Abstract
Acid rain threatens crop yield and nutritional quality, and Ca2+ can regulate plant responses to abiotic stresses. To improve the yield and nutritional quality of crops under acid rain stress, we applied exogenous Ca2+ to regulate nitrogen assimilation in rice seedlings under simulated acid rain stress (pH 4.5 or 3.0), taking yield and nutritional quality of rice as evaluation criteria. We found that Ca2+ (5 mM) maintained the total nitrogen content of rice at the seedling and booting stages to alleviate the inhibitory effect of simulated acid rain on rice yield. Meanwhile, Ca2+ improved the activity of glutamate synthase to eliminate the disruption of glutamine synthetase/glutamate synthase balance under simulated acid rain. It decreased the efficiency of nitrogen assimilation, thereby reducing the inhibition of essential amino acid content in rice. The mitigation effect on simulated acid rain at pH 4.5 was better than that of simulated acid rain at pH 3.0. Overall, Ca2+ may reduce the negative effect of acid rain on the yield and nutritional quality of crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid rain (AR) is caused by air pollution, which is one of three major types of pollution affecting the environment (Xu et al. 2015). AR has spread all over Western Europe, North America, and Asia, mostly affecting rice-growing areas. Of 25 major rice-producing countries, 17 are in Asia (Dugan 2015). Approximately 0.662 billion USD worth of annual crop loss in China is caused by AR (Feng et al. 2002). AR not only affects photosynthesis, antioxidant capacity, H+-ATPase activity of plants (Debnath and Ahammed 2020; Debnath et al. 2021; Hu et al. 2021a, b; Liang et al. 2020), and the quality of agricultural products but also causes environmental stress. For example, heavy metals accumulating in rice grains can cause a health risk (Sanjay et al. 2012; Wu et al. 2016; Zhang et al. 2021). Moreover, AR can decrease the nutritional value of vegetables and wheat (Debnath et al. 2018; Li and Liang 2019; Zhou et al. 2018), threatening human health by causing “hidden hunger” among consumers. To face the challenge of food security and safety, it is crucial to find possible ways to alleviate the negative effect of AR on the yield and quality of crops.
Calcium (Ca) is an essential nutrient for plant growth (Montanaro et al. 2014; Wu et al. 2020). Ca2+ is also a second messenger in plants; it regulates plant adaptation to environmental adversities (Plasencia et al. 2021) and alleviates the decrease in yield and quality of crops under the conditions of salinity, drought, high temperature, and hypoxic stress. (He et al. 2015; Wang et al. 2017). For example, exogenous Ca2+ can alleviate the decrease in the nutritional quality of cucumbers under hypoxic stress (He et al. 2018). However, there is limited information on the effect of exogenous Ca2+ on the nutritional quality of crops subjected to simulated acid rain (SAR) (Li and Liang 2019). Apart from being a part of a balanced diet, rice is the staple food for more than half of the world’s population (Chen et al. 2021; Das et al. 2020). Approximately 20% of the world’s dietary energy supply is provided by rice and its derivatives (FAO/IRRI). Proteins and amino acids are the major contributors to the nutritional quality of rice grains (Sangwongchai et al. 2022). Insufficient intake of essential amino acids can affect human growth, nutrition, fertility, and lifespan (Giordano and Castellineo 1997). Glutamate, the product of nitrogen assimilation, is an important precursor of proteins and amino acids, and the efficiency of nitrogen assimilation seriously affects plant productivity (Hou et al. 2021; Iqbal et al. 2020; Navarro-Leon et al. 2016). Abiotic stress can inhibit the activities of nitrate reductase (NR), glutamine synthetase (GS), and glutamate synthetase (GOGAT), thereby reducing nitrogen assimilation and inhibiting the total protein content of rice (Imran et al. 2021; Zhang et al. 2021). As an adaptation to abiotic stress, glutamate dehydrogenase (GDH) pathway in crops could be enhanced to compensate for the weakening of the GS/GOGAT pathway (Wang et al. 2012). Therefore, the effect of Ca2+on the yield and nutritional quality of rice under SAR treatment should be studied, with a focus on nitrogen assimilation. Moreover, the effect of Ca2+ on the GDH and GS/GOGAT pathways of rice under SAR treatment should be clarified at the gene level. Such studies would eliminate the threat of AR to food security and safety.
Therefore, this study evaluated (1) the effect of exogenous Ca2+ and SAR treatment on yield (weight of 100 grains, seed setting rate, and the number of grains per panicle) and nutritional quality of rice (protein and amino acid content); (2) the relationship between yield, nutritional quality, and total nitrogen content in rice at different growth stages under exogenous Ca2+ and SAR treatment; (3) the role of nitrogen assimilation in regulating protein and amino acid content under exogenous Ca2+ and SAR treatment. This information can replenish the regulatory effect of exogenous Ca2+ on the nutritional quality of crops under adversity and, at the same time, provide a theoretical basis for alleviating “hidden hunger” caused by AR.
Materials and methods
Plants and conditions
The seeds (Wuyou 308, purchased from Wuxi Seed Company, China) were soaked in 0.1% HgCl2 for 10 min, then washed with distilled water three times, and left to germinate in vermiculite at 25 ± 5 °C until the second true leaf came out. The seedlings were transferred to foam boards with 24 holes (two seedlings per hole). The details of the nutrient solution were described by Liang et al. (2021). The light/dark cycle was 14/10 h, and the illumination intensity was 300 µmol·m−2.
The concentrated SAR (pH 1.0) was composed of sulfuric acid and nitric acid in a ratio of 3:1. When rice seedlings grow to four leaves, SAR treatment at the seedling stage began and lasted for 5 days and required leaf spraying and root application. For leaf spraying, the deionized water was adjusted to pH 4.5 and pH 3.0 with the concentrated SAR. The rice leaves were sprayed with SAR (pH 4.5 or 3.0) until water droplets slipped away at 10 am and 6 pm during these 5 days. The nutrient solution was adjusted to pH4.5 (SAR1) and pH3.0 (SAR2) by the concentrated SAR as root application SAR. Adding 5 mM CaCl2 into the nutrient solution was as the single Ca2+ treatment. Ca2+ + SAR (pH4.5/3.0) treatment with SAR (pH 4.5/3.0) spraying leaves and adjusting the pH of the nutrient solution while applying 5 mM CaCl2 to the nutrient solution. The plant materials were measured after harvesting after SAR and Ca2+ treatment for 5 days. The control was sprayed with pH 7.0 deionized water leaves and applied with a nutrient root with pH of 5.6. The SAR experiment was carried out at the seedling, booting, and maturity stages of rice. Each stage was treated for 5 days, with the remainder of the culture under control conditions. The specific processes are shown in Fig. 1.
Determination of the protein content in rice grains
The harvested grains were dried to constant weight, peeled, and ground into powder. The rice powder (0.1 g) was put into tubes, mixed with 1 mL of deionized water at room temperature for 2 h, and then centrifuged at 1000 r·min–1 for 10 min to obtain albumin. The residue was extracted in 1 mL 5% NaCl to obtain globulin with the same conditions as in the previous step. To obtain prolamin and glutelin, the residues were extracted in 1 mL 75% ethanol and 1 mL 0.2% NaOH, respectively. The extraction for each component was repeated three times. The obtained extract was evaluated by the Bradford method for determining protein content (Dogan et al. 2005).
Determination of content of amino acids in rice
According to the method of Huifeng et al. (2010), rice powder (300 mg) and 10 mL 6 mol·L–1 HCl were added to vacuum hydrolysis tubes. The bottles were blown with N2 for three minutes and sealed tightly. Thereafter, the bottles were placed in the oven to digest at 110 °C for 22 h and then removed and left to cool. The digestion solution was distilled to 25 mL and passed through a 0.45-µm inorganic filter membrane. The filtrate was used to determine amino acids content using the amino acid analyzer (Symkam, Germany). The conditions of measurement were as follows: the 3-µm sulfonic acid cationic resin separation column (4.6 mm × 60 mm), gradient elution cycle time of 53 min, separation column temperature of 57 °C, reaction column temperature of 135 °C, the buffer flow rate of 0.40 mL·min–1, the flow rate of 0.35 mL·min–1 for ninhydrin, and the detection wavelength of channel 1 of 570 nm.
Calculation of relative growth rate
Following the method of Torabian et al. (2018), the rice powder was placed in the oven at 105 °C for 30 min and then dried at 80 °C to constant weight after harvesting. The relative growth rate (RGR) was calculated according to the following formula:
where W1: the dry weight before SAR treatmentW2: the dry weight after SAR treatment
Determination of total nitrogen content
The determination was performed according to the method of Barbano et al. (1990) with slight modifications. The dry plant samples (0.5 g) were digested with H2SO4 and H2O2. The samples were transferred into the digestion tubes and mixed with 5 mL of concentrated sulfuric acid. The digestion tubes were then heated in the digestion furnace, and 1–2 mL H2O2 was added to the tubes during the digestion process. The digestion process was completely done when the digestion solution became clear and transparent. The digestion solution was transferred to a test tube containing the final volume (15 mL). The supernatant (1 mL) was added to 1 mL Nessler’s reagent and 2.5 mL of 50 g·L–1 potassium sodium tartrate, and pH was adjusted to 11 by 100 g·L–1 KOH; then, the reaction system was set to 15 mL. The absorbance was measured at 425 nm, and the total nitrogen content of the sample was calculated.
Determination of key enzyme activities for nitrogen assimilation
For analysis of the activity of NR, leaves (1 g) were homogenized in the extract, including 10 mM cysteine, 1 mM EDTA-Na2, and 25 mM phosphate buffer (pH 8.7). Crude extracts were centrifuged at 4000 r·min–1 for 15 min at 4 °C, and the supernatants were enzyme extracts (Hageman and Reed 1980). The enzyme extract (0.2 mL), 100 mM KNO3 (0.5 mL), and 2 mg·mL–1 NADH (0.3 mL) were added to test tubes, and the tubes were incubated at 25 °C for 30 min. Then, trichloroacetic acid (1 mL) was added to stop the reaction. Sulfa reagent (2 mL) and naphthylamine reagent (2 mL) were used to develop the color, and the absorbance was measured at 520 nm after 15 min.
For the analysis of GS activity, leaves (1.0 g) were homogenized in a 3-mL extraction buffer, and the crude extracts were centrifuged at 15,000 × g for 20 min at 4 °C. The supernatants were enzyme extract. The components of the extraction buffer included 50 mM Tris, 2 mM MgSO4·7H2O, 4 mM dithiothreitol, and 400 mM sucrose at pH 8.0. The measurements were taken as follows: 1.6 mL reaction solution containing 100 mM Tris, 80 mM MgSO4·7H2O, and 19 mM L-sodium glutamate was added to enzyme extracts (0.7 mL) and 40 mM adenosine triphosphate solution (0.7 mL) and mixed. The reaction system was incubated at 37 °C for 30 min and then added to 1 mL of color reagent containing 200 mM trichloroacetic acid solution, 600 mM FeCl3·6H2O, and 5% concentrated hydrochloric acid in tubes. The absorbance was measured at 540 nm after the complete development of color. The activity was expressed as OD540 mg–1 protein·h–1 (Zhang et al. 1997).
For the analysis of the activity of GOGAT, the extraction system, same as the GS extraction system used for the determination of glutamine synthetase, was employed. The reaction system contained 20 mM L-glutamine (0.4 mL), 20 mM α-ketoglutarate (0.5 mL), 10 mM KCl (0.1 mL), 3 mM NADH (0.2 mL), enzyme extract (0.3 mL), and 25 mM Tris–HCl (pH 7.6, 1.5 mL) (Lin and Kao 1996). The molar extinction coefficient (value) was measured at 340 nm every 20 s, and each measurement was repeated 11 times. The steady decrease in optical density was considered as the enzyme activity.
To analyze the activity of GDH, leaves (1.0 g) were homogenized in extraction buffer (3 mL), which contained 10 mM Tris–HCl, 1 mM MgCl2, 1 mM EDTA-Na2, and 1 mM 2-mercaptoethanol, with a pH of 7.6. The crude extract was centrifuged at 15,000 × g for 30 min at 4 °C. The supernatant was the enzyme extract. The assay system composing of 100 mM 2-ketoglutarate (0.3 mL), 1 M NH4Cl (0.3 mL), 3 mM NADH (0.2 mL), enzyme extract (1 mL), and 0.2 M Tris–HCl (pH 8.0) was used to make up the reaction system to the total volume (3 mL). The reaction was started by adding the enzyme extract. The extinction value was determined for 3 min at 340 nm. One unit of the enzyme activity was defined as the value of the reduction in 1 OD340 per minute.
qRT-PCR for determination of transcript expression of key enzymes for nitrogen assimilation
Fresh leaves were used to extract total RNA with TRIZOL reagent (Sangon Biotech, Shanghai, China). The RNA quality was assessed by NanoDrop (Thermo Fisher, America). The AMV First Strand cDNA Synthesis Kit (Sangon Biotech, Shanghai, China) was used to perform reverse transcription to obtain cDNA. The reverse transcription setup consisted of 0.25 µL forward primer, 0.25 µL revere primer, 5 µL solution in the kit, 1 µL cDNA, and 3.5 µL ddH2O. The reaction proceeded at 95 °C for 3 min, followed by melting at 95 °C for 6 s, and annealing at 57 °C for 11 s (a total of 40 cycles for melting and annealing). Relative expression abundance was calculated using comparative performance analysis (Optical System software, version 1.0). The detailed information about the forward and revere primers is shown in Table S1.
Statistical analysis
Each treatment was repeated three times. The data were obtained and analyzed by SPSS 18.0 software. The experimental results were presented as mean ± standard deviation (SD). The least significant difference (LSD) was used to compare the mean between different treatment groups (P < 0.05).
Results
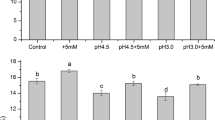
Effect of exogenous Ca 2+ and SAR on rice yield
Figure 2 depicts the effect of exogenous Ca2+ on the weight of 100 seeds (a), seed setting rate (b), and grain number per panicle (c) in rice subjected to SAR. Compared with the control, SAR1 (pH 4.5) treatment had no significant effect on the weight of 100 seeds, seed setting rate, and grain number per panicle (P > 0.05). In contrast, SAR2 (pH 3.0) treatment significantly reduced the values of these three parameters (P > 0.05). The single exogenous Ca2 + treatment had no significant effect on the weight of 100 seeds, seed setting rate, and grain number per panicle (P > 0.05). In the SAR1 (pH 4.5) + Ca2+ treatment group, the weight of 100 seeds was higher than that in the control and SAR1 (pH 4.5) treatment groups (P < 0.05). However, compared with the control and SAR1 (pH 4.5) treatment groups, the seed setting rate and grain number per panicle did not change in the SAR1 (pH 4.5) + Ca2+ treatment group. No significant difference in the weight of 100 seeds, seed setting rate, and grain number per panicle was observed between the SAR2 (pH 3.0) + Ca2+ treatment and control groups (P > 0.05). However, the values for these three parameters were higher in the SAR2 (pH 3.0) + Ca2+ treatment group than in the SAR2 (pH 3.0) treatment group (P < 0.05).
Effect of exogenous Ca 2+ and SAR on the nutritional quality of rice
As depicted in Fig. 3, the content and components of proteins (a) and amino acids (b), especially the content of essential amino acids (c), were the indicators of rice nutritional quality. The rice protein can be classified into albumin, globulin, prolamin, and glutelin. Essential amino acids include threonine, valine, methionine, phenylalanine, leucine, isoleucine, and lysine. Compared with the control group, the content and components of proteins did not change (P > 0.05) in the SAR1 (pH 4.5) treatment group, but the total amino acid content and the contents of six essential amino acids (except methionine) significantly decreased (P < 0.05). In the SAR2 (pH 3.0) treatment group, the contents of total protein, four protein components, total amino acid, and six essential amino acids except isoleucine were lower than those in the control and SAR1 (pH 4.5) treatment groups (P < 0.05). Single Ca2+ treatment had no significant effect on the contents of total protein, total amino acid, and essential amino acids (P > 0.05). In the SAR1 (pH 4.5) + Ca2+ treatment group, the content and components of protein were not significantly different from those in the control and SAR1 (pH 4.5) treatment group (P > 0.05). Moreover, in the SAR1 (pH 4.5) + Ca2+ treatment group, the contents of total amino acids and total essential amino acids did not differ from that in the control group (P > 0.05); however, the contents were higher than those in the SAR1 (pH 4.5) treatment group (P < 0.05). In the SAR2 (pH 3.0) + Ca2+ treatment group, the contents of total protein and the three components (albumin, globulin, and glutenin) were not significantly different from that in the control group (P > 0.05); however, the contents were higher than those in the SAR2 (pH 3.0) treatment group (P < 0.05). Moreover, in the SAR2 (pH 3.0) + Ca2+ treatment group, the contents of prolamin, total amino acids, and six essential amino acids (except isoleucine) were lower than those in the control group (P < 0.05) but higher than those in the SAR2 (pH 3.0) treatment group (P < 0.05).
Effect of exogenous Ca2+ on components of protein (a), content of total amino acid (b), and content of essential amino acid (c) of rice under SAR. Uppercase letters represented significant difference of total amount of protein (or the total amount of essential amino acid) among the treatment groups (P < 0.05), and lowercase letters represented significant difference of protein compositions (or seven essential amino acids) among the treatment groups (P < 0.05)
Effect of exogenous Ca 2+ and SAR on RGR and total nitrogen content of rice at different growth stages
Figure 4 depicts the effect of exogenous Ca2+ on RGR (a) and total nitrogen content (b) of rice under SAR treatment at the seedling, booting, and maturity stages. SAR1 (pH 4.5) treatment did not change RGR and total nitrogen content at the three growth stages; however, the total nitrogen content was higher than that of the control at the seedling stage. SAR2 (pH 3.0) treatment reduced RGR at the seedling and booting stages, as well as reduced total nitrogen content at the seedling, booting, and maturity stages (P < 0.05). In the single exogenous Ca2+ treatment group, RGR and total nitrogen content at three growth stages were not different from those in the control group (P > 0.05). In the SAR1 (pH 4.5) + Ca2+ treatment group, RGR and total nitrogen content at the seedling and booting stages were higher than those in the control and SAR1 treatment groups (P < 0.05), whereas RGR and total nitrogen content at the maturity stage were not different from that in control and SAR1 (pH 4.5) treatment groups (P > 0.05). In the SAR2 (pH 3.0) + Ca2+ treatment group, RGR and total nitrogen content at the seedling and booting stages were higher than that in the SAR2 (pH 3.0) treatment group (P < 0.05); however, RGR at the seedling stage did not differ from that in the control group (P > 0.05). Moreover, in the SAR2 (pH 3.0) + Ca2+ treatment group, RGR and total nitrogen content at the maturity stage did not differ from that in the control and SAR2 (pH 3.0) treatment groups (P > 0.05).
Effect of exogenous Ca 2+ and SAR on the activities of NR , GS , GOGAT, and GDH in rice seedling leaves
Figure 5 illustrates the effect of exogenous Ca2+ on the activities of key enzymes for nitrogen assimilation, including NR (a), GS (b), GOGAT (c), and GDH (d) in rice under SAR treatment. Compared with the control group, NR and GDH activities increased, GOGAT activity decreased (P < 0.05), and GS activity did not change in the SAR1 (pH 4.5) treatment group (P > 0.05). Compared with the control group, NR activity decreased (P < 0.05), and GDH activity increased in the SAR2 (pH 3.0) treatment group; the GDH activity was higher than that in the SAR1 (pH 4.5) treatment group (P < 0.05). In the SAR2 (pH 3.0) treatment group, GS activity was higher than that in the control group (P < 0.05), whereas GOGAT activity was lower than those in the control and SAR1 (pH 4.5) treatment groups (P < 0.05). The single exogenous Ca2+ treatment had no significant effect on the activities of four key enzymes (P > 0.05). In the SAR1 (pH 4.5) + Ca2+ treatment group, NR and GDH activities were higher than that in the control group; the GDH activity was also higher than that in the SAR1 (pH 4.5) treatment group (P < 0.05). In the SAR1 (pH 4.5) + Ca2+ treatment group, GS and GOGAT activities were not different from those in the control group; the GOGAT activity was also higher than that in the SAR1 (pH 4.5) treatment group. In the SAR2 (pH 3.0) + Ca2+ treatment group, NR and GOGAT activities were not different from those in the control group but were higher than those in the SAR2 (pH 3.0) treatment group. Moreover, in the SAR2 (pH 3.0) + Ca2+ treatment group, GS and GDH activities were higher than those in the control group (P < 0.05), whereas GS activity was lower and GDH activity was higher than that in the SAR2 (pH 3.0) treatment group (P < 0.05).
Effect of exogenous Ca 2+ on the expression of genes encoding NR , GS , GOGAT, and GDH
Figure 6 depicts the effect of exogenous Ca2+ on the expressions of genes encoding NR, GS, GOGAT, and GDH in rice under SAR treatment. NR was encoded by OsNR1 and OsNR2 (a), while GS was encoded by three genes; namely, OsGS1;1, OsGS1;2, and OsGS1;3 (b). Moreover, GOGAT was encoded by OsNADH-GOGAT1 and OsNADH-GOGAT2 (c), and GDH was encoded by OsGDH1, OsGDH2, OsGDH3, and OsGDH4 (d). Compared with the control, SAR1 (pH 4.5) treatment upregulated the expressions of OsNR1, OsNR2, OsGDH1, and OsGDH4 in rice seedling leaves (P < 0.05), whereas the treatment downregulated the expressions of OsNADH-GOGAT1, OsNADH-GOGAT2, OsGDH2, and OsGDH3 (P < 0.05). At the same time, SAR1 (pH 4.5) treatment did not affect the expressions of four genes encoding GS (P > 0.05). SAR2 (pH 3.0) treatment downregulated the expressions of OsNR1, OsNR2, OsNADH-GOGAT1, OsNADH-GOGAT2, OsGDH2, and OsGDH3 (P < 0.05), whereas the treatment upregulated the expressions of four genes encoding GS, including OsGDH1 and OsGDH4 (P < 0.05). The single exogenous Ca2+ treatment did not significantly affect the expressions of genes encoding NR, GS, GOGAT, and GDH (P > 0.05). In the SAR1 (pH 4.5) + Ca2+ treatment group, the expressions of OsNR1, OsNR2, OsNADH-GOGAT1, OsGDH1, and OsGDH4 were higher than that in the control and SAR1 (pH 4.5) treatment groups (P < 0.05); however, the expressions of OsNR1 and OsNR2 were not different from that in the SAR1 (pH4.5) treatment group (P > 0.05). In the SAR1 (pH 4.5) + Ca2+ treatment group, the expressions of the four genes encoding GS were similar to that in the control group (P > 0.05). In the SAR2 (pH 3.0) + Ca2+ treatment group, the expressions of OsNR1, OsNR2, OsGS1;1, OsGS1;2, OsGS1;3, OsNADH-GOGAT1, OsNADH-GOGAT2, OsGDH1, and OsGDH4 were higher than that in the control and SAR2 (pH3.0) treatment groups (P < 0.05); however, the expressions of three genes encoding GS were lower than that in the SAR2 (pH 3.0) treatment group (P < 0.05), and the expression of OsNADH-GOGAT1 did not differ from that in the control group (P > 0.05).
Effect of exogenous Ca2+ on expressions of NR genes (OsNR1 and OsNR2), GS genes (OsGS1;1, OsGS1;2, and OsGS1;3), GOGAT genes (OsNADH-GOGAT1 and OsNADH-GOGAT2), and GDH genes (OsGDH1, OsGDH2, OsGDH3, and OsGDH4) in leaves of rice seedlings under SAR. Different letters represented significant difference (P < 0.05)
Discussion
Effect of exogenous Ca 2+ on yield and grain nutritional quality of rice under SAR
The nutritional quality of rice is mainly determined by the protein content and amino acid content, especially essential amino acid content (Sarkadi 2019). Proteins can be divided into albumin, globulin, prolamin, and glutelin, of which glutelin is rich in essential amino acids and is a high-quality protein (Guo et al. 2018). In our experiments, SAR1 (pH 4.5) treatment did not affect 100-seed weight, seed setting rate, and grain number per panicle in rice, while decreased the contents of total amino acids and essential amino acids. SAR2 (pH3.0) treatment decreased the yield and nutritional quality of rice grains, including total amino acids, essential amino acids, total protein, and glutelin content. This indicates that SAR1 (pH 4.5) declined the nutritional quality of rice grains, although it did not affect rice yield. SAR2 (pH 3.0) caused greater damage to the yield and nutritional quality of rice. Exogenous Ca2+ increased the 100-seed weight of rice under SAR1 (pH 4.5) treatment, even higher than the control level, and effectively alleviated the decline in total amino acids and essential amino acids. In the SAR2 (pH 3.0) + Ca2+ treatment, exogenous Ca2+ alleviated decreased yield and the total content of rice protein and amino acids and essential amino acids. However, alleviating the effect of exogenous Ca2+ on total amino acids and essential amino acid contents were limited in the SAR2 (pH 3.0) + Ca2+ treatment. This shows that exogenous Ca2+ can effectively guarantee the yield under SAR(pH 4.5/3.0), but the regulatory effect on the nutritional quality of rice was limited by the intensity of acid rain. As the important nutritional quality of rice (Mirtaleb et al. 2021), essential amino acids are essential for a healthy human diet (Borack and Volpi 2016; Mereu 1967). The addition of exogenous Ca2+ alleviated the decrease of essential amino acids caused by SAR (pH 4.5/3.0), which may help to alleviate the lack of nutrient intake caused by AR and avoid the occurrence of “hidden hunger.”
Effect of exogenous Ca 2+ on growth and total nitrogen content of rice at different growth stages under SAR
The yield and quality of rice are the results of long-term plant growth and development; the sensitivities of different growth stages to environmental changes are different. In addition, nitrogen is an essential macronutrient for the growth and production of nitrogen-containing organic compounds in crops (Han et al. 2020). Therefore, the level of nitrogen in crops has a decisive impact on the productivity and quality of crops (Zhou et al. 2021). Our results show that SAR1 (pH 4.5) treatment had no effect on the growth of rice at all three growth stages and the total nitrogen content in rice at the booting stage and maturity stages, whereas it increased the total nitrogen content in rice at the seedling stage. However, SAR2 (pH 3.0) significantly reduced the growth and total nitrogen content of rice at the seedling and booting stages. This indicates that the growth of rice at the seedling and booting stages was more sensitive to SAR than it was at the maturity stage. It was consistent with other studies that the activity of Mg2+-ATPase in the rice chloroplast was more sensitive to the SAR treatment at the seedling and booting stages than at the maturity stage (Zhang et al. 2018). This may be related to the stronger defense mechanism of rice at the maturity stage. We found that the seed setting rate and number of seed per panicle of rice under pH 4.5 SAR treatment did not change. The reason may be that we treated rice at each stage with SAR for 5 days and then moved them under the control condition to recovery as shown in Fig. 1. Besides, our previous study found that rice can tolerant low acidity of SAR such as pH 4.5 SAR, showing strong tolerance to acid rain stress (Liang et al. 2020). In this study, we also found that pH 4.5 SAR had no significant effect on the relative growth rate of rice at each growth stage, and finally did not change the seed setting rate of rice and number of seed per panicle. Combined with no significant effect on the rice yield and a decrease in the total amino acids and essential amino acids contents of rice under SAR1 (pH 4.5) stress, we found that the maintenance of total nitrogen level in rice under SAR1 (pH 4.5) stress was beneficial for keeping the final yield of rice but cannot maintain the nutritional quality. It may be because SAR1 (pH 4.5) did not inhibit nitrogen absorption but affected the utilization process, which contributed more to forming amino acids. Similar to our result, it has been found that earthworm casts improve amino acid contents in rice, which is related to amino acid metabolism, rather than changes of total nitrogen content in grains (Huang et al. 2018). Combining with the significant decrease in yield and contents of total amino acids, essential amino acids, and total protein in of rice under SAR2 (pH 3.0), it has also been proved again that the decrease in total nitrogen level in in rice at different growth stages was one of the reasons for the decrease in the productivity and quality of rice. Moreover, a positive relationship between the total nitrogen content and the yield of rice exposed to SAR at each growth stage was observed in our experiment. In the SAR1 (pH 4.5) + Ca2+ treatment, the growth and total nitrogen content of rice at the seedling and booting stages were higher than the control. In the SAR2 (pH 3.0) + Ca2+ treatment, the growth increased to the control level, the total nitrogen content was still lower than the control at the seedling stage. In contrast, growth was lower than the control, and total nitrogen content increased to the control at booting stage. This shows that exogenous Ca2+ had the best regulatory effect on the growth at the seedlings stage under SAR. This phenomenon was also found in our previous research about soybeans (Li and Liang 2019). Combined with yield and nutritional quality in the SAR(pH 4.5/3.0) + Ca2+ treatment, we found exogenous Ca2+ alleviated the decrease of yield and the decline of amino acids by promoting the growth and total nitrogen content at the seedling and booting stages. The regulatory effect of Ca2+ may depend on the absorption and utilization of nitrogen.
Effect of exogenous Ca 2+ on activities and genes expression of key enzymes in nitrogen assimilation in rice leaves under SAR
According to a previous analysis on growth and total nitrogen content in rice, we found the regulating effect of exogenous Ca2+ on rice growth at the seeding stage was the strongest among the three growth stages. Hence, we selected rice at the seedling stage to explore the effect of exogenous Ca2+ on the nitrogen assimilation process in which the inorganic nitrogen is converted into organic nitrogen under SAR. NR is the rate-limiting enzyme for assimilation of NO3– (Andrews and Raven 2022). GS/GOGAT is in charge of assimilation of NH4+, while GS activity is more abundant in plants, and GOGAT activity is the key to affecting the GS/GOGAT cycle (Lee et al. 2020). GS/GOGAT is divided into cytoplasmic GS1 and cytoplasmic NADH-GOGAT, responsible for assimilating NH4+ absorbed from soil to glutamate, and plastid GS2 and Fd-GOGAT responsible for assimilating NH4+ produced by photorespiration (Gayatri et al. 2021; Masclaux-Daubresse et al. 2006). GDH is another way to assimilate NH4+ (Liu et al. 2016). In our experiments, SAR1 (pH 4.5) treatment increased expressions of OsNR1 and OsNR2 as well as the activity of NR. In contrast, it did not affect expressions of three genes encoding GS and the activity of GS. However, SAR2 (pH 3.0) decreased expressions of OsNR1 and OsNR2 as well as the activity of NR, increased expressions of three genes encoding GS, and the activity of GS. Both in the SAR(pH 4.5/3.0) treatment, the expressions of OsNADH-GOGAT1 and OsNADH-GOGAT2, and the activity of GOGAT decreased, and the expressions of OsGDH1 and OsGDH4, and the activity of GDH increased; moreover, the activity of GOGAT under SAR2 (pH 3.0) treatment was lower than under SAR1 (pH 4.5) treatment. This shows that SAR2 (pH 3.0) treatment caused more damage to the GS/GOGAT cycle to reduced assimilation of NH4+ and restricted the assimilation and utilization of NO3– by inhibiting the activity of NR, resulting in a greater reduction in the efficiency of nitrogen assimilation than that of SAR1 (pH 4.5). This may be one of the reasons for a more dramatic reduction in nutritional quality under SAR2 (pH 3.0). It was consistent with other studies that the activity of GOGAT is a key factor for the efficiency of the GS/GOGAT cycle (Lee et al. 2020). Moreover, we found that the increase in activity of GS led to a decrease in the nutritional quality of rice. However, it was different from other studies in that the increased activity of GS during the filling stage was found to improve nitrogen metabolism efficiency and promote the synthesis of amino acids and proteins (Zhengxun et al. 2007). It was also found that nanomaterials improve the activity of GS in plants, promoting the synthesis of nitrogen assimilation products (Hu et al. 2021a, b). This difference in results may be related to different growth stages and the responses of species to stress. Similar to our result, it has been found that high-temperature stress increased the activity of GOGAT and reduced the activity of GS, and grain protein content increased (Liang et al. 2011). As the substrate of GDH, the increased NH4+ content may stimulate the activity of GDH (Wei et al. 2021). At the same time, the increase in the activity of GDH may be the mode of action for plants to adapt to SAR(pH 4.5/3.0), which could compensate for the inhibition of GS/GOGAT caused by SAR(pH 4.5/3.0) to a certain extent and alleviate the negative effect of SAR on the efficiency of nitrogen assimilation. The addition of exogenous Ca2+ can maintain the higher expression levels of OsNR1 and OsNR2 and ensure the activity of NR under SAR (pH 4.5/3.0). Moreover, exogenous Ca2+ increased the expression of OsNADH-GOGAT1 and OsNADH-GOGAT2 to maintain the activity of GOGAT to the control level under SAR and increased the expression of OsGDH1 and OsGDH4 to increase the activity of GDH under SAR (pH 4.5/3.0). But under SAR2 (pH 3.0) treatment, the expression of OsGS1;1, OsGS1;2, and OsGS1;3 and the activity of GS were still higher than the control. These indicated that exogenous Ca2+ was beneficial to the activity of NR under SAR and could adjust the balance of the GS/GOGAT cycle and increase the activity of GDH. However, exogenous Ca2+ could not effectively reduce the activity of GS to the control level under SAR2 (pH 3.0), resulting in lower nitrogen assimilation efficiency than in control. Combined with Ca2+ alleviated the decrease of total amino acids, essential amino acids, and protein contents limited by the intensity of SAR2 (pH 3.0), we can speculate that exogenous Ca2+eliminate the disruption of GS/GOGAT balance for maintaining the nutritional quality of rice under SAR. Under other abiotic stresses, the regulatory effects of exogenous Ca2+ on the activities of NR, GOGAT, and GDH were also reported (Liang et al. 2011; Su et al. 2016). Therefore, when the acid rain issue cannot be addressed from the source, exogenous Ca2+ could be an effective way to control the yield and mitigate the reduction in quality. It will be instructive to explore the effect of exogenous Ca2+ on yield and nutritional quality of the crop in field experiments in the future.
Conclusion
Exogenous Ca2+ alleviated the decrease in the yield under SAR (pH 4.5/3.0) treatment and even improved the yield of rice under SAR1 (pH 4.5) treatment. Exogenous Ca2+ alleviated the decrease in the total amino acids and essential amino acid contents, and the degree was limited by the intensity of SAR. The alleviating effect of exogenous Ca2+ on the yield and nutritional quality of rice was achieved by alleviating the decrease of growth and total nitrogen content at the seedling and booting stages. In addition, exogenous Ca2+ could also increase the expression of OsNR1 and OsNR2, as well as OsGDH1 and OsGDH4, to ensure the enhanced NR and GDH activities when SAR was applied, to compensate for the decrease in the efficiency of the GS/GOGAT cycle. The difference in the regulation of rice nutritional quality under the influence of different intensities of SAR was due to the fact that exogenous Ca2+ could not downregulate the expression of the four genes encoding GS under SAR2 (pH 3.0) treatment, resulting in a decrease in the efficiency of the GS/GOGAT cycle. Considering the emission of NOx and SO2 cannot be controlled effectively so far, our findings can provide a theoretical basis for forming effective measures to alleviate the negative effect of acid rain on food security and safety.
References
Andrews M, Raven JA (2022) Root or shoot nitrate assimilation in terrestrial vascular plants-does it matter? Plant and Soil 476:31–62
Barbano DM, Clark JL, Dunham CE, Fleming JR (1990) Kjeldahl method for determination of total nitrogen content of milk: collaborative study. J Assoc Offic Anal Chem 73:849–859
Borack MS, Volpi E (2016) Efficacy and safety of leucine supplementation in the elderly. J Plant Nutr 146:2625–2629
Chen Y, Fan P, Mo Z, Kong L, Tian H, Duan M, Li L, Wu L, Wang Z, Tang X, Pan S (2021) Deep placement of nitrogen fertilizer affects grain yield, nitrogen recovery efficiency, and root characteristics in direct-seeded rice in south china. J Plant Growth Regul 40:379–387
Das P, Adak S, Lahiri Majumder A (2020) Genetic manipulation for improved nutritional quality in rice. Front Genet 11:776
Debnath B, Ahammed G (2020) Effect of acid rain on plant growth and development: physiological and molecular interventions. In: Naeem, M., Ansari, A., Gill, S. (eds) Contaminants in Agriculture. Springer, Cham. https://doi.org/10.1007/978-3-030-41552-5_5
Debnath B, Hussain M, Li M, Lu XC, Sun YT, Qiu DL (2018) Exogenous melatonin improves fruit quality features, health promoting antioxidant compounds and yield traits in tomato fruits under acid rain stress. Molecules 23(8):1868
Debnath B, Sikdar A, Islam S, Hasan K, Li M, Qiu D (2021) Physiological and molecular responses to acid rain stress in plants and the impact of melatonin, glutathione and silicon in the amendment of plant acid rain stress. Molecules 26(4):862
Dogan S, Turan P, Dogan M, Arslan O, Alkan M (2005) Purification and characterization of Ocimum Basilicum L. Polyphenol Oxidase J Agr Food Chem 53:10224–10230
Dugan M (2015) Commodity of the quarter rice. J Agric Food Inf 16:3–10
Feng ZW, Miao H, Zhang FZ, Huang YZ (2002) Effects of acid deposition on terrestrial ecosystems and their rehabilitation strategies in China. J Environ Sci (China) 14:227–233
Gayatri, Venkatesh K, Sinha SK, Roy P, Mandal PK (2022) Molecular characterization of GS2 and Fd-GOGAT homeologues and their biased response to nitrogen stress in bread wheat (Triticum Aestivum L.). J Plant Growth Regul 41:2555–2569
Giordano M, Castellineo P (1997) Correlation between amino acid induced changes in energy expenditure and protein metabolism in humans. Nutrition 13(4):309–312
Guo X, Duan X, Wu Y, Cheng J, Zhang J, Zhang H, Li B (2018) Genetic engineering of maize (Zea mays L.) with improved grain nutrients. J Agric Food Chem 66:1670–1677
Hageman RH, Reed AJ (1980) Nitrate reductase from higher plants. Methods Enzymol 69:270–280
Han X, Wu K, Fu X, Liu Q (2020) Improving coordination of plant growth and nitrogen metabolism for sustainable agriculture. aBIOTECH 1:255–275
He L, Li B, Lu X, Yuan L, Yang Y, Yuan Y, Du J, Guo S (2015) The effect of exogenous calcium on mitochondria, respiratory metabolism enzymes and ion transport in cucumber roots under hypoxia. Sci Rep 5:11391
He L, Yu L, Li B, Du N, Guo S (2018) The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. Bmc Plant Biology 18:180
Hou M, Yu M, Li Z, Ai Z, Chen J (2021) Molecular regulatory networks for improving nitrogen use efficiency in rice. Int J Mol Sci 22(16):9040
Hu H, Hua W, Shen A, Zhou H, Sheng L, Lou W, Zhang G (2021) Photosynthetic rate and chlorophyll fluorescence of barley exposed to simulated acid rain. Environ Sci Pollut R 28:42776–42786
Hu Y, Zhang P, Zhang X, Liu Y, Feng S, Guo D, Nadezhda T, Song Z, Dang X (2021) Multi-wall carbon nanotubes promote the growth of maize (Zea mays) by regulating carbon and nitrogen metabolism in leaves. J Agr Food Chem 69:4981–4991
Huang M, Zhao C, Zhou X, Chen G, Zou Y, Uphoff N (2018) Earthworm responses to cropping rotation with oilseed rape in no-tillage rice fields and the effects of earthworm casts on human-essential amino acid content in rice grains. Appl Soil Ecol 127:58–63
Huifeng N, Jiangfang Q, Zhenghui L, Zhaomiao L, Ganghua L, Qiangsheng W, Shaohua W, Yanfeng D (2010) Distribution of proteins and amino acids in milled and brown rice as affected by nitrogen fertilization and genotype. J Cereal Sci 52:90–95
Imran M, Hussain S, Rana MS, Saleem MH, Rasul F, Ali KH, Potcho MP, Pan S, Duan M, Tang X (2021) Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol Environ Saf 211:111911
Iqbal A, Qiang D, Alamzeb M, Wang X, Gui H, Zhang H, Pang N, Zhang X, Song M (2020) Untangling the molecular mechanisms and functions of nitrate to improve nitrogen use efficiency. J Sci Food Agric 100:904–914
Lee S, Marmagne A, Park J, Fabien C, Yim Y, Kim S-j, Kim T-H, Lim PO, Masclaux-Daubresse C, Nam HG (2020) concurrent activation of osamt1;2 and osgogat1 in rice leads to enhanced nitrogen use efficiency under nitrogen limitation. Plant J 103:7–20
Li Y, Liang C (2019) Exogenous application of ca2+ mitigates simulated acid rain stress on soybean productivity and quality by maintaining nutrient absorption. Environ Sci Pollut R 26:4975–4986
Liang C, Chen L, WangY LJ, Xu G, Li T (2011) High temperature at grain-filling stage affects nitrogen metabolism enzyme activities in grains and grain nutritional quality in rice. Rice Sci 18(3):210–216
Liang C, Ma Y, Li L (2020) Comparison of plasma membrane H+-ATPase response to acid rain stress between rice and soybean. Environ Sci Pollut R 27:6389–6400
Liang C, Zhang Y, Ren X (2021) Calcium regulates antioxidative isozyme activity for enhancing rice adaption to acid rain stress. Plant Sci 306:110876
Lin CC, Kao CH (1996) Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by nacl. Plant Growth Regul 18:233–238
Liu L, Wang J, Han Z, Sun X, Li H, Zhang J, Lu Y (2016) Molecular analyses of tomato GS, GOGAT and GDH gene families and their response to abiotic stresses. Acta Physiologiae Plantarum 38:229
Masclaux-Daubresse C, Reisdorf-Cren M, Pageau K, Lelandais M, Grandjean O, Kronenberger J, Valadier MH, Feraud M, Jouglet T, Suzuki A (2006) Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol 140:444–456
Mereu T (1967) Adequacy of low-phenylalanine diet. Arch Pediat Adol Med 113(5):522–523
Mirtaleb SH, Niknejad Y, Fallah H (2021) Foliar spray of amino acids and potassic fertilizer improves the nutritional quality of rice. J Plant Nutr 44:2029–2041
Montanaro G, Dichio B, Lang A, Mininni AN, Nuzzo V, Clearwater MJ, Xiloyannis C (2014) Internal versus external control of calcium nutrition in kiwifruit. J Plant Nutr Soil Sci 177:819–830
Navarro-Leon E, Barrameda-Medina Y, Lentini M, Esposito S, Ruiz JM, Blasco B (2016) Comparative study of zn deficiency in L. Sativa and B. Oleracea plants: NH4+ assimilation and nitrogen derived protective compounds. Plant Sci 248:8–16
Plasencia FA, Estrada Y, Flores FB, Ortiz-Atienza A, Lozano R, Egea I (2021) The Ca2+ sensor Calcineurin B-like protein 10 in plants: emerging new crucial roles for plant abiotic stress tolerance. Frontiers in Plant Sci 11:599944
Sangwongchai W, Krusong K, Thitisaksakul M (2022) Salt tolerance at vegetative stage is partially associated with changes in grain quality and starch physicochemical properties of rice exposed to salinity stress at reproductive stage. J Sci Food Agri 102:370–382
Sanjay D, Aradhana M, Preeti T, Dave R, Amit K, Sudhakar S, Debasis C, Pabodh Kumar T, Bijan A, Norton GJ, Tripathi RD, Chandra Shekhar N (2012) Arsenic affects essential and non-essential amino acids differentially in rice grains: Inadequacy of amino acids in rice based diet. Environ Int 46:16–22
Sarkadi LS (2019) Amino acids and biogenic amines as food quality factors. Pure Appl Chem 91:289–300
Su H, Li L, Ma H, Lyu D, Sun J (2016) Calcium alleviates temperature stress by regulating nitrogen and respiratory metabolism in malus baccata roots. Int J Agric Biol 18:286–292
Torabian S, Shakiba MR, Nasab ADM, Toorchi M (2018) Exogenous spermidine affected leaf characteristics and growth of common bean under water deficit conditions. Commun Soil Sci Plan 49:1289–1301
Wang H, Zhang M, Guo R, Shi D, Liu B, Lin X, Yang C (2012) Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). Bmc Plant Biol 12:194
Wang G, Bi A, Amombo E, Li H, Zhang L, Cheng C, Hu T, Fu J (2017) Exogenous calcium enhances the photosystem ii photochemistry response in salt stressed tall fescue. Front Plant Sci 8:2032
Wei Y, Xiong S, Zhang Z, Meng X, Wang L, Zhang X, Yu M, Yu H, Wang X, Ma X (2021) Localization, gene expression, and functions of glutamine synthetase isozymes in wheat grain (Triticum Aestivum L.). Front Plant Sci 12:580405
Wu H, Song Z, Wang X, Liu Z, Tang S (2016) Increasing CO2 differentially affects essential and non-essential amino acid concentration of rice grains grown in cadmium-contaminated soils. Environ Pollut 216:86–94
Wu D, Liu Y, Pang J, Yong JWH, Chen Y, Bai C, Han X, Liu X, Sun Z, Zhang S, Sheng J, Li T, Siddique KHM, Lambers H (2020) Exogenous calcium alleviates nocturnal chilling-induced feedback inhibition of photosynthesis by improving sink demand in peanut (Arachis Hypogaea). Front Plant Sci 11:607029
Xu H, Zhang J, Ouyang Y, Lin L, Quan G, Zhao B, Yu J (2015) Effects of simulated acid rain on microbial characteristics in a lateritic red soil. Environ Sci Pollut Res 22:18260–18266
Zhang CF, Peng SB, Peng XX, Chavez AQ, Bennett J (1997) Response of glutamine synthetase isoforms to nitrogen sources in rice (Oryza sativa L) roots. Plant Sci 125:163–170
Zhang F, Hu H, Wang L, Zhou Q, Huang X (2018) Effects of rare earth and acid rain pollution on plant chloroplast ATP synthase and element contents at different growth stages. Chemosphere 194:441–449
Zhang Q, Feng Y-X, Lin Y-J, Yu X-Z (2021) Indigenous proline is a two-dimensional safety-relief valve in balancing specific amino acids in rice under hexavalent chromium stress. J Agric Food Chem 69:11185–11195
Zhengxun J, Chunrong Q, Jing Y, Haiying L, Zhongze P (2007) Changes in activities of glutamine synthetase during grain filling and their relation to rice quality. Rice Sci 14(3):211–216
Zhou Q, Wu X, Xin L, Jiang H, Wang X, Cai J, Jiang D (2018) Waterlogging and simulated acid rain after anthesis deteriorate starch quality in wheat grain. Plant Growth Regul 85:257–265
Zhou T, Chen L, Wang W, Xu Y, Zhang W, Zhang H, Liu L, Wang Z, Gu J, Yang J (2021) Effects of application of rapeseed cake as organic fertilizer on rice quality at high yield level. J Sci Food Agri 102(5):1832–1841
Funding
The authors are grateful for the financial support from National Natural Science Foundation of China (no. 31971407, 31370517), the Natural Science Foundation of Jiangsu Province (BK20161131), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX20_1952).
Author information
Authors and Affiliations
Contributions
Yuanqi Zhang: formal analysis, conceptualization, writing—original draft, writing—review and editing. Chanjuan Liang: writing—review and editing, supervision, project, funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Liang, C. Improving yield and quality of rice under acid rain stress by regulating nitrogen assimilation with exogenous Ca2+. Environ Sci Pollut Res 30, 12085–12097 (2023). https://doi.org/10.1007/s11356-022-22946-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22946-5