Abstract
The presence of cyanotoxins, mainly microcystins (MCs), in surface freshwater represents a serious health risk to aquatic organisms living in the water body, as well as terrestrial animals and plants that are in contact with contaminated water. Consequently, the use of MCs contaminated water for irrigation represents a hazard for cultivated plants and could induce severe economical losses due to crops’ yield reduction. The experimental approach undertaken in this work was exposing Vicia faba seedlings (inoculated with a Rhizobium strain resistant to MCs), to water supplemented with cyanobacterial crude extract containing total microcystins at a concentration of 50 and 100 μg/L (environmental relevant concentrations of MCs dissolved in the raw irrigation water from Lalla Takerkoust Lake-Marrakesh region). After chronic MCs exposure (2 months), biological and physiological parameters (plant growth, nitrogen uptake, mineral assimilation, and oxidative defense mechanisms) were evaluated. The results obtained showed evidence that chronic exposure to cyanobacterial bloom extract containing MCs strongly affected the physiological and biological plants activities; reduction of dry matter, photosynthetic activity, nodule number, and nitrogen assimilation. At the same time, an increase of oxidative stress was observed, as deduced from a significant increase of the activities of peroxidase, catalase, polyphenoloxidase, and phenylalanine ammonia lyase in leaves, roots, and nodules of faba bean plants exposed to cyanotoxins, especially at 100 μg/L of MCs. This experimentation constitutes a simulation of the situation related to cyanotoxins chronic exposure of seedlings—plants via the contaminated irrigation water. For this reason, once should take into consideration the possibility of contamination of agricultural crops and the quality of irrigation water should be by the way monitored for cyanotoxins biohazard.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Morocco, as well as in many countries located in arid and semi-arid areas, irrigation with raw water from lake reservoirs is a common agricultural and horticultural practice, and an important tool for farmers to improve crop yields. This kind of surface water bodies can contain potentially toxic bloom-forming cyanobacteria, and its use as a source for irrigation water can enable a transfer of cyanotoxins into crop plants (Peuthert et al. 2007; Saqrane and Oudra 2009). In the Marrakech region, Lalla Takerkoust reservoir is an aquatic ecosystem known by frequent and massif proliferations of the toxic cyanobacterium Microcystis aeruginosa during late-summer and autumn. The bloom toxicity, diversity of microcystins (MC) variants, and MCs cell quota are being previously monitored since 1994 (Oudra et al. 2001, 2002; El Ghazali et al. 2011). The most common referenced cyanotoxins are microcystins. These cyclic heptapeptides occur as more than 70 different congeners (Zurawell et al. 2005). Among them, microcystin-LR is known to be a potent inhibitor of the protein phosphatase 1 and 2A (PP1 and PP2A) of mammals and higher plants. It has been shown that cyanotoxins are taken up by seedlings (Peuthert et al. 2007) and their toxicity has already been demonstrated in terrestrial plants (Abe et al. 1996; McElhiney et al. 2001; Pflugmacher et al. 2006; Pichardo and Pflugmacher 2010; El Khalloufi et al. 2011; Lahrouni et al. 2012) and aquatic plants (Vassilakaki and Pflugmacher 2007; Kinnear et al. 2008; Saqrane et al. 2009), causing inhibitory effects on development, root growth, and photosynthesis.
First step in cyanotoxins detoxification is the formation of a glutathione conjugate (Pflugmacher et al. 1998). In this context, the activity of glutathione-S-transferase has been shown to increase upon exposition of plants to cyanotoxins (Mitrovic et al. 2004; Pflugmacher et al. 2007).
Furthermore, as a result of exposure to these microcystins, reactive oxygen species (ROS) can be generated (Pflugmacher et al. 2007), which react with other cellular compounds such as lipids, proteins, and DNA (Esterbauer et al. 1991). Plants have the ability to decrease toxins exposure, a well-elaborated antioxidative defense system of plants’ functions relieves the negative effects caused by ROS. The antioxidant defenses comprise enzymatic and nonenzymatic mechanisms. Some of the key enzymes of the antioxidant defense are catalase, peroxidase, and superoxide dismutase (Mitrovic et al. 2004; Pflugmacher et al. 2007; Saqrane et al. 2007). Enzymes of the glutathione–ascorbate cycle, such as glutathione reductase, are also involved in the detoxification of ROS produced upon exposure to cyanotoxins (Pflugmacher 2004; Pflugmacher et al. 2007). Besides redox enzymes, several compounds also participate in alleviating the oxidative stress; the pool of oxidized/reduced glutathione levels are adjusted (Pflugmacher 2004) and phenolic compounds also play an important role in the detoxification of free radicals (Ksouri et al. 2007).
Faba bean (Vicia faba) is an important legume plant that is widely cultivated, generally in Morocco and particularly in the Marrakech region, and used for human and animal nutrition (MADRPM/DERD 2006). It has a high nutritional value as a consequence of its antioxidative capacity, providing high yield of proteins and vitamins (Bond et al. 1985).
The aim of the present study is examining the long-term effects of water contaminated with MCs on the V. faba–Rhizobium leguminosarum symbiosis. In particular, the principal aimed objectives, are to determine the physiological and biochemical responses of faba bean seedlings, induced by irrigation water containing cyanobacterial crude extract [equivalent to 50 and 100 μg/L MCs]. Moreover, the oxidative stress caused by MCs was evaluated by determining peroxidase (POD), catalase (CAT), polyphenoloxidase (PPO), and phenylalanine amonialyase (PAL) activities in faba bean plants exposed and not exposed to MCs. The originality of the experimental approach consists in using a MCs-tolerant rhizobial strain, R. leguminosarum RhOF4 (Lahrouni et al. 2012). According to our knowledge, scarce studies were carried out on the effects of MCs on the oxidative stress and the antioxidant defense of V. faba inoculated with a MCs-tolerant symbiotic rhizobial strain.
Material and methods
Extraction of cyanotoxins
The cyanobacterial bloom material (mainly M. aeruginosa) was collected in September 2005 from Lalla Takerkoust reservoir. The sample was freeze-dried and the lyophilized material was stored at −20 °C for MCs extraction and HPLC analysis. The methodology of extraction was previously described by El Khalloufi et al. (2011). Basically, the bloom was sonicated for 15 min, stirred for 1 h at room temperature, and centrifuged at 22,000×g for 15 min. The supernatant was collected, aliquoted, and stored at −20 °C until use. This raw bloom extract was used for the experiments. Microcystins quantification by high-performance liquid chromatography coupled to a photodiode array detector (HPLC-PDA) in the raw extract was done in a previous work (El Khalloufi et al. 2011), and corresponded to 22.24 μg MCs/mL. The concentration of MCs in the water used for plant irrigation was adjusted to 50–100 μg/L in order to mimic the natural conditions which occur during bloom event in Lalla Takerkoust lake (cells lysis, cyanotoxins release, MCs dissolved fraction). Natural occurring MCs concentrations in this lake were widely studied for several years (Oudra et al. 2001, 2002; El Ghazali et al. 2011).
Faba bean seedlings and growth conditions
Seeds of commercial cultivar of V. faba L. var. aguadulce (widely cultivated in Marrakech region) were surface-sterilized with sodium hypochlorite (6 %) for 10 min, then rinsed several times with sterile distilled water and germinated at 26 °C in moist autoclaved sand. After initial germination, seedlings selected for uniformity, were inoculated with a suspension of R. leguminosarum strain RhOF4, which has been described as cyanotoxins-tolerant rhizobial strain (Lahrouni et al. 2012) and planted (three per pot) in pots containing sterile sand. The sand was previously washed with distilled water and sterilized at 200 °C for 4 h in a muffle furnace. Chronic and continuous exposure to cyanotoxins was started 1 week after transplanting the germinated faba bean seeds into the pots.
Plants were grown for 5 weeks in a glasshouse under natural conditions during October–November 2010 and irrigated with N-free nutrient solution (Rigaud and Puppo 1975). After germination stage, the seedlings were grown in normal conditions (not in contact with cyanotoxins) and afterwards the pots were separated into three experimental groups. The first group was kept without cyanotoxins (served as a control), while the second and the third groups were irrigated with water containing cyanobacterial bloom extract equivalent to 50 and 100 μg/L MCs, respectively. Cyanotoxins concentrations are in accordance with the environmental realistic concentrations detected in Lalla Takerkoust’s water bodies and reflective of possible MCs exposures during the occurrence of natural blooms of M. aeruginosa (El Ghazali et al. 2011).
Plant harvest
After exposure to cyanobacterial cell extract, the plants were harvested at the flowering stage, washed, and separated into two groups for each treatment (control, 50 and 100 μg/L). For the first group, nodules were detached from their roots and counted. After desiccation at 70 °C during 72 h, the dry matter of the different vegetative parts of the plants (shoots, roots, and nodules) was quantified. Nitrogen as well as mineral nutrient content in shoots and roots part were dosed. For the other group, nodules root and leaves were separated, the fresh weight determined, and subsequently submerged in liquid nitrogen and stored at −20 °C for biochemical analysis. The activity of antioxidative enzymes POD, CAT, PPO, and PAL was evaluated in different plant organs.
Mineral nutrients analysis
To determine the inorganic ions Na+, K+, and Ca2+, 0.25 g of dried matter were ashed in a muffle furnace for 6 h at 550 °C, afterwards dissolved in 3 mL of chloridric acid (5 N), and then diluted with distilled water up to 25 mL. These cations were analyzed in a flame-photometer Jen Woy PFP7 according to the procedure described by Saqrane et al. (2009). Nitrogen content in shoots and roots was dosed by the Kjeldahl procedure as described by Lahrouni et al. (2012).
Photosynthetic parameters
Chlorophyll analysis
Three weeks after exposure to Microcystis aqueous extract, chlorophyll (a + b) content was determined to assess the impact of MCs on photosynthetic pigments. Chlorophylls a and b were extracted in the dark, and analyzed following the methodology described by Geider and Osborne (1992). Briefly, 50 mg of leaves were homogenized in 2 mL of aqueous acetone 90 % (v/v). The homogenate was centrifuged (10,000×g for 5 min), and the pellet was extracted twice with 0.5 mL of 90 % acetone. The concentration of chlorophyll a and b was calculated from the absorbance at 664 and 647 nm.
Photosynthetic activity
Photosynthetic activity was assayed by fluorescence in dark-adapted leaves, using a portable fluorometer (Handy Plant Efficiency Analyser, Hansatech Instruments Ltd), after illumination at a light intensity of 3,500 μmole photons m−2 s−1. Photosystem II (PSII) was estimated by the ratio (Fm–F0)/Fm (also known as Fv/Fm) (Maxwell and Johnson 2000), where F0 is the minimum fluorescence, corresponding to the open state of PSII reaction centers, and Fm, the maximum fluorescence, corresponding to a closed state of PSII reaction centers. The fluorescence parameters were calculated automatically using Handy PEA v 1.3 software.
Enzymatic assays
Extraction and assay of POD activity
Samples (100 mg) were ground with 5 % polyvinyl polypyrrolidone (PVPP), and homogenized with 1.5 mL sodium phosphate buffer (0.1 M, pH 7). The samples were centrifuged at 9,000 rpm at 4 °C for 30 min. The supernatants were used as crude enzyme sources to assay POD enzymatic activity according to the method of Venisse et al. (2001) with some modifications. Guaiacol was used as the substrate. Oxidation of guaiacol to tetraguaiacol was monitored spectrophotometrically at 470 nm for 2 min at 30 °C.
Extraction and assay of CAT activity
The method of extraction as described by Gong et al. (2001) was used in the experiment with some modifications. One hundred milligrams of fresh weight of tissue was homogenized in 2.5 mL of 50 mM Tris–HCl buffer (pH 8.5) including 2 mM EDTA and 5 % (w/v) PVPP. The homogenate was centrifuged at 12,000 rpm for 20 min at 4 °C. Supernatant was used for the activity measurement. Catalase (CAT) activity was determined by following the disappearance of H2O2 in the enzyme reaction mixture (Du and Bramlage 1994). The enzyme extract (100 μL) was added to 800 μL assay mixture (50 mM Tris–HCl buffer pH 6.8, containing 5 mM H2O2). The reaction was stopped by adding 100 μL 20 % titanium tetrachloride (in concentrated HCl, v/v) after 10 min at 20 °C. A blank was prepared by addition of 100 μL 20 % titanium tetrachloride at zero time to stop the enzyme activity. The absorbance of the reaction solutions was read at 415 nm against water. CAT activity was determined by comparing absorbance against a standard curve of H2O2 from 0.25 to 2.5 mM.
Extraction and assay of PPO activity
For PPO assay, samples of 100 mg of different tissues were ground with 5 % polyvinyl polypyrrolidone, and homogenized with 2.5 mL sodium phosphate buffer (0.1 M, pH 6.4). The samples were centrifuged at 9,000 rpm at 4 °C for 30 min. The supernatants were used as crude enzyme sources. The PPO activity was determined according to the method of Jiang et al. (2002), by adding 100 μL of enzyme preparation to 1 mL of 10 mM catechol (dissolved in 100 mM sodium phosphate, pH 6.4) as a substrate. Absorbance at 420 nm was measured. The activity was expressed in units (U) per milligram protein. One unit was defined as Δ A420 of 0.01 per minute.
Extraction and assay of PAL activity
Phenylalanine ammonia lyase activity was measured according to the method of Camm and Towers (1973) with some modifications. The reaction mixtures contained 250 μL supernatant with 1 mL 0.1 mol L−1 borate buffer (pH 8.8) and 10 mmol L−1 L-phenylalanine. After incubation of the mixtures at 30 °C for 1 h, the reaction was terminated by the addition of 0.2 mL of 5 N HCl. The increase in absorbance at 290 nm, due to the formation of trans-cinnamate, was measured spectrophotometrically.
Extraction and analysis of phenolic compounds
The extraction of leaf, root, and nodules samples for the determination of total phenolics was made and the total phenolic content (TPC) of extracts was assessed by using the Folin-Ciocalteau phenol reagent method (Singleton and Rossi 1965). To 100 μL of the sample extracts (diluted 1:5 (v/v) with methanol), 1,750 μL of bidistilled water, and 250 μL of Folin-Ciocalteau reagent (diluted 1/3 v/v) were added; after 5 min incubation at room temperature, 500 μL of sodium carbonate (20 % w/v) were added. The extracts were mixed and incubated for 30 min at 30 °C before measuring the absorbance at 760 nm. A mixture of water and reagents was used as a blank. The TPC was expressed as caffeic acid equivalents in milligrams per gram of fresh weight of plant tissue.
Statistical analysis
The experimental design was a randomized complete block. The growth values, the parameters related to nodulation were means of seven replicates per treatment and parameters related to the enzymatic activities were means of five replicates per treatment. Data were analyzed by variance analysis, and the mean separation was achieved by LSD test by the COSTAT software. All numeric differences in the data were considered significantly different at the probability level of P ≤ 0.05.
Results
Plant growth responses and symbiotic performance
The bloom extract used in this work was previously analyzed (El Khalloufi et al. 2011). HPLC-PDA data obtained from the extract of the M. aeruginosa bloom used in this study revealed a mixture of five variants of MCs: DMC-LR (4.20 %); MC-(H4)-YR (4.32 %); MC-LY (8.32 %); MC-FR (9.45 %), and MC-LR (73.71 %). The total MCs content of the bloom extract was 22.24 μg of MC/mL.
After 4 weeks of faba bean exposure to cyanobacterial bloom extract containing 50 and 100 μg/L total MCs, no visual morphological changes were perceptible compared with the controls. At the end of the experiment (5 weeks), the number of leaves and nodes were counted and the length of shoot and root parts were measured. The obtained results showed that there were no significant differences in total nodes and leaves number and shoot length between plants exposed and not exposed to cyanobacterial bloom extract (Table 1). However, the effect of MCs exposure seems to be related with a significant decrease of shoot dry weight (up to 31 % at 100 μg/L total MCs).
The root part appeared to be more sensitive to MCs than the shoot. All the investigated root parameters, root length (RL), root dry weight (RDW), total nodule dry weight (NDW), and the total number of nodules (NN), showed decreased values in the presence of MCs (Table 1). Indeed, a reduction of 19 % in RL was registered after exposure to 100 μg/L cyanotoxins MC, whereas up to 53 % reduction in RDW was observed at this MCs concentration. In addition, remarkable visual changes were noticed on plant roots exposed to MCs. This effect became more evident as the experiment progressed after 18 days; the roots of plants exposed to MCs were dark brown due to necrosis and very short (Fig. 1). Nodulation was very sensitive toward MCs; the reduction of two nodulation parameters, NN and NDW, reached 44 and 36 % at 100 μg/L of MC, respectively (Table 1).
Content of nitrogen and mineral nutrients in plant tissues
Figure 2 illustrates the mineral nutrient (Ca2+, K+, and Na+) contents in V. faba plants treated with cyanotoxins (50 and 100 μg/L total MC) in comparison to the controls (unexposed). A significant decreases in K+ (around 20 %) and Ca2+ (around 30 %) contents in shoots, relative to controls, were observed (Fig. 2a). In the roots, only K+ contents differed significantly between the plants exposed and not exposed to cyanotoxins (up to 20 % reduction in K+ levels in roots). As for Na+, cyanotoxins caused a significant increase of Na+ contents both in shoots and roots of V. faba (between 5 and 15 %). The effect of cyanotoxins on nitrogen content by faba bean plants inoculated with RhOF4 was also established. The total nitrogen contents in both shoots and roots of V. faba were significantly decreased (p < 0.05) especially at exposure to 100 μg/L total MC (Fig. 2c). Nevertheless, this reduction was up to a 10–15 %. Since no N was added to the watering solution (except for the low amount of N present in the cyanobacterial crude extract added to the watering solution), the N content of the plants reflects differences in N2-fixation in root nodules.
Mineral nutrient accumulation in shoot (a) and root (b), and nitrogen assimilation (c) by V. faba L. inoculated with the MCs-tolerant strain RhOF4 exposed to cyanobacterial bloom extract containing 50 and 100 μg/L total MCs, compared to unexposed control plants. Data are means of seven replicates; differences in the data were considered significantly different at the probability level of P ≤ 0.05
Photosynthetic activity
In order to evaluate the effect of cyanotoxins on the photosynthetic activity, two parameters were evaluated, chlorophyll content and chlorophyll fluorescence (Fig. 3). The obtained results showed that exposure of Medicago sativa seedlings to different concentrations of MCs led to photosynthetic disorders. Indeed, 100 μg/L total MC induced significantly lower total chlorophyll “a” and “b” contents than the control plants (Fig. 3a; maximum reductions of 10–15 % were observed). The photosynthesis performance was determined after dark adaptation by analyzing Fv/Fm, which represents the maximum quantum yield of PSII (Maxwell and Johnson 2000). The Fv/Fm value decreased by 10–15 % as the concentration of toxin increased (Fig. 3b). This value was around 0.8 for the control plants; whereas for exposed plants this value was significantly lower (0.740 and 0.658 when faba bean plants were exposed respectively to 50 and 100 μg/L total MC). Values of Fv/Fm lower than 0.8 will reveal the plant stress condition, indicating a particular photo-inhibition phenomenon (Johnson et al. 1993).
Antioxidant defense reaction of rhizobia–V. faba symbiosis under MCs exposure
To provide further evidence for the influence of cyanobacterial secondary metabolites in plant stress, several ROS scavenging enzymes have been determined. The activities of POD and CAT showed marked increases, especially when the plants were exposed to cyanobacterial bloom extract containing 100 μg/L total MCs, indicating a strong increase of the oxidative damage (Fig. 4). The activities of POD and CAT were induced in a dose-dependent manner. At 50 μg/L total MCs, increases of 200–300 % in enzyme activities were found, depending on the enzyme and the specific tissue (Fig. 4). In general, at dose 100 μM total MCs/L, induction of POD and CAT reached 300–400 % relative to control unexposed plants. Moreover, for POD, the highest activity levels were found in nodules, as compared to leaves and roots, whereas CAT showed similar activity in all the tissues.
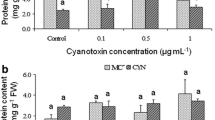
In order to evaluate the effect of cyanotoxins on the detoxification process mediated by phenolic compounds, three parameters were measured; quantification of PPO and PAL activities and phenolics content. In leaves of plants exposed to MCs, PAL activity was strongly induced in a dose-dependent manner (Fig. 5a). Maximum induction was recovered at 100 μg/L (3-fold increased activity). However, in roots and nodules changes were minor or no significantly different. By contrast, the PPO activity showed the maximum induction in nodules (up to 10-fold increased level of activity), whereas the increases of activity in roots and leaves were of three to four times (Fig. 5b). Regarding the concentration of phenolic compounds, there was an increase in all the tissues, which was related to microcystins concentration (Fig. 5c). In leaves and roots, the increase of phenolic compounds reached 1.5–2-fold, whereas in nodules a 2.5-fold increase in phenolic compounds was observed at the maximum MCs concentration.
Activity of phenylalanine ammonia lyase (a), polyphenoloxidase (b), and phenolic compounds content (c) in faba bean plants after 4 weeks exposure to 50 and 100 μg/L total MCs compared to control plants. Significant differences between control and treated plants are indicated by letters a, b, and c (P < 0.05)
Discussion
This study shows the long-term effects of cyanobacterial crude extract containing cyanotoxins on the physiological and biochemical defense reactions of faba bean–rhizobia symbiosis. Two important approaches were undertaken in this work: on the one hand, the experimental design used mimics the real MCs concentration present in raw irrigation water used in the Marrakech region. On the other hand, the Rhizobium strain used for plant inoculation is a MC-tolerant strain. The approach followed in this work is trying to reproduce the natural conditions occurring after cyanobacterial bloom (cell lysis and cyanotoxin release in the water). In our experiments, the raw crude extract is used, instead of pure MCs. In this case, all other substances present in the cell extract are also present in the water, and can affect the plants upon irrigation. Thus, we must also consider that cyanotoxins are only around 1 % of all the substances of the bloom extract (Bláha et al. 2009), so the influence of other substances, besides cyanotoxins, cannot be ruled out. However, this is the “real” situation occurring in water reservoirs.
Several studies have shown that the effect of cyanotoxins on crop plants depends on plant sensitivity, the concentration of cyanotoxins, and the time of exposure. In general, the effect was more apparent on roots than in shoots. In our case, no morphological alterations in the shoot were observed, whereas a marked effect on roots, which looked brown, shorter, and thinner. Similar results were shown in other legumes, such as Phaseolus vulgaris (McElhiney et al. 2001) and in M. sativa (El Khalloufi et al. 2011). Exposure time is a key factor in the extent of the damage. Whereas short exposure times did not affect dry matter of several varieties of bean or rice plants (Pichardo and Plugmacher 2010; Prieto et al. 2011), long-term exposure produced necrosis in leaves of Phaseolus vulgaris (McElhiney et al. 2001) and spinach (Pflugmacher et al. 2007). Plant sensitivity is also critical; while stem malformations, plant browning, and root inhibition occurred in white mustard (Sinapis alba L.) at 5 μg/L MC-LR (Kós et al. 1995), no significant differences were reported in broccoli and mustard exposed up to 10 μg/L MCs (Järvenpää et al. 2007).
Few studies have focused on the effects of the cyanotoxins to rhizobia–legume symbiosis. Nodulation is one of the plant processes most affected by cyanotoxins (El Khalloufi et al. 2011). In a previous work, several Rhizobium strains were isolated, with different tolerance against MCs (Lahrouni et al. 2012). In this work, a MC-tolerant strain of R. leguminosarum was used for plant inoculation in order to minimize the toxic effects of MCs on the plant. The use of resistant Rhizobium strains has been reported to alleviate plant stress caused by different abiotic factors. For instance, heavy-metal-resistant Rhizobium strains help legume plants cultivated in heavy-metals-polluted soils by protecting plant roots (Dary et al. 2010; Reichman 2007; Wani et al. 2008). Analogously, salt-tolerant Rhizobium strains also improve the yield of legumes under salt or drought stress (Ali et al. 2009; Bianco and Defez 2009; Singla and Garg 2006). From a practical point of view, application of a MC-tolerant Rhizobium may improve plant yield and nodulation when plants are irrigated with MCs-containing water. Our results indicate the successful root infection with this particular rhizobial strain (RhOF4), even at the highest MCs concentration, although nodulation was decreased. However, the reduction of 45 % in the NN led to only 15 % reduction in N content. These results suggest that nodules were effective in nitrogen fixation. Other positive effect of inoculation is related to the accumulation of toxic compounds in plants. The use of metal-resistant Rhizobium decreased the amount of metals taken up by plants (Dary et al. 2010; Wani et al. 2008). It could be interesting to test whether the use of a MCs-resistant Rhizobium could decrease the amount of toxins present in plant tissues, thus decreasing the entrance of the toxins into the food chain.
Intoxication with MCs (at 100 μg L−1) also had an adverse effect on photosynthetic activity of faba bean plants, as can be deduced from chlorophyll and Fv/Fm values. Impaired photosynthesis of terrestrial plants by cyanobacterial toxins was detected in many species, such as potato, maize, lens, wheat, pea, spinach, and alfalfa (El Khalloufi et al. 2011; Pflugmacher et al. 2007; McElhiney et al. 2001; Saqrane et al. 2009). This effect was related to the content of chlorophyll (Lichtenthaler 1987; Saqrane et al. 2009) or to impaired carboxylation efficiency (Abe et al. 1996). At the concentrations used in this work, these changes in photosynthesis led to a decrease of 30 % shoot dry matter, although other morphological changes were not seen. It could be possible that the defense mechanisms induced allow the plant to stand these MC concentrations, without being affected.
The presence of cyanotoxins has also effects on plant nutrition. K+ and Ca2+ content decreased whereas Na+ increased. Saqrane et al. (2009) have reported that MCs led to an increase in mineral nutrients content (K+ and Ca2+) in the roots of Lens esculenta, Pisum sativum, Zea mays, and Triticum durum plants. These results indicate that MCs may cause a disruption of the membrane permeability.
The promotion of oxidative stress in plants caused by exposure to cyanobacterial crude extract has been previously shown in different plants. In this work, several enzymes were determined aimed to test whether chronic exposure to bloom extract induced an oxidative stress in faba bean. In order to avoid damaging consequences of hydrogen peroxide, the most stable ROS, plants have evolved various enzymes able to detoxify this compound. Increased activity of antioxidative enzymes such as POD and CAT in all organs of faba bean clearly indicated that oxidative stress is promoted in plants exposed to cyanobacterial crude extract containing MCs. Analogous results were reported spinach (Pflugmacher et al. 2007), bean (Pichardo and Pflugmacher 2010), rape (Brassica napus) and rice (Oryza sativa) (Chen et al. 2004), coontail (Ceratophyllum demersum) and alfalfa (M. sativa) (Pflugmacher 2004; Pflugmacher et al. 2006; El Khalloufi et al. 2011), and in the cyanobacteria Synechocystis sp. (Vassilakaki and Pflugmacher 2007).
PAL and PPO are induced by a variety of stimuli, particularly under conditions of stress, including pathogen attack, wounding, and disease. PAL catalyzes the conversion of phenylalanine to cinnamic acid, which has an antioxidant effect in stress conditions (Archana et al. 2011; Boo et al. 2011). In our experiment, PAL activity in leaves and nodules was significantly altered when plants were watered with the cyanobacterial crude extract, while no changes were distinguished in this enzyme in roots. PPO may be involved in the browning reaction, or alternatively the browning reaction could be a secondary result of other metabolic events (Zhou et al. 2003).
Besides these enzymatic activities, others could be also involved in ROS scavenging, such as SOD, enzymes of the glutathione–ascorbate cycle, or the glutathione-S-transferase, involved in the formation of a glutathione–MC complex (Mitrovic et al. 2004; Pflugmacher et al. 1998, Pflugmacher 2004; Pflugmacher et al. 2007).
Phenolic compounds play an important role in the detoxification of free radicals including O2 ·−, H2O2, ·OH, and 1O2 (Ksouri et al. 2007). At the gateway from primary metabolism, L-phenylalanine ammonia lyase (PAL; EC 4.3.1.5) plays a pivotal role in phenolic synthesis and it has been amply demonstrated that there is a correlation between increased activity of this key enzyme and increases in phenolic compounds in response to different stimuli in plants (Boudet 2007; Sakihama et al. 2002). This could explain the increase of phenol contents in V. faba plants irrigated with cyanobacterial crude extract containing cyanotoxins, similar results to those found in Lemna gibba and alfalfa (Saqrane et al. 2007; El Khalloufi et al. 2011). Besides phenolic compounds, some other substances, such as ascorbate, dehydroascorbate, glutathione, glutathione disulfide, and tocopherol were reported to act as antioxidant scavengers in plants treated with cyanotoxins (Pflugmacher et al. 2007).
Conclusion
The present work clearly shows the oxidative stress induction in V. faba L. var. aguadulce during its symbiosis with a MC-tolerant Rhizobium strain exposed to cyanobacterial crude extract containing MC-LR. The activities of POD, CAT, PPO, and PAL were significantly increased in leaves, roots, and nodules of faba bean plants exposed to cyanobacterial bloom extract containing MCs at 100 μg/L of MCs. Survival of plants under stress conditions is possible if several antioxidants cooperate together with antioxidant enzymes, providing a good defense system and a quick and balanced regeneration of the active reduced forms of the antioxidants.
The cyanotoxins had also a negative effect on growth, nodulation process, nitrogen uptake, and mineral elements assimilation by V. faba–rhizobia symbiosis. The role of selected strains of rhizobia highly tolerant to cyanotoxins may be of great value and a friendly biotechnological pathway in order to improve faba bean productivity and tolerance towards cyanotoxins stress.
Abbreviations
- CAT:
-
Catalase
- Fv/Fm:
-
Ratio of variable to maximum fluorescence–the quantum efficiency of open photosystem II centers
- Fm:
-
Maximum fluorescence
- F0:
-
Minimum fluorescence yield
- HPLC-PDA:
-
High-performance liquid chromatography coupled to a photodiode array detector
- MC:
-
Microcystins
- MC-LR:
-
Microcystin-LR
- NN:
-
Number of nodules
- NDW:
-
Nodule dry weight
- PAL:
-
L-phenylalanine ammonia lyase
- POD:
-
Peroxidase
- PPO:
-
Polyphenoloxidase
- PSII:
-
Photosystem II
- RDW:
-
Root dry weight
- RL:
-
Root length
- TPC:
-
Total polyphenols content
References
Abe T, Lawson T, Weyers JDB, Codd GA (1996) Microcystin-LR inhibits photosynthesis of Phaseolus vulgaris primary leaves: implications for current spray irrigation practice. New Phytol 133:651–658. doi:10.1111/j.1469-8137.1996.tb01934.x
Ali SF, Rawat LS, Meghvansi MK, Mahna SK (2009) Selection of stress-tolerant rhizobial isolates of wild legumes growing in dry regions of Rajasthan, India. ARPN J Agric Biol Sci 4(1):13–18, http://www.arpnjournals.com/jabs/research_papers/rp_2009/jabs_0109_105.pdf
Archana S, Prabakar K, Raguchander T, Hubballi M, Valarmathi P, Prakasam V (2011) Defense response of grapevine to Plasmopara viticola induced by Azoxystrobin and Pseudomonas fluorescens. Int J Sustain Agric 3(1):30–38, http://idosi.org/ijsa/3(1)11/6.pdf
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107. doi:10.1093/jxb/erp140
Bláha L, Babica P, Maršálek B (2009) Toxins produced in cyanobacterial water blooms—toxicity and risks. Interdiscip Toxicol 2(2):36–41. doi:10.2478/v10102-009-0006-2
Bond DA, Lawes DA, Hawtin GC, Saxena MC, Stephens JS (1985) In: Summerfield RJ, Roberts EH (eds) Grain legume crops. Collins, London, pp 199–265
Boo HO, Heob B, Gorinsteinc S, Chond S (2011) Positive effects of temperature and growth conditions on enzymatic and antioxidant status in lettuce plants. Plant Sci 181:479–484. doi:10.1016/j.plantsci.2011.07.013
Boudet AM (2007) Evolution and current status of research in phenolic compounds. Phytochemistry 68:2722–2735. doi:10.1016/j.phytochem.2007.06.012
Camm EC, Towers GHN (1973) Phenylalanine ammonia lyase. Phytochemistry 12:961–973. doi:10.1016/0031-9422(73)85001-0
Chen J, Song L, Dai J, Gan N, Liu Z (2004) Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 43:393–400. doi:10.1016/j.toxicon.2004.01.011
Dary M, Chamber-Pérez MA, Palomares AJ, Pajuelo E (2010) “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater 177:323–330. doi:10.1016/j.jhazmat.2009.12.035
Du Z, Bramlage WJ (1994) Superoxide dismutase activities in senescing apple fruit (Malus domestica Borkh). J Food Sci 59:581–584. doi:10.1111/j.1365-2621.1994.tb05567.x
El Ghazali I, Saqrane S, Saker M, Youness O, Oudra B, Vasconcelos V, Del Campo FF (2011) Caractérisation biochimique et moléculaire d’efflorescences à cyanobactéries toxiques dans le réservoir Lalla Takerkoust (Maroc). Rev Sci l’eau 24(2):117–128. doi:10.7202/1006106ar (In french)
El Khalloufi F, Oufdou K, Lahrouni M, El Ghazali I, Saqrane S, Vasconcelos V, Oudra B (2011) Allelopatic effects of cyanobacteria extracts containing microcystins on Medicago sativa–rhizobia symbiosis. Ecotoxicol Environ Saf 74:431–438. doi:10.1016/j.ecoenv.2010.10.006
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonrnal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Geider RJ, Osborne BA (1992) Algal photosynthesis. Chapman & Hall, New York, OCLC 23082055
Gong Y, Toivonen P, Lau OL, Wiersma PA (2001) Antioxidant system level in Braeburn apple is related to its browning disorder. Bot Bull Acad Sin 42:259–264, http://ejournal.sinica.edu.tw/bbas/content/2001/4/bot424-04.html
Järvenpää S, Lundberg-Niinistö C, Spoof L, Sjövall O, Tyystjärvi E, Meriluoto J (2007) Effects of microcystins on broccoli and mustard, and analysis of accumulated toxin by liquid chromatography–mass spectrometry. Toxicon 49:865–874. doi:10.1016/j.toxicon.2006.12.008
Jiang AL, Tian SP, Xu Y (2002) Effects of controlled atmospheres with high-O2 or high-CO2 concentrations on post harvest physiology and storability of “Napoleon” sweet cherry. Acta Bot Sin 44:925–930, http://www.jipb.net/pubsoft/content/2/2313/X010590(PS2).pdf
Johnson GN, Young AJ, Scholes JD, Horton P (1993) The dissipation of excess excitation energy in British plant species. Plant Cell Environ 16(6):673–679. doi:10.1111/j.1365-3040.1993.tb00485.x
Kinnear SHW, Fabbro LD, Duivenvoorden LJ (2008) Variable growth responses of water thyme (Hydrilla verticillata) to whole-cell extracts of Cylindrospermopsis raciborskii. Arch Environ Contam Toxicol 54:187–194. doi:10.1007/s00244-007-9026-0
Kós P, Gorzó G, Surányi G, Borbély G (1995) Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Anal Biochem 225:49–53. doi:10.1006/abio.1995.1106
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem 45:244–249. doi:10.1016/j.plaphy.2007.02.001
Lahrouni M, Oufdou K, Faghire M, Peix A, El Khalloufi F, Vasconcelos V, Oudra B (2012) Cyanobacterial extracts containing microcystins affect the growth, nodulation process and nitrogen uptake of faba bean (Vicia faba L., Fabaceae). Ecotoxicology 21:681–687. doi:10.1007/s10646-011-0826-7
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. doi:10.1016/0076-6879(87)48036-1
MADRPM/DERD (2006) Statistiques du Ministère de l’Agriculture, du Développement Rural et des Pêches Maritimes, Maroc. http://www.madrpm.gov. (In french)
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51(345):659–668. doi:10.1093/jexbot/51.345.659
McElhiney J, Lawton LA, Leifert C (2001) Investigations into the inhibitory effects of Microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 39:1411–1420. doi:10.1016/S0041-0101(01)00100-3
Mitrovic SM, Pflugmacher S, James KJ, Furey A (2004) Anatoxin-a elicits an increase in peroxidase and glutathione S-transferase activity in aquatic plant. Aquat Toxicol 68:185–192. doi:10.1016/j.aquatox.2004.03.017
Oudra B, Loudiki M, Sbiyyaa B, Martins R, Vasconcelos V, Namikoshi M (2001) Isolation, characterization and quantification of microcystins (heptapeptides hepatotoxins) in Microcystis aeruginosa dominated bloom of Lalla Takerkoust lake reservoir (Morocco). Toxicon 39:1375–1381. doi:10.1016/S0041-0101(01)00093-9
Oudra B, Loudiki M, Sbiyyaa B, Sabour B, Amorim A, Martins R, Vasconcelos V (2002) Detection and variation of microcystin contents of Microcystis blooms in eutrophic Lalla Takerkoust lake (Morocco). Lakes Reserv Res Manag 7:35–44. doi:10.1046/j.1440-1770.2002.00165.x
Peuthert A, Chakrabarti S, Pflugmacher S (2007) Uptake of microcystins-LR and -LF (cyanobacterial toxins) in seedlings of several important agricultural plant species and the correlation with cellular damage (lipid peroxidation). Environ Toxicol 22(4):436–442. doi:10.1002/tox.20266
Pflugmacher S, Wiegand C, Oberemm A, Beattie KA, Krause E, Codd GA, Steinberg CEW (1998) Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: the first step of detoxication. Biochim Biophys Acta 1425(3):527–533
Pflugmacher S (2004) Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin Microcystin-LR. Aquat Toxicol 70:169–178. doi:10.1016/j.aquatox.2004.06.010
Pflugmacher S, Jung K, Lundvall L, Neumann S, Peuthert A (2006) Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environ Toxicol Chem 25:2381–2387
Pflugmacher S, Aulhorn M, Grimm B (2007) Influence of a cyanobacterial crude extract containing microcystin-LR on the physiology and antioxidative defense systems of different spinach variants. New Phytol 175:482–489. doi:10.1111/j.1469-8137.2007.02144.x
Pichardo S, Pflugmacher S (2010) Study of the antioxidant response of several bean variants to irrigation with water containing MC-LR and cyanobacterial crude extract. Environ Toxicol 26:300–306. doi:10.1002/tox.20622
Prieto A, Campos A, Cameàn A, Vasconcelos V (2011) Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol Environ Saf 74:1973–1980. doi:10.1016/j.ecoenv.2011.06.009
Reichman S (2007) The potential use of the legume–rhizobium symbiosis for the remediation of arsenic contaminated sites. Soil Biol Biochem 39:2587–2593. doi:10.1016/j.soilbio.2007.04.030
Rigaud J, Puppo A (1975) Indole-3-acetic acid catabolism by soybean bacteroids. J Gen Microbiol 88:223–228, http://mic.sgmjournals.org/content/88/2/223.full.pdf
Sakihama Y, Cohen MF, Grace SC, Yamasaki H (2002) Plant phenolic antioxidant and pro-oxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 177:67–80. doi:10.1016/S0300-483X(02)00196-8
Saqrane S, Oudra B (2009) CyanoHAB occurrence and water irrigation cyanotoxins contamination: ecological impacts and potential health risks. Toxins 1:113–122. doi:10.3390/toxins1020113
Saqrane S, El Ghazali I, Ouahid Y, El Hassani M, El Hadrami I, Oudra B, Bouarab L, del Campo FF, Vasconcelos V (2007) Phytotoxic effects of cyanobacteria extract on the aquatic plant Lemna gibba: microcystin accumulation, detoxification and oxidative stress induction. Aquat Toxicol 83:284–294. doi:10.1016/j.aquatox.2007.05.004
Saqrane S, Ouahid Y, El Ghazali I, Oudra B, Bouarab L, Del Campo FF (2009) Physiological changes in Triticum durum, Zea mays, Pisum sativum and Lens esculenta cultivars, caused by irrigation with water contaminated with microcystins: a laboratory experimental approach. Toxicon 53:786–796. doi:10.1016/j.toxicon.2009.01.028
Singla R, Garg N (2006) The legume–rhizobia symbiosis under salt stress—a review. Agric Rev 27(1):1–21, http://arccjournals.com/pdf/Reviews/ar-27-1/ar-27-1-001.pdf
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic phospholungistic acid reagents. Am J Enol Vitic 16:144–158, http://ajevonline.org/content/16/3/144.full.pdf
Vassilakaki M, Pflugmacher S (2007) Oxidative stress response of Synechocystis sp. (PCC 6803) due to exposure to microcystin-LR and cell-free cyanobacterial crude extract containing microcystin-LR. J Appl Phycol 20:219–225. doi:10.1007/s10811-007-9222-3
Venisse JS, Gullner G, Brisset MN (2001) Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol 125:2164–2172. doi:10.1104/pp.125.4.2164
Wani PA, Khan MS, Zaidi A (2008) Chromium-reducing and plant growth-promoting Mesorhizobium improves chickpea growth in chromium-amended soil. Biotechnol Lett 30(1):159–163. doi:10.1007/s10529-007-9515-2
Zhou Y, Dahler JM, Underhill SJR, Wills RBH (2003) The enzymes associated with blackheart development in pineapple. Food Chem 80:565–572. doi:10.1016/S0308-8146(02)00375-8
Zurawell RW, Chen H, Burke JM, Prepas EE (2005) Hepatotoxic cyanobacteria: a review of the biological importance of microcystins in freshwater environments. J Toxicol Environ Health 68:1–3. doi:10.1080/10937400590889412
Acknowledgments
This study is financially supported by the International Foundation for Sciences (IFS project F/2826-3F). This work is also carried out within the framework of the Morocco-Spanish collaboration (AECID project n° A1/035873/11).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Lahrouni, M., Oufdou, K., El Khalloufi, F. et al. Physiological and biochemical defense reactions of Vicia faba L.–Rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ Sci Pollut Res 20, 5405–5415 (2013). https://doi.org/10.1007/s11356-013-1535-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1535-y