Abstract
Nowadays, there is great attention to extract natural colorants and food additives from the by-products of food processing industries. In this research, the β-carotene extraction from carrot pomace was investigated using electrohydrodynamic (EHD) pretreatment and ultrasonic process as an innovative extraction technique. To achieve this purpose, the statistical method of Complete Randomized Design (CRD) was used to estimate the effects of EHD time (at six levels of 2.5, 5, 10, 15, 20 and 25 min) and EHD voltage (at five levels of 0, 14, 16, 18, 20 and 22 kV) on the dependent factors of β-carotene content, antioxidant activity, total phenolic content, and extraction yield of the carrot pomace extracts. The ultrasonic extraction was performed at similar procedure with a temperature of 30 °C, power of 500 W, frequency of 20 kHz, and time of 80 min. It was found that the highest values of β-carotene concentration (415.28 ± 0.56 mg/L; P ≤ 0.01), antioxidant activity (90.42 ± 0.48%; P ≤ 0.001), total phenolic content (151.48 ± 76 mg/L; P ≤ 0.001), and extraction yield (9.15 ± 0.25%; P > 0.05) were achieved at EHD time of 20 min. Moreover, the highest recovery of the β-carotene concentration (433.42 ± 1.86 mg/L; P ≤ 0.001), antioxidant activity (92.35 ± 0.52%; P ≤ 0.001), total phenolic content (155.42 ± 7.73 mg/L; P ≤ 0.001), and extraction yield (9.70 ± 0.10%; P ≤ 0.01) of the extracts were obtained at EHD voltage of 20 kV. Fourier transform infrared (FT-IR) spectroscopy displayed no degradation of functional groups of the carrot pomace extracts during the EHD pretreatment. Therefore, the application of EHD pretreatment method leads to higher levels of β-carotene content, antioxidant activity, total phenolic content, and extraction yield from the carrot pomace extracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food colorants are additives, which are used in foods, to improve their attractiveness and marketability or to repair the food color decrease during their preparation and storage [1]. Carotenoids are classified as one of the most essential groups of natural food colorants, which are extensively consumed in the food industry. These colorants have different biological functions because of their high antioxidant properties. Among carotenoids, β-carotene (yellow-red pigment) is one of the most plentiful carotenoids [2]. In the bodies of humans and various animals, this pigment is converted into vitamin A [3, 4]. Furthermore, the β-carotene pigment has some valuable effects on human health such as the prevention of cataract degeneration and cardiovascular diseases, and the reduction of the risk of cancer [2]. Nowadays, the new researches are shifted toward the extraction of carotenoids from various natural resources especially from agricultural by-products which provide considerable economic benefits for the food industry [3].
Carrot is a good source of β-carotene. Therefore, the extraction of β-carotene from the carrot processing waste is promising for a huge economic development in the food industry. Numerous methods such as solvent extraction [5], Soxhlet [6], shaking incubation [3], and microwave [7] have been used to extract β-carotene from diverse agricultural resources. However, the mentioned techniques are time and solvent consuming. Furthermore, their extraction temperature is high that it may lead to β-carotene degradation. In recent years, some new extraction methods such as ultrasound [3,4,5,6,7,8, 10] and enzymatic extraction [2] have been used to extract β-carotene from diverse sources.
A really innovative extraction technique is electrohydrodynamic (EHD) or high voltage electrical discharge (HVED) which has been applied to extract bioactive compounds from some plant sources [8,9,10,11, 13]. The key mechanism of the EHD extraction process is the production of corona wind by applying a high voltage between one or more discharged electrodes (in different shapes of wire, needle, or pin) and a grounded electrode [8, 14]. The air around the surface of the discharge electrode(s) is ionized. The ions are moved toward the grounded electrode and thus, it creates the corona wind [15]. The effect of corona wind in liquids is based on the electrical breakdown which can result in damage to cell structure and increasing the extraction of intracellular components to the solvent [8, 16]. The EHD extraction method is an environmentally friendly procedure, which has numerous advantages such as having a simple equipment system, low solvent consumption, low operation temperature, low acoustic noise, and being lightweight [8, 15].
In this study, the EHD pretreatment with ultrasonic process was recommended to develop the extraction of β-carotene. The ultrasonic waves have frequencies more than the human hearing threshold (more than 16 kHz). In liquid systems, the ultrasound effects are principally related to the cavitation phenomenon. During the ultrasonic process, cavitation bubbles grow and finally unstable bubbles collapse. It produces very high shear forces resulting in breaking the matrix cells and extraction of the cell compounds to the solvent [17].
In this study, for the first time, a multiple-wires-to-plate EHD system has been employed before ultrasonic extraction of β-carotene from carrot pomace powder. The statistical method of Complete Randomized Design (CRD) was used to estimate the effects of EHD time (at six levels of 2.5, 5, 10, 15, 20 and 25 min) and EHD voltage (at five levels of 0, 14, 16, 18, 20 and 22 kV) on the dependent factors of β-carotene content, antioxidant activity, total phenolic content, and extraction yield of the carrot pomace extracts. In summary, the goals of this literature were as follows: (i) examination of the effects of EHD extraction time and EHD extraction voltage on the dependent factors, (ii) investigation of the probable morphological changes of carrot pomace powder during the selected extraction treatments by the scanning electron microscope, and (iii) determination of probable changes of the functional groups of the extracts during the selected EHD extraction treatments by a Fourier Transform Infrared Spectroscopy. The hypothesis of this study is that the EHD pretreatment application could improve the extraction of β-carotene from carrot pomace powder. However, application of long EHD process and very high level of EHD voltage may reduce the concentration of the β-carotene and the antioxidant activity of the carrot pomace extracts.
Materials and methods
Chemicals and reagents
Ethanol with a purity of 96% was bought from Sina Fariman Company (Khorasan Razavi, Iran). β-Carotene standard with a purity of 97% and Folin–Ciocalteu reagent was purchased from Merck Company (Darmstadt, Germany). Moreover, 1,1-diphenyl-2-picrylhydrazil (DPPH radical) was prepared from Sigma Chemical Company (St. Louis, MO, U.S.A).
Sample preparation
The fresh carrots were bought from a farm located in Najaf Abad, Isfahan, Iran. At first, the carrots were washed with urban water to remove the dirt on their surface. Next, the juice extraction was done by a domestic juicer (Model GM 202X.2, General, Japan). Then, carrot pomace was dried by a hot-air convective dryer (Made in Isfahan, Iran) at a wind velocity of 1.5 m/s and a temperature of 45 °C to achieve constant weight. The dried carrot pomace was grinded with a household mill (Model GM 202X.2, General, Japan) and was passed through a 35-mesh sieve. Finally, low-density polyethylene bags were used for packing the fine powder. The samples were stored in a freezer (Model REFST170, Pars, Iran) at a temperature of − 18 °C before performing the extraction treatments.
EHD set-up and EHD pretreatment process
The EHD set-up, used in this research, is shown in Fig. 1. In this set-up, the positive voltage from 0 to 60 kV is provided by a DC high-voltage power supply (Model HV50POC, Fanavaran Nano-Meghyas, Tehran, Iran). The EHD set-up also has a Teflon frame (30 cm × 25 cm), a stainless-steel grounded plate (19 cm × 23 cm), and 5 discharge wire electrodes with the external diameter of 1.2 mm and length of 19.2 cm. The distance between the two neighboring wires was 4 cm and the electrode gap between the grounded plate and the discharge electrodes was 5 cm.
The dried carrot pomace powder (10 g) was mixed with ethanol solvent (100 mL) in an aluminum plate with the dimensions of 16 cm × 16 cm × 2 cm to start the EHD pretreatment. Then, the aluminum plate containing sample and solvent was placed on the grounded electrode of the EHD system. The EHD pretreatment process was applied at various periods of 2, 5, 10, 20, and 25 min and various voltages of 14, 16, 18, 20 and 22 kV and the statistical method of Complete Randomized Design (CRD) was applied to study the effects of these variables on the investigated responses. After EHD pretreatment, the mixture was transferred into a 100 mL glass beaker to be submitted to the ultrasonic extraction process.
Ultrasound extraction process
To perform ultrasound extraction process, the mixture of sample and solvent in the glass beaker was processed by an ultrasonic probe (Model UIP1000hd, Germany) at the temperature of 30 °C, ultrasonic power of 500 W, and ultrasonic frequency of 20 kHz for 80 min. Finally, the extract was filtered by a Whatman paper (No. 40, England) and the following tests were immediately performed on the extract [2].
Physicochemical analysis
Quantification of β-carotene content
The absorptions of the extracts were measured by a UV–Vis spectrophotometer (Model T70, PG, USA) at 450 nm using ethanol as the blank to determine the β-carotene content. The calibration curve of the β-carotene standard at concentrations from 40 to 360 mg/L was prepared to obtain the Eq. 1. Finally, the absorption of each extract was replaced instead of Asample in Eq. 1 to estimate the amount of β-carotene content in mg/L [3]:
Determination of antioxidant activity
The antioxidant activity of the extracts was measured using 1,1-diphenyl-2-picrylhydrazil (DPPH) radical scavenging procedure described by Shahram and Taghian Dinani (2019b) [8]. Briefly, 1 ml of DPPH solution with the concentration of 200 mg/L was mixed with 1 ml of the carrot pomace extract in a test tube. The tube was manually shaken and then it was incubated for 20 min in a dark place at room temperature. Finally, the absorbance value of the mixture of DPPH solution with extract (Asample) was measured using the UV–Vis spectrophotometer at 517 nm. The antioxidant activity of extract based on DPPH scavenging activity (%) was evaluated according to Eq. 2:
where Acontrol was the absorbance of pure DPPH solution (200 mg/L).
Determination of total phenolic content
The Folin–Ciocalteu method described by shahram et al. (2018) was used to measure the total phenolic content of the extracts [18]. For this test, 125 µL extract, 500 µL of distilled water, and 125 µL of Folin–Ciocalteu reagent were mixed in a test tube, and put for 6 min at room temperature. Next, 1.25 mL of sodium carbonate solution (7% (w/v)) and 1 mL distilled water were added to the contents in the test tube and they were mixed thoroughly. The mixture was incubated in a dark place at room temperature for 90 min. After completion of reaction time, the mixture absorbance was measured at 760 nm by the UV–Vis spectrophotometer. The amount of total phenolic content of extracts was examined based on the standard calibration curve of gallic acid solutions at concentrations of 5, 20, 40, 60, 80, 100, 120 and 140 mg/L. By plotting the absorbance of gallic acid solutions versus different concentrations of gallic acid solutions (mg/L), the following formula was prepared:
where Atest was the absorbance of the extracts.
Determination of extraction yield of the carrot extracts
After the extraction process, the solvent of the carrot extract was separated by a rotary vacuum evaporator (Model RV 10B, IKA, Germany) with a rotational speed of 170 rpm at 70 °C. The extraction yield of the extracts was calculated using the following formula:
Fourier transform infrared (FT-IR) spectroscopy
The FT-IR spectra of carrot pomace extracts from selected treatments were obtained according to the procedure described by Shahram and Taghian Dinani (2018) [2]. For this test, after preparation of a potassium bromide (KBr) disk, 1 drop of the extract was poured on the KBr disk by a capillary tube and it was placed in the FT-IR spectrophotometer (Model Spectrum 65, Norwalk, Connecticut, PerkinElmer, U.S.A.). The FT-IR spectra were obtained in the region of 4000–450 cm−1.
Scanning electron microscopy (SEM) images
SEM images were performed to examine the influence of EHD pretreatment time and voltage on the microscopic structure of carrot waste powder. For this purpose, the remained solids gathered after selected EHD-ultrasonic treatments were dried at room temperature. Then, a thin layer of gold was put on the surface of dried solids using a gold sputter coating machine (Ziess, German). Finally, microscopic images were done by a Scanning Electron Microscope (Ziess, German) with a voltage of 10 kV [2].
Statistical design and data analysis
In this research, a Complete Randomized Design (CRD) was applied to study the effects of the EHD pretreatment time (at four levels of 2, 10, 20 and 25 min) and EHD pretreatment voltage (at five levels of 14, 16, 18, 20 and 22 kV) on the investigated responses. The Duncan new multiple range test by SPSS software (version 19) at a confidence level of 95% (P ≤ 0.05) was used to determine differences between the experimental mean values. Using the Pvalue, it is possible to find if the response is significantly influenced by independent factors or not. When the P value of an independent factor for a response is more than 0.05 (P ˃ 0.05), less or equal to 0.05 (P ≤ 0.05), less or equal to 0.01 and more than 0.001 (P ≤ 0.01), less or equal to 0.001 (P ≤ 0.001), the Fvalue of this independent factor for this response is insignificant, significant, very significant, and extremely significant, respectively. The reported results of this research were presented as mean ± standard error (SE) for at least two replications.
Results and discussion
Effect of EHD pretreatment time
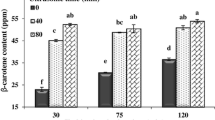
The influence of EHD pretreatment time on the β-carotene content of carrot waste extracts is represented in Fig. 2a. According to the results, the highest (415.28 ± 0.56 mg/L) and the lowest (413.22 ± 0.42 mg/L) contents of β-carotene pigment were achieved at EHD time of 20 min and 25 min, respectively. Figure 2a depicts that the influence of EHD time on the β-carotene content of the extracts was insignificant (P ≤ 0.01). Applying the EHD process results in the generation of turbulence in the solvent, disruption of the cell membrane, and eventually releasing the β-carotene pigment from the sample cells to the extraction solvent [8]. However, the reduction of β-carotene content extraction with increasing EHD time from 20 to 25 min may be due to the saturation of the extraction solvent at longer extraction time [10]. In addition, it can be attributed to the degradation of extracted β-carotene pigments exposed to corona wind during a very long EHD extraction.
Effect of EHD ptretreatment extraction time on the β-carotene concentration, antioxidant activity, total phenolic content, and extraction yield of carrot pomace powder extracts. Data are shown as mean ± SE. Difference of the corresponding alphabets show statistical significance of each treatment (P ≤ 0.05)
It can be seen in Fig. 2b that the effect of EHD pretreatment time on antioxidant activity of carrot waste extracts is significant (P ≤ 0.001). According to this figure, the lowest and the highest antioxidant activity of the extracts (60.41 ± 1.93% and 90.42 ± 0.48%, respectively) were obtained at EHD time of 2.5 min and 20 min, respectively. This is probably due to the fact that EHD pretreatment had a negligible effect on the extraction yield of antioxidant compounds at the beginning of the process. However, increasing the EHD process time leads to further destruction of the cell walls and more release of antioxidant compounds to the solvent.
Figure 2c illustrates the variations of total phenolic content under various EHD extraction time. This figure shows that with an increase in the EHD time from 2.5 min to 25 min, the total phenolic contents improved from 92.70 ± 1.36 mg/L to 150.58 ± 2.28 mg/L, respectively (P ≤ 0.001). The application of EHD process for a long time results in more destruction of the sample cell walls and improving the mass transfer of phenolic compounds from sample to the solvent [8]. This result is in agreement with that reported by Rajha et al. (2014), who indicated that the extraction of polyphenols was increased with the time increase of high-voltage electrical discharges from 0 to 180 min [19].
Figure 2d depicts that the effect of EHD time on the extraction yield of the extracts was not statistically significant (P > 0.05). With respect to Fig. 2d, the lowest and the highest extraction yields of extracts (7.90 ± 0.30% and 9.15 ± 0.25%, respectively) were obtained at EHD time of 2.5 min and 20 min, respectively. In good agreement with this result, Shahram and Taghian Dinani (2019b) reported that the increase of EHD time from 2 to 30 min led to the intensification of the extraction yield of orange pomace powder. These researchers stated that this phenomenon could be because of the corona wind which creates shock waves in the solvent, destructs the cell walls of the sample and, thus, increases the mass transfer rate of cell compounds to the solvent [8].
Consequently, the optimum EHD extraction time was found to be 20 min because the results indicated that the maximum β-carotene concentration, total phenolic content, extraction yield, and antioxidant activity of the carrot pomace extracts were obtained at EHD pretreatment time of 20 min. Therefore, the EHD time of 20 min was used in the next steps for optimization of other experimental parameters such as EHD time.
Effect of EHD pretreatment time on FT-TR spectra of the extracts
The FT-IR spectra of two extraction treatments with EHD pretreatment times of 2.5 min and 20 min at similar conditions (solid to solvent ratio of 1:10 (w/v), EHD pretreatment voltage of 18 kV, electrode gap of 5 cm, the ultrasonic temperature of 30 °C, ultrasonic frequency of 20 kHz, ultrasonic time of 80 min, and ultrasonic power of 500 W) were presented in Fig. 3. In this figure, the small peak at 3820.86 cm−1 signifies the free O–H stretch [2] and the extensive peak of 3377.13 cm−1 displays the O–H stretch [18]. The peak at 2974.10 cm−1 illuminates C–H asymmetric stretch of –CH3 [3] and the peak located at 2900.46 cm−1 indicates CH2 antisymmetric stretch of methyl groups [18]. The peak of 1669.71 cm−1 is associated with the C=C aromatic stretching hydroxyl group [20] and the peak of 1398.44 cm−1 can be attributed to the symmetric stretching C–H group [18]. The absorption peak of 1057.14 cm−1 shows CH3 stretching [21]. The absorption frequency at 882.44 cm−1 illustrates R–CH=CH–R group [21] and the peak of 662.28 cm−1 represents the CH=CH stretching vibration [22]. According to Fig. 3, the comparison of two spectra shows no changes in the type of functional groups of the samples. It shows that application of EHD pretreatment times for 2.5 min and 20 min for β-carotene extraction did not result in the destruction of functional groups of the extracts.
The FTIR spectra of carrot pomace powder extracts from two treatments of EHD time of 2.5 min and EHD time of 20 min at similar extraction conditions (solid to solvent ratio of 1:10 (w/v), EHD pretreatment voltage of 18 kV, electrode gap of 5 cm, ultrasonic time of 80 min, and ultrasonic power of 500 W)
Effect of EHD pretreatment voltage
In this step, the experiments for the investigation of the EHD pretreatment voltage were done at optimal EHD pretreatment time of 20 min. Figure 4a confirms that the EHD voltage had a significant influence on the β-carotene content of the extracts (P ≤ 0.001). It can be observed in this figure that the highest recovery of the β-carotene pigment (433.42 ± 1.86 mg/L) was obtained at the EHD voltage of 20 kV. It is necessary to mention that there was not a significant difference between the two treatments of EHD voltages of 20 kV and 18 kV in terms of the extracted β-carotene content (P > 0.05). The lowest extraction of β-carotene content (340.86 ± 6.28 mg/L) was obtained at EHD voltage of 22 kV (Fig. 4a). As can be seen in Fig. 4a, the EHD voltage of 22 kV treatment had significant difference with all other EHD voltages in terms of the β-carotene content (P ≤ 0.001). Generally, the results obtained in this part of the study indicate that increasing the EHD voltage from 0 to 20 kV increased the β-carotene content of the extracts, and increasing the EHD voltage from 20 to 22 kV decreased this response. This phenomenon can be related to further destruction of the sample cell walls and the increase of the β-carotene pigment release from the carrot pomace to the extraction solvent with increasing the EHD voltage from 0 to 20 kV. However, the decline in β-carotene with further increase in the voltage of the EHD process from 20 to 22 kV can be due to the saturation of solvent with the extracted compounds [10] and the negative effect of severe corona wind produced at a very high level of voltage (22 kV) on the extracted β-carotene. For comparison of the new extraction method of the EHD pretreatment-ultrasonic process with other methods, we can refer to the paper of Mirleli and Taghian Dinani (2018). They studied the extraction of β-carotene pigment from carrot processing waste using the ultrasonic-shaking incubation method. The maximum β-carotene content (53.88 ± 2.61 mg/mL) was obtained at 80 min ultrasonic-120 min shaking incubation treatment [3] which is considerably lower than the extracted β-carotene content in our study. In addition, Purohita and Gogatea (2015) studied the ultrasound-assisted extraction of β-carotene from carrot residue [10]. The mixture of 1% (w/v) of carrot residue powder in ethanol was subjected to ultrasound treatment for 10, 20, 30, 40, 50, and 60 min. Operating conditions during their study were the temperature of 30 °C, duty cycle of 50%, and power dissipation of 100 W. At extraction time of 50 min, maximum extraction yield of β-carotene (74.94%) was obtained. The higher extracted β-carotene content in their study can be due to the different ultrasonic probe and carrot variety.
It is evident from the results in Fig. 4b that the highest antioxidant activity of the extracts (equivalent to 92.35 ± 0.52%) was achieved at EHD voltage of 20 kV and the lowest antioxidant activity of the extracts (equivalent to 72.06 ± 3.87%) was obtained at EHD voltage of 0 kV (extraction without the EHD process application). In other words, the average of antioxidant activity increased significantly (P ≤ 0.001) when the EHD voltage was increased from 0 to 18 kV. However, with further increase in EHD voltage from 20 to 22 kV, the antioxidant activity of extracts declined insignificantly (P > 0.05). In agreement with this result, Shahram and Taghian Dinani (2019) stated that the antioxidant activity of orange pomace extracts improved by increasing EHD voltage from 0 to 18 kV, while it was reduced with the further increase in EHD voltage from 18 to 22 kV [8].
Figure 4c shows the effect of EHD voltage on the total phenolic content of the carrot pomace extracts. As can be seen in this figure, the highest and the lowest recovery of total phenolic compounds (155.42 ± 7.73 mg/L and 79.97 ± 3.44 mg/L, respectively) were achieved at EHD voltages of 20 kV and 14 kV, respectively. There is no statistically significant difference between EHD voltages of 0, 14, and 16 kV in terms of the total phenolic content of the carrot pomace extracts. Furthermore, EHD voltages of 18, 20 and 22 kV do not show a significant difference in terms of the total phenolic content of the carrot pomace extracts (Fig. 4c). The increase of EHD voltage from 0 to 22 kV can lead to severe destruction of the cell walls and, thus, the proliferation of the mass transfer of phenolic compounds from the cells to the solvent [13].
According to Fig. 4d, by increasing the EHD voltage from 0 to 20 kV, the extraction yield of the extracts was significantly increased from 6.10 ± 0.70 to 9.70 ± 0.10% (P ≤ 0.01). However, the average of extraction yield was insignificantly reduced from 9.70 ± 0.10 to 9.30 ± 0.30% by increasing EHD voltage from 20 to 22 kV. Shahram and Taghian Dinani (2019b) stated that, the corona wind results in the generation of shock waves and destroying the cell walls of the sample. Therefore, it leads to the extraction increase of compounds from the damaged cells to the extraction solvent [8]. Generally, the highest recoveries of the β-carotene pigment, antioxidant activity, total phenolic content, and extraction yield of the extracts were obtained at EHD voltage of 20 kV. However, there was insignificant difference between the two treatments of EHD voltages of 20 kV and 18 kV. Therefore, EHD voltage of 18 kV was selected as the optimum EHD voltage in this step.
Effect of EHD pretreatment time on FT-TR spectra of the extracts
The FT-IR spectra of extracts obtained at two EHD pretreatment voltages of 0 kV and 18 kV at similar conditions (solid to solvent ratio of 1:10 (w/v), EHD pretreatment time of 20 min, electrode gap of 5 cm, ultrasonic time of 80 min, and ultrasonic power of 500 W) are illustrated in Fig. 5. In this figure, the small peak at 3838.83 cm−1 indicates the free O–H stretch [2] and the wide peak of 3373.36 cm−1 confirms the O–H stretch [18]. The peaks at 2973.56 cm−1 and 2899.25 cm−1 show CH2 antisymmetric stretch of methyl groups [18]. The peak of 1658.00 cm−1 can be ascribed to C=C aromatic stretching hydroxyl group [20]. The absorption peak of 1056.05 cm−1 represents C–OH stretching band [23]. The absorption frequency at 881.17 cm−1 can be attributed to R–CH=CH–R [21] and the peak of 670.80 cm−1 represents the CH=CH stretching vibration [22]. As can be seen in Fig. 5, the use of EHD pretreatment at a voltage of 18 kV in comparison with omitting this pretreatment did not result in any changes in the types of functional groups of the extracts.
The FTIR spectra of carrot pomace powder extracts from two treatments of EHD voltage of 0 kV and EHD voltage of 18 kV at the similar extraction conditions (solid to solvent ratio of 1:10 (w/v), EHD pretreatment time of 20 min, electrode gap of 5 cm, ultrasonic time of 80 min, and ultrasonic power of 500 W)
Scanning electron microscopy (SEM) images
Figure 6a–c shows the SEM images of (a) the untreated carrot pomace powder, (b) the dried residue of carrot pomace powder after extraction at EHD voltage of 0 kV, and (c) the dried residue of carrot pomace powder after pretreatment at EHD voltage of 18 kV (at similar conditions of solid to solvent ratio of 1:10 (w/v), EHD pretreatment time of 20 min, electrode gap of 5 cm, ultrasonic time of 80 min, and ultrasonic power of 500 W). Based on Fig. 6a, the untreated sample had a smooth surface compared to the two other treatments. Compression of the dried residue of carrot pomace powder after pretreatment at the EHD voltage of 18 kV (Fig. 6c) with the dried residue of carrot pomace powder after pretreatment at the EHD voltage of 0 kV (Fig. 6b) shows that the use of the EHD pretreatment increases the porosity of the cell walls of carrot pomace powder. Shahram and Taghian Dinani (2019) reported that applying the EHD process resulted in more cracks and porosities in the cell walls of the orange pomace powder [8]. Moreover, Maher et al. (2019) stated that the EHD process caused considerable damage in the cell structure of lemon processing waste powder [24]. The results from these researches are in good agreement with the present study.
Conclusion
In this research, the influence of the EHD process before the ultrasonic process for β-carotene extraction from carrot pomace powder was investigated. For this aim, the effects of EHD time and voltage were investigated on the β-carotene content, antioxidant activity, total phenolic content, and extraction yield. The results showed that increasing the EHD time from 2.5 to 20 min increased the β-carotene concentration (P ≤ 0.01) and total phenolic content (P ≤ 0.001). Moreover, the β-carotene concentration (P ≤ 0.001), antioxidant activity (P ≤ 0.001), and extraction yield (P ≤ 0.01) of the extracts were increased initially with increasing the EHD voltage from 0 to 20 kV. However, these responses were decreased with further increase in EHD voltage from 20 to 22 kV. SEM images showed that applying the EHD process led to more degradation of the microscopic structure of the residues, which could increase the recovery of the β-carotene of carrot pomace powder. Therefore, using EHD pretreatment for extraction of bioactive compounds from by-products of food factories can be suggested as a new extraction method with economic benefits. In the next paper, the optimization of the ultrasonic time and ultrasonic power will be studied at these optimized parameters of the EHD pretreatment process.
References
N. Martins, C.L. Roriz, P. Morales, L. Barros, I.C.F.R. Ferreira, Trends Food Sci. Technol. 52, 1–15 (2016)
H. Shahram, S.T. Dinani, S. Taghian, J. Food Process Eng. 42, e13042 (2019)
M. Mirheli, S. TaghianDinani, J. Food Meas. Charact. 12, 1818–1828 (2018)
J. Azmir, I.S.M. Zaidul, M.M. Rahman, K.M. Sharif, A. Mohamed, F. Sahena, M.H.A. Jahurul, K. Ghafoor, N.A.N. Norulaini, A.K.M. Omar, J. Food Eng. 117, 426–436 (2013)
C.C. Teow, V. Den Truong, R.F. McFeeters, R.L. Thompson, K.V. Pecota, G.C. Yencho, Food Chem. 103, 829–838 (2007)
L. Barros, M.J. Ferreira, B. Queirós, I.C.F.R. Ferreira, P. Baptista, Food Chem. 103, 413–419 (2007)
N. Chumnanpaisont, C. Niamnuy, S. Devahastin, Chem. Eng. Sci. 116, 442–451 (2014)
H. Shahram, S.T. Dinani, LWT 111, 23–30 (2019)
Y. Sun, G. Ma, X. Ye, Y. Kakuda, R. Meng, Ultrason. Sonochem. 17, 654–661 (2010)
A.J. Purohit, P.R. Gogate, Sep. Sci. Technol. 50, 1507–1517 (2015)
L.G. Yan, Y. Deng, T. Ju, K. Wu, J. Xi, Food Chem. 256, 350–357 (2018)
S. El Kantar, N. Boussetta, H.N. Rajha, R.G. Maroun, N. Louka, E. Vorobiev, Food Res. Int. 107, 755–762 (2018)
J. Xi, L. He, L. Gong Yan, Food Chem. 230, 354–361 (2017)
S. Taghian Dinani, N. Hamdami, M. Shahedi, M. Havet, D. Queveau, Food Bioprod. Process. 95, 83–95 (2015)
S. Taghian Dinani, M. Havet, J. Clean. Prod. 95, 203–211 (2015)
J.R. Sarkis, N. Boussetta, C. Blouet, I.C. Tessaro, L.D.F. Marczak, E. Vorobiev, Innov. Food Sci. Emerg. Technol. 29, 170–177 (2015)
M. Germec, K. Tarhan, E. Yatmaz, N. Tetik, M. Karhan, A. Demirci, I. Turhan, Biotechnol. Prog. 32, 393–403 (2016)
H. Shahram, S.T. Dinani, M. Amouheydari, J. Food Meas. Charact. 13, 487–498 (2019)
H.N. Rajha, N. Boussetta, N. Louka, R.G. Maroun, E. Vorobiev, Food Res. Int. 65, 462–468 (2014)
S. Rahimpour, S. Taghian, J. Food Meas. Charact. 12, 2394–2403 (2018)
M.M. Kamil, G.F. Mohamed, M.S. Shaheen, J. Am. Sci. 7, 558–572 (2011)
K. Sinha, P. Das Saha, S. Datta, Dyes Pigment. 94, 212–216 (2012)
F. Khalili, S. Taghian Dinani, J. Food Meas. Charact. 12, 974–981 (2018)
M. Maher, S. Taghian Dinani, H. Shahram, J. Food Meas. Charact. (2019). https://doi.org/10.1007/s11694-019-00323-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Both authors declare that they do not have conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salehi, L., Taghian Dinani, S. Application of electrohydrodynamic‐ultrasonic procedure for extraction of β-carotene from carrot pomace. Food Measure 14, 3031–3039 (2020). https://doi.org/10.1007/s11694-020-00542-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00542-w