Abstract
Chromium (Cr) contamination in soil–plant systems poses a pressing environmental challenge due to its detrimental impacts on plant growth and human health. Results exhibited that Cr stress decreased shoot biomass, root biomass, leaf relative water content, and plant height. However, single and co-application of Bacillus subtilis (BS) and arbuscular mycorrhizal fungi (AMF) considerably enhanced shoot biomass (+ 21%), root biomass (+ 2%), leaf relative water content (+ 26%), and plant height (+ 13) under Cr stress. The frequency of mycorrhizal (F) association (+ 5%), mycorrhizal colonization (+ 13%), and abundance of arbuscules (+ 5%) in the non-stressed soil was enhanced when inoculated with combined BS and AMF as compared to Cr-stressed soil. The co-inoculation with BS and AMF considerably enhanced total chlorophyll, carotenoids, and proline content in Cr-stressed plants. Cr-stressed plants resulted in attenuated response in SOD, POD, CAT, and GR activities when inoculated with BS and AMF consortia by altering oxidative stress biomarkers (H2O2 and MDA). In Cr-stressed plants, the combined application of BS and AMF considerably enhanced proline metabolism, for instance, P5CR (+ 17%), P5CS (+ 28%), OAT (− 22%), and ProDH (− 113%) as compared to control. Sole inoculation with AMF downregulated the expression of SIPIP2;1, SIPIP2;5, and SIPIP2;7 in Cr-stressed plants. However, the expression of NCED1 was downregulated with the application of sole AMF. In contrast, the relative expression of Le4 was upregulated in the presence of AMF and BS combination in Cr-stressed plants. Therefore, it is concluded that co-application of BS and AMF enhanced Cr tolerance by enhancing proline metabolism, antioxidant enzymes, and aquaporin gene expression. Future study might concentrate on elucidating the molecular processes behind the synergistic benefits of BS and AMF, as well as affirming their effectiveness in field experiments under a variety of environmental situations. Long-term research on the effect of microbial inoculation on soil health and plant production might also help to design sustainable chromium remediation solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the rhizosphere of plants, arbuscular mycorrhizal fungi (AMF) are significant microorganisms. Their relationship is symbiotic about 80% of terrestrial plants, enhancing the plant’s capacity to acquire essential nutrients and water. The plants reciprocate by providing these fungi with up to 20% of the carbon produced during photosynthesis (Xie et al. 2020). Many research has shown colonization of plants by AMF improves the plant’s ability to withstand non-living environmental stressors by regulating the levels of polyamines and fatty acids, promoting the defense mechanisms against oxidative damage, facilitating adjustments in osmotic balance, directly absorbing water through the mycorrhizal extraradical mycelium, releasing glomalin to stabilize soil particles, and controlling the expression of genes that respond to stress (Augé, 2001; Allen 2007; Kumar et al. 2015). The findings derived from the studies by Wu et al. in 2019, which make references to the studies conducted by Jia-Dong et al. (2019) and Zhang et al. (2019). The investigations undertaken by Zou et al. (2013a, 2013b) and Zou et al. (2021a, 2021b) are also pertinent in this context.
Two specific types of AMFs, Funneliformis mosseae and Paraglomus occultum, have been proven to greatly improve the metabolic processes of sucrose and proline in leaves by overseeing and controlling the actions of enzymes involved in sucrose and proline metabolism. The osmotic equilibrium in the host plant is of utmost significance (Wu et al. 2017). In addition, a research investigation conducted by (Zou et al. 2013a, 2013b) indicates that the incorporation of Glomus versiforme significantly enhances the structure of plant roots and the buildup of soluble sugars in plants exposed to severe salinity. In addition, the introduction of Diversispora spurca has been discovered to alleviate the adverse effects impacts of waterlogging stress (WS) on the growth of citrus plants, precisely modifying their fundamental structure and levels of antioxidant enzymes (Zou et al. (2013a, 2013b). AMF has a crucial function in reducing the harmful effects of abiotic stress on host plants.
Aquaporins (AQPs) are stress-responsive genes that are situated in distinct areas of the nuclear membrane in plants. They enhance the transportation of water (Cheng et al. 2020). AQPs are transmembrane proteins that allow water and small solutes to passively permeate biological membranes (Reinhardt et al. 2016). The abundance and functionality of AQP proteins in cell membranes are vital to determine water uptake in plants, which in turn impacts other physiological processes (Jiang et al. 2020). Five prominent subfamilies of AQPs are X intrinsic proteins (XIPs), small basic intrinsic proteins (SIPs), nodin-26 like intrinsic proteins (NIPs), tonoplast intrinsic proteins (TIPs), and plasma membrane intrinsic proteins (Kapilan et al. (2018)). The AQP subfamilies are found in several locations within plant cells, including symbiotic membranes, vacuole membranes, vacuole generating structures, and protoplasmic membranes (Maurel et al. (2008)). Glycerin, boric acid, urea, carbon dioxide, formaldehyde, hydrogen peroxide, and urea may enter PIP1, which has limited water transport capacity. Contrarily, PIP2 has markedly higher water transportation capability in comparison to PIP1. TIPs demonstrate the greatest water permeability among plant AQPs, along with the ability to transport glycerin and urea. NIPs are involved in the transportation of water and glycerin, as evidenced by Maurel et al. (2008) (Reinhardt et al. (2016), and Kapilan et al. (2018).
AQPs have vital function of controlling the absorption of water, promoting the growth of plants, and influencing the activation different stresses affect stress-related genes (Liang et al. 2013). These proteins aid in the swift transportation of water and different tiny molecules, such as glycerin, amino acids, hydrogen peroxide, polypeptides, and ions, through cell membranes. As a result, they have a role in various aspects of plant development (He et al. 2016). Scientific literature extensively documents that AMF elicit specific patterns of responsiveness in the expression of AQP genes in host plants when they are exposed to unfavorable environmental conditions (Wu et al. 2013a, b) (Zou et al. 2019). An investigation by Ding et al. (2020) revealed that the presence of P. occultum in the roots of trifoliate orange under salt stress (SS) leads to an increase in the expression of PtTIP4;1, while simultaneously reducing the expression of PtTIP1;1, PtTIP1;3, PtTIP2;2, PtTIP1;2, and PtTIP2;1. Moreover, a study conducted by Quiroga et al. (2019) suggests that maize plants that have been exposed to AMF demonstrate enhanced water movement in their root cells. This is attributed to the heightened activity of PIP2 water-responsive scarcity in the soil. Furthermore, the increase in the ability of water flow through the roots of tomato plants due to the mutual relationship with mycorrhizal fungi can be linked to the activation of both the fungal aquaporin GintAQP1 and the plant aquaporin SlPIP1;7 genes during periods of water scarcity (Calvo-Polanco et al. 2014). Patterns of expression AQP in host plants during mycorrhization indicate diverse ways for dealing with abiotic stress, as emphasized by Cheng et al. (2020). It is crucial to acknowledge that prior studies have mostly investigated the regulatory impacts of specific categories of AQPs by AMF. Nevertheless, relying solely on the assessment of mycorrhizas using a particular AQP subfamily may not provide a thorough and all-encompassing outcome (Bárzana et al. 2014). The modification in the ability of host plants to withstand stress caused by mycorrhizas is likely due to the collective impacts of different aquaporins, such as PIPs, TIPs, NIPs, and SIPs (Ruiz-Lozano & Aroca 2017). The research carried out by Bárzana et al. (2014) examined the expression levels of expression of 36 AQP genes in maize, with a specific focus on how they respond to mycorrhizal fungus in drought conditions. The findings indicated that AM symbiosis influenced the expression of 16 AQP genes, including PIPs, TIPs, NIPs, and SIPs. The level of control varied according on drought stress intensity. Thus, the intricate interaction between AMF-AQP-host plant-abiotic stresses requires future research to identify host plant AQP subfamily genes and improve our understanding of mycorrhizal activities.
So, we hypothesis that mycorrhizosphere bacteria play an important role in reducing chromium-induced phytotoxicity in tomato plants by controlling proline metabolism, the antioxidant defense system, and the expression of aquaporin genes. This hypothesis is based on earlier studies indicating that bacterial and fungal symbiosis may improve plant stress tolerance and soil health. By studying the interactions between mycorrhizosphere bacteria and chromium-contaminated soil, we aim to provide insights into sustainable techniques for chromium remediation and plant protection.
Material and methods
Materials and experimental design
The uppermost layer of soil (0–20 cm) with a sandy loamy structure, pH of 7.5, 0.73 m/S electrical conductivity, carbon 9600 mg kg−1, nitrogen 65.98 mg kg−1, phosphorous 18.78 mg kg−1, and potassium 80.67 mg kg−1 was collected from the fields adjacent to the research station. The soil was sterilized by subjecting it to autoclaving at a temperature of 121 °C for a duration of 2 h. The sterilized material was then utilized to fill pots with dimensions of 21 cm in height and 20 cm in diameter, with a total weight of 7 kg of earth in each pot. Seven days before to sowing, an AMF inoculum containing Rhizophagus irregularis in the form of tomato root pieces and spores was supplied at a rate of 50 g, which is approximately equivalent to 700 spores. The AMF inoculum was collected from the research station and composed of tomato roots infected with spores. The pots were moved to a chamber with a photoperiod of 16 h of daylight and 8 h of darkness. Light intensity was set at 300 μmol−2 s−1 and the temperature during the maintained day and night 22/18 °C, respectively. A constant relative humidity of 60–70% was maintained.
Tomato variety Riogrande seeds were acquired from a commercial source. The viable and mature seeds were subjected to surface sterilization by immersing them in a solution of NaClO with 0.1% concentration for 15 min. After that, they went through a Milli-Q water for a duration of 20 min. Our wet sieve and sucrose density centrifugation methods proved AMF spores in the inoculum for spore extraction (Pacioni 1992). Additionally, root portions were stained with an ink/vinegar solution and examined under a microscope to further confirm their presence (Vierheilig et al. 1998). Endophytic bacterium Bacillus subtilis (an extracellular polymeric substance-producing strain, previously used in our study) was also used in this experiment (Shah et al. 2020). For 3 days at 28 °C, the strain was grown in a nutrient broth medium (CM0001; OXOID) with a final concentration of 108 cfu/mL. Tomato plants were inoculated by immersing their seedlings in a bacterial culture and then having 1 mL of inocula poured around each seedling to ensure success. Combinations of treatments included (a) no treatment, (b) Bacillus subtilis, (c) AMF, (d) Bacillus subtilis + AMF, (e) Bacillus subtilis + Cr, (f) AMF + Cr, and (g) Bacillus subtilis + AMF + Cr. Each container’s soil moisture level was measured as its volumetric water content (%) using a MiniTrase time domain reflectometer (TDR, Soil Moisture Equipment Corp, CA, USA). An entirely random design with three duplicates was used to organize the experimental containers. To prevent nutrient shortage, pots were checked and watered regularly (70% ± 5), and a diluted Hoagland solution was added weekly. When plants were put under Cr stress for 50 days, they were carefully removed and taken to the lab. To preserve the root tissue for later use, it was preserved in a formalin:acetic acid:ethanol solution.
Measurement of growth characters
Tomato plants were removed from three separate containers and rinsed with distilled water for each treatment. The root and shoot dry weights were measured. The weight in dry matter of the harvested shoots and roots was recorded right away. Dry weight was obtained by drying materials at 70 °C. Each plant had a sample of its current leaves taken to determine its LRWC. A digital balance was used to determine the weight of newly harvested leaves. The leaves were then stored in 5 mM CaCl2 solution for 24 h to restore turgidity, after which they were weighed once more to determine their turgid weight. The leaves were then dried in an oven at 70 °C for 72 h.
Measurement of photosynthetic pigments
Using the approach given by Arnon (1949), the chlorophyll (a and b) and carotenoid concentrations of fully inflated leaves were calculated. After centrifuging the leaf samples at 12,000g for 10 min, they were incubated in 10 mL of an aqueous 80% acetone solution. The absorbance of the extracts was checked at nm values of 663, 645, and 470. Chlorophyll a, b, and carotenoids were assessed using UV-spectrophotometer (Kyoto measured by the method provided by Karimi and Ghasempour (2019). We used trichloroacetic acid to extract leaf samples (0.5 g) and centrifuged them at 12,000g for 15 min. Finally, 0.5 mL of enzyme supernatant (produced by Shimadzu, Japan) was obtained.
Quantification of hydrogen peroxide (H2O2) and malondialdehyde (MDA)
A phosphate buffer with a pH of 7.0 (0.5 mL) and 1 mM potassium iodide were treated with an H2O2 concentration. Absorption capacity of the reactants were established at 390 nm using H2O2 as the standard. According to Heath and Packer (1968), the level of MDA in tomato leaves was calculated. In short, a 0.5-g leaf sample was mixed with 10 mL of ethanol and centrifuged at 10,000 g for 10 min. Two milliliters of a thiobarbituric acid (TBA) and trichloroacetic acid (TCA) mixture (0.65% and 20%, respectively) were added to 1 mL of enzyme extract. After boiling for 30 min, the concoction was chilled, then centrifuged at 10,000g for another 5 min. Nonspecific absorbance difference between 600 and 532 nm was used to calculate MDA concentration.
Determination of antioxidants enzymes
Fresh tomato leaves were extracted in a 5-mL solution of phosphate buffer containing 50 mM of EDTA (0.2 mM) and PVP-40 (2%) at a pH of 7.8, and the enzyme concentrations were calculated. The homogenate was centrifuged at 4 °C for 10 min at an acceleration of 12,000 times the force of gravity. The supernatant, the liquid leftover after the solids have been removed, was used in an analysis of enzyme function. Similar enzyme extracts were utilized to ascertain the existence of soluble proteins. The activity of superoxide dismutase (EC 1.15.1.1, SOD) was quantified using a method devised by Giannopolitis and Ries (1977). The procedure relies on the photochemical reduction of nitroblue tetrazolium. An enzyme unit is the amount of enzyme required to prevent the photoreduction of nitroblue tetrazolium by 50% when exposed to light at 560 nm. The method for measuring catalase activity (EC1.11.1.6, CAT) was proposed by Aebi in 1984. The rate of decomposition of H2O2 was determined by tracking the change in absorbance at a specific wavelength of 240 nm throughout a period of three minutes. The activity of glutathione peroxidase (EC 1.11.1.9, GPX) was assessed using hydrogen peroxide as the substrate (Ahmad et al. 2018).
Mycorrhizal colonization
We combined two root systems in each plot. The method for cleansing and coloring the root substance was adopted from Vierheilig et al. (1998). The roots were subjected to a temperature of 90 °C for a duration of 5 to 10 min in a solution containing 10% potassium hydroxide (KOH). The object will acquire coloration upon immersion for a duration of 5 min at a temperature of 90 °C in a solution containing black ink with a concentration of 5% in acetic acid. Subsequently, the item should be rinsed with water and subsequently immersed in a solution of 8% acetic acid at ambient temperature for a duration of 25 min. Once more, the roots were rinsed with water, then immersed in undiluted glycerol, and kept at a temperature of 4 °C. The roots were dissected into 1 cm segments for microscopic analysis. Subsequently, 15 specimens were immersed in glycerol and affixed onto a slide using a cover slip. Each sample underwent this technique on four separate occasions. The rates of mycorrhization were assessed by quantifying the number of root segments that exhibited mycorrhizal structures at the root system level (F), evaluating the extent of mycorrhizal colonization at the root system level (M), and enumerating the number of arbuscules at the root system level (A), following the methodology described by Trouvelot et al. (1986).
Total RNA extraction and gene expression assay
Leaf samples were collected from 40-day-old tomato plants, weighing 0.1 g; the MiniBEST Plant RNA Extraction Kit was used for the extraction of total RNA, from Takara, Japan. The extraction process used was that described by the manufacturers. gDNA eraser was used to break down the contaminated DNA. Using the PrimeScript™ RT reagent Kit from Takara, Japan, we synthesized 1 ng of cDNA from 1 ng of RNA. Using the CFX96™ Real-Time System (Bio-Rad, USA), the qRT-PCR experiment was performed in triplicate using SYBR®Premix Ex Taq II (Takara, Japan) according to the manufacturers’ protocols. The 2-CT approach, first described by Livak and Schmittgen in 2001, was used to determine the amount of mRNA that was transcribed. Table S1 in the supplementary materials lists this study’s primers.
Proline metabolized enzyme protocol
The ninhydrin method of Troll and Lindsley in 1955 was used to assess concentration of proline in leaves. Leaf P5CS, OAT, and ProDH activities were checked using the method stated by Zou et al. (2013a, b). Leaf P5CR activity was assessed according to the method used by Chilson et al. (1991) with some minor changes. Fresh leaf samples (0.2 g) were homogenized with 5 mL 100 mM Tris–HCl buffer (pH 7.5) containing 10 mM MgCl2, 10 mM β-mercaptoethanol, 1 mM EDTA, 2% (w/v) polyvinylpolypyrrolidone, and 2 mM phenylmethanesulfonyl fluoride. The homogenized mixture was centrifuged at 20,000 × g for 20 min at 4 °C. With 1 mL of reaction mixture comprising supernatant, enzyme activity was measured, 20 mM proline, 200 mM glycine buffer (pH 10.3), and 15mM NAD+. The absorbance at 340 nm was taken down, and a P5CR unit was defined as the amount of enzyme of 1 μmol NADH during 1 min (U/g FW).
Statistical analysis
To conduct the statistical analysis, Rstudio was utilized (Team 2021). Rstudio’s in-built Shapiro and Bartlett functions were used to check the normality and homoscedasticity of the data. For data that fit the normal distribution assumption, significance was determined using the D’Agostino test of skewedness on the residual variance (Komsta and Novomestky, 2015; package “moments”) and the post hoc Tukey’s honest significant detection test (De Mendiburu, 2017; Tukey’s HSD, p < 0.05, package “agricolae”). PCA and HCA were applied to all the observed variables in the presence of stress, under normal conditions, and after microbial inoculation.
Results
Bacillus subtilis and AMF enhanced Cr-stressed plant phenotypic traits
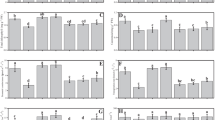
Under Cr stress, the shoot dry weight (SDW) was considerably reduced by − 37% but no significant (p < 0.05) impact on root dry weight (RDW) was observed, in comparison to the control (no Cr; Fig. 1). Furthermore, the sole application of BS and AMF was particularly pronounced within the Cr-stressed soil–plant condition, exerting significant impact on SDW (+ 35% and + 33%) and RDW (+ 21% and + 22%), relative to control (with Cr; Fig. 1a–d). Notably, the treatment of both of BS and AMF yielded a considerable (p < 0.05) increase (+ 21%) in SDW and a slight reduction (− 2%) in RDW being subjected to Cr stress as opposed to control (with Cr; Fig. 1a, b).
Shoot dry weight (a), root dry weight (b), leaf water retention capacity (c), and plant height (d) were analyzed in tomato plants exposed to Cr stress conditions with and without BS and AMF inoculation. To evaluate the significance of the observed differences among treatments, a two-way ANOVA was conducted. The lowercase letters placed above the bars indicate the presence of statistically significant variations between different treatments, with a significance level set at p < 0.05
Additionally, relative to non-stressed plants, Cr stress produced a substantial reduction (− 26%) in leaf water retention capacity (LWRC). Furthermore, under Cr-stressed conditions, LWRC showed a pronounced response to sole BS and AMF applications, contributing to significant enhancements of + 28% and + 26%, respectively, compared to stressed control (with Cr; Fig. 1c). Interestingly, the co-application of BS and AMF further elevated LWRC by + 26% under Cr-stress, in comparison to the control (with Cr; Fig. 1c).
In addition, the presence of Cr stress led to a notable decrease (− 1%) in plant height, relative to the control (no Cr; Fig. 1d). Moreover, the sole application of BS and AMF resulted in substantial rise in plant height by + 44% and + 15%, respectively, in comparison with control (with Cr; Fig. 1d). Significantly, the co-application of BS and AMF had a synergistic effect on increasing plant height (+ 13%) in the presence of Cr stress, in comparison to the control (with Cr; Fig. 1d).
Co-applied Bacillus subtilis and AMF increased colonization and biochemical traits of Cr-stressed plants
In Cr-stressed soil–plant conditions, the sole treatment of AMF elicited significant (one-way ANOVA; p < 0.05) alterations, when compared to the control (no Cr). Specifically, the frequency of mycorrhizal associations (F) demonstrated a substantial reduction (-6%), while mycorrhizal colonization (M) experienced a notable reduction (− 22%), compared to the control (no Cr stress). Moreover, following the sole AMF application, the abundance of arbuscules (A) exhibited a significant decline of − 34%, compared to the control (no Cr). Conversely, the co-application of BS and AMF induced even more pronounced reductions (F (− 9%), M (− 26%), and A (− 28%)) in the Cr-stressed condition, relative to control (no Cr; Fig. 2a–c).
This figure presents the impact of BS and AMF on mycorrhizal associations such as frequency of mycorrhizal associations (a), mycorrhizal colonization (b), and the abundance of arbuscules (c), analyzed using one-way ANOVA and biochemical responses, including total chlorophyll content (d), carotenoid content (e), and proline content (f), assessed via two-way ANOVA in Cr-stressed tomato plants. The lowercase letters above the bars indicate statistically significant differences between various treatments, with a significance level set at p < 0.05
Furthermore, in the presence of Cr stress, a significant (two-way ANOVA; p < 0.05) impact on key biochemical constituents was evident in comparison to the compared to the control (no Cr; Fig. 2d, e). Significantly, a notable decrease was observed in the overall chlorophyll content by − 62%, along with a decrease in carotenoid content by − 10%, relative to the control (no Cr). Conversely, a substantial increase in proline accumulation of + 236% was observed in response to Cr stress, in comparison with control (no Cr). Furthermore, when compared to the control (with Cr), the application of BS and AMF alone significantly increased the total chlorophyll content (+ 66% and + 31%), carotenoid content (+ 20% and + 18%), and proline accumulation (+ 130% and + 77%) in a Cr-stressed soil–plant environment. Similarly, the usage of BS and AMF exhibited significant increase in total chlorophyll (+ 70%), carotenoid content (+ 22%), and proline accumulation (+ 132%), in comparison to the control (with Cr; Fig. 2d, e).
Bacillus subtilis and AMF reduce oxidative stress by enhancing antioxidant enzymes in Cr-stressed plants
In the presence of Cr stress, a significant shift was observed in antioxidant enzyme activity, all relative to the control (no Cr; Fig. 3b–d). Superoxide dismutase (SOD) activity showed a considerable rise of + 44%, while peroxidase (POD) activity demonstrated a notable upregulation of + 103%, respectively, compared to the control (no Cr). Additionally, glutathione reductase (GR) activity increased by + 15% and catalase (CAT) activity showed a remarkable upsurge of + 141%, as a result to Cr stress, in comparison to the control (no Cr). Furthermore, under Cr-stressed soil–plant environment, the application of BS resulted in a significant increase in SOD activity by + 21%, while sole AMF treatment evoked a substantial increase of + 22%, both relative to the stressed control (with Cr). Similarly, under the same conditions (Cr stress), sole application of BS and AMF led to a notable (+ 29% and + 26%, respectively) elevation in POD activity, relative to the control (with Cr; p < 0.05; Fig. 3a,c). To amid Cr stress, the sole application of BS brought about a significant enhancement (+ 82%) in GR activity, while sole AMF treatment exhibited a notable increase by + 71%, relative to the stressed control (with Cr). Additionally, the CAT activity was significantly influenced by the sole application of BS and AMF (+ 13% and + 10%), compared to the control (with Cr; Fig. 3b, d).
This figure provides a visual representation of the impact of BS and AMF on antioxidant levels in Cr-stressed tomato plants. The figure displays the results for the levels of superoxide SOD (a), GR (b), POD (c), CAT (d), H2O2 (e), and MDA (f), all of which were analyzed to investigate the influence of BS and AMF on the antioxidant response. The data presented in this study represent the mean values ± standard deviation (SD) obtained from three replicates, ensuring the reliability and precision of the results. To assess the significance of the observed differences among treatments, a two-way ANOVA was conducted. The lowercase letters positioned above the bars indicate the presence of statistically significant variations between different treatments, with a significance level set at p < 0.05
Moreover, in the context of oxidative stress markers, Cr stress resulted in a notable decrease in hydrogen peroxide (H2O2) levels by − 21%, while the levels of malondialdehyde (MDA) were significantly increased by + 42%, in comparison to the control (no Cr). Moreover, the sole BS and AMF application induces significant reductions (− 21% and − 24%, respectively) in H2O2 content, whereas MDA levels led to noteworthy reductions (BS and AMF induced − 13% and − 6%, reduction, respectively), relative to the control (with Cr; Fig. 3e, f).
Furthermore, the co-application of BS and AMF exhibited a significant (p < 0.05) impact on these enzymatic and oxidative responses, compared to the control (with Cr; Fig. 3e, f). Particularly, under Cr stress, the co-application led to substantial increase in enzymatic activity of SOD (+ 29%), POD (+ 29%), GR (+ 143%), and CAT (+ 51%), all relative to the stressed control (with Cr; Fig. 3a–d). Moreover, combined inoculation (BS + AMF) significantly increases H2O2 levels by + 54%, whereas a significant reduction (− 27%) in MDA level was observed, both compared to the control (with Cr; Fig. 3e, f).
Bacillus subtilis and AMF act synergistically to modulate proline metabolism under Cr stress
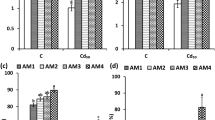
Under Cr stress, the activity of key enzymes involved in proline metabolism exhibited significant (p < 0.05) changes, when compared to the control (no Cr; Fig. 4a–d). Notably, P5CR (pyrroline-5-carboxylate reductase) displayed a substantial increase of + 42%, while P5CS (pyrroline-5-carboxylate synthetase) showed a notable rise of + 55%, relative to the control (no Cr; Fig. 4a, b). Conversely, the enzyme OAT (ornithine aminotransferase) exhibited a minor decline of − 7%, relative to the control (no Cr; Fig. 4c). Additionally, ProDH (proline dehydrogenase) activity displayed a modest increase of + 11%, when compared to the control (no Cr; Fig. 4d).
The figure depicts the impact of Cr stress and the influence of BS and AMF on proline metabolism in tomato plants. It presents the results for P5CR activity (a), P5CS activity (b), OAT (c), and ProDH (e). The data represents the mean values ± standard deviation (SD) obtained from three replicates. Significance among treatment groups was determined by a two-way analysis of variance (ANOVA), and statistically significant differences between treatments are indicated by lowercase letters above the bars (p < 0.05)
The sole treatment of BS and AMF led to a reduction in P5CR activity, showing reduction by -17% and -18%, respectively, relative to the control (with Cr; Fig. 4a). Similarly, following the sole treatment of BS and AMF, a significant decline of -31% was observed in P5CS for both treatments, compared to the control (with Cr; Fig. 4b). In contrast, when BS and AMF were individually inoculated, an opposing trend (+ 12% upsurge for both treatments) emerged in the activity of OAT, when compared to the control with Cr (Fig. 4c). Conversely, the sole introduction of BS led to a significant increase in ProDH activity (+ 42%), while AMF application yielded a more measured + 13% rise, when compared to the control (with Cr; Fig. 4d).
Moreover, co-application of BS and AMF yielded a significant decline in P5CR and P5CS activity, with reductions of − 17% and − 28%, respectively, in contrast to the control (with Cr; Fig. 4a, b). Conversely, a notable increase of + 29% and a substantial upsurge of + 113% in OAT and ProDH activity, respectively, were observed when both BS and AMF were co-applied, relative to the control with Cr (Fig. 4a–d).
Differential response of aquaporin genes in Bacillus subtilis and AMF inoculated plants under Cr stress
In the presence of Cr stress, notable significant (p < 0.05) changes were observed in the expression of key genes involved in ion transport, abscisic acid synthesis and stress response genes, compared to control conditions (no Cr). Specifically, the expression of ion transporter genes SlPIP2;1, SlPIP2;5, and SlPIP2;7 exhibited substantial increases by + 303%, + 323%, and + 198% respectively, compared to the control (no Cr). Additionally, the abscisic acid synthesis gene (NCED1) displayed a significant upregulation of + 217%, respectively, compared to the control (no Cr), whereas the relative expression of stress response gene (Le4) showed a substantial upregulation by + 1203%, compared to the control (no Cr).
Furthermore, relative to control (with Cr), the sole introduction of BS and AMF led to a significant reduction in the relative expression of SlPIP2;1 (− 30% and − 91%), SlPIP2;5 (− 33% and − 65%), and SlPIP2;7 (− 47% for both treatments), compared to the control (with Cr; Fig. 5a–c). Conversely, sole-BS application prompted an increase in the relative expression NCED1 by + 21%, while AMF led to a significant reduction by − 74%, relative to the control (with Cr; Fig. 5d). Moreover, the relative expression of Le4 was markedly suppressed (− 77% and − 78%, respectively) by the sole BS and AMF treatments, in comparison to the control (with Cr; Fig. 5e). Moreover, under Cr-induced stress condition, the co-application of BS and AMF produced synergistic impacts, leading to a downregulation in relative gene expression (SlPIP2;1 (− 62%), SlPIP2;1 (− 65%), SlPIP2;7 (− 46%), NCED1 (− 66%), and Le4 (− 96%), respectively), in comparison to the stressed control (with Cr; Fig. 5a–e).
This figure illustrates the gene expression profiles of ion transporter genes, including SlPIP2;1 (a), SlPIP2;5 (b), and SlPIP2;7 (c), the abscisic acid synthesis gene NCED1 (d), and the stress response gene Le4 (e) in the context of BS, AMF, and under Cr stress. The data represents the mean expression levels ± standard deviation (SD) obtained from three replicates. Significance among different treatments was assessed using a two-way ANOVA, with lowercase letters above the bars indicating statistically significant differences between treatments (p < 0.05)
Multivariate analysis
Principal component analysis (PCA) was employed to elucidate the relationships among plant traits in response to BS and AMF treatments and their impact on Cr uptake. Two principal components, PC1 and PC2, cumulatively accounting for 68.2% of the total variance, revealed distinct trait associations. PC1 (41% variance) highlighted the pivotal role of microbial treatments in promoting plant growth and mycorrhizal interactions, as evidenced by the strong contributions of SDW, RDW, LWRC, and mycorrhizal variables. PC2 (27.2% variance) emphasized the importance of plant physiological responses to stress, with attributes such as total chlorophyll content, carotenoid levels, proline concentration, and antioxidant enzyme activities (SOD, GR, POD, CAT) as prominent contributors (Fig. 6a).
The figure illustrates the outcomes of principal component analysis (PCA) (a) on various parameters in Cr-stressed tomato plants with and without BS and AMF treatment. The heat map (b) visually presents the variations in these parameters, offering valuable insights into the impact of BS and AMF on plant responses under Cr stress. The figure provides a concise and informative representation of how BS and AMF application can influence multiple biochemical and molecular factors in maize plants, promoting enhanced tolerance to Cr stress and overall plant health
Subsequently, hierarchical clustering analysis (HCA) was employed to unveil the inherent relationships and associations among the diverse plant traits and their corresponding genes in response to the treatments involving BS and AMF vis-à-vis Cr uptake. The HCA results were visually represented in the form of a heatmap, which effectively grouped the traits into two primary clusters. Notably, the first major cluster encompassed all the growth-related factors, such as dry shoot weight, dry root weight, plant height, and the mycorrhizal associations. In contrast, the second cluster tightly comprised metabolites, and the respective genes implicated in the study, including genes such as SlPIP2;1, SlPIP2;5, SlPIP2;7, NCED1, and Le4 (Fig. 6b).
Discussion
Cr contamination in soil–plant systems is a pressing environmental issue due to its detrimental effects on plant growth and human health (Hu et al. 2017; Shahid et al. 2017)(Hu et al. 2017). Cr exists in various oxidation states, with hexavalent Cr(VI) and trivalent Cr(III) being the most prevalent forms in soil (Apte et al. 2005). Cr(VI) is known for its high toxicity and mobility (Vázquez et al. 1987), while Cr(III) is less mobile but can become more toxic under certain conditions. Industrial activities, mining, and improper waste disposal contribute to the release of Cr into soil, leading to its accumulation in plants and subsequent entry into the food chain (Scoccianti et al. 2006). The toxicity of hexavalent Cr(VI) and its propensity to accumulate in plants can disrupt cellular processes, impair growth, and induce oxidative stress (Sharma et al. 2020; Ukhurebor et al. 2021; Ulhassan et al. 2022; Zulfiqar et al. 2023).
In recent years, researchers have explored various strategies to mitigate the adverse effects of Cr stress on plants. Among these strategies, the application of beneficial microorganisms such as BS and AMF has gained significant attention. Bacillus subtilis has been shown to enhance nutrient solubilization, improve soil structure, and produce antioxidants, thereby promoting plant growth and reducing oxidative stress (Hashem et al. 2019). AMF, through their symbiotic associations with plant roots, enhances nutrient uptake, improves antioxidant defense systems (Riaz et al. 2021), and influences the speciation of Cr in soil, mitigating its toxic effects. These microorganisms offer promising solutions for sustainable plant growth in Cr-stressed environments. Now, through graphical representation (Fig. 7), we unveil the outcome of our study, showcasing how the dynamic responses of plant traits, genes, and metabolic pathways under BS and AMF treatments contribute to enhanced plant resilience and Cr tolerance in the face of environmental challenges.
Inoculation with Bacillus subtilis and AMF enhanced plant traits under Cr stress
The present study underscores the substantial influence of BS and AMF in mitigating the adverse effect of Cr stress on plant growth and physiological parameters (Fig. 1a–d). This aligns with research emphasizing the role of AMF in enhancing nutrient uptake and improving plant growth during stressful conditions (Smith & Read 2010; Wu & Zou 2009)(Wu & Zou 2009). These outcomes suggest a relationship between BS and AMF that promotes plant growth by enhancing nutrient availability and improving interactions, between roots and soil (Moreira et al. 2020; Wu et al. 2010).
Furthermore, the study emphasizes how BS and AMF play a role, in enhancing the capacity of leaves to retain water when exposed to Cr induced stress (Fig. 4c). This improved ability to retain water can be credited to the influence of BS on soil structure and its ability to hold water well, as the enhanced efficiency of water uptake associated with AMF (Harman et al. 2021; Koza et al. 2022; Smith & Read 2010). The combined treatment of BS and AMF further amplifies the positive effects, potentially indicating their synergistic action in enhancing plant water stress tolerance.
The dynamic growth response in plant height is another noteworthy aspect of the study (Fig. 4d). Sole applications of BS and AMF led to substantial increases in plant height, reflecting their ability to enhance shoot growth. The synergistic effect observed in the combined treatment of BS and AMF, resulting in a + 13% increase in plant height under Cr stress, suggests their complementary actions in promoting overall plant development, which is likely facilitated by improved nutrient availability and water uptake.
Integration of Bacillus subtilis and AMF modulate mycorrhizal colonization and biochemical responses
Intriguingly, the study also investigated the impact of AMF on mycorrhizal associations and biochemical responses under Cr-stressed conditions (Fig. 2a–c). Previous research has shown that AMF can be sensitive to heavy metal stress, leading to altered colonization rates and morphological changes (Gadd 2004; Kapoor et al. 2012). The current study’s findings depicts that the application of AMF under Cr stress led to reductions in mycorrhizal associations, colonization rates, and arbuscule abundance. The introduction of BS alongside AMF resulted in even more pronounced reductions in these parameters, suggesting complex interactions between BS, AMF, and mycorrhizal associations under stress conditions.
Furthermore, the study investigates into the biochemical responses of plants to the application of BS and AMF under Cr stress (Fig. 2d–f). The observed increase in total chlorophyll content, carotenoid content, and proline accumulation is indicative of enhanced photosynthetic capacity, stress tolerance, and osmotic regulation. These findings corroborate previous research that has demonstrated the positive impact of BS and AMF on chlorophyll and carotenoid content under heavy metal stress (Abd_Allah et al. 2018; Porcel et al. 2003; Shi et al. 2018). The significant enhancements in these biochemical constituents upon the combined application of BS and AMF further underline their cooperative effects in promoting plant resilience and growth under challenging conditions.
Co-applied Bacillus subtilis and AMF enhance antioxidant enzymes and reduce oxidative stress
In terms of antioxidant enzyme activities, the sole application of BS and AMF yielded significant increases in SOD and POD activities under Cr stress (Fig. 3a, c). These enzymes are crucial components of the plant’s defense against oxidative stress, as they scavenge reactive oxygen species and mitigate cellular damage (Mittler 2002). The observed enhancements in SOD and POD activities suggest that both BS and AMF treatments contribute to improved oxidative stress tolerance. This aligns with previous research that has highlighted the role of BS and AMF in enhancing antioxidant enzyme activities (Flores-Duarte et al. 2023; Lingua et al. 2013). Additionally, the significant rise in GR and CAT activities upon BS and AMF application further supports the idea of enhanced antioxidant defense mechanisms (Fig. 3b, d) (Noctor & Foyer 1998).
Moreover, the reduction in H2O2 content and MDA levels upon sole BS application underscores their role in minimizing oxidative stress-induced damage. H2O2 and MDA are widely used as indicators of oxidative stress and lipid peroxidation (Wadhwa et al. 2012) (Gill & Tuteja 2010). The significant reductions in these oxidative stress markers upon BS treatment point to their potential in alleviating the harmful effects of Cr-induced oxidative stress (Fig. 3e, f). The study also uncovers the significant impact of combined BS and AMF application on antioxidative responses. The co-application led to substantial increases in SOD, POD, GR, and CAT activities, further highlighting the cooperative effects of BS and AMF in enhancing antioxidant defense mechanisms. Interestingly, the co-application resulted in an increase in H2O2 levels, suggesting a potential role of these growth-promoting agents in regulating signaling pathways associated with oxidative stress (Mittler 2002). The reduction in MDA levels upon co-application reinforces the idea of their collective action in minimizing lipid peroxidation and oxidative stress-induced damage.
Co-inoculation with Bacillus subtilis and AMF modify proline metabolism under Cr stress
Furthermore, the study delves into the modulation of proline metabolism under Cr stress (Fig. 4a–d). Proline is known to accumulate in plants under stress and serves as a compatible solute, protecting cells from osmotic stress and oxidative damage (Hayat et al. 2012; Hossain et al. 2014)(Hayat et al. 2012). The observed alterations in proline metabolism, including changes in P5CR, P5CS, OAT, and ProDH activities, highlight the dynamic responses of this pathway to BS and AMF applications. These changes in proline metabolism could contribute to osmotic adjustment and stress tolerance in plants, aiding plants’ survival under challenging conditions (Signorelli et al. 2021).
Aquaporin genes respond differentially to Bacillus subtilis and AMF under Cr stress
In the context of ion transporter genes, the observed reductions in the expression of SlPIP2;1, SlPIP2;1, and SlPIP2;7 genes upon BS and AMF applications suggest a potential role in ion homeostasis under Cr stress (Fig. 5a–c). These genes encode aquaporin proteins, which are known to regulate water and ion transport across cellular membranes, thereby affecting plant water balance and nutrient uptake (Maurel et al. 2008). The significant decrease in their expression could be a strategy to maintain ion equilibrium and counteract the harmful effects of excess Cr. The contrasting responses between BS and AMF treatments may reflect the complexity of their interactions and their specific roles in ion transport regulation.
Furthermore, the upregulation of the abscisic acid synthesis gene (NCED1) in response to BS application implies a potential involvement of abscisic acid (ABA) in stress signaling and responses (Fig. 5d). ABA is a well-known stress hormone that plays a pivotal role in mediating various stress responses, including stomatal closure and adaptation to environmental challenges (Fujita et al. 2006; Tuteja 2007). The increase in NCED1 expression upon BS treatment suggests a possible contribution of ABA to the plant’s adaptive response to Cr-induced stress. Conversely, the reduction in NCED1 expression upon AMF application could indicate a differential regulatory role of AMF in ABA signaling.
The marked suppression of the stress response gene (Le4) by sole BS and AMF treatments further underscores their regulatory roles in stress adaptation (Fig. 5e). Stress response genes like Le4 are often involved in coordinating cellular responses to various stressors (Jung et al. 2016). The significant reduction in Le4 expression indicates that BS and AMF treatments potentially alleviate the stress-induced transcriptional response, suggesting their capacity to mitigate the effects of Cr stress on plant cells.
The most intriguing finding is the synergistic impact of BS and AMF co-application on gene expression under Cr stress. The observed decrease in the relative expression of ion transporter genes, NCED1 and Le4, in the presence of both BS and AMF indicates their cooperative action in fine-tuning the plant’s stress responses. This synergistic effect suggests that BS and AMF may have complementary roles in modulating gene expression to enhance stress tolerance, potentially involving cross-talk between different signaling pathways.
Co-applied Bacillus subtilis and AMF restrict Cr uptake in plants
The combined use of PCA and HCA has revealed critical insights into the complex interplay of plant traits and genetic responses to BS and AMF treatments regarding Cr uptake. PCA identified two principal components, PC1 and PC2, explaining 68.2% of the variance. PC1 (41% variance) highlighted the role of microbial treatments in promoting plant growth and mycorrhizal interactions, while PC2 (27.2% variance) underscored the importance of plant stress responses. HCA further segregated these traits, distinguishing between growth-related, proline metabolism, mycorrhizal associations, biochemical responses, and molecular components. These findings align with established research on mycorrhizal associations and plant stress responses (Branco et al. 2022; Sachdev et al. 2021), offering insights for enhancing Cr tolerance and uptake in plants for applications in phytoremediation and agriculture.
The application of beneficial microorganisms like BS and AMF represents a promising strategy for promoting plant growth and mitigating the adverse impacts of Cr stress. By harnessing their multiple mechanisms of action, these microorganisms contribute to improved nutrient uptake, antioxidant defense, and detoxification, ultimately fostering sustainable and resilient plant growth in Cr-contaminated environments.
Conclusion
The study highlights the significant potential of BS and AMF in mitigating the detrimental impacts of Cr-induced stress on plant growth and physiological responses. These beneficial microorganisms exhibit multifaceted effects, including enhanced biomass accumulation, improved water retention, and increased antioxidant enzyme activities under Cr-stressed conditions. The synergistic action of BS and AMF is evident in various parameters, such as plant height, antioxidant defense mechanisms, and gene expression responses. Notably, their combined application demonstrates cooperative effects that promote plant resilience and adaptation to Cr stress. This research sheds light on innovative strategies for sustainable plant growth in Cr-contaminated environments by harnessing the potential of BS and AMF to enhance nutrient uptake, antioxidant protection, and detoxification processes.
Data Availability
The data will be avaliable upon request.
Change history
18 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11356-024-32965-z
References
Aebi Hugo (1984) Catalase in vitro. In Methods in enzymology. Academic press. 105:121–126
Ahmad H, Hayat S, Ali M, Liu T, Cheng Z (2018) The combination of arbuscular mycorrhizal fungi inoculation (Glomus versiforme) and 28‐homobrassinolide spraying intervals improves growth by enhancing photosynthesis, nutrient absorption, and antioxidant system in cucumber (Cucumis sativus L.) under salinity. Ecol Evol 8(11):5724–5740
Allah Abd EF, Alqarawi AA, Hashem A, Radhakrishnan R, Al-Huqail AA, Al-Otibi FON, Malik JA, Alharbi RI, Egamberdieva D (2018) Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J Plant Inter 13(1):37–44
Allen MF (2007) Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone J 6(2):291–297
Apte AD, Verma S, Tare V, Bose P (2005) Oxidation of Cr (III) in tannery sludge to Cr (VI): field observations and theoretical assessment. J Hazard Mater 121(1–3):215–222
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris Plant Physiol 24(1):1
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11(1):3–42
Bárzana G, Aroca R, Bienert GP, Chaumont F, Ruiz-Lozano JM (2014) New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol Plant Microbe Interact 27(4):349–363
Branco S, Schauster A, Liao HL, Ruytinx J (2022) Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol 235(6):2158–2175
Calvo-Polanco M, Molina S, Zamarreño AM, García-Mina JM, Aroca R (2014) The symbiosis with the arbuscular mycorrhizal fungus Rhizophagus irregularis drives root water transport in flooded tomato plants. Plant Cell Physiol 55(5):1017–1029
Cheng HQ, Ding YE, Shu B, Zou YN, Wu QS, Kuča K (2020) Plant aquaporin responses to mycorrhizal symbiosis under abiotic stress. Intl J Agric Biol 23:786–794
Chilson OP, Kelly-Chilson AE, Siegel NR (1991) Pyrroline-5-carboxylate reductase in soybean nodules: isolation/partial primary structure/evidence for isozymes. Arch Biochem Biophys 288(2):350–357
Ding Y-E, Fan Q-F, He J-D, Wu H-H, Zou Y-N, Wu Q-S, Kuča K (2020) Effects of mycorrhizas on physiological performance and root TIPs expression in trifoliate orange under salt stress. Arch Agronom Soil Sci 66(2):182–192
Flores-Duarte NJ, Pajuelo E, Mateos-Naranjo E, Navarro-Torre S, Rodríguez-Llorente ID, Redondo-Gómez S, Carrasco López JA (2023) A culturomics-based bacterial synthetic community for improving resilience towards arsenic and heavy metals in the nutraceutical plant Mesembryanthemum crystallinum. Int J Mol Sci 24(8):7003
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9(4):436–442
Gadd GM (2004) Microbial influence on metal mobility and application for bioremediation. Geoderma 122(2–4):109–119
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in Higher Plants Plant Physiol 59(2):309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Harman G, Khadka R, Doni F, Uphoff N (2021) Benefits to plant health and productivity from enhancing plant microbial symbionts. Front Plant Sci 11:610065
Hashem A, Tabassum B, Allah Abd EF (2019) Bacillus subtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci 26(6):1291–1297
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
He F, Zhang H, Tang M (2016) Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 26:311–323
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Hossain MA, Hoque MA, Burritt DJ, Fujita M (2014) Proline protects plants against abiotic oxidative stress: biochemical and molecular mechanisms. In Oxidative damage to plants (pp. 477–522). Elsevier
Hu B, Jia X, Hu J, Xu D, Xia F, Li Y (2017) Assessment of heavy metal pollution and health risks in the soil-plant-human system in the Yangtze River Delta, China. Int J Environ Res Public Health 14(9):1042
Jia-Dong H, Tao D, Hui-Hui W, Ying-Ning Z, Qiang-Sheng W, Kamil K (2019) Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci Hortic 243:64–69
Jiang C, Song X, He H, Chu L, Zhou H, Zhao Y, Xu Y, Zeng W, Lin X, Lu M-Z (2020) Genome-wide identification of plasma membrane aquaporin gene family in Populus and functional identification of PIP1;1 involved in osmotic stress. Environ Exp Bot 179:104200
Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Wigge PA (2016) Phytochromes function as thermosensors in Arabidopsis. Science, 354(6314):886–889
Kapilan R, Vaziri M, Zwiazek JJ (2018) Regulation of aquaporins in plants under stress. Biol Res 51(1):1–11
Kapoor R, Evelin H, Mathur P, Giri B (2012) Arbuscular mycorrhiza: approaches for abiotic stress tolerance in crop plants for sustainable agriculture. In Plant acclimation to environmental stress (pp. 359–401). Springer.
Karimi N, Ghasempour H-R (2019) Salicylic acid and jasmonic acid restrains nickel toxicity by ameliorating antioxidant defense system in shoots of metallicolous and non-metallicolous Alyssum inflatum Náyr. Populat Plant Physiol Biochem 135:450–459
Koza NA, Adedayo AA, Babalola OO, Kappo AP (2022) Microorganisms in plant growth and development: roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms 10(8):1528
Kumar A, Dames JF, Gupta A, Sharma S, Gilbert JA, Ahmad P (2015) Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: a biotechnological perspective. Crit Rev Biotechnol 35(4):461–474
Liang W-H, Li L, Zhang F, Liu Y-X, Li M-M, Shi H-H, Li H, Shang F, Lou C, Lin Q-T (2013) Effects of abiotic stress, light, phytochromes and phytohormones on the expression of OsAQP, a rice aquaporin gene. Plant Growth Regul 69(1):21–27
Lingua G, Bona E, Manassero P, Marsano F, Todeschini V, Cantamessa S, Copetta A, D’Agostino G, Gamalero E, Berta G (2013) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization. Intl J Mol Sci 14(8):16207–16225
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Maurel C, Verdoucq L, Luu D-T, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Moreira H, Pereira SI, Vega A, Castro PM, Marques AP (2020) Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J Environ Manage 257:109982
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49(1):249–279
Pacioni G (1992) 16 wet-sieving and decanting techniques for the extraction of spores of vesicular-arbuscular fungi. Methods Microbiol 24:317–322
Porcel R, Barea JM, Ruiz-Lozano JM (2003) Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol 157(1):135–143
Quiroga G, Erice G, Ding L, Chaumont F, Aroca R, Ruiz-Lozano JM (2019) The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant, Cell Environ 42(7):2274–2290
Team R (2021) RStudio: integrated development for R. RStudio, PBC, Boston, MA. 2020
Reinhardt H, Hachez C, Bienert MD, Beebo A, Swarup K, Voß U, Bouhidel K, Frigerio L, Schjoerring JK, Bennett MJ (2016) Tonoplast aquaporins facilitate lateral root emergence. Plant Physiol 170(3):1640–1654
Riaz M, Kamran M, Fang Y, Wang Q, Cao H, Yang G, Deng L, Wang Y, Zhou Y, Anastopoulos I (2021) Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: a critical review. J Hazard Mater 402:123919
Ruiz-Lozano JM, Aroca R (2017) Plant aquaporins and mycorrhizae: their regulation and involvement in plant physiology and performance. Plant Aquaporins: From Transport to Signaling, 333–353
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10(2):277
Scoccianti V, Crinelli R, Tirillini B, Mancinelli V, Speranza A (2006) Uptake and toxicity of Cr(III) in celery seedlings. Chemosphere 64(10):1695–1703
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D (2020) Chromium bioaccumulation and its impacts on plants: an overview. Plants 9(1):100
Shi Z, Zhang J, Wang F, Li K, Yuan W, Liu J (2018) Arbuscular mycorrhizal inoculation increases molybdenum accumulation but decreases molybdenum toxicity in maize plants grown in polluted soil. RSC Adv 8(65):37069–37076
Signorelli S, Tarkowski ŁP, O’Leary B, Tabares-da Rosa S, Borsani O, Monza J (2021) GABA and proline metabolism in response to stress. Hormones and Plant Response, 291–314.
Smith SE, Read DJ (2010) Mycorrhizal symbiosis. Academic press.
Tuteja N (2007) Abscisic acid and abiotic stress signaling. Plant Signal Behav 2(3):135–138
Ukhurebor KE, Aigbe UO, Onyancha RB, Nwankwo W, Osibote OA, Paumo HK, Siloko IU (2021) Effect of hexavalent chromium on the environment and removal techniques: a review. J Environ Manag 280:111809
Ulhassan Z, Khan I, Hussain M, Khan AR, Hamid Y, Hussain S, Zhou W (2022) Efficacy of metallic nanoparticles in attenuating the accumulation and toxicity of chromium in plants: Current knowledge and future perspectives. Environ Pollut, 120390
Vázquez MD, Poschenrieder C, Barcelo J (1987) Chromium VI induced structural and ultrastructural changes in bush bean plants (Phaseolus vulgaris L). Annals Botany 59(4):427–438
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64(12):5004–5007
Wadhwa N, Mathew BB, Jatawa S, Tiwari A (2012) Lipid peroxidation: mechanism, models and significance. Int J Curr Sci 3(1):29–38
Wu Q, Zou Y (2009) Mycorrhizal influence on nutrient uptake of citrus exposed to drought stress. Phil Agri Sci 92(1):33–38
Wu Q-S, Zou Y-N, He X-H (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304
Wu Q-S, Srivastava AK, Zou Y-N (2013a) AMF-induced tolerance to drought stress in citrus: a review. Sci Hortic 164:77–87
Wu Q-S, Zou Y-N, Huang Y-M (2013b) The arbuscular mycorrhizal fungus Diversispora spurca ameliorates effects of waterlogging on growth, root system architecture and antioxidant enzyme activities of citrus seedlings. Fungal Ecol 6(1):37–43
Wu H-H, Zou Y-N, Rahman MM, Ni Q-D, Wu Q-S (2017) Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci Rep 7(1):42389
Wu Q-S, He J-D, Srivastava A, Zou Y-N, Kuča K (2019) Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol 39(7):1149–1158
Xie M-M, Zou Y-N, Wu Q-S, Zhang Z-Z, Kuča K (2020) Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant Soil Environ 66(6):287–294
Zhang F, Wang P, Zou Y-N, Wu Q-S, Kuča K (2019) Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch Agro Soil Sci 65(9):1316–1330
Zou Y-N, Liang Y-C, Wu Q-S (2013a) Mycorrhizal and non-mycorrhizal responses to salt stress in trifoliate orange: plant growth, root architecture and soluble sugar accumulation. Int J Agric Biol 15(3):565–569
Zou Y-N, Wu Q-S, Huang Y-M, Ni Q-D, He X-H (2013b) Mycorrhizal-mediated lower proline accumulation in Poncirus trifoliata under water deficit derives from the integration of inhibition of proline synthesis with increase of proline degradation. PLoS ONE 8(11):e80568
Zou Y-N, Wu H-H, Giri B, Wu Q-S, Kuča K (2019) Mycorrhizal symbiosis down-regulates or does not change root aquaporin expression in trifoliate orange under drought stress. Plant Physiol Biochem 144:292–299
Zou Y-N, Zhang F, Srivastava AK, Wu Q-S, Kuča K (2021a) Arbuscular mycorrhizal fungi regulate polyamine homeostasis in roots of trifoliate orange for improved adaptation to soil moisture deficit stress. Front Plant Sci 11:600792
Zou YN, Wu QS, Kuča K (2021b) Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol 23:50–57
Zulfiqar U, Haider FU, Ahmad M, Hussain S, Maqsood MF, Ishfaq M, Eldin SM (2023) Chromium toxicity, speciation, and remediation strategies in soil-plant interface: a critical review. Front Plant Sci 13:1081624
Funding
This project was supported by Researchers Supporting Project number (RSP2024R230) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Tariq Shah conceived and designed the experiment; Tariq Shah prepared the materials and data collection; M. Asad assisted in software and statistical analysis of the data; Zeeshan Khan wrote the first draft of the manuscript; Ayesha Imran assisted in the software used for graphical representation of data; S. Khan assisted in materials and reagents preparation; Tahani AA. assisted in review of manuscript and funding; Mohammad Javed Ansari assisted in review, editing, and approval of final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, T., Khan, Z., Alahmadi, T.A. et al. Mycorrhizosphere bacteria inhibit chromium uptake and phytotoxicity by regulating proline metabolism, antioxidant defense system, and aquaporin gene expression in tomato. Environ Sci Pollut Res 31, 24836–24850 (2024). https://doi.org/10.1007/s11356-024-32755-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32755-7