Abstract
Microcystins (MCs) are the most widespread and hazardous cyanotoxins posing a huge threat to agro-ecosystem by irrigation. Some adaptive metabolisms can be initiated at the cellular and molecular levels of plant to survive environmental change. To find ways to improve plant tolerance to MCs after recognizing adaptive mechanism in plant, we studied effects of MCs on root morphology, mineral element contents, root activity, H+-ATPase activity, and its gene expression level in cucumber during exposure and recovery (without MCs) periods. After being exposed to MCs (1, 10, 100 and 1000 μg L−1) for 7 days, we found 1 μg L−1 MCs did not affect growth and mineral elements in cucumber. MCs at 10 μg ·L−1 increased root activity and H+-ATPase activity partly from upregulation of genes (CsHA2, CsHA3, CsHA8, and CsHA9) expression, to promote nutrient uptake. Then, the increase in NO3−, Fe, Zn, and Mn contents could contribute to maintaining root growth and morphology. Higher concentration MCs (100 or 1000 µg L−1) inhibited root activity and H+-ATPase activity by downregulating expression of genes (CsHA2, CsHA3, CsHA4, CsHA8, CsHA9, and CsHA10), decreased contents of nutrient elements except Ca largely, and caused root growing worse. After a recovery, the absorption activity and H+-ATPase activity in cucumber treated with10 μg L−1 MCs were closed to the control whereas all parameters in cucumber treated 1000 μg L−1 MCs were even worse. All results indicate that the increase in H+-ATPase activity can enhance cucumber tolerance to MC stress by regulating nutrient uptake, especially when the MCs occur at low concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The eutrophication of water bodies caused by excessive levels of nutrients such as N and P is one of the environmental pollution challenges for human in the twenty-first century (Sinha et al. 2017). Eutrophic water bodies and climate change lead to large-scale and frequent outbreaks of algae blooms that cause the release of toxic secondary metabolites (cyanobacterial toxins) in water (Codd et al. 2005). Among these cyanotoxins, microcystins (MCs) are the most hazardous groups spreading widely. The occurrence of MCs has been found in Asia, Europe, North America, North Africa, and Scandinavian countries (Merel et al. 2013; Pawlik-Skowronska et al. 2019). To control harmful effects of MCs on human health, the World Health Organization consider 40 μg/kg/day as no observable adverse effect level for MCs in food and 1 µg L−1 as a maximum value for MCs in drinking water (WHO 1998). However, total MC concentrations in surface waters vary from 4 to 50 μg L−1 up to 6500 μg L−1, and even extremely high level of 29,000 μg L−1 in Algeria (Corbel et al. 2014). Agricultural plants could be exposed to MCs when they are irrigated with surface water contaminated with MCs. The presence of MC in irrigation water can cause toxic effects on edible plants and induce food chain contaminations with considerable health risks (Drobac et al. 2017; Liang et al. 2021, 2016; Redouane et al. 2023; Zhu et al. 2018). However, preventing the release of MCs in water is difficult to achieve in the short term by controlling pollution sources because of the complexity of environmental pollution and the involvement of numerous industries (Kraft et al. 2014). The understanding on the adaptive mechanism of plants to MCs could provide guidance for finding some effective ways to reduce the harmful effect of MCs on agricultural production.
The negative impact of MCs on agricultural plants includes inhibiting germination and growth, decreasing photosynthetic capacity, disturbing balance of mineral nutrient, and inducing excessive reactive oxygen species accumulation (Freitas et al. 2015; Ma et al. 2023; Machado et al. 2017; Prieto et al. 2011). MC-induced oxidative stress and cellular damage in plant root may affect the distribution and transport of nutrients in the plants, and then may lead to the decrease in the content of nutrient elements and even inhibit the growth and development of plant (Gutiérrez-Praena et al. 2014). Actually, the absorption of nutrient elements in soil by plant roots plays a decisive role in the content of nutrient elements in plants (Zhang and Liang 2023). In addition, plant membrane plasma H+-ATPase (PM H+-ATPase) plays an irreplaceable role in regulating the secondary transport of plant nutrients across the membrane. For example, transmembrane transport of most of mineral elements (K, Ca, Na, Mg, N and P etc.) depend on PM H+-ATPase that can provide an energy source for transport of nutrient into the cell by extruding positive charges (H+) and thus forming a membrane potential (Zeng et al. 2015; Zhang et al. 2011). Plasma membrane H+- ATPase is also involved in NH4+ promoting phosphorus uptake by rice roots (Zeng et al. 2012). In fact, there is a good correlation between nutrient uptake and PM H+-ATPase activity (Schmidt 2003). And even it has proved the role of PM H+-ATPase activity in plant adapting to abiotic stress by regulating nutrient uptake (Li and Liang 2019; Liang and Zhang 2018; Liu et al. 2018; Zhang et al. 2017). However, there is little information on the toxic mechanism of MCs on nutrient uptake that is vital for plant growth and survival. Besides, few studies on the toxic effects of MCs to plant metabolism are carried out at the genetic level (Wang et al. 2019). The PM H+-ATPase is encoded by a multigene family, and changes in gene expression of the PM H+-ATPase can be considered as its response to environmental stressors such as salinity, heavy metals, low temperature, and acid rain (Janicka-Russak et al. 2012, 2013; Kabala et al. 2013; Liang et al. 2015). Therefore, it could be interesting to reveal the adaptation mechanism of plant to MCs from a new perspective of nutrient uptake regulated by PM H+-ATPase at genetic level. The information will help us to find possible ways for reducing such damage to plants caused by MCs.
To achieve the aims, we firstly focused on studying response of root morphology of cucumber seedlings to MC exposure and recovery without MCs to know the adaptive capacity of cucumber seedlings to MC stress. Secondly, we observed changes in mineral element contents, root activity, PM H+-ATPase activity, and ATP content in cucumber under MC exposure and recovery without MCs to clarify the role of PM H+-ATPase in regulating nutrient uptake for adapting to MC stress. Last, we clarified the effect of MCs on transcriptional expression of PM H+-ATPase genes at level to reveal the reason for changing the activity of PM H+-ATPase to resist MC stress at genetic level. These results can help us to further reveal the adaptive mechanism of plants to MC stress and provide theoretical basis for reducing such harmful effects on plants caused by MCs.
Materials and methods
Plant culture, MC extraction, and MC exposure
Seeds of Cucumis sativus L. “xin jin yan 4” (Shanxi, China) were sterilized and germinated according to the procedures reported in Yan et al. (2002). Selected seedlings in unanimous growth were moved to turnover boxes (6.88 L) filled with Hoagland solution in a chamber (Jiang et al. 2011). The light intensity was 200 μmol m−2 s−1 and kept for 16 h each day. The relative humidity and temperature were kept at 60%, 27 ± 1 °C during the day and 70%, 20 ± 1 °C at night. After the cucumber seedlings were cultured for 30 days, they were treated with MCs. MCs used in our experiments were extracted by the method used in Liang et al. (2016). The concentration of MCs was detected by the Microcystins Plate Kit (Saco, ME). MC extract was diluted with deionized water to the final concentration of MCs at 1, 10, 100, and 1000 μg L−1. Each of the MC solutions (1, 10, 100, and 1000 μg L−1) was taken at the same volume to prepare Hoagland nutrition solution, respectively. The cucumber seedlings cultured in nutrition solution without containing MC were the control group, and the cucumber seedlings cultured in nutrition solution containing MCs (1, 10, 100, and 1000 μg L−1) for 7 days were MC treatment groups. All treatments were done by triplicate (three pots and six cucumber seedlings per pot). On the 8th day, half of cucumber seedlings in each group were collected for further analysis, and the rest of the cucumber seedlings of each group were moved to culture without MC exposure for another 7 days. On the 15th day, all seedlings were collected for analysis.
Observation of root growth and morphology
The length, surface area, volume of root, and the number of root tips as well as root morphology were determined by the root automatism scanning apparatus with WinRHIZO software (version 3.0).
Determination of nutritional elements in cucumber
Contents of mineral elements such as potassium (K), calcium (Ca), manganese (Mn), magnesium (Mg), iron (Fe), copper (Cu), zinc (Zn), and molybdenum (Mo) in cucumber roots and shoots were measured by inductively coupled plasma method. The setting conditions and calculating formula were the same with the method reported by Yuan et al. (2017). Ammonium (NH4+) was determined with the method described by Andreev (2017). The absorbance was measured at 625 nm. Nitrates (NO3−) were determined by the method reported by Miranda et al. (2001). The absorbance of solution was measured by a spectrophotometer at 410 nm. Phosphorus (P) was determined by molybdate blue method (Chang et al. 2009) after persulfate digestion. Absorption was measured at 880 nm. The method was calibrated with blank and five standards prepared by dissolving KH2PO4 in purified water (0.1, 0.2, 0.4, 0.6, and 0.8 g ml−1).
Measurement of root activity
The triphenyl tetrazolium chloride (TTC) method was used to measure root activity (Comas et al. 2000). Fresh cucumber roots (0.5 g) and the extraction buffer containing 0.4% TTC and phosphate buffer (1/15 mol L−1, pH 7.0) were placed into tubes and incubated in water bath at 37 °C for 2 h. Then, the reaction was stopped by adding 2 mL H2SO4 (1 M). After using 4 mL ethanol (95%, v/v) to extract the triphenyl tetrazolium formazane (TTF), the absorbance was measured at 485 nm. Root activity was expressed as TTF production per min on fresh weight basis.
Determination the hydrolytic activity of PM H+-ATPase
Fresh cucumber roots were used to isolate PM vesicles by two-phase partitioning method (Larsson et al. 1987) with some modification (Kłobus and Buczek 1995). Then, plasma membrane vesicles were used to determine hydrolytic PM H+ATPase activity by the method of Gallagher and Leonard (1982) with some modification (Zhao et al. 2015).
Determination of ATP content
According to the report of Liu et al. (2006), cucumber roots (2 g) were put in liquid nitrogen for freezing and grinding powder. Extraction of adenosine phosphates from the powder was preformed according to the procedure reported by Yang et al. (2002). The concentration of ATP was qualified by high performance liquid chromatography under setting conditions described by Zhang et al. (2017).
Analysis of expression of PM H+-ATPase genes
All primers used for expression of PM H+-ATPase genes (CsHA1 ~ CsHA10) in cucumber are shown in Supplementary material Table 1. The total RNA was extracted from fresh cucumber roots (0.3 g) according to the manufacturer’s instructions for TRIzol Reagent kit (Sigma-Aldrich, St. Louis). The total RNA concentration and purity were qualified by NanoDrop Spectrophotometer ND-1000 (Thermo Scientific, Wilmington, DE). Reverse transcription was performed with the method described by Yuan et al. (2017), and PCR reaction conditions for performing real-time quantitative PCR are shown in Supplementary material Tables 2 and 3. Relative expression levels of targeted genes were analyzed by the Optical System software (version 1.0).
Statistical analysis
All data were statistically analyzed by using SPSS16.0 and presented as means ± standard deviation. The significance of differences was analyzed with t test by one-way analysis of variance (P < 0.05).
Results
Effect of MCs on the root morphology of cucumber seedlings
Table 1 shows the change in root morphology parameters (the length, volume and surface area of root, and the number of root tips) of cucumber seedlings with the exposure of MCs, and Fig. 1 reflects images of roots under each treatment. Exposure of 1 μg L−1 MCs did not affect the growth of roots of cucumber seedlings (P > 0.05). When cucumber seedlings were exposed to 10 μg L−1 MCs, roots became smaller compared with the control (Fig. 1C), and root morphology parameters were obviously lower than the control (P < 0.05) (Table 1). After being exposed to high-concentration MCs (100 or 1000 μg L−1), the decrease in root size and morphology parameters were larger depending on the increase in the concentration of MCs. After a recovery period (7 days), the decreased degree in root length and root surface area in cucumber roots treated with MCs 10 or 100 μg L−1 was smaller than those measured on the 8th day although four morphology parameters were still lower than those of the control (P < 0.05). However, the decreased degree in four morphology parameters in cucumber roots treated with MCs 1000 μg L−1 was even larger than that measured on the 8th day.
Accumulation of MCs in cucumber seedlings
After cucumber seedlings were treated with MCs at 1, 10, 10, or 1000 μg L−1 for 7 days, we detected MC accumulation in cucumber shoots and roots except the control (Fig. 2A). The concentration of MCs in shoots and roots of cucumber treated with 1 μg L−1 was 10.9 and 8.15 μg kg−1. The highest concentration of MCs was 115.42 μg kg−1 observed in the roots of cucumber seedlings treated with 1000 μg L−1 MCs. The accumulation of MCs in shoots or roots was positively correlated with the exposure concentration of MCs (R2 = 0.732, R2 = 0.762, P < 0.01) (Table 2), and the accumulated concentration of MCs in roots was higher than that in shoots under the same MC treatment. However, the bioaccumulation factor (BCF) was lowered depending on the increase in concentration of MC exposure (Table 3). After a 7-day recovery (Fig. 2B), the accumulation of MCs in cucumber treated with MCs at 1, 10, 100, or 1000 μg L−1 was still detected, but was lower than that measured during the stress period at the same treatment. Besides, the accumulation of MCs in shoots or roots was still positively in correlation with the exposure concentration of MCs (R2 = 0.770, R2 = 0.838, P < 0.01). However, the accumulation of MCs in shoots was higher than that in roots at the same treatment except 1000 μg L−1 MC treatment, differing from the trend found during the stress period.
Accumulation of MCs in different organs of cucumber seedling during stress (A) and recovery (B) periods. Different lowercase letters represent significantly different accumulation of MCs at P < 0.05 in cucumber roots, and different uppercase letters represent significantly different accumulation of MCs at P < 0.05 in cucumber shoots
Effect of MCs on nutrient element contents in cucumber seedlings
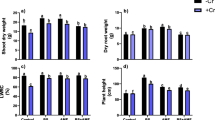
As shown in Fig. 3A, MCs at 1 μg L−1 did not change contents of N, P, K, Ca, and Mg (macronutrient elements) and contents of Zn, Fe, Mn, Cu, and Mo (micronutrient elements) in cucumber seedling (P > 0.05). When cucumber seedlings were exposed to 10 μg L−1 MCs for 7 days, the decrease in Ca, Cu, and Mo contents and the increase in NO3−, Fe, Zn, and Mn contents were observed (P < 0.05), and that no change was found in NH4+, K, and Mg contents. In addition, the increased/decreased degree in roots was larger than shoots. MCs at 100 μg L−1 or 1000 μg L−1 significantly reduced the contents of macronutrient and micronutrient elements in cucumber except that contents of Ca and NH4+ were increased. The decreased degree in Mg, Fe, Cu, and Mo in roots was larger than shoots. After recovery for 7 days (Fig. 3B), the contents of macronutrient and micronutrient elements in cucumber roots exposed to 10 μg L−1 MCs were closed to the control (P > 0.05). However, contents of macronutrients and micronutrients in cucumber roots and shoots exposed to 100 or 1000 μg L−1 MCs were significantly lower than that of the control (P < 0.05).
Effect of MCs on root activity, PM H+-ATPase activity, and ATP concentration in cucumber roots
As shown in Fig. 4, MCs (1 μg L−1) did not change the root activity (Fig. 4A), the activity of PM H+-ATPase (Fig. 4B), and ATP concentration (Fig. 4C) in cucumber roots compared with those of the control (P > 0.05). MCs at 10 μg L−1 increased root activity and PM H+-ATPase activity by 13.85% and 12.83%, but decreased ATP concentration by 14.72% in cucumber roots (P < 0.05). Higher concentrations of MCs (100 or 1000 μg L−1) decreased root activity, PM H+-ATPase activity, and ATP concentration (P < 0.05), and the decreased degree depended on the concentration of MCs. After a recovery for 7 days, root activity, PM H+-ATPase activity, and ATP concentration in cucumber roots with the exposure of 10 μg L−1 MCs were similar with the control level (P > 0.05). However, three parameters in cucumber roots exposed to MCs at 100 or 1000 μg L−1 were still lower than the control, but higher than that measured on the 8th day.
Effect of MCs on root activity (A), PM H+-ATPase activity (B) and ATP content (C) in roots of cucumber seedling. Different uppercase letters represent significant differences among treatment groups (P < 0.05) during the stress period, and different lowercase letters represent significant differences among treatment groups (P < 0.05) during the recovery period
Effects of MCs on expression of PM H+-ATPase gene in cucumber roots
Figure 5 shows relative expression level of genes (CsHA1-CsHA10) coding PM H+-ATPase in roots of cucumber seedlings during the stress and recovery periods. Expression analysis reflects that that seven of the 10 genes were expressed in cucumber roots (Fig. 5), and the expression of CsHA1, CsHA6 and CsHA7 at transcriptional level was not detectable. This may mean that genes that could be expressed in other tissues but silent in roots under our experimental conditions. Compared to the control, the exposure of 1 μg L−1 MCs did not have obvious effect on the relative expression of CsHA2, CsHA5, CsHA8, and CsHA9, while there was slight decrease in expression of CsHA3, CsHA4, and CsHA10 at transcript level. When cucumber roots were exposed to 10 μg L−1 MCs, the relative expression levels of CsHA2, CsHA3, CsHA8, and CsHA9 were increased by 6.45 ~ 20.81% whereas expression levels of CsHA4 and CsHA10 were decreased by 14.37% and 10.14%. After being exposed to MCs at 100 or 1000 μg L−1, the expression levels of all genes were decreased by 55.04 ~ 82.12%, and the inhibition degree depended on the concentration of MCs. After a 7-day recovery, CsHA1, CsHA6 and CsHA7 genes were still not detected in cucumber roots. The expression levels of CsHA3, CsHA4, and CsHA10 genes in cucumber roots exposed to 1 μg L−1 MCs were similar with the control, while expression levels of CsHA2 and CsHA9 in cucumber roots were increased. Expression levels of seven genes in cucumber roots exposed to 10 μg L−1 MCs were not different from those of the control. However, expression levels of seven genes in cucumber roots exposed to MCs at 100 or 1000 μg L−1 were still downregulated. In addition, expression levels of CsHA3, CsHA4, CsHA8, and CsHA9 were even lower than those measured on the 8th day.
Effect of MCs on relative expression of PM H+-ATPase genes in cucumber roots during the stress and recovery periods. Different lowercase letters represent significant differences among treatment groups (P < 0.05) during the stress period, and different uppercase letters represent significant differences among treatment groups (P < 0.05) during the recovery period
Principal component analysis on mineral element contents and PM H+-ATPase activity in cucumber root under MCs exposure
Figure 6 principal component analysis revealed the relationship between mineral element contents and PM H+-ATPase activity in cucumber roots under the exposure of MCs at different concentrations. Samples 1 through 5 were the control, 1, 10, 100, and 1000 μg L−1 MC treatment groups during the stress period, and samples 6 through 10 were the control, 1, 10, 100, and 1000 μg L−1 MC treatment groups during the recovery period. The PM H+-ATPase activity was positively correlated with contents of NO3−, Mg, K, P, Fe, Zn, Mo, and Mn during stress and recovery periods. However, the exposure concentration of MCs was negatively correlated with the activity of PM H+-ATPase, the content of ATP, and concentrations of NO3−, Mg, K, P, Fe, Zn, Mo, and Mn in cucumber seedlings.
Principal components analysis on concentrations of MCs, plasma membrane H+-ATPase activity, ATP content, and nutrition element concentration. Numbers 1–5 are samples of cucumber seedlings treated with 0 (the control), 1, 10, 100 and 1000 μg L−1 MCs during the stress period, and numbers 6–10 are samples of cucumber seedlings treated with 0 (the control), 1, 10, 100, and 1000 μg L−1 MCs during the recovery period
Discussion
Effect of MCs on root morphology and growth of cucumber seedlings
Under environmental stresses, plant could change root morphology to enhance its adaptation to stressors (Grossnickle 2005). Root morphology parameters such as root length, surface area, volume, and number of root tips can reflect the growth condition of plant root under environmental stresses (Fageria and Moreira 2011; Forino et al. 2012). In present experiments, low concentration of MCs (1 μg L−1) did not affect root morphology and growth of cucumber roots. It was not consistent with our previous results that 1 μg L−1 MCs promoted the growth of rice roots (Liang and Wang 2015). It may because different plant species showed different sensitivity to MC exposure. Cucumber roots treated with 10 μg L−1MCs or higher concentration (100 or 1000 μg L−1) were smaller than those of the control (Fig. 1), and all root morphology parameters were lower than those of the control (P < 0.05) (Table 1). The decreased degree in root size and morphology parameters was dependent on the increase in the concentration of MCs. The decrease in the number of root tips was smallest among all morphology parameters in roots treated with 10 μg L−1MCs, showing that plant root could take adaptation to low MC stress. The root tip is the most important part of the plant life activity because it plays a decisive role in root growth, differentiation, and absorption (Yan et al. 2002). The maintenance of root tips was benefit to keep root activity and function under adverse conditions. Li et al. (2015) also found that maize increases their root tips to improve the absorption of nutrients and water from the soil under the condition of lack of nutrition. On the contrary, the decreased degree in the number of root tips was larger than other morphology parameters in roots treated with 100 or 1000 μg L−1 MCs, and the recovery of the number of root tips was also the worse compared to the other parameters after a 7-day recovery. The phenomena indicated that that root tip as the most activity function section of root can reflect the adaptive ability of cucumber to MC stress. Zhang et al. (2016) reported that root tips of rice root can contribute to rice adaptation to acid rain stress, and root morphology plays a role in the root biomass production. After a 7-day recovery, the root system morphology in cucumber treated with 1000 μg L−1 MCs was even worse than that measured during the stress period. It means that high concentration MCs caused the irreversible damage to cucumber roots, and it could be the one of main causes for MC-induced inhibition on plant growth.
Accumulation of MCs in cucumber seedlings
MC accumulation in plants can bring potential danger to animal or human being through food chains. In addition, the data on MC accumulation in different organ of plants are informative to reveal the damage mechanism of MCs on plants. In present experiments, we found that the accumulation of MCs in shoots or roots was positively correlated with the exposure concentration of MCs (Table 2), while the bioaccumulation factor (BCF) of MCs in the root system was decreased by the increase in the exposure concentration of MCs (Table 3). The phenomena show that the low concentration of MCs could be more easily absorbed and accumulated in the roots of cucumber. It could be because the root morphology of cucumber treated with low MCs was maintained (Fig. 1; Table 1), and the absorption of root was not be inhibited badly. However, high MCs (> 100 g L−1) caused cucumber roots shorter and smaller, reduced the number of root tips, and then destroyed root function badly. In addition, we observed that the accumulated concentration of MCs in roots was higher than that in shoots under the same MC treatment. Saqrane et al. (2009) reported that MCs can be accumulated in plant tissues (roots, stem, and leaves) of wheat, soybean, pea, and lentil, and the accumulated content in different tissues depends on plant species and concentration of MC treatment. Plant root is the target organ that MCs attack firstly. After MCs enter the plant root, MCs could face different resistance to transport through the plant, and/or could undergo different metabolic change (Liang and Wang 2015). After a 7-day recovery without MC exposure, MC accumulation in root or shoot of cucumber was still detected and was positively correlated with the concentration of MC exposure. For the same treatment, the concentration of MCs in cucumber was lower than that measured during the stress period. That could be because the continuous growth of cucumber seedlings diluted the concentration of MCs in different organs of cucumber. On the other hand, MCs can combine with glutathione catalyzed by glutathione-S-transferase to produce a new substance (MC-GSH) in the plant (Bittencourt-Oliveira et al. 2016), and then the concentration of MCs in cucumber was decreased. Differing from the phenomena observed during the stress period, the accumulation of MCs in shoots was higher than that in roots at the same treatment except 1000 μg L−1 MC treatment. Combining with recovery of the root system morphology in cucumber treated with 1000 μg L−1 MCs, we inferred that the high concentration of MCs caused severe damage to the root system of cucumber seedlings, and even inhibited the transfer of MCs from root to the aboveground part, and therefore, the MC concentration in the root was higher than that in shoot.
Effect of MCs on contents of mineral elements in cucumber seedlings
Root monograph and growth influence directly the uptake of moisture and nutrients; meanwhile, the contents of nutrient elements in plants affect the development and growth of root because they are components of cell structure material, involved in regulating activities of enzymes, and maintain ion concentration balance, colloidal stability, and charge neutralization (Machado et al. 2017). Therefore, the changes in contents of nutrient elements may be one of the mechanisms of root growth inhibition under stressful conditions. In the present study, the low concentration of MCs (1 μg L−1) did not affect the contents of nutrient elements in cucumber seedlings, being consistent with the study of Freitas et al. (2015). This could be one of the reasons that low concentration of MCs (1 μg L−1) did not affect root growth and monograph. MCs at 10 μg L−1 increased contents of NO3−, Fe, Zn, and Mn in cucumber seedlings, indicating that MCs at 10 μg L−1 promoted the absorption and utilization of some nutrient elements although the root growth was inhibited (Table 1). The increase in these nutrient elements can contribute to synthesize more substances such as antioxidants (Cu/Zn-SOD, Mn-CAT) or to participate in catalytic reactions of antioxidative enzymes (CAT, POD) for enhancing the resistance of plants to MC exposure (Gu and Liang 2020). Thus, the fewer amounts of nutrient elements can be used to synthesize substances for plant growth, and the root growth was inhibited. Machado et al. (2017) also found a significant increase in Mn content in carrots exposed to MC-LR for 14 days. In addition, the decreased/increased degree in nutrient contents in roots was larger than in shoots, indicating that the inhibition on growth caused by 10 μg L−1 MCs was mainly because of the decrease in absorption ability of roots. However, high-concentration MCs (100 μg L−1 or 1000 μg L−1) significantly reduced contents of nutrient elements in cucumber roots and shoots except Ca and NH4+. This means that both decreases in absorption of root and transport from root to shoot were reasons why high-concentration MCs (100 μg L−1 or 1000 μg L−1) inhibited cucumber growth badly. In addition, the decrease in bioaccumulation factor (BCF) of MCs in cucumber treated with higher concentration MCs (Table 3) also proved that high-concentration MCs caused severe damage to absorption function of root system. Some studies reported that 100 μg L−1 MCs cause mineral elements decreased in faba bean and lettuce (Freitas et al. 2015; Lahrouni et al. 2013). However, contents of Na and K are increased after exposure to a crude extract containing 2220–22,240 μg L−1 MCs for 30 days (El Khalloufi et al. 2011). We also found that contents of Ca and NH4+ in cucumber seedlings treated with high-concentration MCs were increased. It has been proved that variations in cytosolic free Ca2+ concentration might be involved in plant response to different kinds of abiotic stresses. The concentration of free Ca2+ is very low at the normal condition, but can be instantly increased to bind with CAM for regulating signal transduction in plants under stressful conditions (Jaffe et al. 1975). The significant increase in NH4+ may be because the high accumulation of MCs in cucumber seedlings accelerated protein breakdown. On the other hand, the high accumulation of MCs in cucumber seedlings could hinder the formation of amino acids and reduce the transformation of NH4+. The high content of NH4+ has toxic effect on plant growth.
Effect of MCs on root activity, PM H+-ATPase activity, and ATP content in cucumber roots
Nutrient uptake in plant root is a complex and comprehensive process. The adaptive mechanism in plant roots to stressful environment involves many aspects such as morphology, kinetics, physiology, and biochemistry. Root activity can reflect the capacity of root for absorbing water and nutrients. Plasma membrane H+-ATPase plays an important role in providing the driving force for ion transport across membranes because it can form a transmembrane electrochemical potential gradient by hydrolyzing ATP to pump H+ out of the cell membrane (Crawford and Glass 1998). In the present study, low-concentration MCs (1 μg L−1) did not affect root activity, plasma membrane H+-ATPase activity, and ATP content in roots of cucumber seedlings (Fig. 4A and B), and contents of nutrient elements in cucumber showed no difference from the control (Fig. 3). After being treated with 10 μg L−1 MCs for 7 days, the increase in root activity and PM H+-ATPase activity and the decrease in ATP content were observed. The results indicate that 10 μg L−1 MCs stimulated the capacity of root to absorb water and nutrient elements. In addition, the driving force for transmembrane transport of minerals elements comes from the proton-driving potential whose formation depends on PM H+-ATPase hydrolyzing ATP (Dawood et al. 2012). The increase in hydrolytic activity of PM H+-ATPase induced by10 μg L−1 MCs can provide more the proton gradient or proton motive force for ion uptake by hydrolyzing more ATP. It could be one of causes for increase in NO3−, Fe, Zn, and Mn in cucumber (Fig. 3). P-type ATPases, including PM H+-ATPase, have been implicated in the transport of multiple ions, including protons, Ca, Mn, Mo, Cu, and phospholipids (Palmgren and Harper 1999). For example, the active transport of NO3− in the presence of more than one H+ requires H+-ATPase on the cytoplasmic membrane to provide energy and protons, and the absorption of both ammonium nitrogen and nitrate nitrogen is closely related to PM H+-ATPase (Glass 2002). In addition, the difference in absorption of between ammonium nitrogen and nitrate nitrogen in rice root main is mainly manifested in the change of the quantity of H+-ATPase protein (Di et al. 2007). It has been recognized that the activation of PM H+- ATPase is the main physiological mechanism for plants to adapt to Fe deficiency (Rabotti and Zocchi 2006). Zn deficiency simulates the increase in transport activity of PM H+-ATPase depending on the hydrolysis of ATP (Pinton et al. 1993). Hence, the increase in NO3−, Fe, Zn, and Mn in cucumber treated with 10 μg L−1 MCs could be related with the increase in hydrolytic activity of PM H+-ATPase, showing adaptive response to MC stress. After a recovery, the root activity and the hydrolytic activity of PM H+-ATPase were maintained at the control level, probably being beneficial to the restoration of growth and contents of mineral elements in cucumber treated with 10 μg L−1 MCs. These phenomena also show the role of nutrient uptake regulated by PM H+-ATPase activity in adaptable mechanism of cucumber seedlings to MC stress. High-concentration MCs (100 μg L−1 or 1000 μg L−1) decreased root activity, the hydrolytic activity of PM H+-ATPase, and the ATP content in roots of cucumber seedlings (Fig. 4A and B), and the dramatic decrease in contents of mineral elements was observed as well (Fig. 3). The decrease in root activity and PM H+-ATPase activity induced by higher contractions MCs could be one of factors decreasing contents of mineral elements. PM H+-ATPase is one of key enzymes located in plasma membrane, and the decrease in PM H+-ATPase activity could be resulted from the changes in membrane fluidity and integrity caused by high-concentration MCs inducing oxidative stress in cells of plant (Gu and Liang 2020; Wang et al. 2014). Besides, excessive H2O2 accumulation caused by high-concentration MCs could directly participate in regulating the activity PM H+-ATPase to mediate cell growth through wall relaxation (Majumdar and Kar 2021). On the other hand, the destruction of MCs on plasma membrane integrity may aggravate the reduction in contents of mineral elements due to oxidative stress. Based on the principal component analysis between contents of nutrient elements and activity of plasma membrane H+-ATPase (Fig. 6), we also found that the activity of H+-ATPase was positively correlated with ATP content, NO3−, P, Zn, Mg, Mn, Na, Mo, K, and Fe. In addition, there was a significant negative correlation between the concentration of MC exposure and plasma membrane H+-ATPase activity, ATP content, NO3−, P, Zn, Mg, Mn, Na, Mo, K, and Fe while a positive correlation between the concentration of MCs exposure and Ca, NH4+. Hence, plasma membrane H+-ATPase can be involved in the adaptive mechanism in cucumber to MC stress by regulating energy source for nutrient uptake of cucumber root.
Effects of MCs on PM H+-ATPase gene expression in cucumber seedlings
Usually, plasma membrane H+-ATPase activity is regulated by the transcriptional level, post-translational modification, and membrane environment (Arango et al. 2003). In addition, the high expression of plasma membrane H+-ATPase at transcription level is crucial for the increase in the activity of plasma membrane H+-ATPase by increasing plasma membrane H+-ATPase concentration (Janicka-Russak et al. 2008; Liang et al. 2020). To clarify the changes in the activity of plasma membrane H+-ATPase in cucumber roots exposed to MC stress, we detected the expression of cucumber H+-ATPase genes (CsHA1-CsHA10) at transcriptional level by using RT-PCR. In the present study, three (CsHA1, CsHA6, and CsHA7) of 10 genes in cucumber roots in the control and other treatments were not detected. That the expression level of genes of plasma membrane H+-ATPase could be different under environmental stresses (Duby and Boutry 2009; Gaxiola et al. 2007). Janicka-Russak et al. (2013) found that accumulation of the CsHA1 transcript is induced by NaCl exposure and is not expressed at detectable levels in roots of control plants. Under low temperature stress, six of the isoforms are CsHA2, CsHA3, CsHA4, CsHA8, CsHA9, and CsHA10 expressed in cucumber roots (Janicka-Russak et al. 2012). In our experiments, MCs at different concentrations changed the express of seven genes (CsHA2, CsHA3, CsHA4, CsHA5, CsHA8, CsHA9, and CsHA10) at transcriptional level in cucumber roots. Being exposed to 10 μg L−1 MCs, relative expression levels of genes CsHA2, CsHA3, CsHA8, and CsHA9 at transcriptional level were increased whereas relative expression levels of genes CsHA4 and CsHA10 were decreased. It indicates that the high expression of genes CsHA2, CsHA3, CsHA8, and CsHA9 at transcriptional level could cause the increase in activity of plasma membrane H+-ATPase. For plants under stressful environment, maintaining ionic equilibrium and replenishing lost substances are very important. Maintaining active transport of nutrient elements across the plasma membrane requires increased generation of a proton gradient by plasma membrane H+-ATPase (Michelet and Boutry 1995). Therefore, the high expression of genes CsHA2, CsHA3, CsHA8, and CsHA9 at transcriptional level can contribute to increasing the activity of plasma membrane H+-ATPase for maintaining active transport of mineral elements (NO3−, Fe, Zn, and Mn), being helpful in the adaption of cucumber to MC stress conditions. Zeng et al. (2012) also reported that the upregulation of five isoforms of PM H+-ATPase in rice root at transcription level can be the fundamental reason for the increase in hydrolytic activity of H+-ATPase by increasing the concentration of H+-ATPase. Moreover, the upregulation of these isoforms could be the link between the enhancement of P uptake by NH4+ nutrition and PM H+-ATPase activity. Hence, we speculated that the four isoforms of PM H+-ATPase in cucumber root played a crucial role in the adaptive response of nutrient uptake to MC stress. High concentrations of MCs (100 μg L−1 and 1000 μg L−1) downregulated the expression of seven PM H+-ATPase genes at transcriptional level in cucumber roots to decrease the activity of PM H+-ATPase. It could be because the excessive accumulation of reactive oxygen species induced by high concentrations of MCs can cause genetic damage to transcription of PM H+-ATPase genes (Kim et al. 2013). Previous studies found that cucumber is more sensitive to MC stress than rice by comparing activities of antioxidative enzymes and protein phosphatases in cucumber and rice (Gu and Liang 2020; Ma et al. 2023). The decrease in PM H+-ATPase activity by downregulating expression of PM H+-ATPase genes may be another cause that cucumber cannot resist high-concentration MC stress because PM H+-ATPase can provide proton driving force for transporting nutrients and other substances to maintain plant growth (Janicka-Russak 2011). After a 7-day recovery, the relative expression of seven genes at transcriptional level in cucumber roots treated with 10 μg L−1 MCs was close to the control level whereas those in cucumber roots treated with 100 or 1000 μg L−1 MCs were still lower than those of the control. Liang et al. (2020) also found the similar phenomena that acid rain at pH4.5 promote the transcription regulation to maintain H+-ATPase activity higher in soybean for resisting stress whereas acid rain at pH3.0 caused irreversible inhibition on transcription express of H+-ATPase and decrease H+-ATPase activity in soybean. It can be inferred that higher contractions MCs induced irreversible inhibition on transcription expression of PM H-ATPase genes to decrease H+-ATPase activity, and then could cause the driving force for transmembrane transport of mineral elements decreased. This may be one of reasons for nutritional deficiency and abnormal growth in cucumber under high-concentration MC stress.
Conclusion
Low-concentration MCs (1 μg L−1) did not affect root growth and physiological function in cucumber whereas higher concentrations of MCs (100 μg L−1 or 1000 μg L−1) caused irreversible inhibition on root growth and nutrient uptake by decreasing PM H+-ATPase activity. In addition, MCs at 10 μg L−1 increased PM H+-ATPase activity by inducing upregulation expression of CsHA2, CsHA3, CsHA8, and CsHA9 in cucumber roots to promote the uptake of mineral elements (NO3−, Fe, Zn, and Mn) for maintaining root growth and morphology. It was demonstrated that the adaptive response of nutrient uptake in cucumber regulated by plasma membrane H+-ATPase was limited by high-concentration MCs. Taking measures to enhance plant tolerance to MC stress by overexpressing PM H+-ATPase could be better than diluting irrigation water to lower the risk of MCs for crops, especially where water shortage occurs. Furthermore, the adaptive mechanism of plant to MC stress needs to be further studied during the whole growth period of crops in the farmland.
Data availability
Not applicable.
References
Andreev IM (2017) Emerging evidence for potential role of Ca2+-ATPase-mediated calcium accumulation in symbiosomes of infected root nodule cells. Funct Plant Biol 44:955–960
Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216:355–365
Bittencourt-Oliveira MdC, Cordeiro-Araujo MK, Chia MA, de Toledo Arruda-Neto JD, de Oliveira ET, dos Santos F (2016) Lettuce irrigated with contaminated water: photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol Environ Saf 128:83–90
Chang C, Hu Y, Sun S, Zhu Y, Ma G, Xu G (2009) Proton pump OsA8 is linked to phosphorus uptake and translocation in rice. J Exp Bot 60:557–565
Codd GA, Morrison LF, Metcalf JS (2005) Cyanobacterial toxins: risk management for health protection. Toxicol Appl Pharmacol 203:264–272
Comas LH, Eissenstat DM, Lakso AN (2000) Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol 147:171–178
Corbel S, Mougin C, Bouaïcha N (2014) Cyanobacterial toxins: modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 96:1
Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3:389–395
Dawood M, Cao FB, Jahangir MM, Zhang GP, Wu FB (2012) Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J Hazard Mater 209:121–128
Di T-j, Zhu Y-y, Qiu M-h, Kan J, Zhang X-m, Xu G-h, Shen Q-r (2007) Response of plasma membrane H+-ATPase of rice root to ammonium and nitrate nutrition. Chin J Rice Sci 21:360–366
Drobac D, Tokodi N, Kiprovski B, Malencic D, Vazic T, Nybom S, Meriluoto J, Svircev Z (2017) Microcystin accumulation and potential effects on antioxidant capacity of leaves and fruits of Capsicum annuum. J Toxicol Environ Health-Part a-Current Issues 80:145–154
Duby G, Boutry M (2009) The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflügers Archiv-European J Physiol 457:645–655
El Khalloufi F, Oufdou K, Lahrouni M, El Ghazali I, Saqrane S, Vasconcelos V, Oudra B (2011) Allelopatic effects of cyanobacteria extracts containing microcystins on Medicago sativa-Rhizobia symbiosis. Ecotoxicol Environ Saf 74:431–438
Fageria NK, Moreira A (2011) The role of mineral nutrition on root growth of crop plants. Adv Agron 110:251–331
Forino LMC, Castiglione MR, Bartoli G, Balestri M, Andreucci A, Tagliasacchi AM (2012): Arsenic-induced morphogenic response in roots of arsenic hyperaccumulator fern Pteris vittata. J Hazard Mater s235–236, 271–278
Freitas M, Azevedo J, Pinto E, Neves J, Campos A, Vasconcelos V (2015) Effects of microcystin-LR, cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture on growth, oxidative stress and mineral content in lettuce plants (Lactuca sativa L.). Ecotoxicol Environ Saf 116:59–67
Gallagher SR, Leonard RT (1982) Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiology 70:1335–40
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214
Glass DMA (2002) The regulation of nitrate and ammonium transport systems in plants. J Exp Bot 53:855–864
Grossnickle SC (2005) Importance of root growth in overcoming planting stress. New Forest 273–294:273–294
Gu Y, Liang C (2020) Responses of antioxidative enzymes and gene expression in Oryza sativa L and Cucumis sativus L seedlings to microcystins stress. Ecotoxicol Environ Saf 193:110351
Gutiérrez-Praena D, Campos A, Azevedo J, Neves J, Freitas M, Guzmán-Guillén R, Cameán AM, Renaut J, Vasconcelos V (2014) Exposure of Lycopersicon Esculentum to microcystin-LR: effects in the leaf proteome and toxin translocation from water to leaves and fruits. Toxins 6:1837–1854
Jaffe LA, Weisenseel MH, Jaffe LF (1975) Calcium accumulations within the growing tips of pollen tubes. J Cell Biol 67:488
Janicka-Russak M, Kabała K, Burzyński M, Kłobus G (2008) Response of plasma membrane H+-ATPase to heavy metal stress in Cucumis sativus roots. J Exp Bot 59:3721–3728
Janicka-Russak M (2011): Plant plasma membrane H+-ATPase in adaptation of plants to abiotic stresses. In: Shanker A (Editor), Abiotic stress response in Plants-physiological, biochemical and genetic perspectives. InTech, Rijeka, Croatia, pp. 197–218
Janicka-Russak M, Kabala K, Wdowikowska A, Klobus G (2012) Response of plasma membrane H+-ATPase to low temperature in cucumber roots. J Plant Res 125:291–300
Janicka-Russak M, Kabala K, Wdowikowska A, Klobus G (2013) Modification of plasma membrane proton pumps in cucumber roots as an adaptation mechanism to salt stress. J Plant Physiol 170:915–922
Jiang J, Gu X, Rui S, Wang X, Yang L (2011) Microcystin-LR induced oxidative stress and ultrastructural alterations in mesophyll cells of submerged macrophyte Vallisneria natans (Lour.) Hara. J Hazard Mater 190:188–196
Kabala K, Janicka-Russak M, Anklewicz A (2013) Mechanism of Cd and Cu action on the tonoplast proton pumps in cucumber roots. Physiol Plant 147:207–217
Kim H-S, Oh J-M, Luan S, Carlson JE, Ahn S-J (2013) Cold stress causes rapid but differential changes in properties of plasma membrane H+-ATPase of camelina and rapeseed. J Plant Physiol 170:828–837
Kłobus G, Buczek J (1995) The role of plasma membrane oxidoreductase activity in proton transport. J Plant Physiol 146:103–107
Kraft B, Tegetmeyer HE, Sharma R, Klotz MG, Ferdelman TG, Hettich RL, Geelhoed JS, Strous M (2014) The environmental controls that govern the end product of bacterial nitrate respiration. Science 345:676–679
Lahrouni M, Oufdou K, El KF, Baz M, Lafuente A, Dary M, Pajuelo E, Oudra B (2013) Physiological and biochemical defense reactions of Vicia faba L.-Rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ Sci Pollut Res 20:5405–5415
Larsson C, Widell S, Kjellbom P (1987) [52] Preparation of high-purity plasma membranes. Methods Enzymol 148C:558–568
Li Y, Liang C (2019) Exogenous application of Ca2+ mitigates simulated acid rain stress on soybean productivity and quality by maintaining nutrient absorption. Environ Sci Pollut Res 26:4975–4986
Li Z, Philip D, Neuhaeuser B, Schulze WX, Ludewig U (2015) Protein dynamics in young maize root hairs in response to macro- and micronutrient deprivation. J Proteome Res 14:3362–3371
Liang C, Ge Y, Su L, Bu J (2015): Response of plasma membrane H+-ATPase in rice (Oryza sativa) seedlings to simulated acid rain. Environ Sci Pollut Res, 535–545
Liang C, Wang W (2015) Response and recovery of rice (Oryza sativa) seedlings to irrigation with microcystin-contaminated water. Environ Earth Sci 73:4573–4580
Liang C, Wang W, Wang Y (2016): Effect of irrigation with microcystins-contaminated water on growth, yield and grain quality of rice (Oryza sativa). Environ Earth Sci 75
Liang C, Zhang B (2018) Effect of exogenous calcium on growth, nutrients uptake and plasma membrane H+-ATPase and Ca2+-ATPase activities in soybean (Glycine max) seedlings under simulated acid rain stress. Ecotoxicol Environ Saf 165:261–269
Liang C, Ma YJ, Li LR (2020) Comparison of plasma membrane H+-ATPase response to acid rain stress between rice and soybean. Environ Sci Pollut Res 27:6389–6400
Liang C, Ma XD, Liu HY (2021) Effect of microcystins at different rice growth stages on its yield, quality, and safety. Environ Sci Pollut Res 28:13942–13954
Liu H, Jiang Y, Luo Y, Jiang W (2006) A simple and rapid determination of ATP, ADP and AMP concentrations in pericarp tissue of litchi fruit by high performance liquid chromatography. Food Technol Biotechnol 4:531–534
Liu H, Ren X, Zhu J, Wu X, Liang C (2018) Effect of exogenous abscisic acid on morphology, growth and nutrient uptake of rice (Oryza sativa) roots under simulated acid rain stress. Planta 248:647–659
Ma X, Gu Y, Liang C (2023) Adaptation of protein phosphatases in Oryza sativa and Cucumis sativus to microcystins. Environ Sci Pollut Res 30:7018–7029
Machado J, Azevedo J, Freitas M, Pinto E, Almeida A, Vasconcelos V, Campos A (2017) Analysis of the use of microcystin-contaminated water in the growth and nutritional quality of the root-vegetable, Daucus carota. Environ Sci Pollut Res 24:752–764
Majumdar A, Kar RK (2021) Transcriptional co-regulation of plasma membrane H+-ATPase and NADPH oxidase during root growth. Plant Gene 26:100272
Merel S, Walker D, Chicana R, Snyder S, Baurès E, Thomas O (2013) State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ Int 59:303–327
Michelet B, Boutry M (1995) The plasma membrane H+-ATPase (a highly regulated enzyme with multiple physiological functions). Plant Physiol 108:1
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide-Biol Chem 5:62–71
Palmgren MG, Harper JF (1999) Pumping with plant P-type ATPases. J Exp Bot 50:883–893
Pawlik-Skowronska B, Toporowska M, Mazur-Marzec H (2019) Effects of secondary metabolites produced by different cyanobacterial populations on the freshwater zooplankters Brachionus calyciflorus and Daphnia pulex. Environ Sci Pollut Res 26:11793–11804
Pinton T, Cakmak I, Marschner H (1993) Effect of zinc deficiency on proton fluxes in plasma membrane-enriched vesicles isolated from bean roots. J Exp Bot 44:623–630
Prieto A, Campos A, Camean A, Vasconcelos V (2011) Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol Environ Saf 74:1973–1980
Rabotti G, Zocchi G (2006) Plasma membrane-bound H+-ATPase and reductase activities in Fe-deficient cucumber roots. Physiol Plant 90:779–785
Redouane E, Tazart Z, Lahrouni M, Mugani R, Elgadi S, Zine H, Zerrifi SE, Haida M, Martins JC, Campos A, Oufdou K, Vasconcelos V, Oudra B (2023) Health risk assessment of lake water contaminated with microcystins for fruit crop irrigation and farm animal drinking. Environ Sci Pollut Res 30:80234–80244
Saqrane S, Ouahid Y, EIGhazali I, Oudra B, Bouarab L, del Campo FF (2009) Physiological changes in Triticum durum, Zea mays, Pisumsativum and Lens esculenta cultivars, caused by irrigation with water contaminated with microcystins: a laboratory experimental approach. Toxicon 53:786–796
Schmidt W (2003) Iron homeostasis in plants: sensing and signaling pathways. J Plant Nutr 26:2211–2230
Sinha E, Michalak AM, Balaji V (2017) Eutrophication will increase during the 21st century as a result of precipitation changes. Science 357:405–408
Wang Q, Wang X, Zhang S, Zong W (2019) Molecular mechanism for the discrepant inhibition of microcystins on protein phosphatase 1. Environ Sci Pollut Res 26:21774–21783
Wang W, Deng Y, Zou H, Liang C (2014) Effects of microcystins on growth and antioxidant system of rice roots. Environ Sci China 35:1468–1472
WHO (1998) Guidelines for drinking-water quality: health criteria and other supporting information. World Health Organ
Yan F, Zhu Y, Müller C, Zörb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129:50–63
Yang NC, Ho WM, Chen YH, Hu ML (2002) A convenient one-step extraction of cellular ATP using boiling water for the luciferin-luciferase assay of ATP. Anal Biochem 306:323–327
Yuan L, Wang C, Huang X, Tang L, Hou J, Liu F, Wang L, Zhu S (2017) The Regulation of 24-epibrassinolide on AsA-GSH cycle and xanthophylls homeostasis in chloroplast of cucumber under Ca(NO3)2 stress. Acta Horticulturae Sinica 44:881–890
Zeng H, Liu G, Kinoshita T, Zhang R, Zhu Y, Shen Q, Xu G (2012) Stimulation of phosphorus uptake by ammonium nutrition involves plasma membrane H+ ATPase in rice roots. Plant Soil 357:205–214
Zeng H, Di T, Zhu Y, Subbarao GV (2015) Transcriptional response of plasma membrane H+-ATPase genes to ammonium nutrition and its functional link to the release of biological nitrification inhibitors from sorghum roots. Plant Soil 398:301–312
Zhang B, Bu J, Liang C (2016) Root morphology and growth regulated by mineral nutrient absorption in rice roots exposed to simulated acid rain. Water Air Soil Pollut 227:457
Zhang B, Bu J, Liang C (2017) Regulation of nitrogen and phosphorus absorption by plasma membrane H+-ATPase in rice roots under simulated acid rain. Int J Environ Sci Technol 14:101–112
Zhang R, Liu G, Wu N, Gu M, Zeng H, Zhu Y, Xu G (2011) Adaptation of plasma membrane H+ ATPase and H+ pump to P deficiency in rice roots. Plant Soil 349:3–11
Zhang Y, Liang C (2023) Improving yield and quality of rice under acid rain stress by regulating nitrogen assimilation with exogenous Ca2+. Environ Sci Pollut Res 30:12085–12097
Zhao B, Liu P, Wang W, Sun J, Ma H (2015) Effects of 5-aminolevulinic acid on the AsA-GSH cycle in grape leaves under salt stress. Plant Physiol J 51:385–390
Zhu J, Ren X, Liu H, Liang C (2018) Effect of irrigation with microcystins-contaminated water on growth and fruit quality of Cucumis sativus L. and the health risk. Agric Water Manag 204:91–99
Funding
The authors are grateful for the financial support from National Natural Science Foundation of China (No. 31971407), and the Natural Science Foundation of Jiangsu Province (BK20161131).
Author information
Authors and Affiliations
Contributions
Chanjuan Liang: conceptualization, writing-original draft, writing-review and editing, supervision, project, funding acquisition. Jiuzheng Zhu: formal analysis, investigation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, C., Zhu, ·. Role of root plasma membrane H+-ATPase in enhancing Cucumis sativus adaptation to microcystins. Environ Sci Pollut Res 31, 20133–20148 (2024). https://doi.org/10.1007/s11356-024-32371-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32371-5