Abstract
Main conclusion
Application of proper ABA can improve acid tolerance of rice roots by balancing endogenous hormones and promoting nutrient uptake.
Abstract
Abscisic acid (ABA) has an important signaling role in enhancing plant tolerance to environmental stress. To alleviate the inhibition on plant growth and productivity caused by acid rain, it is crucial to clarify the regulating mechanism of ABA on adaptation of plants to acid rain. Here, we studied the effects of exogenously applied ABA on nutrients uptake of rice roots under simulated acid rain (SAR) stress from physiological, biochemical and molecular aspects. Compared to the single SAR treatment (pH 4.5 or 3.5), exogenous 10 μM ABA alleviated the SAR-induced inhibition of root growth by balancing endogenous hormones (abscisic acid, indole-3-acetic acid, gibberellic acid and zeatin), promoting nutrient uptake (nitrate, P, K and Mg) in rice roots, and increasing the activity of the plasma membrane H+-ATPase by up-regulating expression levels of genes (OSA2, OSA4, OSA9 and OSA10). However, exogenous 100 μM ABA exacerbated the SAR-caused inhibition of root growth by disrupting the balance of endogenous hormones, and inhibiting nutrient uptake (nitrate, P, K, Ca and Mg) through decreasing the activity of the plasma membrane H+-ATPase. These results indicate that proper concentration of exogenous ABA could enhance tolerance of rice roots to SAR stress by promoting nutrients uptake and balancing endogenous hormones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid rain pollution is one of the serious environmental challenges in current world (Reis et al. 2012). The rapid development of urbanization and industrialization has increased the use of fossil fuels and consequently increased the emission of SO2 and NOx, resulting in the globalization of acid rain pollution (Mohan and Kumar 1998; Bo et al. 2000). Europe, North America and Asia are the three large acid rain regions in the world (Abbasi et al. 2013). Acid rain not only causes massive economic losses, but also has adverse effects on terrestrial ecosystem and agricultural production activities that rely on natural climatic conditions (Likens et al. 1996; Zhang et al. 2017b). Acid rain has severe negative effects on plant growth, shape formation, yield and quality, and even causes plants death. The damage mechanism of acid rain on plants involves destroying the ultrastructure of chloroplasts, inhibiting photosynthetic capacity, inducing membrane lipid peroxidation, and causing loss of nutrient levels in plants (Kumaravelu and Ramanujam 1998; Wen et al. 2011; Wu and Liang 2017). Acid rain also indirectly causes serious damage to plant roots by causing soil acidification and releasing base ions. As the main part of the land-ecosystem, plants can be considered as the biggest victim of acid rain pollution (Imran et al. 2014; Ramlall et al. 2015). Finding possible ways to alleviate the damage on plants caused by acid rain is worthy of consideration.
Abscisic acid (ABA) as trace signal molecule plays a vital role in regulating seed embryo development, seed dormancy, fruit ripening and stress tolerance improvement (Matilla et al. 2015; Ju et al. 2017). ABA enhance plant tolerance to abiotic stress as an endogenous hormone or by exogenous application (Li et al. 2003; Wang et al. 2007, 2011). Previous studies proved that exogenous ABA improved cold stress tolerance in Cynodon dactylon and Elymus nutans by increasing the antioxidant enzyme activity (Cheng et al. 2016; Fu et al. 2017), improved the adaption of Oryza sativa under alkaline stress by reducing membrane damage (Wei et al. 2015), and improved acid tolerance in Oryza sativa by enhancing the photosynthetic rate (Wu and Liang 2017). The regulating mechanisms of ABA on plants under the adversity of stress involve an enhanced selective absorption of ions to keep the ions in the cell in balance, suppresses stomatal opening by inhibiting K+ outflow channel, up-regulates expression of genes coding antioxidant enzymes, alters osmoregulation by increasing the content of proline, soluble sugar, soluble protein (Ruiz-Lozano et al. 2009; Sripinyowanich et al. 2013; Wei et al. 2015; Guajardo et al. 2016; Fu et al. 2017).
Nutrient elements are essential to maintain physiological metabolisms for plant growth and development. Negative effects of environmental stresses on morphology and growth of plant roots can be one of the main reasons for disturbing water metabolism, nutrient exchange and hormone synthesis, and inhibiting plant growth, biomass accumulation and final yield (Russell 1979; Ericsson 1995; Zhang et al. 2016). The plasma membrane H+-ATPase is the dominant enzyme that provides energy for nutrient transport into the cell by generating electrochemical gradients (Palmgren 2001). In our previous study (Zhang et al. 2017a), we found that the plasma membrane H+-ATPase plays a role in the adaptability of rice under acid rain stress by regulating the absorption of nitrogen and phosphorus. The regulation of nutrients uptake is important to clarify the tolerance of plants to acid rain stress. However, there is little information on the effect of exogenous ABA on nutrients uptake of plants under acid rain stress although it would be important to reveal these mechanisms. Hence, it is interesting to clarify the regulating mechanisms of exogenous ABA on plant tolerance to acid rain stress from a new perspective of plasma membrane H+-ATPase on nutrients uptake of plant roots. Based on the findings, developing new strategies to cope with acid rain stress and to guarantee world food security might be possible.
To understand the regulatory mechanisms of exogenous ABA on nutrient uptake in rice roots under simulated acid rain (SAR) stress, we focused on (1) the adaptation of root morphology and growth; (2) the response of endogenous hormones (ABA, indole-3-acetic acid, gibberellic acid and zeatin), and (3) the regulation mechanism of plasma membrane H+-ATPase on nutrient uptake. These data will help us to further understand the regulating effect of ABA on enhancing tolerance of plants under acid rain, and provide the theoretical basis to find ways for reducing the damage induced by acid rain.
Materials and methods
Plant materials and treatments
Sterilized seeds of rice (‘Huaidao 8’; Xishan Seed Company, Wuxi, China) were germinated at 25 ± 1 °C after soaking for 12 h. Then sprouted seeds were cultured in vermiculite for 25 days. Seedlings were cultured in conventional nutrition solution (pH 5.5) according to Zhu et al. (2009). Rice seedlings were grown in a chamber at 20–25 °C with 13 h/11 h (day/night) photoperiod and 70–80% humidity. The nutrient solution was renewed every 3 days. Four-leaf age of rice seedlings were used for experiment.
Rice seedlings were treated as shown in Table 1 according to Wu and Liang (2017). For SAR treatment, the rice nutrient solution was adjusted to pH 4.5 or 3.5, and the leaves were sprayed with SAR (pH 4.5 or 3.5). Pre-experiments (data shown in electronic supplementary material) on the effects of ABA (0, 0.1, 1, 10 and 100 μM) on chlorophyll content, root growth and biomass of rice seedlings for 5 days showed that 10 μM ABA promoted chlorophyll content, root activity and biomass, while 100 μM ABA had a significant negative effect. ABA at 0.1 and 1 μM did not significantly affect the three indices mentioned above in rice seedlings. To explore the composite effect of ABA and acid rain on growth and physiology of rice roots, we chose the promotion and inhibitory effect of ABA concentration at 10 and 100 μM, respectively. Rice seedlings were sprayed with ABA (10 and 100 μM) in the afternoon. After 5 days, some rice seedlings were collected for determination. The remaining seedlings grew under control conditions (without SAR and exogenous ABA) for further 5 days, and were then collected for determinations. All treatments were done in triplicate.
Determination of root biomass
Fresh rice roots were washed with distilled water until no dirt was observed, and were cleaned by filter paper for determining the fresh weight (FW). The dry weight (DW) of the roots was determined after drying at 80 °C for 12 h in an oven (Sedmak and Grossberg 1977).
Scanning and determination of root morphology
A root automatic scanner (Epson Perfection V700 Photo, Long Beach, CA) with software WinRHIZO 2009 (Regent Instruments, Quebec, Canada) was used to check root morphological characteristics (including root total length, surface area, and volume) determination.
Determination of endogenous hormone content
Root samples were prepared for endogenous hormone content determination according to Hou et al. (2008). Rice roots (3 g) were homogenized in 10 mL methanol for 12 h at 4 °C. After being centrifuged (20,000g) for 20 min at 4 °C, supernatants collected and the remaining residue was repeatedly extracted three times as described above. Supernatants were purified in a rotary evaporator (RE-100, Bibby Sterlin LTD, Stone Staffordshire, UK) at 40 °C. About 0.2 g of polyvinylpyrrolidone was added to concentrate to remove impurities. The supernatant after centrifugation was dried with a vacuum freeze dryer. Dried samples were dissolved in 10 mL methanol and analyzed by high-performance liquid chromatography according to Wu and Liang (2017).
Determination of nutrient element content
Dried roots (0.1 g) were digested in 8 mL oxidizing solution (15 M HNO3 and 9 M H2O2, v/v) for 30 min at 2600 kPa (80 psi) in a MDS-2000 microwave oven (CEM Corp., Matthews, NC, USA). Samples were diluted with 25 mL deionized water. Contents of elements (K, Ca and Mg) in samples were measured by atomic absorption spectroscopy (Hermans et al. 2010). The nitrate content was determined by the salicylic acid method according to Miranda et al. (2001). The ammonium content was measured according to Scheiner (1976). The phosphorus content was measured according to Sumner (1944).
Hydrolytic activity of plasma membrane H+-ATPase
The plasma membrane was obtained by two-phase partitioning method according to Klobus and Buczek (1995). The protein concentration was measured according to Bradford (1976). The H+-ATPase activity of the membrane was divided in the presence and absence of inhibitors (Na3VO4) according to Wakeel et al. (2010). After 30 min of hydrolysis, the amount of Pi was measured to characterize the hydrolytic activity of plasma membrane H+-ATPase.
RNA isolation and quantitative real-time PCR (qRT-PCR)
To assess the expression level of 10 genes (OSA1, OSA2, OSA3, OSA4, OSA5, OSA6, OSA7, OSA8, OSA9 and OSA10) encoding plasma membrane H+-ATPase, specific primers for each gene were analyzed by real-time PCR. The primer sequence of each gene was the same as that reported by Zhang et al. (2017a). Total RNA was isolated and yield determined using NanoDrop Spectrophotometer ND-1000 (Thermo Scientific, Wilmington, DE, USA). The amplification program was as follows: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 40 s. The PCR product was identified by agarose gel electrophoresis. Relative expression levels of genes were determined using the method according to Livak and Schmittgen (2001). In the present study, we selected four representative treatments (control, pH 3.5 SAR, 10 μM ABA, pH 3.5 SAR + 10 μM ABA) for qRT-PCR.
Statistical analysis
All data are presented as means ± SD (standard deviation). One-way analysis of variance (ANOVA) with LSD test was used to analyze the differences between different treatments. Data were statistically analyzed using SPSS16.0 at a significance level of P < 0.05 (Ke et al. 2003).
Results
Effects of exogenous ABA on morphology and growth of rice roots exposed to SAR
The morphology and growth indices (FW, DW, surface area, volume and total length) of rice roots exposed to SAR and ABA are shown in Fig. 1 and Table 2. After 5 days of exposure, SAR at pH 4.5 or 3.5 resulted in sparse and slender rice roots, and decreased FW, DW, surface area, volume and total length of roots compared to the control. Exogenous 10 μM ABA increased the density, number of adventitious roots under pH 4.5 SAR (Fig. 1a1, a5), and caused a higher FW, DW, surface area, volume and total length of rice roots compared to plants exposed to pH 4.5 SAR, even the values were not obviously different from the control (Table 2). Similarly, exogenous 10 μM ABA increased the density and number of adventitious roots under pH 3.5 SAR (Fig. 1a2, a6), and growth indices (FW, DW, surface area, volume and total length) of the roots treated with the combination of pH 3.5 SAR and exogenous 10 μM ABA were significantly higher than those exposed to pH 3.5 SAR although they were lower than those of the control (Table 2). The morphology and growth indices of rice roots observed in combined treatment of pH 4.5 SAR and 10 μM ABA were better than those in combined treatment with pH 3.5 SAR and 10 μM ABA. On the contrary, exogenous 100 μM ABA decreased the number of adventitious roots under SAR (pH 4.5 or 3.5) (Fig. 1a1, a2, a7, a8), and aggravated the decrease in growth indices (FW, DW, surface area, volume and total length) of roots exposed to SAR (pH 4.5 or 3.5) (Table 2). After 5 days of recovery, the growth indices of rice roots in the single SAR treatment (pH 4.5 or 3.5) were higher than those of the exposure period, but still lower than those of the control (Table 2). The density and number of adventitious roots and the growth indices (FW, DW, surface area, volume and total length) of rice roots treated with pH 4.5 SAR and exogenous 10 μM ABA were still at the control level (Fig. 1b, b5; Table 2), and treated with pH 3.5 SAR and exogenous 10 μM ABA were still lower than the control, but they were higher than those of the exposure period (Fig. 1a6, b, b6; Table 2). However, the density and number of adventitious roots and the growth indices (FW, DW, surface area, volume and total length) of rice roots treated with SAR (pH 4.5 or 3.5) and exogenous 100 μM ABA were even lower than those of the exposure period (Fig. 1a7, a8, b7, b8; Table 2).
The morphology of rice roots under different treatments during the exposure period (a) and during the recovery period (b). Control (a), pH 4.5 SAR (a1), pH 3.5 SAR (a2), 10 μM ABA (a3), 100 μM ABA (a4), pH 4.5 + 10 μM ABA (a5), pH 3.5 + 10 μM ABA (a6), pH 4.5 + 100 μM ABA (a7), pH 3.5 + 100 μM ABA (a8). Control (b), pH 4.5 SAR (b1), pH 3.5 SAR (b2), 10 μM ABA (b3), 100 μM ABA (b4), pH 4.5 + 10 μM ABA (b5), pH 3.5 + 10 μM ABA (b6), pH 4.5 + 100 μM ABA (b7), pH 3.5 + 100 μM ABA (b8). Photographs were taken on the fifth day of treatment and the fifth day of recovery (without SAR and exogenous ABA). Three roots were scanned repeatedly for each treatment
Effects of exogenous ABA on endogenous hormone levels in rice roots exposed to SAR
Table 3 shows the changes of endogenous hormone (ABA, IAA, GA3 and ZT) contents in rice roots exposed to SAR and exogenous ABA. After 5 days of exposure, the levels of endogenous hormones (ABA, IAA, GA3 and ZT) in the roots were decreased under SAR (pH 4.5 or 3.5) compared to the control. The amount of endogenous hormones (ABA, IAA, GA3 and ZT) of the roots treated with pH 4.5 SAR and exogenous 10 μM ABA was higher than in controls, and treated with pH 3.5 SAR and exogenous 10 μM ABA the levels were lower than in controls. However, the hormone level provided by the combined treatment was higher than that exposed to single SAR (pH 4.5 or 3.5) treatment, respectively. Exogenous 100 μM ABA increased the endogenous ABA content in rice roots under SAR (pH 4.5 or 3.5), but aggravated the decrease in contents of endogenous hormone (IAA, GA3 and ZT). After 5 days of recovery, levels of endogenous hormones (ABA, IAA, GA3 and ZT) in rice roots treated with SAR (pH 4.5 or 3.5) and exogenous 10 μM ABA were higher than those of the exposure period. However, the contents of endogenous hormones (ABA, IAA, GA3 and ZT) treated with SAR (pH 4.5 or 3.5) and exogenous 100 μM ABA were even lower than those of the exposure period.
Effects of exogenous ABA on nutrient elements contents in rice roots exposed to SAR
The changes of the amount of nutrients (nitrate, ammonium, P, K, Ca and Mg) in rice roots exposed to SAR and exogenous ABA are presented in Table 4. After 5 days of exposure, contents of nitrate, P, K and Mg in the roots decreased under SAR (pH 4.5 or 3.5), whereas contents of ammonium and Ca increased compared to the controls. Exogenous 10 μM ABA increased the contents of nutrient elements (nitrate, P, K and Mg) in rice roots under pH 4.5 SAR by 34.9, 30.8, 20.3 and 29.4%, respectively, and increased the contents of nutrient elements (nitrate, P, K and Mg) in the roots exposed to pH 3.5 SAR by 14.6, 20.9, 26.7 and 19.6%. Whereas exogenous 100 μM ABA decreased the level of nutrients (nitrate, P, K, Ca and Mg) in rice roots under SAR (pH 4.5 or 3.5), it aggravated the accumulation of ammonium in the roots caused by SAR. After 5 days of recovery, contents of nutrient elements (nitrate, P, K and Mg) in rice roots treated with SAR (pH 4.5 or 3.5) and 10 μM ABA were still higher than those treated with the single SAR, while the ammonium and Ca content were just the opposite. However, contents of nutrients (nitrate, P, K, Ca and Mg) in rice roots treated with SAR (pH 4.5 or 3.5) and exogenous 100 μM ABA were still lower than those of the single SAR treatment, but the ammonium content was still significantly higher than that of the single SAR exposure.
Effects of exogenous ABA on plasma membrane H+-ATPase activity and gene expression levels in rice roots exposed to SAR
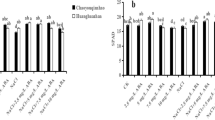
Figures 2 and 3 show changes in plasma membrane H+-ATPase activity and the expression levels of genes (OSA1–OSA10) coding plasma membrane H+-ATPase in rice roots exposed to SAR and exogenous ABA. After 5 days of exposure, compared to control, the activity of the plasma membrane H+-ATPase in the roots under SAR (pH 4.5 or 3.5) significantly increased (Fig. 2a), and the expression level of genes (OSA1, OSA3, OSA4, OSA5, OSA6, OSA7, OSA8, OSA9 and OSA10) in the roots exposed to pH 3.5 SAR increased (Fig. 3a). Exogenous 10 μM ABA significantly increased the activity of the plasma membrane H+-ATPase in rice roots compared to the control. The activity of the plasma membrane H+-ATPase treated with SAR (pH 4.5 or 3.5) and 10 μM ABA was higher than that of the control and the single SAR (pH 4.5 or 3.5) (Fig. 2a). Exogenous 10 μM ABA increased the expression levels of genes (OSA2, OSA4, OSA9 and OSA10) in rice roots under pH 3.5 SAR (Fig. 3a). However, the activity of the plasma membrane H+-ATPase treated with SAR (pH 4.5 or 3.5) and 100 μM ABA was lower than that of the control and the single SAR (pH 4.5 or 3.5) (Fig. 2a). After 5 days of recovery, the activity of the plasma membrane H+-ATPase in the roots under SAR (pH 4.5 or 3.5) was lower than that of the exposure period (Fig. 2b). The expression levels of genes (OSA1, OSA2, OSA3, OSA5, OSA6, OSA8 and OSA9) in the roots under SAR at pH 3.5 were lower than those of the exposure period as well (Fig. 3b). The activity of the plasma membrane H+-ATPase in rice roots treated with the combination of SAR (pH 4.5 or 3.5) and 10 μM ABA was still significantly higher than that of the control and those of the single SAR (pH 4.5 or 3.5) exposure (Fig. 2b). The expression levels of genes (except OSA1) in rice roots treated with pH 3.5 and 10 μM ABA were still higher than those exposed to pH 3.5 SAR (Fig. 3b). However, the activity of the plasma membrane H+-ATPase in the roots treated with the combination of SAR (pH 4.5 or 3.5) and 100 μM ABA was significantly lower than that of a single SAR (pH 4.5 or 3.5) exposure and that of the exposure period (Fig. 2b).
Discussion
Effects of exogenous ABA on morphology and growth of rice roots exposed to SAR
Plant roots as the primary organ to perceive stress signals response to environmental stress by altering their morphology (density and number of adventitious roots, total length, surface area and volume) and biomass (FW and DW) (Fageria and Moreira 2011; Forino et al. 2012). During the exposure period, the density and number of adventitious roots, total length, surface area, volume and biomass of rice treated with SAR (pH 4.5 or 3.5) and exogenous 10 μM ABA were higher than those just treated with SAR (Fig. 1a1, a2, a5, a6; Table 2), indicating that exogenous 10 μM ABA alleviated the negative effect of SAR on root growth. The increase in density and number of adventitious roots, total length, volume and surface area of rice root regulated by exogenous 10 μM ABA will contribute to the accumulation of biomass because root morphology and root biomass accumulation are positively correlated (Imada et al. 2010). The alleviation effect of exogenous ABA on the growth of rice roots was also found in alkaline stress conditions by reducing cell membrane damage and Na+/K+ ratio (Wei et al. 2015). In contrast, the growth indices of rice roots treated with SAR (pH 4.5 or 3.5) and 100 μM ABA were lower than those treated with the single SAR (Fig. 1a1, a2, a7, a8; Table 2), showing that exogenous 100 μM ABA aggravated the inhibition of the growth of the roots by SAR. This means that the alleviation effect of exogenous ABA on the inhibition of root growth caused by acid rain depended on the concentration of ABA. During the recovery period, the growth indices (density and number of adventitious roots, total length, surface area, volume and biomass) of rice roots treated with SAR (pH 4.5 or 3.5) and 10 μM ABA were better than those of the single SAR and the exposure period (Fig. 1; Table 2). However, the growth indices of rice roots treated with SAR (pH 4.5 or 3.5) and 100 μM ABA were even lower than those of the single SAR and the exposure period (Fig. 1; Table 2). This might occur because exogenous ABA still exerts the alleviation or aggravation effect after removal of the stress, and the change of root morphology treated with high concentration of ABA and SAR (pH 4.5 or 3.5) cannot be recovered after a 5-day recovery (Fig. 1). Combined with previous results (Wu and Liang 2017), indicating that the application of 10 μM ABA stimulated the self-recovery of rice, and the inhibition of rice recovery during the recovery period after application of 100 μM ABA, the damage may be beyond the own tolerance range of rice. The regulating effect of exogenous ABA on tolerance of plants under stress will also vary with different crops, varieties, growth stages and culture conditions (Achuo et al. 2006; Sripinyowanich et al. 2013; Palma et al. 2014). Sripinyowanich et al. (2013) found that 50 μM ABA maximal increased the survival rate of salt-sensitive rice under salt stress and 100 μM ABA had no effect on the survival rate, while 100 μM ABA increased the survival rate of salt-tolerant rice to the greatest extent.
Effects of exogenous ABA on endogenous hormone contents in rice roots exposed to SAR
Plant hormones (ABA, IAA, GA3 and ZT) are key players in regulating plant growth, development and morphogenesis process, and play an important role in plant stress tolerance and stress signal transduction (López et al. 2008; Zhao et al. 2012). ABA is a growth-inhibiting hormone, and IAA, GA3 and ZT are growth-promoting hormones (Yan and Chen 2017). In our experiments, exogenous 10 μM ABA increased contents of endogenous hormones (ABA, IAA, GA3 and ZT) in rice roots under SAR (Table 3). Possibly, the exogenous 10 μM ABA could activate the hormone signaling pathway to increase the growth-promoting hormones (IAA, GA3 and ZT) levels (Busov et al. 2008). The increase of endogenous ABA in rice roots could result from the accumulation of ABA in leaves which were transported from roots through the phloem according to our previous study (Wu and Liang 2017).
The increase of endogenous ABA content by the application of 10 μM ABA in rice roots (Table 3) contributed to improve rice tolerance under acid rain stress because there is a significant positive correlation between the accumulation of ABA and the enhancement of plant tolerance (Gomez-Cadenas et al. 1999; Luo et al. 2011). The increase of growth-promoting hormones (IAA, GA3 and ZT) by the application of exogenous 10 μM ABA (Table 3) could cause the number and volume of cells to increase by promoting cell division, elongation and differentiation (López et al. 2008). This might be one of reasons why exogenous 10 μM ABA alleviated SAR-induced inhibition on root growth (Fig. 1; Table 2). However, exogenous 100 μM ABA decreased the contents of endogenous hormones (IAA, GA3 and ZT) in rice roots under SAR (pH 4.5 or 3.5) (Table 3), indicating that high concentration of exogenous ABA may accelerate the degradation of endogenous hormones (IAA, GA3 and ZT) by increasing the activity of some enzymes such as peroxidase (POD) and IAA oxidase, and up-regulating the transcription level of its catabolic genes (Jansen et al. 2001; Seo et al. 2006; Oh et al. 2007; Zentella et al. 2007). The decrease in IAA, GA3 and ZT and an increase in endogenous ABA in rice roots treated with 100 μM exogenous ABA and SAR (pH 4.5 or 3.5) (Table 3) indicated that the application of 100 μM exogenous ABA disrupted the balance of endogenous hormones in the roots, exacerbated the SAR-induced negative effects on endogenous ABA hormones accumulation, and then aggravated the SAR-induced inhibition on root growth (Fig. 1; Table 2). During the recovery period, the levels of endogenous hormones (ABA, IAA, GA3 and ZT) in rice roots treated with SAR (pH 4.5 or 3.5) and 10 μM ABA were not only higher than those of the single SAR exposure (pH 4.5 or 3.5), but also higher than those measured during the exposure period (Table 3). This phenomenon suggests that exogenous 10 μM ABA promoted the restoration of root morphology and growth under SAR stress by increasing the contents of endogenous hormones (ABA, IAA, GA3 and ZT) thus promoting the division and elongation of root cells (Fig. 1; Table 2). On the contrary, contents of endogenous hormones (ABA, IAA, GA3 and ZT) of rice roots treated with SAR (pH 4.5 or 3.5) and 100 μM ABA were significantly lower than those of the single SAR application, and even lower than those of the exposure period (Table 3). Analyzing the morphology and growth in rice roots measured during the recovery period (Fig. 1; Table 2), we inferred that the decrease in growth-promoting hormones contents (IAA, GA3 and ZT) was one of the main causes for inhibiting restoration of the roots.
Effects of exogenous ABA on nutrient element contents in rice roots exposed to SAR
Nutrient elements play a vital role in improving quality, yield and enhancing plant stress tolerance (Maathuis et al. 2009; Khoshgoftarmanesh et al. 2010). Exogenous 10 μM ABA alleviated the decrease in nutrients such as nitrate, P, K and Mg in rice roots under acid rain (Table 4), beneficial for stimulating the growth of root hairs and adventitious roots (Fig. 1), which optimized the root morphology and biomass accumulation (Table 2). The alleviation of the decrease in root surface area by the application of exogenous 10 μM ABA (Table 2) favored to enhance the nutrients (nitrate, P, K and Mg) uptake (Table 3), which is conducive to maintain the nutrient supply in leaves for photosynthesis (Machado et al. 2017; Wu and Liang 2017), to maintain osmotic regulation and normal carbohydrate metabolism (Koricheva et al. 1997; Marcinska et al. 2013; Zhang et al. 2016). Exogenous 10 μM ABA also alleviated an excessive accumulation of Ca and ammonium under acid rain stress (Table 4) by promoting the excessive Ca2+ transfer from roots to leaves (Wu and Liang 2017). A reduced rhizospheric acidification resulted from excessive ammonium-induced secretion of large amounts of H+ (Britto and Kronzucker 2002; Alvarez-Pizarro et al. 2011; Coskun et al. 2013). Thus, the inhibition of growth of rice roots under SAR was alleviated by the application of exogenous 10 μM ABA (Fig. 1; Table 2). On the contrary, exogenous 100 μM ABA aggravated the decrease in nitrate, P, K, Ca and Mg in rice roots under SAR (Table 4), and root growth (density and number of adventitious roots, length, volume, surface area) and biomass accumulation were worse (Fig. 1; Table 2). Exogenous 100 μM ABA exacerbated ammonium accumulation under SAR. It might be one of the reasons for the decrease of zeatin in rice roots (Table 3), because high concentration of ammonium can inhibit the synthesis of cytokinin (Walch-Liu et al. 2000). During the recovery period, contents of nitrate, P, K and Mg in rice roots treated with SAR (pH 4.5 or 3.5) and 10 μM ABA were still higher than those treated with the single SAR; whereas, contents of ammonium and Ca were still lower than those treated with the single SAR (Table 4). These changes were beneficial to maintaining the physiological metabolic activity of rice roots in the recovery period. Thus, the application of 10 μM ABA facilitated the recovery of rice roots growth (Fig. 1; Table 2). However, levels of nutrients (nitrate, P, K, Ca and Mg) in rice roots treated with SAR (pH 4.5 or 3.5) and 100 μM ABA were still lower than those of the single SAR exposure, whereas the ammonium content was still higher than that of the single SAR (Table 4) exposure. This could be one of the important reasons why the damage caused by the combination of SAR (pH 4.5 or 3.5) and 100 μM ABA to growth of rice roots was irreversible (Fig. 1; Table 2).
Effects of exogenous ABA on plasma membrane H+-ATPase activity in rice roots exposed to SAR
The plasma membrane H+-ATPase is the dominant enzyme that provides the driving force for absorbtion of nutrients and ion transport (Arango et al. 2003; Santi et al. 2003). The plasma membrane H+-ATPase in rice is encoded by 10 isozyme genes (OSA1-10) divided into five subfamilies I (OSA1, 2, 3), II (OSA5, 7), III (OSA9), IV (OSA4, 6, 10) and V (OSA8) (Michelet and Boutry 1995). The activity of the H+-ATPase is related with transcription regulation, post-translational modulation of the plasma membrane H+-ATPase and substrate (ATP) content (Oufattole et al. 2000; Zhang et al. 2017a). After 5 days of exposure, exogenous 10 μM ABA significantly increased the activity of the plasma membrane H+-ATPase in rice roots under SAR (pH 4.5 or 3.5) (Fig. 2a), and increased the expression levels of the plasma membrane H+-ATPase genes (OSA2, OSA4, OSA9 and OSA10) under pH 3.5 SAR (Fig. 3a). These results show that the increase of the plasma membrane H+-ATPase activity by the application of 10 μM ABA under SAR may result from up-regulation of subfamilies I (OSA2), III (OSA9) and IV (OSA4, OSA10) because the activity of the H+-ATPase is related to the expression of genes coding H+-ATPase at the transcription level (Janicka-Russak and Klobus 2007; Liang et al. 2015). Combined with the increase in IAA content in the same treatment (Table 4), it can be inferred that the increase in IAA can also contribute to activate the plasma membrane H+-ATPase as an allosteric agent. Shen et al. (2006) found that IAA activates the plasma membrane H+-ATPase of soybean for signal transduction under phosphorus deficiency stress. The increase in the plasma membrane H+-ATPase activity by applying exogenous 10 μM ABA (Fig. 2a) was beneficial in supplying sufficient energy for the transport of ions (Palmgren 2001; Zhang et al. 2017a), and maintaining the contents of nitrates, P, K and Mg in rice roots (Table 4). On the contrary, exogenous 100 μM ABA significantly decreased the activity of the plasma membrane H+-ATPase in rice roots under SAR (pH 4.5 or 3.5) (Fig. 2a). It was unfavorable for pumping excessive intracellular H+ to the outside of cells (Janicka-Russak et al. 2013), and transporting ions of nutrient (nitrate, P, K, Ca and Mg) in rice roots (Table 4). The significant decrease in the activity of the plasma membrane H+-ATPase in rice roots treated with exogenous 100 μM ABA and SAR (pH 4.5 or 3.5) could result from the damage of the H+-ATPase conformation, and the function caused by exogenous 100 μM ABA might aggravate SAR-induced lipid peroxidation (Gong et al. 2003; Liang et al. 2015). During the recovery period, the activity of the plasma membrane H+-ATPase in rice roots treated with SAR (pH 4.5 or 3.5) and 10 μM ABA was still significantly higher than that of the single SAR exposure (Fig. 2b). The expression levels of genes (except OSA1) coding the plasma membrane H+-ATPase in rice roots treated with pH 3.5 SAR and 10 μM ABA were still higher than those of the single pH 3.5 SAR treatment (Fig. 3b). This means that the up-regulation of gene transcription levels still contributed to increase the activity of the plasma membrane H+-ATPase. This could be one of main reasons for alleviating the loss of nutrients (nitrate, P, K and Mg) in rice roots during the recovery period (Table 4). On the contrary, the activity of the plasma membrane H+-ATPase in rice roots treated with SAR (pH 4.5 or 3.5) and 100 μM ABA was significantly lower than that of the control, and even lower than that measured during the exposure period. It shows that the trans-membrane transport of ions was still blocked because of the decrease in the activity of the plasma membrane H+-ATPase, resulting in the inhibition of uptake of nutrients (nitrate, P, K, Ca and Mg) in the roots during the recovery period (Table 4). This might be one of the main reasons why combination of SAR (pH 4.5 or 3.5) and 100 μM ABA caused the irreversible damage of morphology and growth of rice roots (Fig. 1; Table 2).
Conclusion
In this study, we demonstrate that low concentration of exogenous ABA (10 μM) alleviated the negative effects of acid rain on rice growth and morphology by increasing the contents of endogenous hormones (ABA, IAA, GA3 and ZT), promoting uptake of nitrate, P, K and Mg and decreasing the contents of ammonium and Ca. The increase in the activity of the plasma membrane H+-ATPase by applying exogenous 10 μM ABA contributed to maintain the uptake of nutrients in rice roots under SAR. However, high concentration of exogenous ABA (100 μM) aggravated the negative effect of SAR on morphology, growth, and nutrient uptake in rice roots. These results will help us to further understand that the application of exogenous ABA at proper concentration can relieve the damage on plants by acid rain through regulating endogenous hormones and nutrients uptake, and provide a possible way to alleviate or eliminate the negative effects of acid rain on plants. The regulating effect of exogenous ABA on the tolerance of plants to acid rain needs to be determined in different crops, varieties, growth stages and culture conditions in field experiments before it will be applied for agriculture. In addition, more treatments (intermediate treatments of ABA between 10 and 100 μM) could also be informative to identify the effective concentration range of ABA for enhancing plant tolerance to SAR.
Author contribution statement
Hongyue Liu made the draft on main text, figures and tables and collected some data for this manuscript. Xi Wu did some experiments and collected some data for this manuscript. Xiaoqian Ren and Jiuzheng Zhu made some of figures and tables and modified the main text. Dr. Chanjuan Liang designed experiments, made the outline, and revised main text for this manuscript.
Abbreviations
- GA:
-
Gibberellic acid
- IAA:
-
Indole-3-acetic acid
- SAR:
-
Simulated acid rain
- ZT:
-
Zeatin
References
Abbasi T, Poornima P, Kannadasan T, Abbasi SA (2013) Acid rain: past, present, and future. Int J Environ Eng 5(3):229–272
Achuo EA, Prinsen E, Hofte M (2006) Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol 55(2):178–186
Alvarez-Pizarro JC, Gomes-Filho E, Prisco JT, Grossi-De-Sá MF, de Oliveira-Neto OB (2011) NH4 +-stimulated low-K+ uptake is associated with the induction of H+ extrusion by the plasma membrane H+-ATPase in sorghum roots under K+ deficiency. J Plant Physiol 168(14):1617–1626
Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216(3):355–365
Bo KL, Hong SH, Dong SL (2000) Chemical composition of precipitation and wet deposition of major ions on the Korean peninsula. Atmos Environ 34(4):563–575
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159(6):567–584
Busov VB, Brunner AM, Strauss SH (2008) Genes for control of plant stature and form. New Phytol 177(3):589–607
Cheng Z, Jin R, Cao M, Liu X, Chan Z (2016) Exogenous application of ABA mimic 1 (AM1) improves cold stress tolerance in bermudagrass (Cynodon dactylon). Plant Cell Tiss Org 125(2):231–240
Coskun D, Britto DT, Li M, Becker A, Kronzucker HJ (2013) Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4 + toxicity in plant roots. Plant Physiol 163(4):1859–1867
Ericsson T (1995) Growth and shoot: root ratio of seedlings in relation to nutrient availability. Plant Soil 168–169(1):205–214
Fageria NK, Moreira A (2011) The role of mineral nutrition on root growth of crop plants. Adv Agronomy 110:251–331
Forino LMC, Castiglione MR, Bartoli G, Balestri M, Andreucci A, Tagliasacchi AM (2012) Arsenic-induced morphogenic response in roots of arsenic hyperaccumulator fern Pteris vittata. J Hazard Mater 235:271–278
Fu J, Wu Y, Miao Y, Xu Y, Zhao E, Wang J, Sun H, Liu Q, Xue Y, Xu Y, Hu T (2017) Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci Rep 7:39865. https://doi.org/10.1038/srep39865
Gomez-Cadenas A, Verhey SD, Holappa LD, Shen QX, Ho THD, Walker-Simmons MK (1999) An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc Natl Acad Sci USA 96(4):1767–1772
Gong H, Chen K, Chen G, Zhu X, Wang S, Zhang C (2003) Effects of gradual drought on the fatty acid composition of polar lipids, H+-ATPase AND 5’-AMPase activities in the plasma membranes of two spring wheat leaves. Acta Phytoecol Sinica 27(4):459–465
Guajardo E, Correa JA, Contreras-Porcia L (2016) Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 243(3):767–781
Hermans C, Vuylsteke M, Coppens F, Craciun A, Inze D, Verbruggen N (2010) Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol 187(1):119–131
Hou S, Zhu J, Ding M, Lv G (2008) Simultaneous determination of gibberellic acid, indole-3-acetic acid and abscisic acid in wheat extracts by solid-phase extraction and liquid chromatography-electrospray tandem mass spectrometry. Talanta 76(4):798–802
Imada S, Yamanaka N, Tamai S (2010) Contribution of root growth responses to leaf traits and relative growth rate of Populus alba under different water-table conditions. Trees-Struct Funct 24(6):1163–1172
Imran MA, Hussain S, Hussain M, Ch MN, Meo AA (2014) Effect of simulated acid rain (SAR) on some morphochemical aspects of mash (Vigna mungo l.). Pak J Bot 46(1):245–250
Janicka-Russak M, Klobus G (2007) Modification of plasma membrane and vacuolar H+-ATPases in response to NaCl and ABA. J Plant Physiol 164(3):295–302
Janicka-Russak M, Kabala K, Wdowikowska A, Klobus G (2013) Modification of plasma membrane proton pumps in cucumber roots as an adaptation mechanism to salt stress. J Plant Physiol 170(10):915–922
Jansen MA, Re VDN, Tan MY, Prinsen E, Lagrimini LM, Thorneley RN (2001) Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiol 126(3):1012–1023
Ju S, Yin N, Wang L, Zhang C, Wang Y (2017) Effects of silicon on Oryza sativa L. seedling roots under simulated acid rain stress. PLoS One 12(3):e0173378. https://doi.org/10.1371/journal.pone.0173378
Ke L, Wong TWY, Wong AHY, Wong YS, Tam NFY (2003) Negative effects of humic acid addition on phytoremediation of pyrene-contaminated sediments by mangrove seedlings. Chemosphere 52(9):1581–1591
Khoshgoftarmanesh AH, Schulin R, Chaney RL, Daneshbakhsh B, Afyuni M (2010) Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. A review. Agron Sustain Dev 30(1):83–107
Klobus G, Buczek J (1995) The role of plasma-membrane oxidoreductase activity in proton transport. J Plant Physiol 146(1–2):103–107
Koricheva J, Roy S, Vranjic JA, Haukioja E, Hughes PR, Hanninen O (1997) Antioxidant responses to simulated acid rain and heavy metal deposition in birch seedlings. Environ Pollut 95(2):249–258
Kumaravelu G, Ramanujam MP (1998) Effect of simulated acid rain on nodulation and nitrogen metabolism in Vigna radiata cultivars. Biol Plant 41(3):445–450
Li C, Junttila O, Heino P, Palva ET (2003) Different responses of northern and southern ecotypes of Betula pendula to exogenous ABA application. Tree Physiol 23(7):481–487
Liang C, Ge Y, Su L, Bu J (2015) Response of plasma membrane H+-ATPase in rice (Oryza sativa) seedlings to simulated acid rain. Environ Sci Pollut R 22(1):535–545
Likens GE, Driscoll CT, Buso DC (1996) Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272(5259):244–246
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25(4):402–408
López MA, Bannenberg G, Castresana C (2008) Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol 11(4):420–427
Luo L, Xu F, Weng H, Hong S, Duan L, Li Z (2011) Inducing effects and its biological mechanisms of ABA on the chilling resistance of sweet pepper seedlings. Acta Bot Boreali-Occident Sin 31(1):94–100
Maathuis FJM, Salt DE, Williams L (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12(3):250–258
Machado J, Azevedo J, Freitas M, Pinto E, Almeida A, Vasconcelos V, Campos A (2017) Analysis of the use of microcystin-contaminated water in the growth and nutritional quality of the root-vegetable, Daucus carota. Environ Sci Pollut R 24(1):752–764
Marcinska I, Czyczylo-Mysza I, Skrzypek E, Grzesiak MT, Janowiak F, Filek M, Dziurka M, Dziurka K, Waligorski P, Juzon K, Cyganek K, Grzesiak S (2013) Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int J Mol Sci 14(7):13171–13193
Matilla AJ, Carrillo-Barral N, del Carmen Rodriguez-Gacio M (2015) An update on the role of NCED and CYP707A ABA metabolism genes in seed dormancy induction and the response to after-ripening and nitrate. J Plant Growth Regul 34(2):274–293
Michelet B, Boutry M (1995) The plasma-membrane H+-ATPase (a highly regulated enzyme with multiple physiological functions). Plant Physiol 108(1):1–6
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide-Biol Ch 5(1):62–71
Mohan M, Kumar S (1998) Review of acid rain potential in India: future threats and remedial measures. Curr Sci India 75(6):579–593
Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee H, Sun T, Kamiya Y, Choi G (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19(4):1192–1208
Oufattole M, Arango M, Boutry M (2000) Identification and expression of three new Nicotiana plumbaginifolia genes which encode isoforms of a plasma-membrane H+-ATPase, and one of which is induced by mechanical stress. Planta 210(5):715–722
Palma F, Lopez-Gomez M, Tejera NA, Lluch C (2014) Involvement of abscisic acid in the response of Medicago sativa plants in symbiosis with Sinorhizobium meliloti to salinity. Plant Sci 223:16–24
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol 52:817–845
Ramlall C, Varghese B, Ramdhani S, Pammenter NW, Bhatt A, Berjak P, Sershen (2015) Effects of simulated acid rain on germination, seedling growth and oxidative metabolism of recalcitrant-seeded Trichilia dregeana grown in its natural seed bank. Physiol Plant 153(1):149–160
Reis S, Grennfelt P, Klimont Z, Amann M, ApSimon H, Hettelingh JP, Holland M, LeGall AC, Maas R, Posch M, Spranger T, Sutton MA, Williams M (2012) From acid rain to climate change. Science 338(6111):1153–1154
Ruiz-Lozano JM, Del MAM, Bárzana G, Vernieri P, Aroca R (2009) Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and non mycorrhizal maize plants through regulation of PIP aquaporins. Plant Mol Biol 70(5):565–579
Russell RS (1979) Plant root systems: their function and interaction with the soil. Field Crop Res 2(4):177–179
Santi S, Locci G, Monte R, Pinton R, Varanini Z (2003) Induction of nitrate uptake in maize roots: expression of a putative high-affinity nitrate transporter and plasma membrane H+-ATPase isoforms. J Exp Bot 54(389):1851–1864
Scheiner D (1976) Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res 10(1):31–36
Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem 79(1–2):544–552
Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun T, Koshiba T, Kamiya Y, Yamaguchi S, Nambara E (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48(3):354–366
Shen H, Chen J, Wang Z, Yang C, Sasaki T, Yamamoto Y, Matsumoto H, Yan X (2006) Root plasma membrane H+-ATPase is involved in the adaptation of soybean to phosphorus starvation. J Exp Bot 57(6):1353–1362
Sripinyowanich S, Klomsakul P, Boonburapong B, Bangyeekhun T, Asami T, Gu H, Buaboocha T, Chadchawan S (2013) Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): the role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ Exp Bot 86(2):94–105
Sumner JB (1944) A method for the colorimetric determination of phosphorus. Science 100(2601):413–414
Wakeel A, Hanstein S, Pitann B, Schubert S (2010) Hydrolytic and pumping activity of H+-ATPase from leaves of sugar beet (Beta vulgaris L.) as affected by salt stress. J Plant Physiol 167(9):725–731
Walch-Liu P, Neumann G, Bangerth F, Engels C (2000) Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J Exp Bot 51(343):227–237
Wang T, Zhang X, Li C (2007) Growth, abscisic acid content, and carbon isotope composition in wheat cultivars grown under different soil moisture. Biol Plant 51(1):181–184
Wang Y, Ma F, Li M, Liang D, Zou J (2011) Physiological responses of kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regul 64(1):63–74
Wei L, Lv B, Wang M, Ma H, Yang H, Liu X, Jiang C, Liang Z (2015) Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol Bioch 90:50–57
Wen K, Liang C, Wang L, Hu G, Zhou Q (2011) Combined effects of lanthanum ion and acid rain on growth, photosynthesis and chloroplast ultrastructure in soybean seedlings. Chemosphere 84(5):601–608
Wu X, Liang C (2017) Enhancing tolerance of rice (Oryza sativa) to simulated acid rain by exogenous abscisic acid. Environ Sci Pollut R 24(5):4860–4870
Yan A, Chen Z (2017) The pivotal role of abscisic acid signaling during transition from seed maturation to germination. Plant Cell Rep 36(5):689–703
Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, Sun T-P (2007) Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19(10):3037–3057
Zhang B, Bu J, Liang C (2016) Root morphology and growth regulated by mineral nutrient absorption in rice roots exposed to simulated acid rain. Water Air Soil Poll. https://doi.org/10.1007/s11270-016-3151-1
Zhang B, Bu J, Liang C (2017a) Regulation of nitrogen and phosphorus absorption by plasma membrane H+-ATPase in rice roots under simulated acid rain. Int J Environ Sci Te 14(1):101–112
Zhang Y, Li Q, Zhang F, Xie G (2017b) Estimates of economic loss of materials caused by acid deposition in China. Sustainability 9(4):488. https://doi.org/10.3390/su9040488
Zhao M, Han Y, Feng Y, Li F, Wang W (2012) Expansins are involved in cell growth mediated by abscisic acid and indole-3-acetic acid under drought stress in wheat. Plant Cell Rep 31(4):671–685
Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant, Cell Environ 32(10):1428–1440
Acknowledgements
The authors are grateful for the financial support from the Natural Science Foundation of Jiangsu Province (No. BK20161131), the National Natural Science Foundation of China (31000245, 31370517) and Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX17_1485).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Ren, X., Zhu, J. et al. Effect of exogenous abscisic acid on morphology, growth and nutrient uptake of rice (Oryza sativa) roots under simulated acid rain stress. Planta 248, 647–659 (2018). https://doi.org/10.1007/s00425-018-2922-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-2922-x