HIPEF (35 kV/cm, 1800 μs, 200 Hz, 4 μs) and TT (90 °C, 60 s) on sensorial attributes (colour, flavour and overall acceptance) of MJ compared with the non-treated. Similar overall acceptance and flavour between treated and non-treated MJ were observed. Mosqueda-Melgar et al. (2012) observed no differences in flavour and overall acceptance comparing fresh fruit juices and those treated by HIPEF and TT. On the other hand, colour values in HIPEF and thermally treated MJ were alike. Nevertheless, significant differences (p ≤ 0.05) in colour perception of non-treated MJ (5.6 ± 1.6) compared with the HIPEF treated (7.2 ± 1.8) were detected. The reduction of oxidative enzyme activity in HIPEF-treated MJ might avoid the loss of colour. Also, the possible release of natural pigments due to the electroporation effect in HIPEF treatment could explain the great colour score of HIPEF-treated MJ given by the consumers.
Since HIPEF-treated MJ at 1800 μs led to a significant reduction of L. innocua population and enzymatic activity as well as fresh-like physicochemical characteristics, sensory evaluation of MJ treated by HIPEF and TT at day of processing, and further quality analysis along the storage were carried out at 35 kV/cm, 1800 μs, 200 Hz, 4 μs.
Storage Stability of Mango Juice
Microbial Evaluation
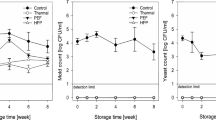
Initial counts of moulds and yeasts in non-treated MJ were 4.22 ± 0.58 log10 CFU/mL, while those of psychrophilic bacteria were 1.74 ± 0.15 log10 CFU/mL. HIPEF or TT effectively reduced microbial loads of the juice up to the detection limit just after processing (day 0) (Fig. 5). During storage, it was observed that moulds and yeasts population increased earlier than psychrophilic bacteria in treated and non-treated MJ. No microbial growth in HIPEF-treated MJ was detected during the first 2 weeks of storage, while the TT MJ did not show microbial growth along the entire storage time. Microbial counts for HIPEF- and thermal-treated MJ was lower than 6 log10 CFU/mL until days 59 and 75, respectively, whereas non-treated MJ exceed those counts at day 23.
Moulds and yeasts (a) and psychrophilic bacteria (b) growth in mango juice treated by HIPEF treatment (35 kV/cm, 1800 μs, 4-μs bipolar pulses at 200 Hz) or thermal treatment (90 °C, 60 s) compared with the non-treated throughout storage at 4 °C during 75 days. Limit of microbial shelf-life at 6 log CFU/mL (dashed line)
Diverse studies have suggested that microorganisms are inactivated because of electroporation and electrofusion phenomena during the HIPEF treatment (Buckow et al. 2013). Nevertheless, a microbial growth in HIPEF-treated MJ could be attributed to a non-complete inactivation of microorganisms (Mosqueda-Melgar et al. 2007). HIPEF treatment enabled to extend the lag phase of MJ microbial population, hence, the recovery of injured microorganisms and germination of those sporulated was delayed. Timmermans et al. (2011) and Elez-Martínez et al. (2006) observed no growth of moulds and yeasts in HIPEF-treated orange juice at 25 and 35 kV/cm, respectively, during 20 and 56 days. Although Timmermans et al. (2011) achieved similar microbial stability compared with the present study, it must be noted that the treatment temperature used was 56 °C, whereas present results were obtained without exceeding 40 °C.
Enzyme Activity
At the beginning of storage, RA of HIPEF-treated (35 kV/cm for 1800 μs with bipolar pulses of 4 μs at 200 Hz) MJ were 70.0 ± 5.1, 69.9 ± 4.9 and 46.3 ± 10.2% for PPO, LOX and POD, respectively. The application of thermal treatment to MJ significantly reduced activity of PPO and POD up to 55.5 ± 0.5 and 20.7 ± 1.0 at day 0 (Table 1). The PPO and POD molecular structures, which contain a prosthetic group in their structure, have been reported to be specially affected by pH, temperature and electric fields (Luo et al. 2010). Otherwise, RALOX after thermal treatment increased at day of processing and LOX appeared to be less thermo-sensible. During storage, a severe increase of RAPOD in non-treated MJ was observed, whereas PPO and LOX activities were slightly reduced. Probably, the increase of POD activity might be assigned to the cell release of POD substrate (organic hydroperoxides), which enable the enzyme-substrate contact (Vervoort et al. 2011).
Both electrochemical and thermal effects associated with HIPEF and TT could result in changes in the structure and conformation of enzymes, which may lead to inactivation (Huang et al. 2012; Timmermans et al. 2011). However, the appearing of isoenzymes and incomplete inactivation might explain the fluctuations of enzymatic activity in TT and HIPEF-treated MJ along the storage. RA of PPO and POD in MJ treated by TT and HIPEF had drastically decrease from day 16 until the end of storage. Among oxidative enzymes, RAPOD of 25.1 ± 3.5% (day 75) and 17.0 ± 4.4% (day 49) was the lowest in MJ treated by TT and HIPEF, respectively. Consistently, literature has reported that POD seemed to be more susceptible to HIPEF than other enzymes and is associated with the modification of the α-helix structure (Leong and Oey 2014). These results are inconsistent with the complete POD inactivation during 56 days reported by Elez-Martínez et al. (2006) in orange juice after HIPEF treatment (35 kV/cm for 1000 μs with bipolar pulses of 4 μs at 200 Hz). However, other authors described a progressive decrease of RAPOD in HIPEF-treated orange juice (23 kV/cm, 90 Hz, monopolar pulses of 2 μs and 130 L/h) along 58 days (Vervoort et al. 2011).
In contrast, significant RALOX reduction in treated MJ required long storage time. Both TT and HIPEF treatments reduced significantly in more than 50% of the initial activity of LOX at the end of storage. Similar to other studies, a retarded decrease of the RALOX was observed (Espachs-Barroso et al. 2006; Zhao et al. 2007). According to Aguiló-Aguayo et al. (2010), LOX protein chain could undergo changes and a development of resistant isoforms in HIPEF-treated fruit juices. Thus, the conformational changes in LOX structure might delay the reduction of the activity throughout storage time.
It is known that HIPEF and thermal enzyme inactivation mechanisms are related to the unfolding of proteins due to changes in their secondary structure (Salvia-Trujillo et al. 2011). Also, a weak affinity of enzyme-substrate complex might describe the decrease of RA in HIPEF-treated MJ during the storage. Another hypothesis for reducing enzymatic activity in HIPEF-treated MJ throughout storage would be the formation of aggregates as a result of a strong polarization of the protein molecules and hydrophobic interactions or covalent bonds (Luo et al. 2010). Therefore, the protein aggregation along the storage could reduce the enzymatic reaction by avoiding the substrate from fitting the active site of the enzyme.
Physicochemical Parameters
pH and TSS values remained stable throughout the storage and no statistical differences among treatments were observed. pH average values for non-treated, TT and HIPEF-treated MJ were 3.7 ± 0.1, 3.76 ± 0.04 and 3.7 ± 0.1, respectively. The mean values of TSS for non-treated, TT and HIPEF-treated MJ were 9.4 ± 0.9, 10.72 ± 0.52 and 8.53 ± 1.62, respectively. In contrast with the obtained results, Timmermans et al. (2011) observed a TSS increase in HIPEF-treated orange juice (23 kV/cm and 90 Hz) after 58 days of refrigerated storage. Differences might be attributed to the use of lower electric field compared with that of the present study; hence, less reduction of enzymatic activity might lead deleterious quality process as increment of turbidity and TSS.
L* values of the non-treated, HIPEF-treated and TT MJ at day 0 were 39.78 ± 0.01, 38.87 ± 0.52 and 40.34 ± 0.25, respectively (Table 2). During storage, non-treated MJ rapidly declined L*, whereas a slightly decreased in HIPEF-treated MJ was observed. L* values of thermal-treated MJ were preserved along the storage. On the other hand, initial h° values of non-treated (106.57 ± 0.26), TT (107.4 ± 0.6) and HIPEF-treated (108.03 ± 0.38) MJ were not significantly different. Along the storage, h° of non-treated MJ decreased; hence loss of yellow colour might occur. TT and HIPEF treatment maintained similar h° in MJ throughout the storage. The loss of L* and h° could be associated with the formation of dark colour compounds and reduction of yellow colour in beverages due to the non-enzymatic browning reactions (Pathare et al. 2012). According to other studies, the loss of colour in non-treated MJ might be related with the oxidative reactions mostly triggered by residual activity of POD and PPO (Timmermans et al. 2011; Wibowo et al. 2015). In this sense, the increase of RAPOD observed in non-treated MJ probably conducted the deterioration of colour. Differently, all treated MJ significantly reduced the activity of POD and PPO; hence, enzymatic browning was avoided. Therefore, treated MJ preserved the yellow colour of fresh mango juice.
Bioactive Compounds and Antioxidant Activity
The effects of processing and storage time on bioactive compounds and antioxidant activity of MJ are shown in Fig. 6. Considering total carotenoid content, TT and HIPEF-treated MJ showed a decrease of 17 and 13%, respectively, compared with non-treated MJ at the beginning of the storage (Fig. 6a). Carotenoids compounds are thermo-labile; hence, heat processing leads to significant higher losses in TT MJ than those HIPEF treated. Differently, an electroporation on the cell membrane, which enable the releasing of carotenoids among other compounds, in HIPEF-treated MJ could occur. Oxidative reactions promoted by enzymes, light or oxygen could affect rapidly the carotenoids released in TT or HIPEF-treated MJ, which could explain the subsequently decline of carotenoids content (Soliva-Fortuny et al. 2009). According to Odriozola-Serrano et al. (2009) oxidation may occur by self-oxidation, where alkylperoxyl radicals are formed, and these radicals attack the double bonds resulting in formation of epoxides. Thus, the severity of oxidation depends on the structure of carotenoids and the environmental conditions. However, during storage period, HIPEF-treated MJ reached 2.2 times more carotenoids than those heat treated (Fig. 6a). Similarly, other studies described great retention of carotenoids in HIPEF-treated compared with heat-treated orange juice during storage (Buckow et al. 2013). Total phenolic compounds in MJ varied from 560.1 ± 17.9 (non-treated) to 333.8 ± 27.8 (HIPEF-treated) and 529.6 ± 15.4 μg (TT) of gallic acid/mL at processing day (day 0). Similarly to Santhirasegaram et al. (2015), no significant difference in the phenolic concentration after thermal treatment compared with non-treated MJ was observed immediately after processing. Although other authors have also reported that after HIPEF treatment the phenolic content is reduced, the mechanism is not well known (Rawson et al. 2011). The interaction with other compounds such as solutes resulting from the high electric field and long treatment time applied could create aggregations reducing the content of phenolic compounds (Soliva-Fortuny et al. 2009).
Total phenolics decreased in non-treated MJ along the storage (Fig. 6b). Otherwise, total phenolic compound concentration increased in MJ treated by HIPEF throughout the storage. Indeed, HIPEF-treated MJ (683.79 ± 0.50 μg GAE/mL) showed the highest phenolic concentration compared with TT MJ at day 59. Phenolic compounds are formed in plant products via the action of phenylalanine ammonia-lyase (PAL) in the phenylpropanoid metabolism (Patthamakanokporn et al. 2008). This response is initiated when the plant recognises a stimulus at the cellular level. It could be hypothesised that HIPEF induced PAL activity and may influence the voltage-gated ion channels and increase the membrane permeability for Ca2+ at the cellular level, followed by a rapid influx of Ca2+ through cation channels. Through this process, Ca2+-dependent protein kinase phosphorylates PAL, which regulates the phenylpropanoid metabolism (Vallverdú-Queralt et al. 2012). On the other hand, the loss of phenolic compounds in non-treated fruit juice was also observed by Patthamakanokporn, Puwastien et al. (2008) who attributed the decrease of phenolics during the storage to deleterious enzymes such as PPO. After analysing the data obtained in this work, it was observed that there was a negative correlation (r = − 0.74) between the activity of PPO and the content of phenolics. This result seems to indicate the importance of TT and HIPEF treatments in reducing RAPPO. Thus, decreasing of PPO activity, which uses phenolic compounds for the oxidative processes to trigger on quinones, was mainly associated with increasing in phenolics (Cheema and Sommerhalter 2015).
Initial antioxidant capacity in MJ was 20.4 ± 1.3, 18.7 ± 0.4 and 17.9 ± 0.4% of DPPH inhibition for HIPEF, TT and non-treated MJ, respectively. The enhancement of radical-scavenging activity in HIPEF-treated MJ might be attributed to the stress response of antioxidant compounds. During storage, the antioxidant capacity of MJ depleted irrespective of the treatment applied (Fig. 6c). It is remarkable that both total carotenoid content and antioxidant capacity rapidly decreased in TT and non-treated MJ along the storage. Our results for HIPEF-treated MJ were in accordance with Odriozola-Serrano et al. (2008) who observed a significant loss of antioxidant capacity as storage time increased in HIPEF-treated tomato juice (35 kV/cm, 100 Hz and 1500 μs of treatment time). In many plant species, a good relationship between antioxidant activity and total phenolics was noted. Contrarily, no correlation between total phenolic compounds and antioxidant capacity in treated MJ was observed. Thus, antioxidant capacity in MJ during refrigerated storage could be related to other bioactive compounds such as vitamin C, which could be easily affected by oxidative deleterious reactions (Buckow et al. 2013).
Conclusions
HIPEF treatment at 35 kV/cm, 4 μs-bipolar pulses, 200 Hz and 1800 μs proved to be feasible in the reduction of L. innocua population to pasteurisation levels in mango juice while enzymatic activity of PPO, LOX and POD was reduced up to 70, 53 and 44% RA, respectively, and fresh-like physicochemical properties were maintained. The native flora stability of HIPEF-treated mango juice was assured throughout 59 days at 4 °C. On the other hand, LOX activity of HIPEF-treated mango juice was halved along the storage. Also, the POD and PPO enzymatic activities in HIPEF-treated mango juice were lower than in those untreated throughout storage. The reduction of PPO enabled a significant increase of the phenolic content in HIPEF-treated mango juice during 59 days. Differently, antioxidant capacity and carotenoid content of all evaluated mango juices decreased gradually throughout storage period. However, bioactive compounds in mango juice were better retained after HIPEF than thermal treatments. The beneficial effect of the HIPEF treatment was noticeable over the storage period with enhanced phenolic content and maintaining fresh-like characteristics of mango juice.
References
Aguilar-Rosas, S. F., Ballinas-Casarrubias, M. L., Nevarez-Moorillon, G. V., Martin-Belloso, O., & Ortega-Rivas, E. (2007). Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. Journal of Food Engineering, 83(1), 41–46.
Aguiló-Aguayo, I., Sobrino-López, Á., Soliva-Fortuny, R., & Martín-Belloso, O. (2008). Influence of high-intensity pulsed electric field processing on lipoxygenase and β-glucosidase activities in strawberry juice. Innovative Food Science & Emerging Technologies, 9(4), 455–462.
Aguiló-Aguayo, I., Soliva-Fortuny, R., & Martín-Belloso, O. (2010). High-intensity pulsed electric fields processing parameters affecting polyphenoloxidase activity of strawberry juice. Journal of Food Science, 75(7), C641–C646.
Amiali, M., Ngadi, M. O., Raghavan, V. G. S., & Nguyen, D. H. (2006). Electrical conductivities of liquid egg products and fruit juices exposed to high pulsed electric fields. International Journal of Food Properties, 9(3), 533–540.
Anthon, G. E., & Barrett, D. M. (2003). Thermal inactivation of lipoxygenase and hydroperoxytrienoic acid lyase in tomatoes. Food Chemistry, 81(2), 275–279.
Buckow, R., Ng, S., & Toepfl, S. (2013). Pulsed electric field processing of orange juice: A review on microbial, enzymatic, nutritional, and sensory quality and stability. Comprehensive Reviews in Food Science and Food Society, 12(5), 455–467.
Cheema, S., & Sommerhalter, M. (2015). Characterization of polyphenol oxidase activity in Ataulfo mango. Food Chemistry, 171, 382–387.
Chen, Y., Yu, L. J., & Rupasinghe, H. P. V. (2013). Effect of thermal and non-thermal pasteurisation on the microbial inactivation and phenolic degradation in fruit juice: A mini-review. Journal of the Science of Food and Agriculture, 93(5), 981–986.
Cortés, C., Esteve, M. J., & Frígola, A. (2008). Color of orange juice treated by high intensity pulsed electric fields during refrigerated storage and comparison with pasteurized juice. Food Control, 19(2), 151–158.
Cserhalmi, Z., Sass-Kiss, Á., Tóth-Markus, M., & Lechner, N. (2006). Study of pulsed electric field treated citrus juices. Innovative Food Science & Emerging Technologies, 7(1–2), 49–54.
Elez-Martínez, P., Aguiló-Aguayo, I., & Martín-Belloso, O. (2006). Inactivation of orange juice peroxidase by high-intensity pulsed electric fields as influenced by process parameters. Journal of the Science of Food and Agriculture, 86(1), 71–81.
Elez-Martínez, P., Soliva-Fortuny, R. C., & Martín-Belloso, O. (2006). Comparative study on shelf life of orange juice processed by high intensity pulsed electric fields or heat treatment. European Food Research and Technology, 222(3–4), 321–329.
Espachs-Barroso, A., Van Loey, A., Hendrickx, M., & Martín-Belloso, O. (2006). Inactivation of plant pectin methylesterase by thermal or high intensity pulsed electric field treatments. Innovative Food Science & Emerging Technologies, 7(1–2), 40–48.
Falade, K. O., Babalola, S. O., Akinyemi, S. O. S., & Ogunlade, A. A. (2004). Degradation of quality attributes of sweetened Julie and Ogbomoso mango juices during storage. European Food Research and Technology, 218(5), 456–459.
FAO. (2003). Tropical fruits.
FAO. (2012). FAOSTAT.
Food and Drug Administration. (2004). Guidance for industry: Juice HACCP hazards and controls guidance first edition. http://www.fda.gov/Food/%0AGuidanceComplianceRegulatoryInformation/GuidanceDocuments/Juice/%0Aucm072557.htm#ftn1
Huang, K., Tian, H., Gai, L., & Wang, J. (2012). A review of kinetic models for inactivating microorganisms and enzymes by pulsed electric field processing. Journal of Food Engineering, 111(2), 191–207.
Hunter, R. S. (1987). The measurement of appearance (Vol. 5). New York: Wiley.
Jiménez-Sánchez, C., Lozano-Sánchez, J., Segura-Carretero, A., & Fernández-Gutiérrez, A. (2017). Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Critical Reviews in Food Science and Nutrition, 57(3), 501–523.
Leong, S. Y., & Oey, I. (2014). Effect of pulsed electric field treatment on enzyme kinetics and thermostability of endogenous ascorbic acid oxidase in carrots (Daucus carota cv. nantes). Food Chemistry, 146, 538–547.
Luo, W., Zhang, R. B., Wang, L. M., Chen, J., & Guan, Z. C. (2010). Conformation changes of polyphenol oxidase and lipoxygenase induced by PEF treatment. Journal of Applied Electrochemistry, 40(2), 295–301.
Mercadante, A. Z., & Rodriguez-Amaya, D. B. (1998). Effects of ripening, cultivar differences, and processing on the carotenoid composition of mango. Journal of Agricultural and Food Chemistry, 46(1), 128–130.
Morales-de la Peña, M., Salvia-Trujillo, L., Rojas-Graü, M. A., & Martín-Belloso, O. (2010). Impact of high intensity pulsed electric field on antioxidant properties and quality parameters of a fruit juice-soymilk beverage in chilled storage. LWT-Food Science and Technology, 43(6), 872–881.
Mosqueda-Melgar, J., Raybaudi-Massilia, R. M., & Martín-Belloso, O. (2012). Microbiological shelf life and sensory evaluation of fruit juices treated by high-intensity pulsed electric fields and antimicrobials. Food and Bioproducts Processing, 90(2), 205–214.
Mosqueda-Melgar, J., Raybaudi-Massilia, R., & Martín-Belloso, O. (2007). Influence of treatment time and pulse frequency on salmonella Enteritidis, Escherichia coli and Listeria monocytogenes populations inoculated in melon and watermelon juices treated by pulsed electric fields. International Journal of Food Microbiology, 117(2), 192–200.
Nanjundaswamy, A. M. (1998). In R. E. Litz (Ed.), The mango botany, production and uses (509–544). Wallingford, UK: CAB International.
Odriozola-Serrano, I., Soliva-Fortuny, R., Hernández-Jover, T., & Martín-Belloso, O. (2009). Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chemistry, 112(1), 258–266.
Odriozola-Serrano, I., Soliva-Fortuny, R., & Martín-Belloso, O. (2008). Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innovative Food Science & Emerging Technologies, 9(3), 272–279.
Pathare, P. B., Opara, U. L., & Al-Said, F. A.-J. (2012). Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology, 6(1), 36–60.
Patthamakanokporn, O., Puwastien, P., Nitithamyong, A., & Sirichakwal, P. P. (2008). Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. Journal of Food Composition and Analysis, 21(3), 241–248.
Quitão-Teixeira, L. J., Aguiló-Aguayo, I., Ramos, A. M., & Martín-Belloso, O. (2007). Inactivation of oxidative enzymes by high-intensity pulsed electric field for retention of color in carrot juice. Food and Bioprocess Technology, 1(4), 364–373.
Rawson, A., Patras, A., Tiwari, B. K., Noci, F., Koutchma, T., & Brunton, N. (2011). Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Research International, 44(7), 1875–1887.
Robles-Sánchez, R. M., Rojas-Graü, M. A., Odriozola-Serrano, I., González-Aguilar, G. A., & Martín-Belloso, O. (2009). Effect of minimal processing on bioactive compounds and antioxidant activity of fresh-cut “Kent” mango (Mangifera indica L.) Postharvest Biology and Technology, 51(3), 384–390.
Salvia-Trujillo, L., Morales-de la Peña, M., Rojas-Graü, M. A., & Martín-Belloso, O. (2011). Microbial and enzymatic stability of fruit juice-milk beverages treated by high intensity pulsed electric fields or heat during refrigerated storage. Food Control, 22(10), 1639–1646.
Sánchez-Moreno, C., Plaza, L., Elez-Martínez, P., De Ancos, B., Martín-Belloso, O., & Cano, M. P. (2005). Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. Journal of Agricultural and Food Chemistry, 53(11), 4403–4409.
Santhirasegaram, V., Razali, Z., George, D. S., & Somasundram, C. (2015). Effects of thermal and non-thermal processing on phenolic compounds, antioxidant activity and sensory attributes of Chokanan mango (Mangifera indica L.) juice. Food and Bioprocess Technology. doi:10.1007/s11947-015-1576-y.
Schieber, A., Ullrich, W., & Carle, R. (2000). Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innovative Food Science and Emerging Technologies, 1(2), 161–166.
Sharma, S., Singh, A. K., Kaushik, S., Sinha, M., Singh, R. P., Sharma, P., et al. (2013). Lactoperoxidase: Structural insights into the function, ligand binding and inhibition. International Journal of Biochemistry Molecular Biology, 4(3), 108–128.
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1998). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology.
Soliva-Fortuny, R., Balasa, A., Knorr, D., & Martín-Belloso, O. (2009). Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends in Food Science and Technology, 20(11–12), 544–556.
Timmermans, R. A. H., Mastwijk, H. C., Knol, J. J., Quataert, M. C. J., Vervoort, L., Van der Plancken, I., et al. (2011). Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice. Part I: Impact on overall quality attributes. Innovative Food Science & Emerging Technologies, 12(3), 235–243.
Timmermans, R. A. H., Nierop Groot, M. N., Nederhoff, A. L., van Boekel, M. A. J. S., Matser, A. M., & Mastwijk, H. C. (2014). Pulsed electric field processing of different fruit juices: Impact of pH and temperature on inactivation of spoilage and pathogenic micro-organisms. International Journal of Food of Microbiology, 173, 105–111.
Vallverdú-Queralt, A., Oms-Oliu, G., Odriozola-Serrano, I., Lamuela-Raventos, R. M., Martín-Belloso, O., & Elez-Martínez, P. (2012). Effects of pulsed electric fields on the bioactive compound content and antioxidant capacity of tomato fruit. Journal of Agricultural and Food Chemistry, 60(12), 3126–3134.
Vásquez-Caicedo, A. L., Schilling, S., Carle, R., & Neidhart, S. (2007). Effects of thermal processing and fruit matrix on β-carotene stability and enzyme inactivation during transformation of mangoes into purée and nectar. Food Chemistry, 102(4), 1172–1186.
Vega-Mercado, H., Martín-Belloso, O., Qin, B.-L., Chang, F. J., Marcela Góngora-Nieto, M., Barbosa-Cánovas, G. V., & Swanson, B. G. (1997). Non-thermal food preservation: Pulsed electric fields. Trends in Food Science & Technology, 8(5), 151–157.
Vervoort, L., Van der Plancken, I., Grauwet, T., Timmermans, R. A. H., Mastwijk, H. C., Matser, A. M., et al. (2011). Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice: Part II: Impact on specific chemical and biochemical quality parameters. Innovative Food Science & Emerging Technologies, 12(4), 466–477.
Wibowo, S., Grauwet, T., Gedefa, G. B., Hendrickx, M., & Van Loey, A. (2015). Quality changes of pasteurised mango juice during storage. Part I: Selecting shelf-life markers by integration of a targeted and untargeted multivariate approach. Food Research International, 78, 396–409.
Wouters, P. C., Dutreux, N., Smelt, J. P. P. M., & Lelieveld, H. L. M. (1999). Effects of pulsed electric fields on inactivation kinetics of Listeria innocua. Applied and Environmental Microbiology, 65(12), 5364–5371.
Zhang, Y., Gao, B., Zhang, M., Shi, J., & Xu, Y. (2010). Pulsed electric field processing effects on physicochemical properties, flavor compounds and microorganisms of longan juice. Journal of Food Processing and Preservation, 34(6), 1121–1138.
Zhao, W., Yang, R., Lu, R., Tang, Y., & Zhang, W. (2007). Investigation of the mechanisms of pulsed electric fields on inactivation of enzyme: Lysozyme. Journal of Agricultural and Food Chemistry, 55(24), 9850–9858.
Acknowledgements
This work was supported by the University of Lleida (Spain) and financed by Tecnológico de Monterrey, Mexico (Research Chair Funds CAT-200 and CDB081).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salinas-Roca, B., Elez-Martínez, P., Welti-Chanes, J. et al. Quality Changes in Mango Juice Treated by High-Intensity Pulsed Electric Fields Throughout the Storage. Food Bioprocess Technol 10, 1970–1983 (2017). https://doi.org/10.1007/s11947-017-1969-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1969-1