Abstract
Protocatechuic acid (PCA), a phenolic compound found in teas, fruits, and vegetables, is widely recognized with its antioxidant and anti-inflammatory activities. Here, we verified the protective role of PCA on carrageenan (CGN)-induced paw edema in mice. Forty-five male Swiss albino mice were assigned into five groups: control group, CGN-injected group (1% w/v), PCA (25 mg/kg) + CGN group. PCA (50 mg/kg) + CGN group and diclofenac sodium (20 mg/kg) + CGN group. PCA and diclofenac sodium were administered orally for 5 consecutive days prior to the CGN injection. PCA pretreatment notably decreased the volume of the developed edema and alleviated the histopathological alterations induced by carrageenan. Additionally, PCA administration enhanced the cellular antioxidant capacity as demonstrated by the increased levels of catalase, superoxide dismutase, and reduced glutathione, in addition to the decreased malondialdehyde level in the edematous tissue. Interestingly, PCA administration was able significantly to suppress the developed inflammatory response upon carrageenan injection as indicated by the decreased levels and expression of pro-inflammatory cytokines and mediators including tumor necrosis factor alpha, interleukin-1 beta, interleukin-6, inducible nitric oxide synthase, nitric oxide, cyclooxygenase-II, prostaglandin E2, monocyte chemoattractant protein-1, myeloperoxidase and nuclear factor kappa B. These results collectively confirm the protective effect of PCA against carrageenan-induced paw edema owing to its antioxidant and anti-inflammatory characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammation is an innate immunity response of the body toward tissue injury, involving complicated series of reactions that can be triggered by a variety of agents such as toxins and bacteria (Adegbaju et al. 2020). Acute inflammation is mainly associated with fluid exudation, neutrophil infiltration, and vasodilation coupled with pain and tissue damage (Medzhitov 2008). During acute inflammation, several immune cells are recruited at the site of injury. In order to encounter tissue damage, these activated cells overproduce free radicals and inflammatory molecules (Khafaga et al. 2021; Zhang et al. 2020). Additionally, imbalance between pro-oxidants and antioxidants, lipid peroxidation, nitric oxide over release are essential events during the inflammatory state (Ahmad et al. 2012). Inflammation is mainly treated by non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids (Abbate et al. 2016). Although these anti-inflammatory medications can treat a variety of inflammatory diseases, their application exhibit negative side effects. Hepatorenal complications and gastrointestinal impairments are widely recorded with NSAIDs, while glucocorticoids cause cardiotoxicity, neuronal deficits, dermal impairment osteoporosis, and obesity (Harirforoosh et al. 2013; Oray et al. 2016). Therefore, finding anti-inflammatory drugs with minimum adverse reactions and more efficiency are required. Carrageenan-mediated paw edema in experimental animals is widely used as acute inflammatory model to examine the potential anti-inflammatory property of novel agents.
Due to their wide medicinal applications, naturally occurring phenolic compounds have received a great attention (Abdel-Daim et al. 2020; Abugomaa and Elbadawy, 2020; Alsharif et al. 2020; El Okle et al. 2018). PCA (protocatechuic acid) is a phenolic compound found in vegetables (onion, Allium cepa L.), fruits (grapes, Vitis vinifera), and other plants and spices such as rosemary (Rosmarinus officinalis L.) (Kakkar and Bais, 2014). Previous reports demonstrated that PCA has several biological and pharmacological properties (Kassab et al. 2021; Li et al. 2021). Lende et al. (2011) showed the anti-inflammatory and analgesic activity of PCA against paw edema and arthritis murine models. It has been demonstrated that PCA isolated from Sansevieria roxburghiana showed hypoglycemic effect and inhibited cardiac oxidative damage and inflammation associated with the development of animal diabetic model (Bhattacharjee et al. 2017). Moreover, PCA administration restored the activity of neuronal anti-oxidative proteins and suppressed neuronal inflammation upon cadmium intoxication (Al Olayan et al. 2020). Additionally, PCA extracted from leaves of Trianthema portulacastrum was found to attenuate dermal wounds via inhibiting oxidative and inflammatory responses (Yadav et al. 2017). Furthermore, PCA showed hepatoprotective activity following cisplatin exposure through enhancing antioxidant molecules and downregulating pro-inflammatory cytokines (Habib et al. 2021).
Hence, the current study was designed to examine the anti-edematous effect of PCA administration through evaluating the redox hemostasis and inflammatory status along with histopathological changes following carrageenan injection in mice.
Materials and methods
Chemicals
Protocatechuic acid (CAS Number: 99–50-3, purity ≥ 97.0%) and carrageenan (CAS number: 11114–20-8) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Whereas, diclofenac sodium (Declophen) was obtained from Pharco Pharmaceuticals, Alexandria, Egypt. The used reagents were of high analytical grade.
Animals and experimental design
Forty-five adult male Swiss albino mice aged between 10 and 12 weeks, weighing 20–25 g were obtained from the King Fahd for Medical Research animal house at King Abdul Aziz University in Jeddah, Saudi Arabia. Animals were adapted for 1 week before being included in the study under standard laboratory conditions (12 h of light, 12 h of darkness) (humidity: 50 ± 10%) in a temperature-controlled room (22 ± 2 °C). Mice were arbitrarily categorized into the following 5 groups (nine mice in each group):
-
Control group: mice received orally normal saline solution for 7 successive days.
-
Carrageenan (CGN)-injected group: mice were injected into the left hind paw’s sub-plantar tissues with 1% w/v carrageenan dissolved in normal saline (0.1 ml) (Zhang et al. 2020).
-
Protocatechuic acid + carrageenan (PCA-L + CGN): mice orally treated with PCA (25 mg/kg) for 7 days prior to CGN injection (Al Olayan et al. 2020; Lende et al. 2011).
-
Protocatechuic acid + carrageenan (PCA-H + CGN): mice orally treated with PCA (50 mg/kg) for 7 days prior to CGN injection (Al Olayan et al. 2020; Lende et al. 2011).
-
Diclofenac sodium + carrageenan (DIC + CGN): mice orally treated with DIC (20 mg/kg) according to (Zhang et al. 2020) before the CGN injection.
Protocatechuic acid and diclofenac sodium (an anti-inflammatory reference drug) were dissolved in normal saline. The paw tissue thickness was assessed before and after carrageenan injection (at 2, 4, 6, and 8 h). The measurements were estimated as the difference in paw volume (ml) and were compared to the same animal’s right hind paw. Mice were sacrificed after 8 h,, and paw tissue samples were collected and divided into two parts. One part was homogenized in an ice-cold medium containing 50 mM Tris–HCl (pH 7.4) and centrifuged at 500 × g for 10 min at 4 °C to produce a 50% (w/v) homogenate. The biochemical measurements were performed on the paw supernatant, while the histopathological changes and molecular studies were performed on the second sample part.
Assessment of oxidative stress markers in the paw tissue
Malondialdehyde (MDA), a lipid peroxidation marker, was quantified in the paw supernatant based on the method described by Ohkawa et al. (1979). The reduced glutathione (GSH) level was determined according to the method of Ellman (1959). The superoxide dismutase (SOD) in addition to catalase (CAT) activities was measured, according to the methods of Nishikimi et al. (1972) and Aebi (1984), respectively.
Assessment of inflammatory mediators in the paw tissue
Tumor necrosis factor alpha (TNF-α, Cat. No: CSB-E04741m), interleukin-1 beta (IL-1β, Cat. No: CSB-E04621m), interleukin-6 (IL-6, Cat. No: CSB-E04640r), interleukin-8 (IL-8, Cat. No: CSB-E13052B), cyclooxygenase-2 (COX-II, Cat. No: CSB-E12910m), prostaglandin 2 (PGE2, Cat. No: CSB-PA040059), and monocyte chemoattractant protein-1 (MCP-1, Cat. No: CSB-E07430m), nuclear factor kappa B (NF-ĸB P65, Cat. No: CSB-E12108m) concentrations were assessed in paw tissue supernatant by ELISA kits obtained from CUSABIO Life Sciences, Wuhan, China, according to the manufacturer’s instructions. Interleukin-8 (IL-8, Cat. No: MBS7606860) was purchased from MyBiosource, San Diego, California, USA. Nitric oxide (NO) level in paw supernatant was assessed in accordance with the protocol defined by Green et al. (Green et al. 1982). Myeloperoxidase (MPO) activity in the inflamed tissue was quantified using Bradley et al. (Bradley et al. 1982) method with slight modifications. Homogenates were frozen and thawed three times before being centrifuged for 10 min at 10,000 × g, 4 °C. About 200 μl of the supernatant was added to 2.8 ml of 0.05 M (pH = 6) phosphate buffer and 1 ml of 1.6 mM o-dianisidine hydrochloride containing H2O2, 0.0005% (v/v). The absorbance change was measured at 460 nm, and MPO activity was expressed as U/mg protein.
Gene expression
The total RNA from the paw tissues was extracted and then reverse-transcribed into cDNA in accordance with the protocol described by Abdel Moneim (2016). qRT-PCR was used to determine the mRNA levels of iNOS (inducible nitric oxide synthase) and Ccl1 (C–C motif chemokine ligand 1) using the QuantiTect SYBR Green PCR kit (Qiagen, Germany) and the Applied Biosystems 7500 Real-Time PCR device. The housekeeping gene GAPDH was used for data normalization. Table 1 shows the primer sequences used for GAPDH, iNOS and Ccl1. The comparative delta CT (2−ΔΔCT) method was used to assess the relative expression levels of genes.
Histopathological examination
Paw tissue samples were collected and fixed in 4% neutral formaldehyde for 24 h. Tissue was then paraffinized and sliced into 4-μm-thick sections. To determine the histological alterations in the different groups, the sections were stained with hematoxylin and eosin (H&E) and examined under a light microscope.
Statistical analysis
The results were depicted as mean ± SD (standard deviation). SPSS version 17 was used to perform the data analysis. To determine the differences between the groups, a one-way analysis of variance (ANOVA) was used, followed by a post hoc Duncan’s test. The level of significance was set at less than 0.05 (p < 0.05).
Results
Effect of PCA pre-treatment on carrageenan-induced paw edema
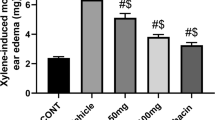
The increase in paw volume in a time-dependent manner was one of the first signs that appeared after carrageenan intraplantar injection. This increase was significant relative to the control (p < 0.05). PCA pretreatment at doses of 25 and 50 mg/kg caused a significant reduction in the left-hind paw volume compared to the carrageenan group (p < 0.05). The reference drug, diclofenac sodium (20 mg/kg), also reduced post-carrageenan edema significantly, and its effect was very similar to that recorded following PCA administration at the higher dose (50 mg/kg) (Fig. 1).
Effect of PCA pre-treatment on carrageenan-induced histological alterations
As shown in Fig. 2, carrageenan causes sub-epidermal edema (black line), neutrophil infiltration (red arrow), and disruption of tissue structure (red star). However, PCA pretreatment notably attenuated these alterations. Similarly, diclofenac sodium reduced the histological injury in paw tissues of carrageenan-injected mice.
Effect of protocatechuic acid (PCA) and diclofenac sodium (DIC) histopathological alterations in the paw tissue following carrageenan injection. A: control group, B: CGN-injected group, C: PCA (25 mg/kg) + CGN, D: PCA (50 mg/kg) + CGN, and E: DIC (20 mg/kg) + CGN. Scale bar = 80. Red arrows point to the neutrophil infiltration. Red stars point to the disruption of tissue structure. The white line indicates the width of edema
Effect of PCA pre-treatment on oxidative stress parameters of edematous tissue in carrageenan-induced paw edema
To assess the impact of PCA on the antioxidant capacity in carrageenan-injected mice, the MDA and GSH contents in addition to the SOD and CAT activities were determined in the edematous tissue. Carrageenan injection markedly reduced the levels of CAT, SOD, and GSH and increased the MDA level as compared to the control mice (Fig. 3). On the contrary, PCA and diclofenac sodium pretreatment significantly increased the levels of CAT, SOD, and GSH as well as decreased the MDA level in the edematous tissue of carrageenan injected mice. Interestingly, the higher dose of PCA (50 mg/kg) showed a better improvement in the antioxidant capacity as compared to the reference drug (diclofenac sodium 20 mg/kg).
Effect of PCA pre-treatment on inflammatory mediators in carrageenan-induced paw edema
In comparison to control mice, a significant increase in TNF-α, IL-1β, IL-6, and IL-8 levels was observed as well as Ccl1 expression level in the inflamed tissue following carrageenan injection. PCA pretreatment at doses of 25 mg/kg and 50 mg/kg reduced these elevated cytokine levels significantly. The reference drug, diclofenac sodium (20 mg/kg), decreased these cytokines levels substantially as well, and its effect was superior to that of the two PCA doses (Fig. 4).
As shown in Fig. 5, carrageenan significantly increases the COX-II activity and PGE2 level (p < 0.05) in the carrageenan-injected mice. PCA pretreatment extensively attenuated these elevations in a dose-dependent manner. Similarly these PCA doses (25 mg/kg and 50 mg/kg) decreased the expression of iNOS and NO level in comparison with the CGN group. As expected, diclofenac sodium at a dose of 20 mg/kg significantly reduced COX-II, PGE2, iNOS, and NO levels in the inflamed tissue.
Effect of protocatechuic acid (PCA, 25 and 50 mg/kg) and diclofenac sodium (DIC) on COX-II activity and PGE2 along with mRNA expression of iNOS and NO level in mice following carrageenan (CGN)-induced paw edema. Results are depicted as mean ± SD (n = 9); a P < 0.05 against control mice; b P < 0.05 against carrageenan (CGN)-treated mice
Figure 6 illustrates that the CGN injection has substantially elevated MPO activity and MCP1 level as compared to the control group; reflecting neutrophil infiltration at the inflamed skin. Interestingly, both doses of PCA in addition to diclofenac sodium significantly reduced these inflammatory indices.
Effect of protocatechuic acid (PCA, 25 and 50 mg/kg) and diclofenac sodium (DIC) on MPO activity and MCP1 level along with Ccl1 mRNA expression in mice following carrageenan (CGN)-induced paw edema. Results are depicted as mean ± SD (n = 9); a P < 0.05 against control mice; b P < 0.05 against carrageenan (CGN)-treated mice
In carrageenan-injected mice, the level of NF-κB p65, which regulates a number of inflammatory genes, increased. PCA and diclofenac sodium both reduced this level substantially, and the effect of the higher dose of PCA (50 mg/kg) was close to that of the reference drug (diclofenac sodium, 20 mg/kg) (Fig. 7).
Discussion
Inflammation is a normal body response toward any pathogens, injured tissue and toxins. Undesired adverse reactions are coupled with long-term consumption of the available anti-inflammatory medications (Lokman et al. 2022). Therefore, finding safe and more efficient alternative is currently mandatory. Carrageenan-induced acute inflammatory response (edematous state) in experimental animals is used to examine the efficiency of the novel anti-inflammatory molecules. Here, the potential anti-edematous role of PCA (25 and 50 mg/kg) was investigated following carrageenan injection in mice. Carrageenan injection enhances excessive release of different pro-inflammatory mediators and potentiated vasodilatation, resulting in the development of paw and the increase in its size which is recorded after 8 h. Additionally, microscopic examination revealed alterations in paw tissue architecture, and the accumulation of infiltrating inflammatory cells and increased inter-fiber space were observed; these findings are in agreement with the previous report (Zhang et al. 2020). Interestingly, PCA administration decreased significantly the volume of the developed paw edema after carrageenan injection and decreased the infiltrated immune cells at the site of injury; this effect may be attributed to its anti-oxidative characteristics.
Oxidative stress is a fundamental mechanism implicated in the pathogenesis of paw edema following carrageenan application. In the current study, a disturbance in the redox status was recorded as characterized by the elevated MDA and the declined GSH, SOD, and CAT activities. Previous reports demonstrated the overproduction of ROS following carrageenan administration. These active molecules affect the plasma membrane integrity and enhance its lipoperoxidation (Zhang et al. 2020), which may explain the increased MDA (lipid peroxidation marker) in the present study. GSH, SOD, and CAT are among the main quenching cellular antioxidants; their depletion indicates the development of oxidative stress and cellular impairments. It has been suggested that blocking lipoperoxidation and the enhancement of cellular antioxidant capacity may alleviate the oxidative challenge associated with the development of paw edema. Interestingly, PCA administration was able to suppress oxidative insults following carrageenan application through inhibiting lipoperoxidation and enhancing the depleted antioxidant proteins. The antioxidative activity of PCA was reported in earlier studies. Bhattacharjee et al. (2017) showed that PCA inhibited ROS production, lipoperoxidation, and protein carbonylation, and increased GSH along with antioxidant enzymes (SOD, CAT, GR, GPx) in the myocardial tissue of diabetic rats. PCA was also found to protect hepatic tissue against oxidative damage by decreasing MDA level and enhancing level of GSH and SOD in response to cisplatin exposure (Habib et al. 2021). Moreover, Al Olayan et al. (2020) stated that PCA provided neuroprotection against cadmium intoxication through enhancing cellular antioxidant gene expression and decreasing lipid peroxidation. The authors attributed these effects to its ability to upregulate nuclear-related factor 2 mRNA expression.
Carrageenan injection triggered excessive release of different inflammatory mediators in the paw skin as characterized by the increased COX-II activity and its product (PGE2) accompanied by upregulation of iNOS mRNA expression and its product (NO). The secretion of pro-inflammatory cytokines and the release of various inflammatory mediators such as COX-II and PGE2 are major events implicated during acute inflammatory responses. The elevated PGE2 reflects the overactivation of COX-II resulting in the progression of inflammation symptoms including redness, fever, swelling, and pain (Zhang et al. 2020). iNOS is upregulated in response to overproduction of pro-inflammatory cytokines, and its upregulation is coupled with NO formation which further interact with superoxide anions and produce highly reactive peroxynitrite radicals resulting in oxidative and inflammatory responses (Alsharif et al. 2020). NO is also known to inflammatory responses such as vasodilation, increased vascular permeability, exudate formation, and prostaglandin synthase activation (Zhang et al. 2020). PCA administration was found to decrease levels and mRNA expression of the increased COX-II, PGE2, iNOS, and NO in the inflamed tissue. The anti-inflammatory property of PCA has been discussed previously in different experimental models. Min et al. (2010) observed that PCA downregulated COX-II and iNOS and diminished their products (PGE2 and NO) in RAW 264.7 cells treated with lipopolysaccharide. In addition, PCA showed anti-inflammatory activity following doxorubicin through downregulation of COX-II and iNOS in the renal tissue (Molehin et al. 2019).
Excessive amounts of inflammatory mediators including TNF-α, IL-1β, IL-6, and IL-8 along with elevated levels of NF-κB were recorded following carrageenan injection. These results are in line with previous studies (Haddadi and Rashtiani, 2020). Cross-talk between oxidative stress and inflammatory response has been confirmed in inflammatory conditions. Overproduction of ROS is strongly related to the development of inflammation upon carrageenan injection through activating immune cells and secretion of pro-inflammatory mediators. Additionally, higher lipid peroxidation product has been linked with oversecretion of pro-inflammatory cytokines (Ou et al. 2019). Macrophages and lymphocytes accumulate at the inflammation site and enhance releasing pro-inflammatory cytokines. IL-1β is pro-inflammatory cytokines that exert pleiotrophic effects on a variety of cells and play key roles in acute and chronic inflammatory and autoimmune disorders (Ren and Torres, 2009). IL-6 is produced immune cells in response to infections and tissue injuries and contributes to host defense through the stimulation of acute phase responses, hematopoiesis, and immune reactions (Tanaka et al. 2014). IL-8 is a chemoattractant cytokine produced by a variety of tissues and cells. It attracts and activates neutrophils at the sites of inflammatory (Bickel 1993). The increased TNF-α and IL-1β during inflammatory response cause hyperalgesia and inflammatory pain (Kadetoff et al. 2012). It has been reported that crosslink between the inflammatory mediators is demonstrated following carrageenan application. Carrageenan enhances phosphorylation of ERK1/2, JNK, and p38 which trigger the secretion of TNF-α. Consequently, TNF-α enhances secretion of IL-1β, IL-6, COX-II, and PGE2 in addition to upregulation of iNOS expression (Ou et al. 2019). NF-κB is transcriptional inflammatory mediator that regulates the activity and differentiation of immune cells in addition to the production of production of different inflammatory markers, which may explain the elevation in the examined inflammatory mediators following carrageenan injection (Almeer et al. 2019).
Therefore, agents that are able to deactivate NF-κB may be applied as anti-inflammatory drugs. Interestingly, PCA administration following carrageenan suppressed markedly the development of inflammation and its associated events. Former studies revealed the anti-inflammatory characteristics of PCA in different experimental protocols. PCA also was found to decrease the overproduced pro-inflammatory cytokines in the cortical tissue upon cadmium exposure (Al Olayan et al. 2020). Moreover, PCA suppressed the secreted TNF-α, IL-6, and IL-1β. The authors attributed this effect to the deactivation of and NF-κB signaling following LPS-mediated acute lung injury (Wei et al. 2012). Additionally, Crespo and collaborators (Crespo et al. 2017) demonstrated that PCA inhibited inflammatory response associated with colitis partially through decreasing pro-inflammatory cytokines and suppressing NF-κB activity. PCA administration blockades the activities of COX-II, iNOS, and NF-κB in mouse epidermis following the exposure to 12-O-tetradecanoylphorbol-13-acetate (Cichocki et al. 2010). In another report, PCA downregulated the expression of COX-II and iNOS in the kidney tissue after the treatment with doxorubicin (Molehin et al. 2019). PCA was reported to attenuate production and expressions of TNF-α, IL-1β, and iNOS, COX-II, and PGE2 via the regulation of NF-kB and MAPK activation in LPS- treated RAW 264.7 (Min et al. 2010).
In the current study, carrageenan injection increased significantly MPO activity and MCP-1 level in the skin paw tissue. These results reflect the migration and infiltration of neutrophils and the degree of tissue damage at the inflamed tissue. These results are in agreement with previous reports (Abdel-Lateff et al. 2020; Almeer et al. 2019). Among other treatments (Zhang and Mejia, 2020), PCA was found to decrease the production of pro-inflammatory cytokines and MCP-1 level. This effect has been attributed to the attenuation of NF-κB and JNK/MAPK-mediated inflammatory responses in adipocytes treated by LPS. Additionally, PCA protected against methotrexate-induced hepatorenal tissues through suppressing inflammatory mediators including MPO, NO, TNF-α, and IL-1β (Owumi et al. 2019). Moreover, PCA deactivated MPO and decreased the increased NO, TNF-α, and IL-1β in the hypothalamic, testicular, and epididymal tissues following furan exposure.
Conclusion
The obtained findings clarified that PCA supplementation elicited a significant inhibitory effect against carrageenan injection-mediated paw edema. PCA markedly augment the cellular antioxidant capacity, decreased pro-inflammatory cytokine production, and improved the histopathological changes in the paw skin. The anti-inflammatory activity of PCA is potentiated mainly via deactivation of iNOS, COX-II, and NF-κB. These results collectively point to the potential usage of PCA as an alternative anti-oxidative an anti-inflammatory agent.
Data availability
Available upon request.
References
Abbate GM, Sacerdote P, Amodeo G, Mangano A, Levrini L (2016): Experimentally induced pulpal lesion and substance P expression: effect of ketoprofen—a preliminary study. Int J Dent 2016
Abdel-Daim MM, Dawood MA, Elbadawy M, Aleya L, Alkahtani S (2020) Spirulina platensis reduced oxidative damage induced by chlorpyrifos toxicity in Nile tilapia (Oreochromis niloticus). Animals 10:473
Abdel-Lateff A, Alarif WM, Algandaby MM, Alburae NA, Abdel-Naim AB (2020) Euryops arabicus displays anti-inflammatory activities in experimental models. J Ethnopharmacol 247:112278
Abdel Moneim AE (2016) Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE 11:e0158965
Abugomaa A, Elbadawy M (2020) Olive leaf extract modulates glycerol-induced kidney and liver damage in rats. Environ Sci Pollut Res 27:22100–22111
Adegbaju OD, Otunola GA, Afolayan AJ (2020) Anti-inflammatory and cytotoxic evaluation of extracts from the flowering stage of Celosia argentea. BMC Complement Med Ther 20:1–7
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad T, Shinkafi TS, Routray I, Mahmood A, Ali S (2012) Aqueous extract of dried flower buds of Syzygium aromaticum inhibits inflammation and oxidative stress. J Basic Clin Pharm 3:323
Al Olayan EM, Aloufi AS, AlAmri OD, Ola H, Moneim AEA (2020) Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: role of oxidative stress, inflammation and apoptosis. Sci Total Environ 723:137969
Almeer RS, Hammad SF, Leheta OF, Abdel Moneim AE, Amin HK (2019) Anti-inflammatory and anti-hyperuricemic functions of two synthetic hybrid drugs with dual biological active sites. Int J Mol Sci 20:5635
Alsharif KF, Almalki AA, Al-Amer O, Mufti AH, Theyab A, Lokman MS, Ramadan SS, Almeer RS, Hafez MM, Kassab RB (2020) Oleuropein protects against lipopolysaccharide-induced sepsis and alleviates inflammatory responses in mice. IUBMB Life 72:2121–2132
Bhattacharjee N, Dua TK, Khanra R, Joardar S, Nandy A, Saha A, De Feo V, Dewanjee S (2017) Protocatechuic acid, a phenolic from Sansevieria roxburghiana leaves, suppresses diabetic cardiomyopathy via stimulating glucose metabolism, ameliorating oxidative stress, and inhibiting inflammation. Front Pharmacol 8:251
Bickel M (1993) The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol 64:456–460
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Investig Dermatol 78:206–209
Cichocki M, Blumczyńska J, Baer-Dubowska W (2010) Naturally occurring phenolic acids inhibit 12-O-tetradecanoylphorbol-13-acetate induced NF-κB, iNOS and COX-2 activation in mouse epidermis. Toxicology 268:118–124
Crespo I, San-Miguel B, Mauriz JL, Ortiz de Urbina JJ, Almar M, Tuñón MJ, González-Gallego J (2017) Protective effect of protocatechuic acid on TNBS-induced colitis in mice is associated with modulation of the SphK/S1P signaling pathway. Nutrients 9:288
El Okle OS, El Euony OI, Khafaga AF, Lebda MA (2018) Thiamethoxam induced hepatotoxicity and pro-carcinogenicity in rabbits via motivation of oxidative stress, inflammation, and anti-apoptotic pathway. Environ Sci Pollut Res 25:4678–4689
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Habib SA, Suddek GM, Rahim MA, Abdelrahman RS (2021) The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice. Life Sci 277:119485
Haddadi R, Rashtiani R (2020) Anti-inflammatory and anti-hyperalgesic effects of milnacipran in inflamed rats: involvement of myeloperoxidase activity, cytokines and oxidative/nitrosative stress. Inflammopharmacology 28:903–913
Harirforoosh S, Asghar W, Jamali F (2013) Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci 16:821–847
Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E (2012) Evidence of central inflammation in fibromyalgia—increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 242:33–38
Kakkar S, Bais S (2014): A review on protocatechuic acid and its pharmacological potential. Int Sch Res Notices 2014
Kassab RB, Theyab A, Al-Ghamdy AO, Algahtani M, Mufti AH, Alsharif KF, Abdella EM, Habotta OA, Omran MM, Lokman MS (2021): Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ Sci Pollut Res, 1–14
Khafaga AF, El-Kazaz SE, Noreldin AE (2021) Boswellia serrata suppress fipronil-induced neuronal necrosis and neurobehavioral alterations via promoted inhibition of oxidative/inflammatory/apoptotic pathways. Sci Total Environ 785:147384
Lende AB, Kshirsagar AD, Deshpande AD, Muley MM, Patil RR, Bafna PA, Naik SR (2011) Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology 19:255
Li Z, Liu Y, Wang F, Gao Z, Elhefny MA, Habotta OA, Moneim AEA, Kassab RB (2021) Neuroprotective effects of protocatechuic acid on sodium arsenate induced toxicity in mice: role of oxidative stress, inflammation, and apoptosis. Chem-Biol Interact 337:109392
Lokman MS, Zaafar D, Althagafi HA, Abdel Daim MM, Theyab A, Hasan Mufti A, Algahtani M, Habotta OA, Alghamdi AA, Alsharif KF (2022): Antiulcer activity of proanthocyanidins is mediated via suppression of oxidative, inflammatory, and apoptotic machineries. J Food Biochem, e14070
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Min S-W, Ryu S-N, Kim D-H (2010) Anti-inflammatory effects of black rice, cyanidin-3-O-β-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol 10:959–966
Molehin OR, Adeyanju AA, Adefegha SA, Oyeyemi AO, Idowu KA (2019): Protective mechanisms of protocatechuic acid against doxorubicin-induced nephrotoxicity in rat model. J Basic Clin Phys Pharmacol 30
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS (2016) Long-term side effects of glucocorticoids. Expert Opin Drug Saf 15:457–465
Ou Z, Zhao J, Zhu L, Huang L, Ma Y, Ma C, Luo C, Zhu Z, Yuan Z, Wu J (2019) Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed Pharmacother 118:109347
Owumi S, Ajijola I, Agbeti O (2019) Hepatorenal protective effects of protocatechuic acid in rats administered with anticancer drug methotrexate. Hum Exp Toxicol 38:1254–1265
Ren K, Torres R (2009) Role of interleukin-1β during pain and inflammation. Brain Res Rev 60:57–64
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6:a016295
Wei M, Chu X, Jiang L, Yang X, Cai Q, Zheng C, Ci X, Guan M, Liu J, Deng X (2012) Protocatechuic acid attenuates lipolysaccharide-induced acute lung injury. Inflammation 35:1169–1178
Yadav E, Singh D, Yadav P, Verma A (2017) Attenuation of dermal wounds via downregulating oxidative stress and inflammatory markers by protocatechuic acid rich n-butanol fraction of Trianthema portulacastrum Linn. in Wistar albino rats. Biomed Pharmacother 96:86–97
Zhang H, Shang C, Tian Z, Amin HK, Kassab RB, Abdel Moneim AE, Zhang Y (2020): Diallyl disulfide suppresses inflammatory and oxidative machineries following carrageenan injection-induced paw edema in mice. Mediat Inflamm 2020
Zhang Q, de Mejia EG (2020) Protocatechuic acid attenuates adipogenesis-induced inflammation and mitochondrial dysfunction in 3T3-L1 adipocytes by regulation of AMPK pathway. J Funct Foods 69:103972
Funding
Self-funding.
Author information
Authors and Affiliations
Contributions
The author has performed all the research items.
Corresponding author
Ethics declarations
Ethical approval
All the experimental protocols, including the use of animals, were approved by the Committee of Research Ethics for Laboratory Animal Care, Umm Al-Qura University (approval no. MATRR0200).
Consent to participate
Not applicable.
Consent for publication
Consented.
Conflict of interest
The author declares no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albarakati, A.J.A. Protocatechuic acid counteracts oxidative stress and inflammation in carrageenan-induced paw edema in mice. Environ Sci Pollut Res 29, 56393–56402 (2022). https://doi.org/10.1007/s11356-022-19688-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19688-9